Summary:
We report a very rare type of tumor in the left nasal ala in an elderly patient. An 81-year-old Saudi woman known to have hypertension, osteoporosis, and rheumatoid disease (who had been compliant to her medications) presented with a 0.5-cm fixed, firm, round well-defined nodule on the left ala of the nose (with crusting, erosion, and telangiectasia of the overlying skin), whose size had been gradually increasing for 2 years. The patient underwent excisional biopsy, and the specimen was sent for a histopathologic analysis. Macroscopic examination showed a round tan-white homogenous nodule, measuring 0.6 × 0.5 × 0.5 cm3. Microscopic examination revealed a fairly circumscribed unencapsulated dermal lesion, featuring basaloid cells with peripheral palisading, and focal stromal clefting. The final diagnosis of basal cell carcinoma with sebaceous differentiation was made. The patient was managed with Mohs surgery with clear margins, and full-thickness skin graft was done. Four months after surgery, the patient had a recurrence, which was managed with a surgical excision (with 4-mm margin) and covered by a full-thickness skin graft.
Basal cell carcinoma is the most common skin cancer in humans. It is responsible for around 75% of nonmelanoma skin cancers and almost 25% of all cancers in the United States.1 Studies have shown that the incidence of basal cell carcinoma is increasing from 3% to 10% annually.2 This is commonly seen in elderly patients in sun-exposed areas, and affects men more than women.1
There are 4 major types of basal cell carcinoma (nodular, superficial, morpheaform, and fibroepithelial), which may occur distinctively or in combination.2 Ulcers may be seen in any type of basal cell carcinoma, but are seen most commonly with nodular basal cell carcinoma.1
Exposure to ultraviolet radiation and having fair skin along with light eye color and light hair color are well-known risk factors.1 Certain ethnic groups are at increased risk, such as northern European descendants. Only 1.8% of basal cell carcinoma occurs in dark-skinned individuals, which is due to the dispersion of melanin and melanosomes, which protect against harmful ultraviolet radiation.
A review of literature on basal cell carcinoma with sebaceous differentiation was done using Pubmed, Google Scholar, Web of Science, and Cochrane. The keywords used were basal cell carcinoma, sebaceoma, sebaceous differentiation, and folliculosebaceous neoplasm. Only 4 case reports with the same histopathologic type of basal cell carcinoma were reported on Pubmed and Google Scholar, and were included in this literature review.
CASE REPORT
Herein we present a case of an 81-year-old Saudi woman known to have hypertension, osteoporosis, and rheumatoid disease, who had been compliant to her medications. She had a medical history of stroke, knee effusions, and disc prolapse. She was referred to the plastic surgery clinic, complaining of a painless solitary nodule (Fig. 1) on the left side of the nose for 2 years, whose size was increasing gradually over 2 years. On physical examination, a 0.5-cm fixed, firm, round well-defined nodule was found on the left ala of the nose with crusting, erosion, and telangiectasia of the overlying skin. No cervical nor supraclavicular lymphadenopathy was noted on physical examination. The patient underwent an excisional biopsy, and the specimen was sent for a histopathologic analysis. Macroscopic examination showed a round tan-white homogenous nodule (size: 0.6 × 0.5 × 0.5 cm3)
Fig. 1.
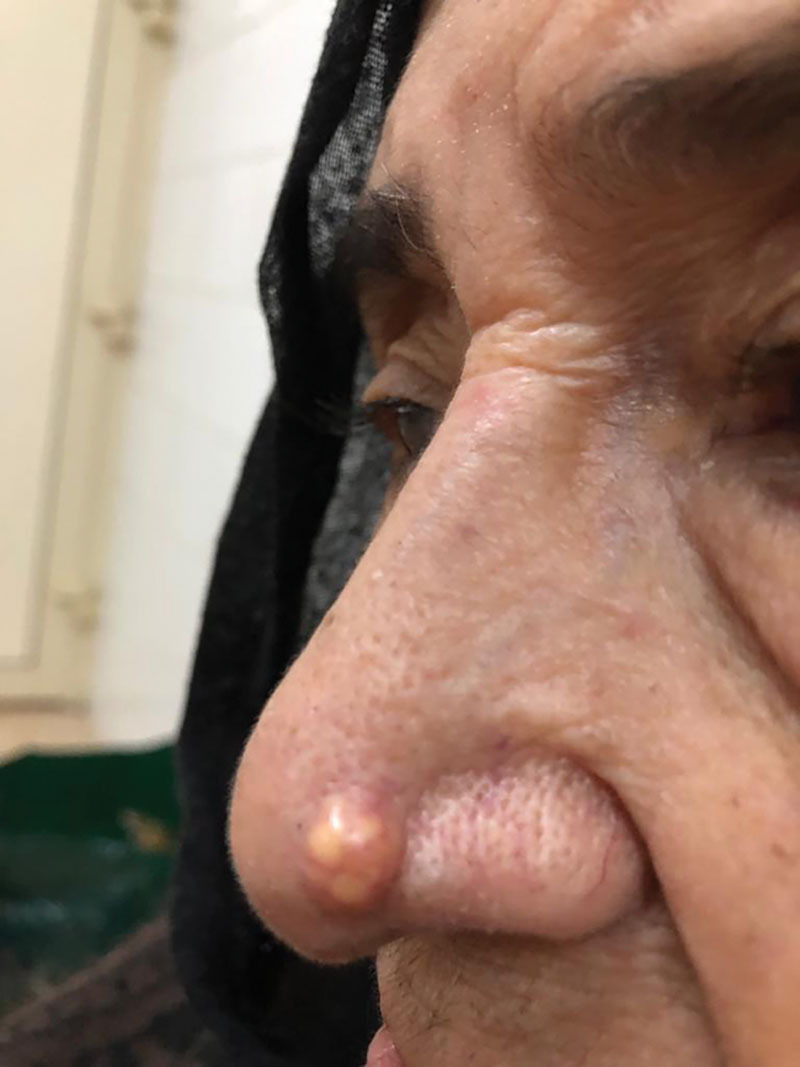
Preoperative picture of the patient with a left nasal nodule.
Microscopic examination revealed a fairly circumscribed unencapsulated dermal lesion, featuring basaloid cells with peripheral palisading, and focal stromal clefting. Scattered aggregates of sebaceocytes with large multivacuolated cytoplasm were seen in between (Fig. 2). No significant cytological atypia or mitosis could be appreciated. Surgical resection margins of the nodule were all negative. Immunohistochemical staining was positive for BCL-2 and β-catenin. EMA and CEA were both negative. D2-40 (podoplanin) was focally positive in the basaloid cells (Fig. 3).
Fig. 2.
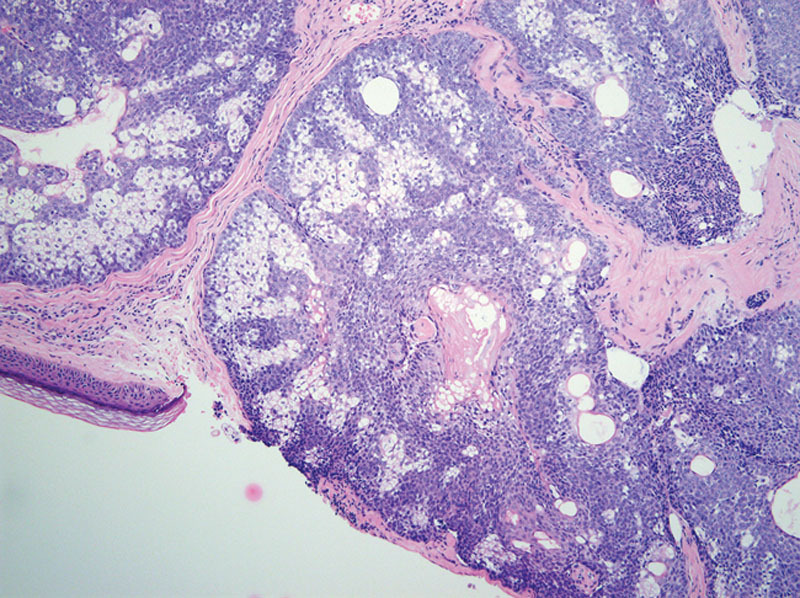
Histologic images showing dermal nodules of basaloid cells admixed with sebaceocytes. H&E ×200.
Fig. 3.
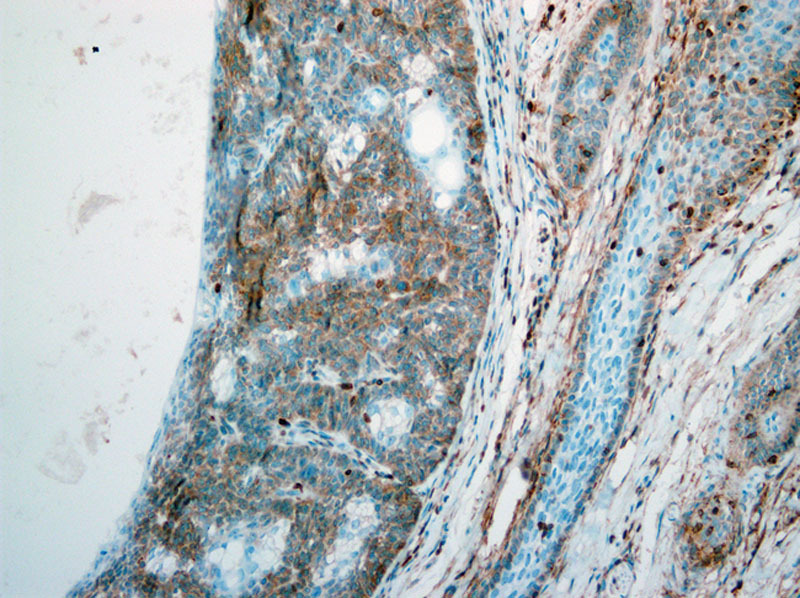
D2-40 immunohistochemical staining featuring focal and weak positivity of the basaloid cells. ×200.
The patient was managed with Mohs surgery and the area covered with a full-thickness skin graft. After 4 months from the Mohs surgery, the patient presented to the clinic with signs of recurrence in the same location (Fig. 4). During physical examination, a 0.4-cm ulcerated irregular nodule with pearly white borders was found on the left ala of the nose. An excisional biopsy was done, and the diagnosis revealed recurrence of the basal cell carcinoma (BCC) with sebaceous differentiation. The patient was then managed with surgical excision of the lesion with a 4-mm margin. The patient has been on follow-up for 18 months and will continue through the follow-up for 5 years to make sure there is no recurrence of the tumor.
Fig. 4.
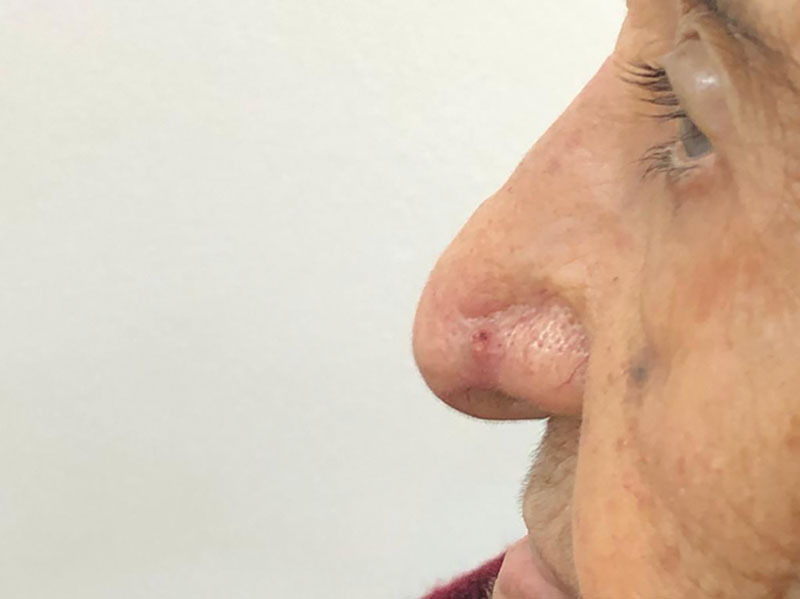
Postoperative picture of the patient, showing signs of recurrence.
DISCUSSION
Three studies have been reported in the literature on basal cell carcinoma with sebaceous, apocrine, or follicular differentiation. Basal cell carcinoma is a malignant neoplasm derived from abnormal folliculosebaceous-apocrine germinative cells.3 It usually arises in middle-aged adults in their 4th or 5th decades of life and is more commonly seen in men.1 Basal cell carcinoma usually occurs above the Onghren’s line, which is a line that joins the medial canthus of the eye to mandibular angle.4
The histopathologic differential diagnosis of a dermal tumor with basaloid and vacuolated cells include sebaceoma, sebaceous carcinoma, and BCC with sebaceous differentiation. Sebaceous carcinoma tends to be aggressive and has a high chance of distant metastases than both sebaceoma and BCC with sebaceous differentiation.5 Sebaceoma microscopically is formed of well-circumscribed dermal tumor formed of sebaceous lobules of sebaceocytes with peripheral basaloid cells, that comprises > 50% of the lesion, with no evidence of cytological atypia, significant mitosis or has an infiltrative growth pattern.6
Nonetheless, BCC may show different or several adnexal differentiation, and when sebaceous differentiation is found, it can be challenging.6 It is usually a poorly circumscribed neoplasm that reaches into the dermis layer, composed of columnar basaloid cells with elongated peripherally located nuclei. Occasional retraction clefts and sebaceous duct-like structures are present.7 Sebaceocytes appearing as vacuolated cells will be present within the neoplasm, displaying foamy, bubbly cytoplasm with starry or scalloped nuclei.8 Sebaceous carcinoma, on the contrary, is essentially made up of sebaceocytes with varying proportions, showing mainly an irregular growth pattern with infiltration, prominent atypia, and marked mitotic figures, some of which may be atypical ones.6
Many immunohistochemical markers have been used to distinguish between sebaceoma, sebaceous carcinoma, and BCC, but no specific marker differentiates them.9 EMA is strongly positive in sebaceous carcinoma, whilst negative in BCC. EMA and AR are positive in sebaceous carcinoma. Sebaceoma shows low mitotic index Ki 67, and p53 staining in the basaloid cells, unlike sebaceous carcinoma, where it has positive and random staining of both.10 BCL-2 is strongly expressed in BCC and is generally found to be decreased or absent in sebaceous carcinoma.9
A study explored the utility of D2-40 (podoplanin) in differentiating benign from malignant sebaceous neoplasm. However, strong and limited expression of the basaloid cells indicates a benign sebaceous proliferation such as sebaceoma.9 In contrast, negative results or focal positivity in the basaloid cells are seen in sebaceous carcinoma in a much haphazard and strong manner than in BCC.9
Both location and size are known to be risk factors for BCC. In general, BCC occurring on the head and neck are more likely to recur than those that occur on the extremities and trunk.11 There is the high-risk area (called area H), which includes the eyelids, eyebrows, periorbital, nose, lips, central face, mandible, chin, preauricular and postauricular sulci, temple, and ear. These are known as the “mask areas” of the face. The hands, feet, and genitalia are also considered to be high-risk areas. The neck, scalp, pretibial, along with the cheeks and forehead are medium-risk areas referred to as “area M.”12 The trunk and extremities are low-risk areas referred to as “area L.” Low-risk tumors are BCC lesions occurring in “area L” and are less than 20 mm in size, or lesions occurring in “area M” and are less than 10 mm in size.11 BCC tumors considered as highly risky are those occurring on “area H” independent of size along with BCC lesions occurring in “area L” and “area H” and are sized more than 20 mm and 10 mm, respectively.
Size is also a risk factor that is related to location. According to a 27-year retrospective review of 5755 BCCs by the Skin and Cancer Unit of the New York University School of Medicine,13 recurrences were significantly more common when tumors occurring in high-risk areas were 6 mm or more in diameter or when tumors occurring in medium-risk areas were 10 mm in diameter.
Other risk factors for BCC include immunosuppression such as organ transplant. The incidence of BCC in patients who had organ transplant is 5–10 times higher than the general population.11 Patients who had undergone radiotherapy for benign conditions were at an increased risk of having BCC, along with BCC that invades the perineum that is more likely to recur and be large in size compared with BCC that does not invade the perineum.
The association between age and BCC is controversial. One multivariate analysis showed that there is a direct relationship between increased age and likelihood of recurrence of BCC.14 On the other hand, another multivariate analysis with a very large database of 71,924 patients with BCC showed that patients younger than 40 years were more likely to have a recurrence during their first diagnosis.15 BCC tumors that have well-defined borders and are primary have the least risk of recurrence are considered low-risk tumors, whereas tumors that have ill-defined borders and are recurring are considered to be high-risk ones.11
When it comes to management, the goal is to remove the tumor and to maximize functional and aesthetic outcomes. Factors to be considered are: location of the tumor, size, histology, primary or recurrent, and degree of risk. There are currently 4 forms of management: medical, destructive, radiation, and surgery.10 Medical treatment is generally used for patients who cannot tolerate surgery or radiative therapy. Generally, 5-fluorouracil cream is applied topically, which is effective for low-risk basal cell carcinoma.10,16
Radiotherapy is reserved for elderly patients aged beyond 60 years, with a cure rate of 92%. It is preferentially used when the tumor is reaching up to 15 mm in size and is located in a high-risk location (such as eyelids, ears, nose, or skin around the eyes). It can also be used for treating tumors in an intermediate risk location (such as the cheek, chin, forehead, scalp, and neck) and where the tumor is 20 mm in size. Radiative therapy is contraindicated in verrucous cancer and certain genetic diseases that increase the risk of skin cancer, such as xeroderma pigmentosum. Osteitis and skin necrosis are side effects of radiative therapy, which limit its uses.17
Curettage and electrodesiccation (C&E) is reserved for tumors with low risk, where curettage removes the tumor that is visible to the naked eye, whereas electrodesiccation removes the residual tumor cells. C&E is contraindicated in hair-bearing areas and tumors extending to subcutaneous layers. The overall cure rate when using C&E is 74%.17 Laser phototherapy is used when the basal cell carcinoma is confined to the epidermis and papillary dermis. A major disadvantage is the inability to evaluate the surgical margins.16 Photodynamic therapy is used in premalignant lesions, where photosensitizing drugs are activated by light to create oxygen-free radicals, which destroy tumor cells.16,17 Cryosurgery is cooling the malignant tissues to −40° C to destroy tumor cells. It is contraindicated in patients who have cold intolerance such as patients with hypothyroidism, morpheaform basal cell carcinoma, recurrent tumors, and cosmetically sensitive areas such as the face. Side effects of cryosurgery include edema for 4–6 weeks and permanent pigment loss.16
The surgical margins for BCC depend on the work of Wolf and Zitelli.18 Their analysis concludes that well-circumscribed BCC lesions less than 2 cm in diameter excised with a 4-mm clinical margin should result in complete removal in more than 95% of the cases. This also applies to re-excision of BCC lesions occurring in “area L regions” if there are positive margins obtained after an initial excision with postoperative margin assessment. If the lesion can be successfully excised with the recommended margins, then skin grafting, linear closure, and second intention healing are all suitable reconstructive approaches. However, if the BCC occurs in “area H regions,” then wider surgical margins must be taken into account and increased recurrence rate should be expected.
Mohs micrographic surgery is the preferred surgical technique for high-risk BCC because it allows for intraoperative analysis of 100% of the excision margin. Two meta-analyses published in 1989 associated Mohs micrographic surgery with a 5-year recurrence rate of 1.0% for primary BCC, and 5.6% if it was recurrent.19,20 In both these meta-analyses, the recurrence rate for patients who had undergone Mohs surgery was lower than that for surgical excision (10.1% and 17.4% for primary and recurrent BCC, respectively), and lower than the recurrence rate for any other treatment modality (cryotherapy, radiotherapy, and curettage and electrodessication). An alternative to Mohs surgery is excision with complete circumferential peripheral and deep-margin assessment using intraoperative frozen section but only if it includes a complete assessment of all deep and peripheral margins.11
Mohs surgery is an excellent option for these patients because it ensures complete removal of the tumor. In Mohs surgery, there is repeated excision until all positive margins are tumor-free.4 Mohs surgery (also known as Mohs micrographic surgery) includes sequential horizontal excision, which is done using topographic mapping of the lesion.21 Indications of Mohs surgery include tumors that have a high recurrence, lesions in cosmetic sensitive areas such as periorbital, periauricular, and paranasal areas, and lesions with poorly delineated margins.21
When it comes to managing basal cell carcinoma, many different features need to be taken into consideration, such as the age of the patient, presence of comorbidities, and the type of BCC. In general, BCC with sebaceous differentiation is not considered to be aggressive distally because it rarely metastasizes to different organs, but it is aggressive locally because it has a high recurrence rate and therefore can be managed with Mohs surgery.6,8 BCC with sebaceous differentiation is said to be benign, with a generally good prognosis and a metastatic rate of less than 0.1% to bones, lungs, and lymph nodes but a high 5-year recurrence rate of 30%–50%.22 There is no clinical feature that distinguished BCC with sebaceous differentiation. It is a strictly histopathologic diagnosis that must be diagnosed by biopsy.
CONCLUSIONS AND RECOMMENDATIONS
Basal cell carcinoma with sebaceous differentiation is a very rare type of differentiation of a common tumor that has a good prognosis, with a metastatic rate of less than 0.1% but a high 5-year recurrence rate of 30%–50%.
It must be distinguished from similar tumors such as sebaceoma and sebaceous carcinoma with basaloid cells. Undoubtedly, histopathologic features remain the mainstay in their differentiation, and thereby, selecting the proper modality of management.
The number of cases reported is less than a handful; so no guidelines exist in the management of such cancer. We recommend frequent follow-up due to the high recurrence rate.
Our suggestion is a follow-up plan every 3 months for the first year and then every 6 months for the second year and then yearly afterward because of the high recurrence rate of 30%–50%.
We recommend Mohs surgery due to the high recurrence rate of this tumor.
PATIENT CONSENT
The patient provided written consent for the use of her image.
Footnotes
Published online 3 December 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Bickers DR, Lim HW, Margolis D, et al. American Academy of Dermatology Association; Society for Investigative Dermatology. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. [DOI] [PubMed] [Google Scholar]
- 2.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–2269. [DOI] [PubMed] [Google Scholar]
- 3.Misago N, Mihara I, Ansai S, et al. Sebaceoma and related neoplasms with sebaceous differentiation: a clinicopathologic study of 30 cases. Am J Dermatopathol. 2002;24:294–304. [DOI] [PubMed] [Google Scholar]
- 4.Choi JH, Kim YJ, Kim H, et al. Distribution of Basal cell carcinoma and squamous cell carcinoma by facial esthetic unit. Arch Plast Surg. 2013;40:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tambe S, Save S, Nayak C. Basal cell carcinoma with sebaceous differentiation. Indian Dermatol Online J. 2018;9:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulton EH, Kaley JR, Gardner JM. Skin adnexal tumors in plain language: a practical approach for the general surgical pathologist. Arch Pathol Lab Med. 2019;143:832–851. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar K. Basal cell carcinoma with sebaceous differentiation: a rare case report. MOJ Clin Med Case Rep. 2016;5:227–228. [Google Scholar]
- 8.Misago N, Suse T, Uemura T, et al. Basal cell carcinoma with sebaceous differentiation. Am J Dermatopathol. 2004;26:298–303. [DOI] [PubMed] [Google Scholar]
- 9.Yang HM, Cabral E, Dadras SS, et al. Immunohisto-chemical expression of D2-40 in benign and malignant sebaceous tumors and comparison to basal and squamous cell carcinomas. Am J Dermatopathol. 2008;30549–554. [DOI] [PubMed] [Google Scholar]
- 10.Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCCN Guidelines 2020. [Internet]. Nccn.org. 2020. [cited 9 July 2020]. Available at: https://www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=1206
- 12.Boeta-Angeles L, Bennett RG. Miller SJ, Maloney ME, eds. Features associated with recurrence (basal cell carcinoma). In: Cutaneous Oncology Pathophysiology, Diagnosis, and Management. 1998:Malden, MA: Blackwell Science; 646–656. [Google Scholar]
- 13.Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. Part 1: Overview. J Dermatol Surg Oncol. 1991;17:713–718. [DOI] [PubMed] [Google Scholar]
- 14.Cheretis C, Angelidou E, Dietrich F, et al. Prognostic value of computer-assisted morphological and morphometrical analysis for detecting the recurrence tendency of basal cell carcinoma. Med Sci Monit. 2008;14:MT13–MT19. [PubMed] [Google Scholar]
- 15.Milan T, Pukkala E, Verkasalo PK, et al. Subsequent primary cancers after basal-cell carcinoma: a nationwide study in Finland from 1953 to 1995. Int J Cancer. 2000;87:283–288. [PubMed] [Google Scholar]
- 16.Gloster HM, Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741–60; quiz 761. [DOI] [PubMed] [Google Scholar]
- 17.Pelucchi C, Di Landro A, Naldi L, et al. Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology (GISED). Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935–944. [DOI] [PubMed] [Google Scholar]
- 18.Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340–344. [PubMed] [Google Scholar]
- 19.Rowe DE, Carroll RJ, Day CL., Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–328. [DOI] [PubMed] [Google Scholar]
- 20.Rowe DE, Carroll RJ, Day CL., Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424–431. [DOI] [PubMed] [Google Scholar]
- 21.Cottel WI, Proper S. Mohs’ surgery, fresh tissue technique: our technique with a review. J Dermatol Surg Oncol. 1982;8:576. [DOI] [PubMed] [Google Scholar]
- 22.Raasch BA, Buettner PG, Garbe C. Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol. 2006;155:401–407. [DOI] [PubMed] [Google Scholar]


