Supplemental Digital Content is available in the text.
Abstract
Background:
The first phase of this study showed that ART FILLER Universal filler (AFU; FILORGA Laboratories) and ART FILLER Fine lines (AFFL) were non-inferior to JUVÉDERM Ultra 3 (Allergan) and FIRST LINES PureSense (Teoxane), respectively. The clinical benefits of AFU and AFFL on nasolabial folds and crow’s feet persisted until at least Day 180. This article reports results from an open-label extension phase that assessed the tolerability and efficacy of AFU and AFFL for up to 18 months based on clinical evaluation and ultrasound high-frequency imaging.
Methods:
Eligible subjects were enrolled at D180 and assessed on D270, D360, and D540. The primary outcome measured was local tolerability. Secondary outcomes measured included: proportion of subjects in whom the severity of nasolabial folds and crow’s feet remained at least 1 point below the baseline measurement (Lemperle scale); general safety; Global Aesthetic Improvement Scale scores by subjects and investigators; wrinkle volumes; and skin thickness by high-frequency ultrasound.
Results:
Adverse events were consistent with the product information and the initial study. No serious adverse events were recorded. In exploratory analyses, wrinkle correction with AFU and AFFL is sustained for at least 18 months: 48.4% and 98.3% of subjects respectively still showed at least a 1-point decrease in the mean Lemperle score compared with the baseline. The benefits were sustained irrespective of whether subjects received additional injections. Modifications in wrinkle volume and skin thickness at D540 were statistically significant compared with the baseline.
Conclusion:
AFU and AFFL were well tolerated and, in exploratory analyses, showed a sustained efficacy for at least 18 months.
INTRODUCTION
The glycosaminoglycan hyaluronic acid (HA), an essential component of the dermis,1–3 is highly hydrophilic, which accounts for the effectiveness of HA dermal fillers in aesthetic indications, such as the correction of crow’s feet and nasolabial folds.4–6 The rheological characteristics of the HA in the filler, the volume injected, and the area treated directly influence the clinical improvement and duration of effect.7,8
The first phase of this study evaluated the efficacy and safety over 180 days (6 months) of ART FILLER Universal filler (AFU; FILORGA Laboratories, Paris, France; HA 25 mg/ml) for nasolabial folds (moderate-to-deep wrinkles), and ART FILLER Fine lines (AFFL; FILORGA Laboratories HA 20 mg/mL) for crow’s feet (fine superficial wrinkles). Both AFU and AFFL are formulated with a unique combination of 3 sizes of hyaluronic acid chains (“Tri-Hyal”) to give the desired ratio of very-long chain to long chain, cross-linking rate, and free HA concentration to achieve the desired rheological characteristics. AFFL is formulated to have a very soft texture and low volumizing power, to provide good spreading and tissue integration to smooth and plump delicate areas. AFU is formulated to have volumizing and sculpting potential, and is an easy-to-shape gel that can correct medium-to-deep wrinkles. AFU and AFFL both contain 0.3% lidocaine hydrochloride to reduce any discomfort during injection. The study showed that profilometric evaluations and high-frequency ultrasound imaging supported the results obtained with the widely used clinical scoring system (split-faced, blinded evaluation).9
The first phase of the study also showed that AFU and AFFL were non-inferior to JUVÉDERM Ultra 3 (Allergan) and FIRST LINES PureSense (Teoxane), respectively.9 The benefits of AFU and AFFL on nasolabial folds and crow’s feet were maintained until at least Day 180 (D180). Objective measurements of skin thickness and wrinkle volume as well as Lemperle score10 and Global Aesthetic Improvement Scale (GAIS) confirmed the efficacy of AFU and AFFL. None of the subjects experienced a serious adverse event.9
This article reports the results from an open-label extension study that assessed AFU and AFFL for up to 18 months (D540). The main objective was to assess local tolerability. Exploratory analyses of secondary efficacy endpoints were also performed.
METHODS
The first phase of the study enrolled female or male healthy subjects (≥19 years of age) with no upper age limit. Subjects had a Fitzpatrick phototype of I–IV with a Lemperle score10 on both sides of the face of 3 or 4 for nasolabial folds and 2 for crow’s feet. Patients had not received any corrective cosmetic procedure (surgery, botulinum toxin, or filler injections) for at least 12 months before the first phase or during the study and had never received a non-resorbable filler. Patients did not have any contraindications for HA injections. Subjects were randomly assigned to receive AFU or JUVÉDERM Ultra 3 for nasolabial folds or AFFL or FIRST LINE Pure Sense for crow’s feet.9
At the end of the first phase (D180), a non-dependent dermatologist from the evaluation center (GREDECO, Group for Research and Evaluation in DErmatology and COsmetology, Paris, France) offered subjects who completed the initial phase the opportunity to enter the 18-month extension phase. These subjects had previously selected a convenient aesthetic practitioner (the injector), who continued to administer the injections during the extension phase and performed follow-up visits.
Subjects signed a new informed consent form to cover the extension phase. The entire study complies with the Declaration of Helsinki guidelines on human biomedical research (1975) and was approved by the local Ethics Committee (Comité de Protection des Personnes, Ile-de-France VI [Pitié Salpêtrière University Hospital, Paris, France]). The study was registered with and approved by the National Agency for the Safety of the Medications and Health Products (ANSM, France, L’Agence Nationale de Sécurité du Médicament et des Produits de Santé, ID-RCB: 2014-A00306-41) and conducted in full accordance with French and European regulations.
After D180, subjects were seen at the following visits:
Day 270 (9 months) ± 7 days by the same injectors and the independent evaluation center (the GREDECO team).
Day 360 (12 months) ± 7 days by the same injectors and the independent evaluation center.
Day 540 (18 months) ± 7 days (last visit) by the independent evaluation center only.
At D180 and D270, subjects could receive another open-label treatment for nasolabial folds or crow’s feet using AFU and AFFL, respectively according to the manufacturer’s instructions (“reinjection”). Subjects who received a reinjection were asked to complete a daily diary over 14 days to score each of the following local symptoms and signs: bruising; redness; swelling; spontaneous pain; pain on pressure; itching; or any other adverse event. These events were scored from 0 (absent) to 3 (severe). Practitioners contacted subjects by telephone approximately 72 hours after the injection and evaluated the subject face-to-face 14 days after the injection. The evaluation center recorded local tolerance data 30 days after the injections.
Subjects were asked not to undergo any other injectable or cosmetic procedures during the entire study. The injector could recommend that the subject apply a simple emollient or hydrating cream to the face.
The injector (on D180, D270, and D360) and an independent blinded evaluator at GREDECO (on D270, D360, and D540) recorded the 4 IGAIS scale (investigator GAIS) and the four Lemperle scale scores10: right and left crow’s feet, and right and left nasolabial folds. The safety assessment was performed by both the injectors (on D270 and D360) and the evaluation center (on D270, D360, and D540) through careful skin examination. Subjects noted SGAIS (subject GAIS) scores in the study notebook at D270, D360, and D540.
The independent blinded evaluator (GREDECO) took standardized high-definition photographs of the nasolabial folds and crow’s feet. The volume of the wrinkles (V/mm2) was determined by profilometric evaluation (Skinstation). High-frequency ultrasound imaging (20 MHz; Monaderm) produced 3D visualizations to measure the dermal thickness (mm) and density (%)11 and investigate the presence or absence of possible inflammatory nodules.
Analysis
Efficacy was assessed by change from baseline based on the Lemperle score at D180, D270, D360, or D540. A 1-point decrease was considered to be a clinically significant result.12,13
Wrinkle volume and skin thickness were compared for nasolabial folds and crow’s feet, separately for each side, using an univariate analysis of variance. As the differences in the baseline values between the 2 sides of the face were not statistically significant,9 the analysis plan compared each side separately. Secondary efficacy criteria were compared using the univariate analysis of variance or Mann-Whitney U nonparametric tests.
RESULTS
Subject Disposition
The first phase of the study enrolled 63 subjects (6 men and 57 women). Among the 57 subjects who finished the first phase, 36 subjects agreed to participate in the extension phase, but only 35 were enrolled (Fig. 1). Most subjects were evaluated at D270 (35 subjects) and D360 (34 subjects), but 31 subjects finished the study at D540. Six subjects stopped during the extension phase and were lost to follow-up, declined to continue, or withdrew because of medical reasons unrelated to the study.
Fig. 1.
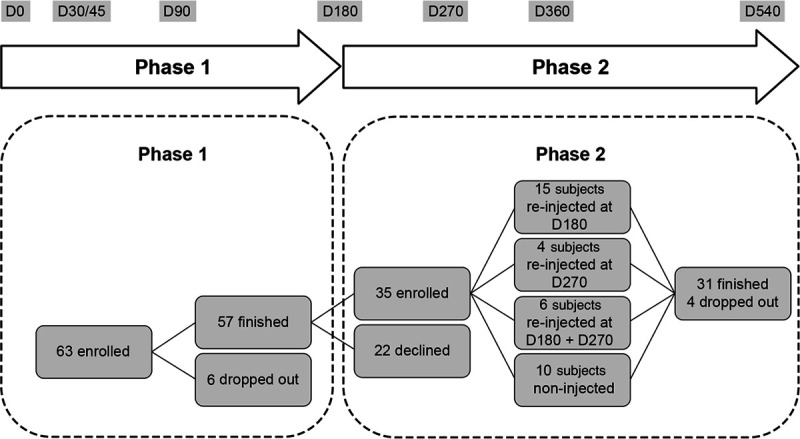
Study design.
Reinjection
Of the 35 subjects enrolled in the extension phase, 15 were reinjected with AFU or AFFL once at D180 (6 months) and 4 subjects once at D270 (9 months). Six subjects were injected twice at D180 as well as D270 (Fig. 1) while 10 subjects did not have any re-injection in extension phase. During the entire study, 25 subjects who received AFU, AFFL, or control at D0 received new injections with AFU or AFFL (15 subjects for nasolabial folds only, 4 for crow’s feet only and 6 for both). The D180 reinjections were well tolerated in the first phase.9
The subjects re-injected at D270 were seen 30 days later. The worst Lemperle score was “moderately deep wrinkles” for 1 nasolabial fold out of 68 nasolabial fold injections (34 subjects for both sides). IGAIS scores improved in all cases. The worst SGAIS score was “worsened” in 2 cases for crow’s feet. During the extension phase, the changes observed in subjects who received additional injections were consistent with those seen in the entire cohort (Fig. 2).
Fig. 2.
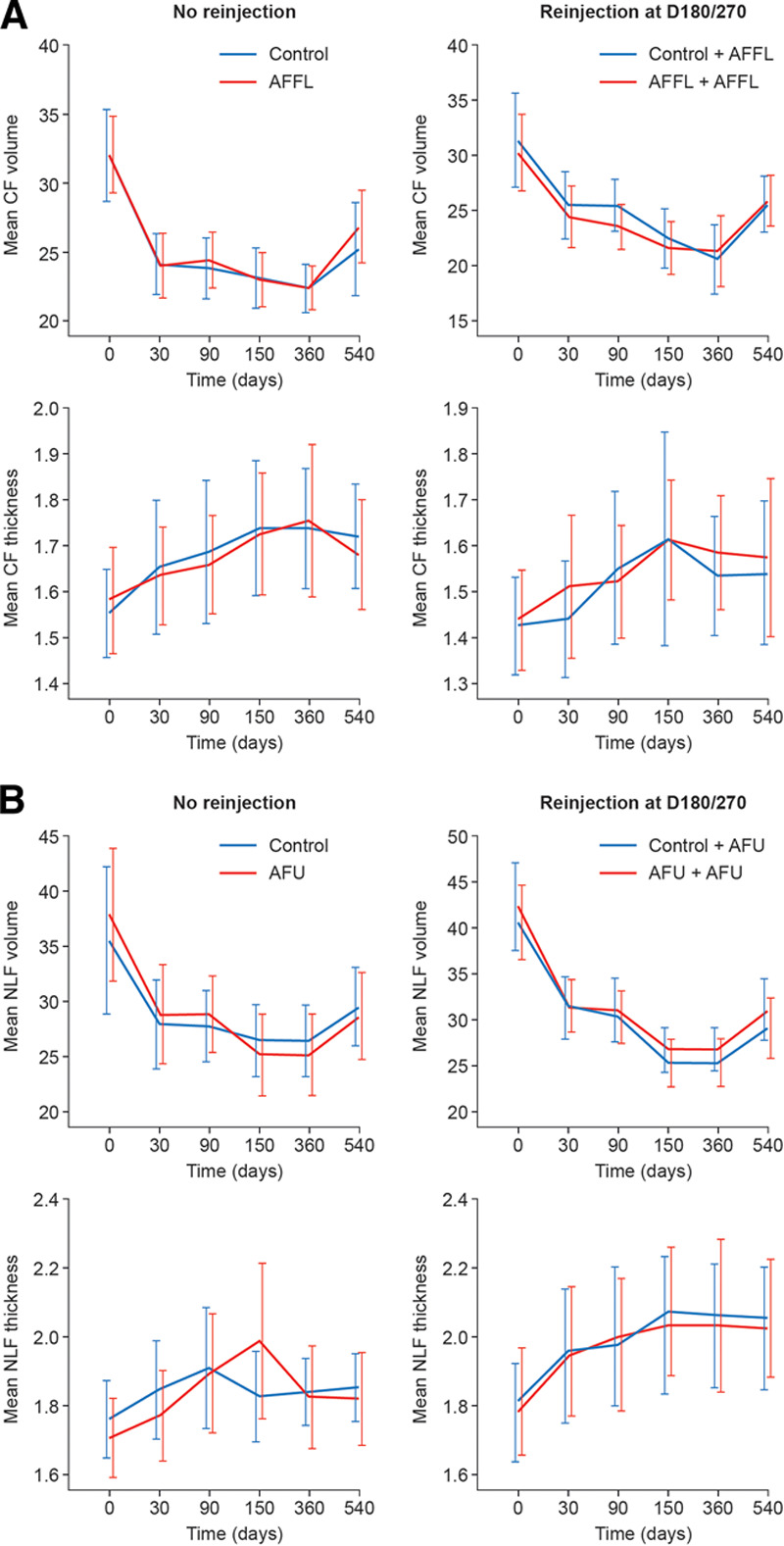
Evolution of wrinkle volume and dermal thickness for crow’s feet (A) and nasolabial folds (B) from D0 to D540. CF, crow’s feet; D, day; NLF, nasolabial folds.
Adverse Events
No unexpected or severe adverse events were detected during the extension phase. Figure 3 summarizes the adverse events noted by the injectors. (See table, Supplemental Digital Content 1, which displays adverse events. http://links.lww.com/PRSGO/B517.) The rosacea-like appearance reported by the subject at D270 (injected by control filler at D0) was not recorded or confirmed either by the injector at D360 or the independent evaluator at D360 and D540. The independent evaluator noted overcorrection of the left nasolabial fold in this subject.
Fig. 3.
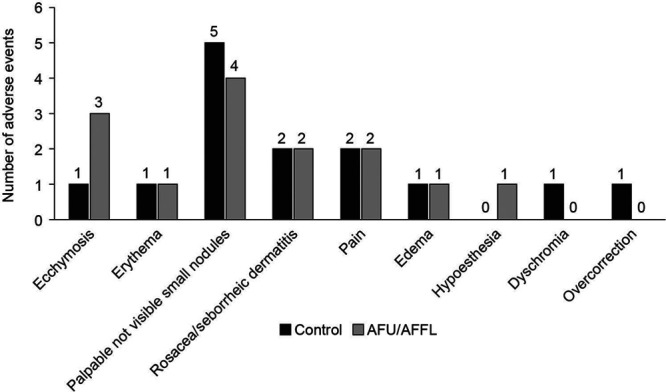
Local adverse events.
In addition, at D360 the injectors reported seborrheic dermatitis, mild pain on palpation, and mild overcorrection separately in 3 subjects. In reinjected subjects, the injector noted mild edema and overcorrection, each in 1 subject. However, high-frequency dermal ultrasound did not identify any inflammatory nodules at any time.
At D270, the independent evaluator noted palpable but invisible nodules of the crow’s feet in 3 subjects treated at D0 with control filler and 2 treated with AFFL. A nodule was also noted in the nasolabial fold of 1 patient. None of these subjects was reinjected at D180.
On D360, the independent evaluator noted 4 cases of palpable but invisible nodules of the crow’s feet (3 control and 1 AFFL), which were the same cases as reported on D270 (2 subjects with left-side palpable invisible nodules and 1 with palpable invisible nodules on both sides). In addition, 1 case of dyschromia was observed: a patient who received the control product exhibited nodules on both sides and dyschromia only on the left side.
Efficacy Analyses at Each Follow-up Evaluation
D270:
Nasolabial folds.
The independent blind evaluator and the injectors recorded that 70% of the subjects showed “no wrinkle” or “very shallow wrinkles,” while 27.9% were recorded as showing “shallow wrinkles.” The worst Lemperle score was “moderately deep wrinkles” for only 1 nasolabial fold on the one side. Neither the independent blind evaluator nor the injectors assessed any of the nasolabial folds as worsened based on IGAIS at D270 compared with D180 (Fig. 4A). (See table, Supplemental Digital Content 2, which displays Lemperle and GAIS scores for the Nasolabial Fold and Crow’s Feet. Phase 1 was a randomized controlled phase for 6 months and phase 2 was the extension phase till 18 months. http://links.lww.com/PRSGO/B518.)
Fig. 4.
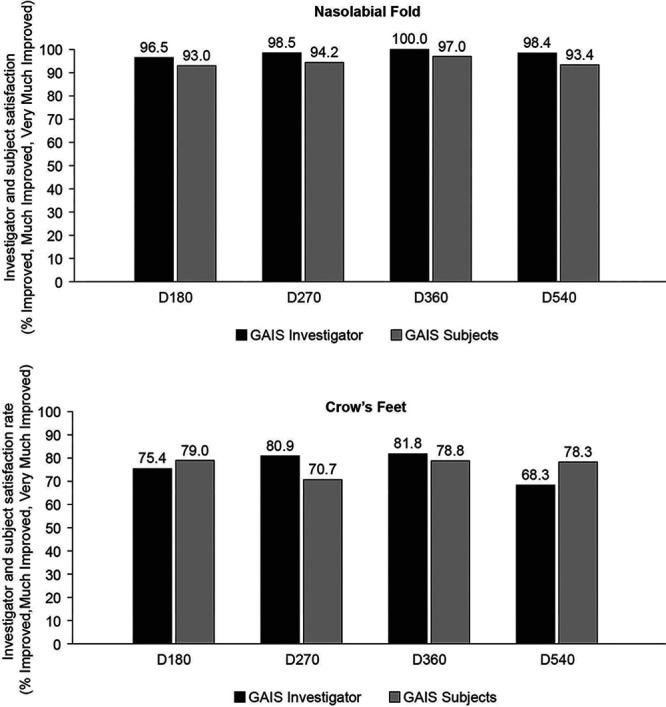
Investigator and subject satisfaction rate (%) evaluated by GAIS for the nasolabial folds (A) and crow’s feet (B) at 6, 9, 15, and 18 months post injection.
Crow’s feet.
The independent blind evaluator and the injectors recorded 58.8% of the subjects as showing “no wrinkle” or “very shallow wrinkles,” while 41.2% were recorded as showing “shallow wrinkles”. Neither the independent blind evaluator nor the injectors assessed any of the crow’s feet wrinkles as worsened based on IGAIS compared with D180. Two subjects noted a worsening of crow’s feet based on SGAIS scores recorded in their diaries at D270 compared with those at D180 (Fig. 4B). (See table, Supplemental Digital Content 2, which displays Lemperle and GAIS scores for the nasolabial fold and crow’s Feet. Phase 1 was a randomized controlled phase for 6 months, and phase 2 was the extension phase till 18 months. http://links.lww.com/PRSGO/B518.) Neither of these subjects were reinjected at D180.
D360:
Nasolabial folds.
On D360, 78.7% of the subjects were recorded as showing “no wrinkle” or “very shallow wrinkles,” while 19.7% were recorded as “shallow wrinkles.” The worst Lemperle score reported by the independent evaluator was “moderately deep wrinkles” for 1 nasolabial fold on the one side. The worst GAIS score noted by the independent evaluator, the injectors, and the subjects was “no change” at D360 compared with at D270 for nasolabial folds (Fig. 4A). (See table, Supplemental Digital Content 2, which displays Lemperle and GAIS scores for the Nasolabial Fold and Crow’s Feet. Phase 1 was a randomized controlled phase for 6 months, and phase 2 was the extension phase till 18 months. http://links.lww.com/PRSGO/B518.)
Crow’s feet.
On D360, 62.1% of the subjects were recorded as showing “no wrinkle” or “very shallow wrinkles,” while 37.9% were recorded as showing “shallow wrinkles.” The worst GAIS scores noted by the independent evaluator, the injectors and the subjects was “no change” at D360 compared with D270 for crow’s feet (Fig. 4B). (See table, Supplemental Digital Content 2, which displays Lemperle and GAIS scores for the Nasolabial Fold and Crow’s Feet. Phase 1 was a randomized controlled phase for 6 months, and phase 2 was the extension phase till 18 months. http://links.lww.com/PRSGO/B518.)
D540:
Nasolabial folds.
On D540, 62.9% of the subjects were recorded as “no wrinkle” or “very shallow wrinkles,” while 27.4% were recorded as showing “shallow wrinkles.” The worst Lemperle score recorded by the independent evaluator was “moderately deep wrinkles” for 6 nasolabial folds (9.7%). The worst IGAIS and SGAIS scores recorded by the independent evaluator and the subjects was “no change” (1.7% and 6.7%, respectively) at D540 compared with at D360 (Fig. 4A). (See table, Supplemental Digital Content 2, which displays Lemperle and GAIS scores for the Nasolabial Fold and Crow’s Feet. Phase 1 was a randomized controlled phase for 6 months and phase 2 was the extension phase till 18 months. http://links.lww.com/PRSGO/B518.)
Crow’s feet.
On D540, 48.4% of the subjects were recorded as showing “no wrinkle” or “very shallow wrinkles,” while 51.6% were recorded as showing “shallow wrinkles.” No worsening based on Lemperle score was recorded by the independent evaluator. The worst IGAIS and SGAIS scores recorded by the independent evaluator and the subjects was “no change” (31.7% and 21.7%, respectively) at D540 compared with at D360 (Fig. 4B). (See table, Supplemental Digital Content 2, which displays Lemperle and GAIS scores for the Nasolabial Fold and Crow’s Feet. Phase 1 was a randomized controlled phase for 6 months, and phase 2 was the extension phase till 18 months. http://links.lww.com/PRSGO/B518.)
Trends in Scores
Evaluation of mean Lemperle scores revealed an overall sustained decrease in all subjects, including those who were reinjected, on D180 and D270. Compared with the baseline, the mean Lemperle score for crow’s feet decreased on average by 0.61 ± 0.71. Furthermore, 48.4% (30 of 62) of crow’s feet showed a 1-point decrease. The mean Lemperle score for nasolabial folds decreased by 2.1 ± 0.73 and 98.3% (61 of 62) showed a 1-point decrease compared with the baseline. Mean Lemperle score reductions in subjects who received additional injections on D180 and D270 were consistent with the overall improvement (Fig. 2).
Compared with the baseline, changes in wrinkle volume (measured by profilometric analysis) and dermal thickness (measured by high-frequency ultrasound study 20 MHz) remained statistically significant at D540. Overall, changes are more substantial for nasolabial folds than crow’s feet (Fig. 2). For crow’s feet and nasolabial folds, a strong and sustained reduction in wrinkle volumes was observed throughout follow-up until D360, with a moderate increase at D540 versus D360 (D540–D360: 4.0 ± 3.9 for crow’s feet and D540–D360: 3.8 ± 3.9 ml for nasolabial folds), as illustrated in Figure 5 in subjects who did not receive reinjection at D180 or D270.
Fig. 5.
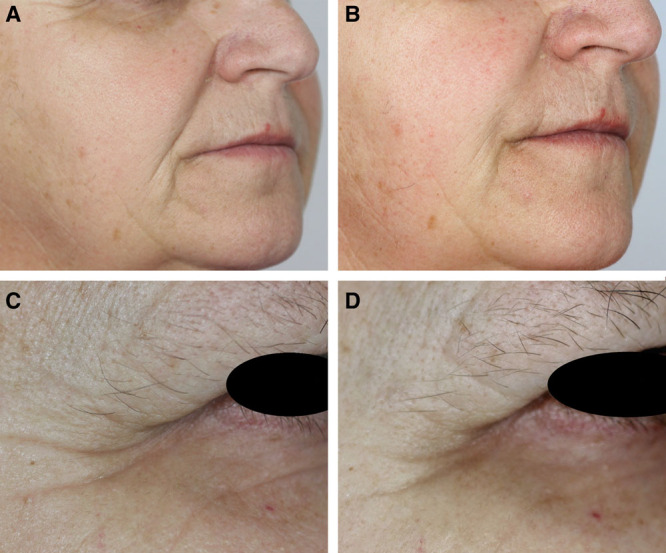
Examples of the sustained efficacy of AFU in the nasolabial folds (A, B) and AFFL in crow’s feet (C, D). Neither patient was reinjected at D180 or D270. Panel A: Day 0, nasolabial folds. Panel B: Day 540, nasolabial folds. Panel C: Day 0, crow’s feet. Panel D: Day 540, crow’s feet.
Dermal thickness steadily increased from D0 to D360, the thickness then reaching a plateau with no significant change between D360 and D540. (See table, Supplemental Digital Content 3, which displays wrinkle volume measured by SkinStation and skin thickness measured by High Frequency Ultrasound. http://links.lww.com/PRSGO/B519.) High-frequency dermal ultrasound showed significant increases in collagen and new collagen density with all fillers until at least D540. New collagen synthesis was more marked for crow’s feet than for nasolabial folds (Fig. 6).
Fig. 6.
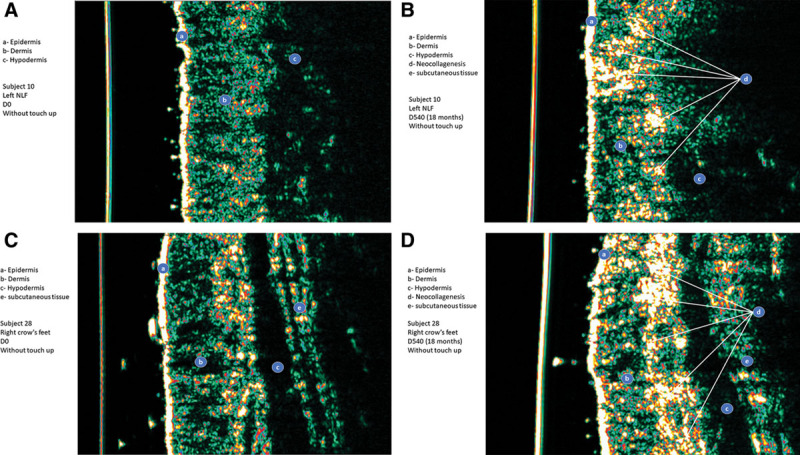
High-frequency ultrasound study showed significant increases of skin thickness and skin density after 18 months of intradermal injections of AFU in the nasolabial folds (A, B) and AFFL in crow’s feet (C, D). Panel A: Day 0, nasolabial folds, Subject No. 10. Panel B: Day 540, nasolabial folds, Subject No. 10. Panel C: Day 0, crow’s feet, Subject No. 28. Panel D: Day 540, crow’s feet, Subject No. 28.
DISCUSSION
The first phase of this study demonstrated that AFU and AFFL are non-inferior to JUVÉDERM Ultra 3 and FIRST LINES PureSense, respectively.9 The clinical benefits of AFU and AFFL on nasolabial folds and crow’s feet were maintained until at least D180. Measurements of the skin thickness and wrinkle volume, the Lemperle score,10 and GAIS confirmed the efficacy of AFU and AFFL.9
This extension phase showed that AFU and AFFL are effective and well tolerated during the 18 months after treatment. None of the subjects experienced a serious adverse event in either the initial9 or extension phase. Adverse events were consistent with the product’s “instruction for use.” In the first phase, redness, swelling, and pain on palpation were the most frequently self-reported adverse events during the first 5–6 days after injection. Injection site bruising and edema were mainly transitory and occurred immediately after treatment. Adverse events were not apparent between D30/45 and D180.9 Although the number of reinjected subjects is small, there were no additional tolerability issues associated with the reinjections on D180 and D270.
In particular, long-term treatment with AFU and AFFL did not seem to be associated with inflammatory nodules, the most common late adverse event either in the initial or in the extension phase. This AE could be associated with resorbable HA fillers.9 The overall prevalence is about 0.1% and most studies are inadequately powered to detect the incidence of inflammatory nodules. This study used centralized and blinded high-frequency dermal ultrasound evaluation of each injection site. The sensitivity and specificity of high-frequency dermal ultrasound to detect inflammatory nodules are well established,14–19 and this sensitive method revealed no inflammatory nodules during the 18-month follow-up.
Exploratory analyses show that the wrinkle correction produced by AFU and AFFL seen in the initial phase9 is sustained clinically for at least 18 months. Indeed, 48.4% and 98.3% of subjects treated with AFU and AFFL respectively still showed at least a 1-point decrease in mean Lemperle score versus baseline, which is considered to be a clinically significant outcome.12,13 The benefits were sustained irrespective of whether subjects received additional injections (Fig. 5). Compared with the baseline, changes in wrinkle volume and skin thickness at D540 were marked and were found to be statistically significant, which confirms the long-term efficacy of AFU and AFFL. Overall, changes are more substantial for nasolabial folds than for crow’s feet, which may reflect the differences in the fillers’ formulation and variations in skin mechanics between the sites.
As the lifetime of HA-based fillers is 12–14 months, the only rationalization for this long-term efficacy might be the tissue effects of “Tri-Hyal” technology. The probable slow release of the non-cross-linked HA (trapped among the very-long chain and long chain cross-linked HA) could provide more suitable conditions for fibroblasts, while the cross-linked portion of HA would persist in the tissue. The combination of long and very-long chain HA means the level of cross-linker required in the formulation is optimized and may explain the comparative safety of these fillers.
As discussed previously,9 the approach taken in this study could be a good model to compare the long-term clinical results of an intradermal filler by combining instrumental measurements performed on the same zone20 with clinical improvement assessed using the Lemperle scale.10 The latter is widely used and well validated, with good inter- and intra-observer consistency.12,13 Centralized profilometric evaluation of wrinkles and high-frequency ultrasound imaging of the skin could provide objective support for the clinical evaluations as well as assessing how the fillers are incorporated in situ and react with the tissue. Profilometric evaluations measured directly the wrinkle depth before and after the treatment, offering a more precise quantitative assessment than clinical scoring. High-frequency ultrasound imaging of the skin measured the interaction of the filler with the tissue (to assess tissue reactions around the filler, granuloma formation, etc) and the density of the dermis, which correlates directly with new collagenesis.14–19
CONCLUSIONS
AFU and AFFL were well tolerated and, in exploratory analyses, showed a sustained efficacy for at least 18 months. These long-term results may be due to the “Tri-Hyal” formulation used in this study. The method used in this study reinforced several validated and objective instrumental approaches and represents, in the authors’ view, a reliable approach to the long-term assessment of HA fillers.
ACKNOWLEDGMENTS
The study was funded by Laboratoires FILLMED (previously FILORGA MEDICAL). The authors acknowledge the assistance of Expert2Expert and the editorial support of Mark Greener and MedSense, which are also funded by Laboratoires FILLMED. The authors thank Jean-Charles Kerihuel, MD, Biostatistician and Chief Executive Officer of the Société VERTICAL, Paris, who performed the statistical analysis. A special thanks to Mr. Selatin Acar and Mr. Karim NADRA, whose contribution in this study was essential and deeply appreciated.
Supplementary Material
Footnotes
Published online 9 December 2020.
Disclosure: Patrick Trevidic, Pierre Andre, Laurent Benadiba, Jean-Jacques Deutsch, Olivier Galatoire, Philippe Garcia, Anne Grand-Vincent, and Sylvie Boisnic all received a fee for conducting this study. Catherine Salomon was an employee at Laboratoires FILORGA during the study. Ferial Fanian is a salaried employee, as the Scientific Director of Laboratoires FILL-MED.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com
REFERENCES
- 1.Nusgens BV. Acide hyaluronique et matrice extracellulaire: Une molecule primitive? Ann Dermatol Venereol. 2010;137:S3–S8. [DOI] [PubMed] [Google Scholar]
- 2.Gall Y. Acide hyaluronique: Structure, metabolisme et implication dans la cicatrisation [in French]. Ann Dermatol Venereol. 2010;137:S30, S3–9.. [DOI] [PubMed] [Google Scholar]
- 3.Price RD, Berry MG, Navsaria HA. Hyaluronic acid: The scientific and clinical evidence. J Plast Reconstr Aesthet Surg. 2007;60:1110–1119. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan Allemann I, Baumann L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin Interv Aging. 2008;3:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monheit GD, Prather CL. Juvéderm: A hyaluronic acid dermal filler. J Drugs Dermatol. 2007;6:1091–1095. [PubMed] [Google Scholar]
- 6.Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: Results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatol Surg. 2011;37:768–775. [DOI] [PubMed] [Google Scholar]
- 7.Edsman K, Nord LI, Ohrlund A, et al. Gel properties of hyaluronic acid dermal fillers. Dermatol Surg. 2012;387 Pt 21170–1179. [DOI] [PubMed] [Google Scholar]
- 8.Kablik J, Monheit GD, Yu L, et al. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. 2009;35:302–312. [DOI] [PubMed] [Google Scholar]
- 9.Trevidic P, Andre P, Benadiba L, et al. Prospective, split-face, randomized, long-term blinded objective comparison of the performance and tolerability of two new hyaluronic acid fillers. Dermatol Surg. 2017;43:1448–1457. [DOI] [PubMed] [Google Scholar]
- 10.Lemperle G, Holmes RE, Cohen SR, et al. A classification of facial wrinkles. Plast Reconstr Surg. 2001;108:1735–50.. [DOI] [PubMed] [Google Scholar]
- 11.Kleinerman R, Whang TB, Bard RL, et al. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. 2012;67:478–487. [DOI] [PubMed] [Google Scholar]
- 12.Baumann LS, Shamban AT, Lupo MP, et al. JUVEDERM vs. ZYPLAST Nasolabial Fold Study Group. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: A multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33suppl 2S128–S135. [DOI] [PubMed] [Google Scholar]
- 13.Goodman GJ, Bekhor P, Rich M, et al. A comparison of the efficacy, safety, and longevity of two different hyaluronic acid dermal fillers in the treatment of severe nasolabial folds: A multicenter, prospective, randomized, controlled, single-blind, within-subject study. Clin Cosmet Investig Dermatol. 2011;4:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovagnorio F, Drudi FM, Valentini C, et al. Ultrasonography in follow-up of soft tissue augmentation of the face with synthetic materials: A pilot study. Acta Radiol. 2004;45:746–750. [DOI] [PubMed] [Google Scholar]
- 15.Young SR, Bolton PA, Downie J. Use of high-frequency ultrasound in the assessment of injectable dermal fillers. Skin Res Technol. 2008;14:320–323. [DOI] [PubMed] [Google Scholar]
- 16.Indrizzi E, Moricca LM, Pellacchia V, et al. Biomaterial implantation in facial esthetic diseases: Ultrasonography monitor follow-up. J Craniofac Surg. 2008;19:1098–1103. [DOI] [PubMed] [Google Scholar]
- 17.Rallan D, Harland CC. Ultrasound in dermatology—basic principles and applications. Clin Exp Dermatol. 2003;28:632–638. [DOI] [PubMed] [Google Scholar]
- 18.Grippaudo FR, Mattei M. High-frequency sonography of temporary and permanent dermal fillers. Skin Res Technol. 2010;16:265–269. [DOI] [PubMed] [Google Scholar]
- 19.Grippaudo FR, Mattei M. The utility of high-frequency ultrasound in dermal filler evaluation. Ann Plast Surg. 2011;67:469–473. [DOI] [PubMed] [Google Scholar]
- 20.Turlier V, Rouquier A, Black D, et al. Assessment of the clinical efficacy of a hyaluronic acid-based deep wrinkle filler using new instrumental methods. J Cosmet Laser Ther. 2010;12:195–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


