Abstract
Background:
Soft tissue sarcomas are rare neoplasms that can occur on any part of the body. The operative position for the resection is determined depending on the site of the soft tissue sarcomas; intraoperative repositioning may be needed for reconstruction. We present the profunda femoris artery perforator (PAP) flap harvest technique (wherein the flap can be used in any position), and suggest that the PAP flap transfer can eliminate the need for intraoperative repositioning.
Methods:
From December 2018 to January 2020, 7 patients with an average age of 68 years underwent reconstructions using a PAP flap after wide resection of STS. The mean defect size was 11.3 × 16.5 cm (range, 5.5–25 × 11–26 cm). The location of the defects was the medial thigh in 2 patients, the posterior thigh in 1, the popliteal fossa in 1, the groin in 1, and the buttock in 2. The PAP flap was elevated in the supine “frog-leg” position, the prone position, the jack-knife position, or the lateral “crisscross” position; the lateral decubitus position with the donor lower extremity on the bottom.
Results:
Of the 7 cases, the operations were performed in the supine “frog-leg” position in 3 cases, the prone position in 2 cases, the jack-knife position in 1 case, and the lateral “crisscross” position in 1 case. There were no intraoperative position changes in all cases. The mean size of the PAP flap was 8.7 × 19.9 cm (range, 6–11 × 17–24 cm). One patient had donor site dehiscence, which was treated conservatively. The PAP flaps survived completely in all cases. The mean follow-up period was 10.5 months (range, 6–17 months).
Conclusion:
Since the PAP flap elevation is feasible in every position, the PAP flap can be considered a versatile reconstruction option after sarcoma resection.
INTRODUCTION
Soft tissue sarcomas (STS) are rare neoplasms that can occur on any part of the body. A good operative position is important for surgeons to achieve wide resection with an adequate margin, and is determined depending on the site of STS. Intraoperative repositioning is needed for reconstruction after the tumor resection, and depends on the flap choice. However, intraoperative repositioning can cause some unfavorable results such as a longer operating time, a higher risk of infection, and changes in hemodynamic parameters.1 To avoid unnecessary intraoperative repositioning, reconstructive surgeons must consider not only the size and shape of the flap that can be harvested from a donor site, but also the intraoperative position during the flap harvest.
Subsequent to the development of the skin flap based on proximal musculocutaneous perforators from the adductor magnus muscle, which was called an “adductor flap” by Angrigiani et al. in 2001, the profunda femoris artery perforator (PAP) flap has gained popularity in microsurgical reconstruction, due to some reports elucidating its anatomy and clinical applications.2–6
In this article we describe an institutional experience of 7 consecutive patients receiving reconstruction using the PAP flap after sarcoma resection without an intraoperative position change. We suggest that the PAP flap transfer is a versatile solution for sarcoma reconstruction, which can be used on any part of the body, while eliminating the need for an intraoperative position change.
PATIENTS AND METHODS
From December 2018 to January 2020, 7 patients (5 men and 2 women) with a mean age of 68 years (range, 45–80) underwent reconstructions using a PAP flap after wide resection of STS. The mean defect size was 11.3 × 16.5 cm (range, 5.5–25 × 11–26 cm). The location of the defects was the medial thigh in 2 patients, the posterior thigh in 1, the popliteal fossa in 1, the groin in 1, and the buttock in 2. The histological diagnoses were myxoid liposarcoma in 2 patients, extraskeletal myxoid chondrosarcoma in 1, undifferentiated pleomorphic sarcoma in 1, spindle cell sarcoma in 2, and solitary fibrous tumor in 1. Patients’ demographic data are given in Table 1.
Table 1.
Summary of Patient Characteristics and Outcome
| Patient No. | Age (y) | Sex | Defect Location | Free/Pedicled | Position | Defect Size (cm) | Histology | Pedicle Length (cm) | Pedicle Diameter (mm) Artery/vein |
Recipient Vessels | Size of Skin Paddle (cm) | Complications/Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Male | Medial thigh | Free | Supine frog-leg | 11 × 11 | Myxoid liposarcoma | 5 | 1.5/1.5, 1.8 | Descending genicular | 9 × 22.5 | |
| 2 | 67 | Male | Popliteal fossa | free | Prone | 6 × 20 | Extraskeletal myxoid chondrosarcoma | 4.5 | 1.2/1.2 | Descending genicular | 8 × 24 | |
| 3 | 80 | Male | Medial thigh | Pedicled | Supine frog-leg | 10 × 14 | Undifferentiated pleomorphic sarcoma | 10.5 | N/A | N/A | 9 × 18 | |
| 4 | 75 | Male | Groin | Free | Supine frog-leg | 9 × 13 | Spindle cell sarcoma | 8.5 | 2.2/2.0, 2.0 | Ascending branch of LFCA | 11 × 20 | Donor-site dehiscence |
| 5 | 63 | Female | Buttock | Free | Lateral crisscross | 25 × 26 | Myxoid liposarcoma | 8 | 1.5/2.0, 2.0 | Pedicle of the ALT flap | 10.5 × 20 | Two other flaps were transferred |
| 6 | 77 | Male | Posterior thigh | Free | Prone | 13 × 16 | Spindle cell sarcoma | 3.5 | 1.5/2.0, 3.5 | PAP | 8 × 17 | |
| 7 | 69 | Female | Buttock | Pedicled | Jack-knife | 5.5 × 16 | Solitary fibrous tumor | 4.5 | N/A | N/A | 6 × 18 | A gracilis flap was transferred |
| Average | 68 | 11.3 × 16.5 | 6.3 | 1.5/2.0 | 8.7 × 19.9 |
Surgical Technique
In this case series, the PAP flap was elevated in the supine “frog-leg” position, the prone position, the jack-knife position, or the lateral “crisscross” position.
Supine Frog-leg Position
For the elevation of the PAP flap in the supine position, we propose the supine frog-leg position. This is the supine position with the donor leg abducted and externally rotated at the hip joint and flexed at the knee joint (Fig. 1). In this position, an anterior approach is used for the dissection of the perforators. The adductor longus muscle is marked. A hand-held doppler ultrasound is used to detect the locations of the perforators from the profunda femoris artery. The PAP flap is designed vertically. A longitudinal incision, which is placed posterior to the anterior edge of the gracilis muscle, is made down to the deep fascia along the anterior border of the flap. Subfascial flap elevation above the deep fascia is then performed anterior-to-posteriorly until the perforators from the profunda femoris artery are identified. After choosing a sizable perforator, pedicle dissection is continued until a desirable length of the pedicle is achieved. An incision is made along the posterior border of the skin paddle, and the PAP flap is elevated, including subcutaneous fat tissue.5
Fig. 1.

Supine frog-leg position for PAP flap elevation. The supine position with the donor leg abducted and external rotated at the hip joint and flexed at the knee joint.
Prone Position
For the elevation of the PAP flap in the prone position, we propose the prone position with the legs slightly abducted at the hip joint and extended at the knee joint (Fig. 2). In this position, a posterior approach is used for the dissection of the perforators. The adductor longus muscle is marked before taking the prone position. The flap design is the same as that of the supine frog-leg position. A longitudinal incision is made down to the deep fascia along the posterior border of the flap. Subfascial flap elevation above the deep fascia is then performed posterior-to-anteriorly until the perforators from the profunda femoris artery are identified. After choosing a sizable perforator, pedicle dissection is continued until a desirable length of the pedicle is achieved. An incision is made along the anterior border of the skin paddle, and the PAP flap is elevated, including subcutaneous fat tissue.
Fig. 2.

Prone position for PAP flap elevation. The prone position with the legs slightly abducted at the hip joint and extended at the knee joint.
Lateral Crisscross Position
For the elevation of the PAP flap in the lateral decubitus position, we propose the lateral “crisscross” position. This is the lateral decubitus position with the donor lower extremity on the bottom. The donor leg is slightly flexed at the knee joint. The other leg is further flexed at the hip and lies parallel to the donor leg. Using foam wedges or pillows, the patient’s hip and knee joint of the contralateral lower extremity can be flexed to obtain a favorable surgical position.7 (Figs. 3, 6A). In this position, an anterior approach is used for the dissection of the perforators as in the supine frog-leg position.
Fig. 3.

Lateral crisscross position for PAP flap elevation. The lateral “crisscross” position; the lateral decubitus position with the donor lower extremity on the bottom. The donor leg is slightly flexed at the knee joint. The other leg is further flexed at the hip and lies parallel to the donor leg.
When harvesting the flap in the prone or lateral positions, the orientation of the anatomical structures should be occasionally confirmed. Otherwise, the elevation technique is relatively similar in both cases but does come with a learning curve.
RESULTS
Of the 7 cases, 5 patients underwent a single-stage reconstruction, and 2 patients underwent a secondary reconstruction. The mean size of the defect was 11.3 × 16.5 cm (range, 5.5–25 × 11–26 cm), and all defects were sufficiently covered. The mean size of the PAP flap was 8.7 × 19.9 cm (range, 6–11 × 17–24 cm). Of the 7 cases, the operations were performed in the supine “frog-leg” position in 3 cases, the prone position in 2 cases, the jack-knife position in 1 case, and the lateral “crisscross” position in 1 case. There were no intraoperative position changes in all cases. The PAP flap survived completely in all cases. Neither anastomosis complications nor infections were encountered. Regarding donor site complications, donor site dehiscence was seen in 1 case, which was treated conservatively. The postoperative course was uneventful in 6 cases. The mean follow-up period was 10.5 months (range, 6–17 months). These findings are summarized in Table 1.
Case Reports
Case 1 (Prone position)
A 77-year-old male suffered from a soft tissue sarcoma on the left posterior thigh (Fig. 4A). Surgical wide resection followed by immediate reconstruction using a free PAP flap from the right thigh was planned. Surgical wide resection with a 3-cm margin was performed. The defect after tumor ablation was 13 × 16 cm (Fig. 4B). An 8 × 17 cm PAP flap was elevated and transferred to the defect (Fig. 4C). The pedicle of the PAP flap was anastomosed to the profunda femoris artery perforator and its vena comitans. All procedures were performed in the prone position. The flap survived completely, and no postoperative complications were seen (Fig. 5). The patient could ambulate freely after 1 week.
Fig. 4.
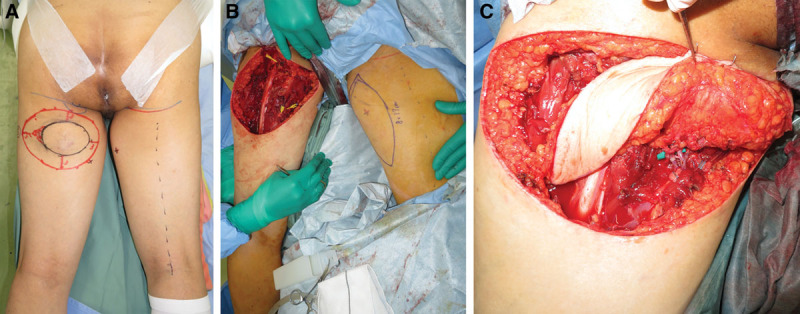
A 77-year-old man suffered from a soft tissue sarcoma on the left posterior thigh. A, Preoperative view. B, Intraoperative view after surgical wide resection. The defect after tumor ablation was 13 × 16 cm. C, Intraoperative view after microsurgical anastomosis. The pedicle of the PAP flap were anastomosed to the profunda femoris artery perforator and its vena comitans.
Fig. 5.

A 77-year-old man suffered from a soft tissue sarcoma on the left posterior thigh. A, Intraoperative view after flap inset. B, Postoperative view at 3 months after the surgery.
Case 2 (Lateral crisscross position)
A 63-year-old woman suffered from a large soft tissue sarcoma on the right buttock (Fig. 6A). Surgical wide resection followed by immediate reconstruction was performed. Surgical wide resection with a 1- to 2-cm margin, including the gluteal major muscle, was performed. The defect after tumor ablation was 25 × 26 cm (Fig. 6B). A 10 × 18 cm lumbar artery perforator flap, a 12 × 25 cm anterolateral thigh (ALT) flap, and a 10.5 × 20 cm PAP flap were transferred to the defect (Fig. 7A). The pedicle of the ALT flap, and the descending branch of the lateral femoral circumflex artery and vein were anastomosed to the inferior gluteal artery and vein. The pedicle of the PAP flap was anastomosed to the distal part of the lateral femoral circumflex artery and vein of the ALT flap. The donor site of the PAP flap was closed primarily, and that of the ALT flap was closed with skin grafting.
Fig. 6.

A 63-year-old woman suffered from a large soft tissue sarcoma on the right buttock. A, Preoperative view. B, Intraoperative view after surgical wide resection. The defect after tumor ablation was 25 × 26 cm.
Fig. 7.

A 63-year-old woman suffered from a large soft tissue sarcoma on the right buttock. A, Intraoperative view after flap inset. Yellow arrow: lumbar artery perforator flap; blue arrow: ALT flap; red arrow: PAP flap. B, Postoperative view at 6 months after the surgery.
All procedures were performed in the lateral “crisscross” position. The flap survived completely, and no postoperative complications were seen. There were no donor site morbidities either in the left medial thigh or in the right lateral thigh (Fig. 7B). The patient could ambulate freely after 3 weeks without any weakness. The range of motion at postoperative 6 months were 120 degree for hip flexion, 15 degree for hip extension, and 45 degree for hip abduction. The manual muscle test grades at the postoperative 6 months were 5 for hip flexion and 5 for hip abduction.
DISCUSSION
Sarcomas are rare malignancies, accounting for less than 1% of new cancer diagnoses. STS occur most often in the extremities; upper and lower extremity STS account for 12% and 28%, respectively, whereas the STS occurring on the trunk account for 10% of all STS. The thigh is the most common location of STS, accounting for 44% of all extremity STS.8 Reconstructive surgical techniques such as free or local flap transfers, including limb-sparing procedures, are essential for improving patients’ quality of life. Some recent studies have reported that free flap transfers have a safety profile similar to that of reconstruction using a local flap, owing to the current improvements in microsurgical techniques and instruments.9–14 Some review articles have concluded that reconstruction using a vascularized flap after tumor resection is a reliable, safe and necessary technique. Complications following a free flap transfer are usually manageable.15 Free tissue transfer allows radical oncosurgical resections and enables adequate defect coverage, together with preservation of function and aesthetics.16 In the setting of extremity sarcoma, a recent study using the National Cancer Database has shown that the ratio of amputation is only 8% and that of limb salvage is 92%.17 In our case series, 4 patients suffered from sarcoma on a lower extremity, and limb salvage was achieved using the PAP flap.
The PAP flap has recently become one of the favored options for reconstructive surgery, due to some reports elucidating its anatomy and clinical applications.2–6 Skin flaps in the posterior medial thigh area were first reported by Conway and Griffith18 and by Conway and Kraissl.19 Subsequent to the development of a skin flap based on proximal musculocutaneous perforators from the adductor magnus muscle, which was called an “adductor flap” by Angrigiani et al. in 2001, the posterior medial thigh donor site has gained popularity in microsurgical reconstruction.2 In 2012, Allen et al. described breast reconstruction using the PAP flap.3 Initially, flap harvesting was performed in a prone position. The supine “frog-leg” position was later adopted to eliminate the need for repositioning. Scaglioni et al. reported head and neck reconstruction using the posteromedial thigh flap in 20155 and various kinds of flap design of the posteromedial thigh area in 2017.20,21
Intraoperative repositioning is associated with a higher risk of infection and changes in hemodynamic parameters. Flap selection that needs a position change may discourage a 2-team approach, with resultant prolongation of anesthesia time.1 In the setting of the surgery for STS, because STS can occur on any part of the body, an operative position that allows for sufficiently wide resection is extremely important; the position should be determined by the STS site, not the donor site of a flap. Therefore, to avoid intraoperative repositioning, reconstructive surgeons should be familiar with the options for flap harvesting in each position.
One of the greatest advantages of the PAP flap is that a relatively large volume of flexible skin paddle and fat tissue with a long pedicle can be harvested from an inconspicuous donor site. There are numerous works that have reported that the PAP flap can be harvested both in the prone position and in the supine “frog-leg” position.2–4,22 In addition, we have discovered that the PAP flap can be harvested in the lateral “crisscross” position, as mentioned above. Therefore, our clinical experience substantiated the feasibility of the PAP flap elevation in every position. An ALT flap, a latissimus dorsi flap, a rectus abdominis flap, a deep inferior epigastric artery perforator flap, a superficial inferior epigastric artery flap, and a superficial circumflex iliac artery perforator are commonly used for microsurgical reconstruction after sarcoma resection.23–27 However, an ALT flap, a rectus abdominis flap, a deep inferior epigastric artery perforator flap, a superficial inferior epigastric artery flap, or a superficial circumflex iliac artery perforator flap cannot be harvested in the prone position. A large LD flap cannot be harvested in the supine position.1 Moreover, it is difficult to harvest a rectus abdominis flap, a deep inferior epigastric artery perforator flap, and a superficial inferior epigastric artery flap in the lateral decubitus position. Therefore, the PAP flap is unique in that it can be elevated in every operative position. We strongly believe that the PAP flap transfer is a versatile solution for sarcoma reconstruction, which can be used on any part of the body, while eliminating the need for an intraoperative position change. To the best of our knowledge, this article presents the first report of the surgical technique of PAP flap elevation in every position and the use of the PAP flap for the reconstruction after sarcoma resection.
The reconstruction for a large defect, over 20 × 20 cm, after wide resection is challenging and sometimes necessitates a double flap transfer. When reconstruction for a large defect is performed with the patient in the lateral “crisscross” position, a double flap transfer using a PAP flap from the bottom of the medial thigh and an ALT flap from the contralateral thigh is an option, as described in the case report. When reconstruction is performed with the patient in the prone position, a double PAP flap transfer from bilateral thigh is one of the options.28
The main limitation of the PAP flap is issues accompanying its donor site closure. Closure of the donor site after the PAP flap elevation must be performed under maximum tension, which can result in scar widening. Trying to prevent this complication will limit the amount of procured tissue.29 The greatest limitation of this study is the paucity of cases. No firm conclusions can be drawn from a series of only 7 patients. For more rigorous statistical analysis, a study with a large number of patients is warranted.
CONCLUSION
With the feasibility of the PAP flap elevation in every position and its minimal donor site morbidity, the PAP flap can be considered a possible reconstruction option after sarcoma resection.
Footnotes
Published online 17 December 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Forte AJ, Oliver JD, McRae M, et al. Use of the subscapular system by maintaining unilateral decubitus placement without repositioning in microvascular free tissue transplantation. Microsurgery. 2020;40:125–129. [DOI] [PubMed] [Google Scholar]
- 2.Angrigiani C, Grilli D, Thorne CH. The adductor flap: a new method for transferring posterior and medial thigh skin. Plast Reconstr Surg. 2001;107:1725–1731. [DOI] [PubMed] [Google Scholar]
- 3.Allen RJ, Haddock NT, Ahn CY, et al. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129:16e–23e. [DOI] [PubMed] [Google Scholar]
- 4.Karakawa R, Yoshimatsu H, Fuse Y, et al. The correlation of the perforators and the accessory saphenous vein in a profunda femoris artery perforator flap for additional venous anastomosis: a cadaveric study and clinical application. Microsurgery. 2020;40:200–206. [DOI] [PubMed] [Google Scholar]
- 5.Scaglioni MF, Kuo YR, Yang JC, et al. The posteromedial thigh flap for head and neck reconstruction: anatomical basis, surgical technique, and clinical applications. Plast Reconstr Surg. 2015;136:363–375. [DOI] [PubMed] [Google Scholar]
- 6.Karakawa R, Yoshimatsu H, Tanakura K, et al. An anatomical study of the lymph collecting vessels of the medial thigh and clinical applications of lymphatic vessels preserving profunda femoris artery perforator (LpPAP) flap using pre- and intra-operative indocyanine green (ICG) lymphography. J Plast Reconstr Aesthet Surg. 2020;73:1768–1774. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimatsu H, Inoue K, Tanakura K, et al. Lateral crisscross position for lymphaticovenular anastomosis: comfortable for both the patient and the surgeon. J Reconstr Microsurg. 2019;35:e3–e4. [DOI] [PubMed] [Google Scholar]
- 8.Hui JY. Epidemiology and etiology of sarcomas. Surg Clin North Am. 2016;96:901–914. [DOI] [PubMed] [Google Scholar]
- 9.Ring A, Kirchhoff P, Goertz O, et al. Reconstruction of soft-tissue defects at the foot and ankle after oncological resection. Front Surg. 2016;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López JF, Hietanen KE, Kaartinen IS, et al. Primary flap reconstruction of tissue defects after sarcoma surgery enables curative treatment with acceptable functional results: a 7-year review. BMC Surg. 2015;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zook E, Russel R, Asaadi M. A comparative study of free and pedicle flaps for lower extremity wounds. Ann Plast Surg. 1986;17:21–33. [DOI] [PubMed] [Google Scholar]
- 12.Serafin D, Geiordiage N, Smith D. Comparison of free flaps with pedicle flaps for lower extremity wounds. Plast Reconstr Surg. 1977;59:492–499. [PubMed] [Google Scholar]
- 13.Cordeiro PG, Neves RI, Hidalgo DA. The role of free tissue transfer following oncologic resection in the lower extremity. Ann Plast Surg. 1994;33:9–16. [DOI] [PubMed] [Google Scholar]
- 14.Penna V, Iblher N, Momeni A, et al. Free tissue transfer in reconstruction following soft tissue sarcoma resection. Microsurgery. 2011;31:434–440. [DOI] [PubMed] [Google Scholar]
- 15.Kontogeorgakos VA, Eward WC, Brigman BE. Microsurgery in musculoskeletal oncology. Eur J Orthop Surg Traumatol. 2019;29:271–278. [DOI] [PubMed] [Google Scholar]
- 16.Viñals JM, Rodrigues TA, Sildenikova DP, et al. Indications of microsurgery in soft tissue sarcomas. J Reconstr Microsurg. 2012;28:619–625. [DOI] [PubMed] [Google Scholar]
- 17.Abarca T, Gao Y, Monga V, et al. Improved survival for extremity soft tissue sarcoma treated in high-volume facilities. J Surg Oncol. 2018;117:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway H, Griffith BH. Plastic surgery for closure of decubitus ulcers in patients with paraplegia; based on experience with 1,000 cases. Am J Surg. 1956;91:946–975. [DOI] [PubMed] [Google Scholar]
- 19.Conway H, Kraissl CJ. The plastic surgical closure of decubitus ulcers in patients with paraplegia. Surg Gynecol Obstet. 1947;85:321–332. [PubMed] [Google Scholar]
- 20.Scaglioni MF, Chen YC, Lindenblatt N, et al. The vertical posteromedial thigh (vPMT) flap for autologous breast reconstruction: a novel flap design. Microsurgery. 2017;37:371–376. [DOI] [PubMed] [Google Scholar]
- 21.Scaglioni MF, Eder M, Giovanoli P. The use of inverted-L posteromedial thigh (L-PMT) flap for autologous breast reconstruction: a case report. Microsurgery. 2018;38:558–562. [DOI] [PubMed] [Google Scholar]
- 22.Haddad K, Obadia D, Hunsinger V, et al. [Breast reconstruction with profunda artery perforator flap in lithotomy position. Surgical technique]. Ann Chir Plast Esthet. 2016;61:217–222. [DOI] [PubMed] [Google Scholar]
- 23.Nemir S, Mericli AF, Adelman DM, et al. A reconstructive algorithm of oncologic defects of the upper trunk and shoulder girdle: factors predicting complexity and outcomes. J Surg Oncol. 2020;122:283–292. [DOI] [PubMed] [Google Scholar]
- 24.Crowley TP, Atkinson K, Bayliss CD, et al. The surgical management of sarcomas of the chest wall: a 13-year single institution experience. J Plast Reconstr Aesthet Surg. 2020;73:1448–1455: [DOI] [PubMed] [Google Scholar]
- 25.Dadras M, Koepp P, Wallner C, et al. Predictors of oncologic outcome in patients with and without flap reconstruction after extremity and truncal soft tissue sarcomas. J Plast Reconstr Aesthet Surg. 2020;73:1239–1252 [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto S, Arikawa M, Kagaya Y. The use of lower abdominal perforator flaps in soft-tissue reconstruction after sarcoma resection. Microsurgery. 2020;40:353–360. [DOI] [PubMed] [Google Scholar]
- 27.Koshima I, Nanba Y, Tsutsui T, et al. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast Reconstr Surg. 2004;113:233–240. [DOI] [PubMed] [Google Scholar]
- 28.Haddock NT, Cho MJ, Teotia SS. Comparative analysis of single versus stacked free flap breast reconstruction: a single-center experience. Plast Reconstr Surg. 2019;144:369e–377e. [DOI] [PubMed] [Google Scholar]
- 29.Dayan JH, Allen RJ., Jr. Lower extremity free flaps for breast reconstruction. Plast Reconstr Surg. 2017;140(5S Advances in Breast Reconstruction):77S–86S. [DOI] [PubMed] [Google Scholar]


