Abstract
Background:
Collagenase clostridium histolyticum-aaes (CCH) enzymatically releases fibrous septa that contribute to the skin dimpling characteristic of cellulite. Long-term safety/duration of efficacy (durability) results from an open-label extension (OLE) of a randomized, double-blind, placebo-controlled trial (RCT) evaluating CCH efficacy/safety for moderate-to-severe cellulite of the buttocks or posterolateral thighs in women was assessed. Efficacy/safety of CCH treatment/retreatment during OLE was also evaluated.
Methods:
After RCT unblinding, women could enroll in OLE for assessment of long-term CCH durability (observation only, up to day 720) or CCH treatment/retreatment, the latter in women with moderate-to-severe buttock/posterolateral thigh cellulite [Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS) and Patient Reported PCSS (PR-PCSS) scores of 3/4; Hexsel Cellulite Severity Scale score ≤13]. A treatment/retreatment course comprised 1 or 2 courses of 3 sessions (0.84-mg CCH injected at days 1, 22, and 43). CCH efficacy/safety was assessed at baseline, days 22, 43, 71, and quarterly at day 360.
Results:
Of the 259 OLE participants, 53 were observed for long-term CCH durability. For those who were ≥2-level composite responders during RCT (≥2-point CR-PCSS/PR-PCSS score improvements), CCH effect was durable (scores did not reach RCT baseline levels) in all women on days 180 (19/19), 360 (16/16), and 720 (7/7). Of the 200 women receiving CCH treatment/retreatment, more than 75% had ≥1-level improvement in patient and clinical assessments at day 71. The most common adverse events were injection-site bruising and pain.
Conclusions:
CCH treatment provided durable improvement in moderate-to-severe buttock/thigh cellulite and was generally well tolerated. Repeated CCH exposure did not increase adverse event risk or reduce efficacy.
INTRODUCTION
Cellulite is a condition characterized by dimpled contour alterations of the skin that affects approximately 80%–98% of postpubertal women.1,2 Although the pathophysiology of cellulite is not fully understood, multiple factors may play a role, including the quantity, type, and orientation of fibrous septa3,4; age-related decreasing dermal thickness3; subcutaneous inflammation and microvascular dysfunction5,6; and superficial and deep adipose tissue architecture.3,4 Cellulite is a major cosmetic concern for many women, and numerous treatment approaches have been considered to minimize its appearance.7,8 These interventions have included topical agents; mechanical stimulation; acoustic wave therapy, or the application of laser, light, or radiofrequency energy; and subcision.7,8 Collagenase clostridium histolyticum-aaes (CCH; QWO, Endo Aesthetics LLC, Malvern, Pa.) is a combination of 2 purified bacterial collagenolytic enzymes that disrupt targeted collagen structures under physiologic conditions and was approved in the United States in July 2020 for the treatment of moderate-to-severe cellulite in the buttocks of adult women.9,10 A different formulation of collagenase clostridium histolyticum (Xiaflex, Endo Pharmaceuticals Inc., Malvern, Pa.) is approved in the United States for the treatment of collagen-associated disorders (ie, Dupuytren contracture with a palpable cord or Peyronie’s disease with a palpable plaque and penile curvature deformity ≥30 degrees at the start of therapy) in adults.11 For both formulations, the mechanism of action of CCH is enzymatic disruption of the fibrous septa to create a contour leveling effect.12 The efficacy and safety of CCH for the treatment of cellulite have been reported in a phase 2 randomized, double-blind, placebo-controlled trial (RCT) in women with moderate-to-severe cellulite on the buttocks or posterolateral thighs.12 Adult women received a subcutaneous injection of CCH for cellulite in up to 3 treatment sessions (each separated by 21 days; 0.84 mg per session) that significantly improved the appearance of cellulite versus placebo by Day 71, based on both clinician and patient ratings, and was well tolerated. Women completing the RCT could subsequently enroll in an open-label extension (OLE) that evaluated the long-term durability of improvements in cellulite appearance achieved with CCH treatment during the RCT (up to ~2 years after first RCT CCH dose), as well as the long-term safety and efficacy of open-label CCH treatment and retreatment for ~1 year. Results of the OLE are presented herein.
MATERIALS AND METHODS
Study Design
An OLE of a previous phase 2 RCT12 was conducted from October 2016–June 2018 (ClinicalTrials.gov identifier: NCT02942160). The primary objective of the OLE (ie, the current study) was to assess the long-term safety of CCH for cellulite, and secondary objectives were to evaluate the durability of response over 12 months and to evaluate the efficacy and safety of retreatment. Women who completed the double-blind RCT could enroll in the OLE trial for observation only (ie, no additional CCH treatment). After RCT unblinding, and if they met eligibility criteria, women could receive open-label CCH treatment for cellulite, with subcategorization of participants based on exposure (see disposition in Results):
1) RCT → OLE exposure (first CCH course in RCT and second CCH course in OLE): (a) in the same area—ie, CCH administered in the same area of cellulite treated with CCH in the double-blind RCT (if cellulite severity scores of the RCT-treated area were at RCT baseline levels or worse) or (b) administered to a different area of cellulite—ie, CCH administered to an area of cellulite different from that treated with CCH in the double-blind RCT. Both scenarios (same or different treatment area) were considered CCH Treatment Course 1 in this study.
2) First OLE exposure (placebo in RCT and first CCH course in OLE): CCH administered in the same area or different area of cellulite treated with placebo in the double-blind RCT (CCH Treatment Course 1).
3) OLE → OLE reexposure (the first and second CCH courses in OLE): ≥28 days after the last treatment on day 43 of CCH Treatment Course 1 in the OLE, CCH administration in the same area of cellulite or in a different area of cellulite (CCH Treatment Course 2)
The study was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and with the ethical principles of the Declaration of Helsinki. The protocol was approved by a central institutional review board (Quorum Review, Inc, Seattle, Wash.), and all participants provided written informed consent.
Study Population
Nonpregnant women aged ≥18 years who completed the RCT and provided written informed consent to participate in the OLE trial were eligible for the study. To receive CCH treatment, women had moderate-to-severe cellulite [Clinician Reported Photonumeric Cellulite Severity Scale (CR-PCSS) and Patient Reported Photonumeric Cellulite Severity Scale (PR-PCSS) scores of 3–4 and a Hexsel Cellulite Severity Scale (CSS) score ≤13] of the buttocks or posterolateral thighs and were willing to apply sunscreen to any treatment area (area treated during RCT or OLE) before each sun exposure to ensure that tanned skin would not confound efficacy assessments.
Women were excluded from the study if they received liposuction on the side of the body chosen for treatment or received injections, or had radiofrequency treatments, laser treatment, surgery, or manual and/or laser- or vacuum-assisted subcision within the selected treatment area during the previous 12 months. Women were also excluded if they received Endermologie (Endo-Systems LLC, Fort Lauderdale, Fla.) or similar treatments within the selected treatment area during the previous 6 months, massage therapy within the selected treatment area during the previous 3 months, or creams to minimize cellulite appearance within the previous 2 weeks. Women with a recent medical history of stroke or bleeding, prior use within 1 week of treatment, or current or anticipated use of anticoagulant or antiplatelet therapy (except ≤150 mg daily aspirin), or known systemic allergy to collagenase were also excluded.
Treatment
After RCT unblinding, each woman with moderate-to-severe cellulite could receive a maximum of 2 courses of CCH treatment overall (see relevant text below), administered during the RCT and/or OLE. Women treated with placebo [composed of same components as CCH, excluding the active ingredient (ie, collagenase)] in the RCT could have 1 area treated with CCH and a subsequent qualifying area treated with CCH in the OLE, if eligible, following completion of the first course of CCH treatment for cellulite. Each treatment course comprised 3 treatment sessions [days 1, 22 (± 3 days), and 43 (± 3 days)] of CCH 0.84 mg (3.6 mL total volume), unless no dimples were apparent in the treatment area on days 22 or 43. The choice of dimples to be treated in the selected area of the buttocks or posterolateral thighs was at the discretion of the investigator, similar to what would occur in a real-world clinical-practice setting. Dimples were required to be well defined and evident when the woman was in a relaxed, standing position. Using the same administration technique as in the RCT,12 CCH was administered during each of the 3 sessions as up to 12 subcutaneous injections, each delivered in three 0.1-mL aliquots. One aliquot was injected perpendicular to the skin, and 2 aliquots were angled at ~45 degrees cephalad-caudad of the perpendicular axis. (See Video [online], which demonstrates the author’s injection technique.)
Video 1. In person injection. Video 1 from “Efficacy, Safety, and Durability of Response of Collagenase Clostridium Histolyticum-aaes for the Treatment of Cellulite”.
Assessments
Digital images of the treatment area were taken under standardized conditions, including setup and lighting, to assess cellulite severity. The treatment area was photographed (standing with relaxed gluteus muscles) using the Vectra H1 imaging system (Canfield Scientific, Inc., Parsippany, N.J.). Standardized parameters for each photographic session included wearing garments in a standing position before the session (ie, to prevent garment impressions), feet positioning, relaxed posture, flash lighting, reference photograph on screen to assist with alignment, distance between camera and treatment area (2 convergent laser beams were aligned on the surface), and camera height.
For women receiving CCH treatment during the OLE trial, efficacy was assessed at baseline via digital images [up to 14 days before the start of treatment (day 1)]; days 22 (± 3 days), 43 (± 3 days), and 71 (+ 5 days); and at 3-month intervals using the validated PR-PCSS (Fig. 1). Women independently and privately evaluated the treatment area, using the PR-PCSS, by viewing the treatment area digital images on standardized computer monitors. The validated CR-PCSS was conducted by the study investigators using live assessments (ie, not digital images). Women receiving CCH treatment were also assessed at baseline and at days 71 (+ 5 days) and 360 (± 7 days) using the validated photonumeric Hexsel CSS (live assessment),13 the Investigator Global Aesthetic Improvement Scale (I-GAIS), and the Subject Global Aesthetic Improvement Scale (S-GAIS).
Fig. 1.
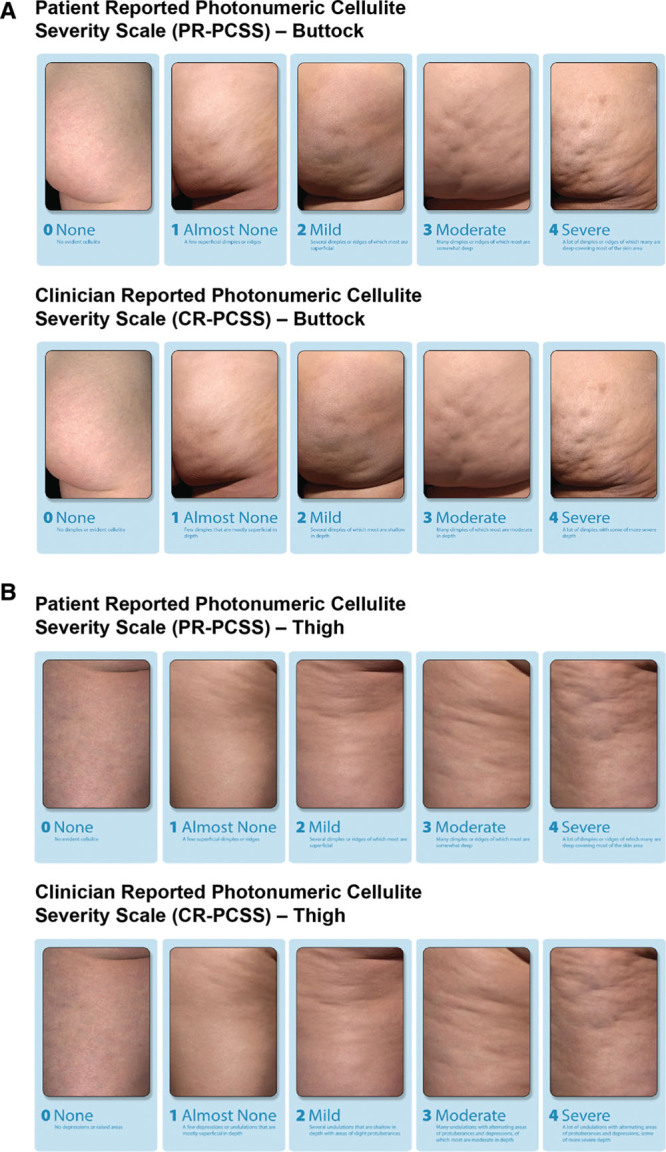
Assessment of cellulite severity using the Patient Reported and Clinician Reported Photonumeric CSSs for the buttocks (A) and thighs (B). Reprinted with permission from Auxilium Pharmaceuticals, LLC. © 2017. All rights reserved.
For assessment of durability (no additional CCH treatment during the OLE trial), cellulite severity was assessed using the CR-PCSS and PR-PCSS, with assessments conducted starting 90 days after day 1 of the double-blind RCT [days 90 (± 7 days), 180 (± 7 days), 270 (± 7 days), 360 (± 7 days), 540 (± 30 days), and 720 (± 30 days)]. In women who received CCH treatment for cellulite in the OLE trial, durability was assessed at 90-day (± 7 days) intervals up to day 360 (± 7 days) for each treatment area, using the CR-PCSS and PR-PCSS. Durable responders were defined as women whose CR-PCSS and PR-PCSS ratings did not return to baseline (pretreatment) scores (or worse).
Treatment-emergent adverse events (AEs) were monitored throughout the study. In the observation population, levels of binding AUX-I and AUX-II antibodies were measured at day 360 ± 7 days or early termination visit. For those who received CCH treatment during the OLE, these antibody levels were measured on day 1 (before injection) and day 71 + 5 days or early termination visit. The observation population was defined as all women who participated in the RCT and enrolled in the OLE trial. The safety population was defined as all women who received ≥1 dose of CCH during the OLE trial. The efficacy population was defined as all women in the safety population who had a baseline and ≥1 post-treatment CR-PCSS and PR-PCSS assessment. The durability population was defined as women who were CCH responders in the RCT and/or OLE trial (improvement of ≥1 level in both CR-PCSS and PR-PCSS from baseline at day 71).
RESULTS
A total of 259 women from the RCT were enrolled in the OLE trial. For the 200 women with moderate-to-severe cellulite who received ≥1 course of CCH treatment for cellulite in the OLE (Treatment Course 1; Fig. 2), 112 (56.0%) had previously received placebo in the RCT, and 88 (44.0%) were treated with CCH in the RCT [in the current study, receiving CCH treatment in the same area (n = 6; 3.0%) or a different area (n = 82; 41.0%) than that treated with CCH in the RCT]. Most of the 200 women were White (86.5%) and had a body mass index of ≥25 kg/m2 (71.5%); the mean age was 48.0 years (Table 1). A total of 75 of 112 women received a second CCH cellulite treatment course during the OLE trial [Treatment Course 2 (2 in the same area of cellulite and 73 in a different area)]. Forty-four of 200 women (22.0%) treated with CCH for cellulite during the OLE trial were discontinued from the study (Fig. 2).
Fig. 2.
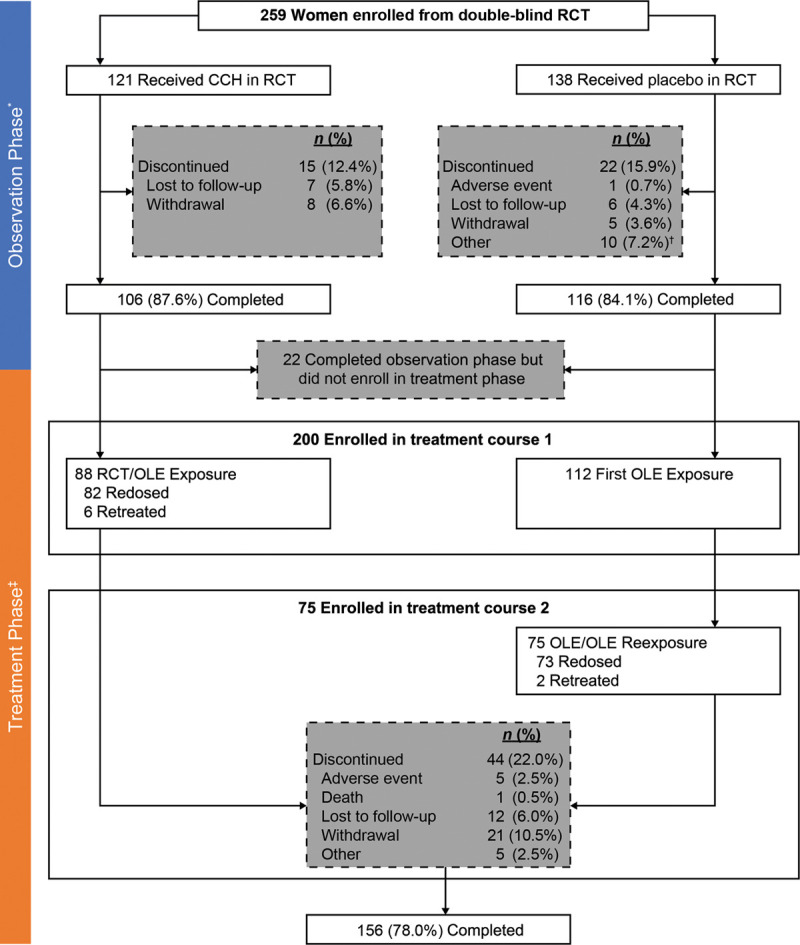
Patient disposition. *Observation Phase defined as the time period from RCT screening to first CCH treatment for cellulite in the OLE trial or to the end of OLE trial, if no treatment was received in OLE. †Included screening failures, women who declined to receive CCH in the OLE, study center closure during patient enrollment, or women noncompliant with study visits. ‡For women treated with CCH for cellulite in the RCT or OLE trial, the treatment area was assessed through Days 720 and 360, respectively, at 3-month intervals.
Table 1.
Baseline Demographics and Characteristics of Women Treated with CCH for Cellulite during the OLE
| Parameter | Women (n = 200)* |
|---|---|
| Mean age ± SD, y (range) | 48.0 ± 11.1 (19–71) |
| Race, n (%) | |
| White | 173 (86.5) |
| Black | 25 (12.5) |
| Asian | 2 (1.0) |
| Mean BMI ± SD, kg/m2 (range) | 29.3 ± 6.8 (16–56) |
| BMI category, n (%) | |
| Obese (≥30 kg/m2) | 69 (34.5) |
| Overweight (25 to <30 kg/m2) | 74 (37.0) |
| Normal (18.5 to <25 kg/m2) | 56 (28.0) |
| Underweight (<18.5 kg/m2) | 1 (0.5) |
*Received ≥1 dose of open-label CCH.
BMI, body mass index.
Safety
CCH for cellulite treatment was generally well tolerated. Of the 259 women enrolled, 59 reported ≥1 AE during the Observation Phase (from screening to the first treatment course or end of study if no treatment was received) of the OLE trial. There was one serious AE (spontaneous abortion) reported; the woman was treated with placebo during the RCT, and the serious AE occurred before CCH administration in the OLE study. In addition, there was 1 discontinuation due to an AE during observation; the woman had received placebo during the RCT.
For the 200 women treated with CCH for cellulite during the OLE trial (1 or 2 treatment courses), 182 (91.0%) women reported ≥1 AE; the most common treatment-emergent AEs were injection-site bruising and injection-site pain (Table 2). Mean duration (± SD) of the most common treatment-related AEs was 18.9 ± 22.0 days for 168 patients who reported injection-site bruising and 12.5 ± 16.7 days for 111 patients who reported injection-site pain. Few women (3.0%) discontinued due to an AE. None of the 4 serious AEs reported (appendicitis, breast cancer, hypertensive crisis, and vehicular-related death) were considered by investigators to be related to CCH treatment (Table 2). A total of 163 women received 2 CCH treatment courses for cellulite [n = 88 received the first CCH course during RCT and the second course during the OLE trial (Treatment Course 1); n = 75 received both CCH courses during the OLE trial (Treatment Courses 1 and 2)], and the AE profile was further assessed by course (Table 3). In general, the overall AE profiles were similar after each treatment course, and the AE profiles of these women were consistent with those observed in the population of 200 women. However, of note, the incidence of any AEs and AEs of injection-site bruising and injection-site pain decreased with the additional CCH treatment course (ie, Treatment Course 2). One woman (0.5%) treated with placebo during the RCT experienced a hypersensitivity reaction (ie, mild generalized rash) 1 day after the first dose of CCH for cellulite in the OLE, that was considered by investigators to be related to CCH treatment. The rash resolved following treatment with 25-mg oral diphenhydramine as needed, and CCH treatment was discontinued.
Table 2.
AE Profile among Women Treated with CCH for Cellulite during the OLE
| Women with an AE | Women, n (%) (n = 200)* |
|---|---|
| ≥1 AE | 182 (91.0) |
| ≥1 Serious AE | 4 (2.0)† |
| ≥1 AE leading to discontinuation | 6 (3.0) |
| Most common AEs (≥5.0% of women) | |
| Injection-site bruising | 170 (85.0) |
| Injection-site pain | 113 (56.5) |
| Injection-site nodules | 39 (19.5) |
| Injection-site pruritus | 33 (16.5) |
| Injection-site swelling | 19 (9.5) |
| Injection-site discoloration | 18 (9.0) |
| Upper respiratory tract infection | 11 (5.5) |
*Received ≥1 dose of CCH in OLE.
†1 woman had 1 event each of appendicitis, breast cancer, hypertensive crisis, or vehicular-related death; none were considered by investigators to be related to CCH treatment.
Table 3.
AE Profile among Women Who Received 2 CCH Treatments for Cellulite*
| Women with an AE | Women, n (%) | |
|---|---|---|
| First CCH Treatment (n = 163)† | Second CCH Treatment (n = 163)† | |
| ≥1 AE | 150 (92.0) | 138 (84.7) |
| Most common AEs (≥5.0% of women for either treatment) | ||
| Injection-site bruising | 138 (84.7) | 124 (76.1) |
| Injection-site pain | 84 (51.5) | 70 (42.9) |
| Injection-site pruritus | 20 (12.3) | 30 (18.4) |
| Injection-site nodules | 26 (16.0) | 17 (10.4) |
| Injection-site discoloration | 15 (9.2) | 7 (4.3) |
| Injection-site swelling | 7 (4.3) | 10 (6.1) |
*88 of 163 women received the first CCH treatment for cellulite during the double-blind study and the second treatment during the OLE; 75 women received both CCH treatments for cellulite during the OLE.
†Up to 3 treatment visits, each separated by 21 days.
Immunogenicity Testing
Data were obtained for both binding (ie, anti-drug) antibodies and neutralizing antibodies (binding antibody subset that neutralizes activity) related to AUX-I and AUX-II. Of the 19 women in the observation population in the current study (OLE) who received CCH treatment for cellulite during the RCT and were seropositive at day 71 of the RCT, 89.5% (n = 17) and 84.2% (n = 16) were seropositive for binding AUX-I and AUX-II antibodies, respectively, at day 360. For a subset of women who were seropositive at day 71 of the RCT and tested for neutralizing antibodies at day 360 of the OLE, 72.2% (13/18) and 39.1% (9/23) were positive for neutralizing AUX-I and AUX-II antibodies, respectively. Of the 200 women who received CCH treatment in Treatment Course 1, 99.4% (n = 161) and 100% (n = 162) were seropositive for binding AUX-I and AUX-II antibodies, respectively, at day 360. All women (100%) who were re-exposed to CCH (2 CCH treatment courses; n = 163) were seropositive for binding AUX-I and AUX-II antibodies on day 360 of the second treatment course. A decrease in the percentage of women who tested positive for neutralizing antibodies to AUX-I and AUX-II was observed between day 71 and day 360 post-treatment following 1 or 2 treatment courses with CCH (Table 4). No clinically relevant laboratory abnormalities or physical findings were observed in women who were seropositive for binding AUX-I and AUX-II antibodies or in women with neutralizing antibodies compared with seronegative women.
Table 4.
Seropositive Women with Neutralizing Antibodies following CCH Treatment for Cellulite
| Women with Neutralizing Antibodies, n/n (%) | 1 CCH Treatment Course | 2 CCH Treatment Courses | ||
|---|---|---|---|---|
| Day 71 | Day 360 | Day 71 | Day 360 | |
| AUX-I | 36/43 (83.7) | 18/37 (48.6) | 36/38 (94.7) | 15/36 (41.7) |
| AUX-II | 18/43 (41.9) | 5/42 (11.9) | 25/35 (71.4) | 3/35 (8.6) |
Durability of Response
A total of 53 women who were treated with CCH for cellulite in the double-blind RCT were included in the durability population. All 53 women were RCT ≥1-level composite responders (ie, ≥1-level improvement from RCT baseline in both CR-PCSS and PR-PCSS), of whom 45 had OLE assessments through 360 days. Nineteen of the women were RCT ≥2-level composite responders to CCH (ie, ≥2-level improvement from RCT baseline in both CR-PCSS and PR-PCSS), of whom 16 had OLE assessments through 360 days. All [100% (7/7)] ≥2-level composite responders (ie, women with improvement from baseline of ≥2 levels of severity in both the CR-PCSS and PR-PCSS) and 91.3% (21/23) of ≥1-level composite responders (ie, women with improvement from baseline of ≥1 levels of severity in both the CR-PCSS and PR-PCSS) had a durable response for up to 720 days after initial dosing in the RCT (Fig. 3). Example photographic images show durability of response through Day 720 in women with a 2-level composite response as a result of RCT CCH treatment of cellulite in the buttock and thigh (Fig. 4). For the ≥1-level composite responders, CCH response was durable in 94.3% at 180 days (n = 50/53), 95.6% at 360 days (n = 43/45), 95.7% at 540 days (n = 22/23), and 91.3% at 720 days (n = 21/23). The durability of treatment response seen in both ≥2- and ≥1-level composite responders who received treatment in the RCT was comparable to that of women receiving CCH treatment for cellulite during the OLE trial (Fig. 3). Of the 124 evaluable women in the OLE trial who received CCH treatment for cellulite, 100% of both ≥2-level and ≥1-level composite responders maintained a durable response at days 180 (≥2-level, 26/26; ≥1-level, 124/124) and 360 (≥2-level, 21/21; ≥1-level, 114/114).
Fig. 3.
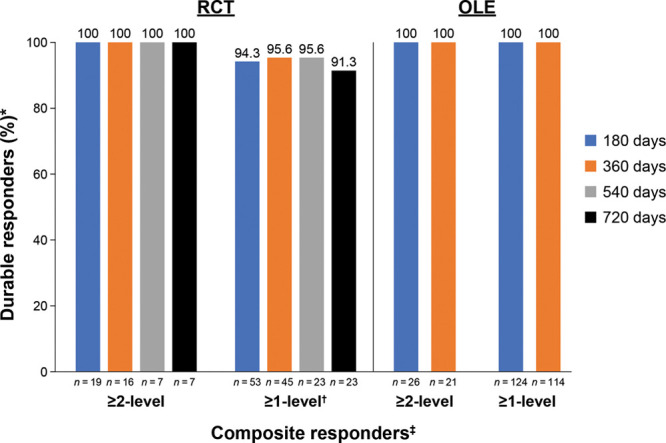
Durability of response to CCH treatment for cellulite. *Women whose CR-PCSS and PR-PCSS ratings did not return to baseline scores (or worse). †At days 180, 360, 540, and 720, there were 3, 2, 1, and 2 women, respectively, who did not achieve a durable response. ‡Women with ≥1-level and ≥2-level improvements from baseline in both CR-PCSS and PR-PCSS ratings.
Fig. 4.
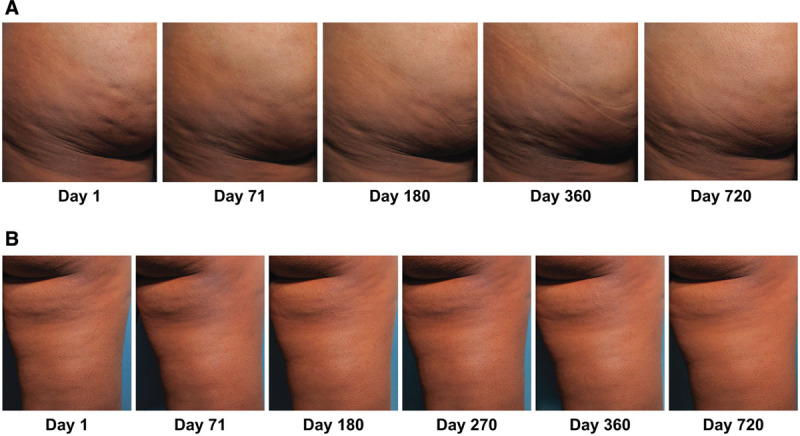
Photographs of a 2-level composite responder to CCH treatment (ie, 2-level improvement from RCT baseline at day 71 in both CR-PCSS and PR-PCSS) in the buttock (A) and thigh (B), with durability of response through Day 720. Reprinted with permission from Endo Pharmaceuticals Inc. © 2020 All rights reserved.
Efficacy
Of the 200 women who received ≥1 dose of CCH treatment for cellulite in the OLE trial, 193 had a baseline and ≥1 post-treatment cellulite severity assessment and were included in the efficacy population (Table 5). A total of 162 women received 2 CCH treatment courses for cellulite across the RCT and OLE trials and had evaluable efficacy data. At day 71, a >75% response rate for the population receiving CCH for 1 or 2 treatment courses was observed for each of the following measures: ≥1-level improvement in the CR-PCSS, ≥1-level improvement in the PR-PCSS, and I-GAIS and S-GAIS ratings of “very much improved”/“much improved”/“improved” (Tables 5 and 6). Improvement from baseline at day 71 was also observed in the CSS score (Tables 5 and 6) after 1 or 2 CCH treatment courses. After 1 or 2 CCH treatment courses, improvements observed at day 71 for all assessments were maintained through day 360 (Tables 5 and 6).
Table 5.
Improvement in Cellulite Severity among Women Treated with CCH for Cellulite during the OLE*
| Parameter | 1 CCH Treatment Course (n = 193) | ||
|---|---|---|---|
| Area treated, n | |||
| Buttock | 103 | ||
| Thigh | 90 | ||
| Baseline values, mean (SD) | |||
| CR-PCSS | 3.3 (0.4) | ||
| PR-PCSS | 3.4 (0.5) | ||
| CSS | 10.5 (1.4) | ||
| Post-Treatment Efficacy | Day 71 (n = 187) | Day 180 (n = 174) | Day 360 (n = 163) |
| CR-PCSS | |||
| Mean change from baseline (SD) | −1.0 (0.8) | −1.0 (0.8) | −1.0 (0.8) |
| Women with ≥2-level improvement, n (%) | 46 (24.6) | 37 (21.3) | 40 (24.5) |
| Women with ≥1-level improvement, n (%) | 139 (74.3) | 127 (73.0) | 121 (74.2) |
| PR-PCSS | |||
| Mean change from baseline (SD) | −1.1 (0.8) | −1.1 (0.8)† | −1.0 (0.8) |
| Women with ≥2-level improvement, n (%) | 53 (28.3) | 57 (32.6)† | 47 (28.8) |
| Women with ≥1-level improvement, n (%) | 148 (79.1) | 126 (72.0)† | 116 (71.2) |
| Mean change from baseline in CSS (SD) | −2.2 (2.2) | NR | −2.4 (2.4) |
| I-GAIS score, n (%) | |||
| Rating of “very much improved”/“much improved”/“improved” | 146 (78.1) | NR | 120 (73.2)‡ |
| S-GAIS rating, n (%) | |||
| Rating of “very much improved”/“much improved”/“improved” | 144 (77.0) | NR | 108 (66.3) |
| Patient satisfaction, n (%) | |||
| Rating of “very satisfied”/”satisfied” | 118 (63.1) | NR | 81 (49.7) |
NR, not reported.
*Women who received ≥1 dose of CCH for cellulite who had a baseline and ≥1 post-treatment CR-PCSS and PR-PCSS assessment.
†n = 175.
‡n = 164.
Table 6.
Improvement in Cellulite Severity after the First and Second CCH Treatment Courses for Cellulite*
| Parameter | First CCH Treatment (n = 162)† | Second CCH Treatment (n = 162)† | ||||
|---|---|---|---|---|---|---|
| Area treated, n | ||||||
| Buttock | 91 | 95 | ||||
| Thigh | 71 | 67 | ||||
| Baseline values, mean (SD) | ||||||
| CR-PCSS | 3.3 (0.5) | 3.2 (0.4) | ||||
| PR-PCSS | 3.4 (0.5) | 3.3 (0.5) | ||||
| Hexsel CSS | 10.7 (1.4) | 10.5 (1.4) | ||||
| Post-Treatment Efficacy | Day 71 | Day 180 | Day 360 | Day 71 | Day 180 | Day 360 |
| CR-PCSS | (n = 162) | (n = 160) | (n = 149) | (n = 159) | (n = 154) | (n = 142) |
| Mean change from baseline (SD) | −0.9 (0.8) | −0.9 (0.8) | −1.1 (0.9) | −1.0 (0.8) | −1.0 (0.8) | −0.9 (0.8) |
| Women with ≥2-level improvement, n (%) | 39 (24.1) | 31 (19.4) | 44 (29.5) | 40 (25.2) | 36 (23.4) | 28 (19.7) |
| Women with ≥1-level improvement, n (%) | 106 (65.4) | 105 (65.6) | 108 (72.5) | 115 (72.3) | 114 (74.0) | 103 (72.5) |
| PR-PCSS | (n = 162) | (n = 159) | (n = 147) | (n = 159) | (n = 155) | (n = 143) |
| Mean change from baseline (SD) | −1.2 (0.8) | −0.9 (0.9) | −1.0 (0.8) | −1.1 (0.8) | −1.0 (0.8) | −1.0 (0.8) |
| Women with ≥2-level improvement, n (%) | 55 (34.0) | 39 (24.5) | 39 (26.5) | 43 (27.0) | 43 (27.7) | 35 (24.5) |
| Women with ≥1-level improvement, n (%) | 124 (76.5) | 101 (63.5) | 111 (75.5) | 124 (78.0) | 111 (71.6) | 102 (71.3) |
| Hexsel CSS | (n = 162) | NR | (n = 149) | (n = 159) | NR | (n = 143) |
| Mean change from baseline (SD) | −2.1 (2.2) | −2.4 (2.5) | −2.2 (2.3) | −2.4 (2.4) | ||
| I-GAIS score, n (%) | (n = 162) | NR | (n = 149) | (n = 159) | NR | (n = 142) |
| Rating of “very much improved”/“much improved”/“improved” | 128 (79.0) | 108 (72.5) | 121 (76.1) | 102 (71.8) | ||
| S-GAIS rating, n (%) | (n = 162) | NR | (n = 148) | (n = 159) | NR | (n = 143) |
| Rating of “very much improved”/“much improved”/“improved” | 125 (77.2) | 101 (68.2) | 118 (74.2) | 94 (65.7) | ||
| Patient satisfaction, n (%) | (n = 162) | NR | (n = 148) | (n = 159) | NR | (n = 143) |
| Rating of “very satisfied”/”satisfied” | 105 (64.8) | 83 (56.1) | 101 (63.5) | 75 (52.4) | ||
NR, Not reported.
*Women who received ≥1 dose of CCH for cellulite who had a baseline and ≥1 post-treatment CR-PCSS and PR-PCSS assessment.
†Women who received 2 CCH treatment courses for cellulite, n = 87 from RCT and OLE (Treatment Course 1) and n = 75 from the OLE trial alone (Treatment Courses 1 and 2). One woman did not have a PR-PCSS score at Day 71 (Treatment Course 2) and was excluded from the efficacy analysis.
DISCUSSION
The results of this OLE trial of CCH for the treatment of cellulite further outline the safety and efficacy of CCH and support and extend the results of the RCT.12 CCH treatment of moderate-to-severe cellulite provided ≥1-level improvement in clinical and patient ratings in the majority (>75%) of women treated. CCH treatment provided durable improvement in cellulite appearance based on a follow-up of ~2 years. CCH efficacy, as assessed by the change in CR-PCSS and PR-PCSS, was similar after 1 or 2 treatment courses, which implies that tachyphylaxis was not observed following reexposure with CCH.
CCH treatment for cellulite was generally well tolerated, with a low rate (3.0%) of discontinuations due to AEs. The most common AEs were injection-site related, including injection-site bruising. During the trial, blood-thinning medications (ie, anticoagulant or antiplatelet therapy, except for low-dose aspirin) were not permitted to help mitigate the bruising intensity. Median duration was ≤15 days for the treatment-related AEs of injection-site pain and bruising. The safety profile (types and incidence of AEs) after Treatment Course 2 was comparable to that for Treatment Course 1 and to the overall safety profile observed in this OLE trial. In 163 women who received a second course of CCH treatment (eg, re-exposure to CCH), the second treatment course did not increase the incidence, seriousness, severity, or the relatedness of the AEs. A general safety concern for therapeutic proteins is the risk of triggering an immune response, which can result in the formation of anti-drug antibodies. These antibodies could negatively impact the clinical efficacy and safety profile by neutralizing therapeutic activity and/or driving hypersensitivity reactions during treatment.14 Of note, no severe hypersensitivity reactions to CCH were reported in this OLE trial, and the number of women with neutralizing antibodies declined over the course of the study. Importantly, there were no apparent clinical differences in efficacy outcomes or AE profile after seroconversion, and no clinically relevant findings of concern were observed in women seropositive for binding AUX-I and/or binding AUX-II antibodies or in women with neutralizing antibodies. In addition, CCH efficacy was similar after 1 or 2 treatment courses, which implies that tachyphylaxis was not observed following reexposure to CCH.
Strengths of the study are that it was a long-term (~2 years) multicenter study with an opportunity to assess efficacy, safety, and long-term durability following repeated exposure to CCH injection. Another strength is the inclusion of women with moderate-to-severe cellulite. In addition, the study includes multiple rating scales for cellulite, encompassing both clinical and patient perspectives, to assess CCH treatment of cellulite. Several different cellulite rating scales have been used in clinical trials, including the Hexsel CSS, I-GAIS, and S-GAIS, but there is currently no standardized, universally accepted measure of cellulite severity. The CR-PCSS and PR-PCSS, included in this study, were developed in accordance with the US Food and Drug Administration guidance on the use of patient-reported outcome measures,15 which emphasizes the importance of using validated instruments. The CR-PCSS has been shown to correlate with the Hexsel CSS and the I-GAIS, and the PR-PCSS also showed a significant correlation with patient ratings of aesthetic changes in the S-GAIS.16 In addition, the 2 scales have been shown to correlate with each other, both overall and in different target regions (buttocks and posterolateral thighs).16 Limitations of the study are the open-label study design, the lack of a comparator group, the small number of patients treated in the RCT with data for the long-term durability assessments, and the patient dropout rate, which is typically seen with long-term follow-up studies.
Further research of CCH for the treatment of cellulite is warranted, and two phase 3 studies of CCH for the treatment of cellulite of the buttocks have been completed (ClinicalTrials.gov identifiers: NCT03446781 and NCT03428750). In conclusion, this long-term study supports that CCH for cellulite is efficacious, generally well tolerated, and provides durable improvement as a treatment for cellulite in women for up to 2 years postinjection. Repeated exposure to CCH injection for cellulite does not appear to increase the risk of AEs, result in hypersensitivity reactions, or reduce treatment efficacy.
ACKNOWLEDGMENTS
Technical editorial and medical writing assistance was provided, under the direction of the authors, by Mary Beth Moncrief, PhD, and Julie B Stimmel, PhD, Synchrony Medical Communications, LLC, West Chester, Pa. Funding for this assistance was provided by Endo Pharmaceuticals Inc., Malvern, Pa.
Footnotes
Published online 23 December 2020.
These data were presented in part at the Orlando Dermatology Aesthetic and Clinical Conference, January 18–21, 2019, Orlando, Fla.; at the 43rd Annual Hawaii Dermatology Seminar; February 17–22, 2019, The Big Island, Hawaii; and at Vegas Cosmetic Surgery, June 5–8, 2019, Las Vegas, Nev.
Disclosures: Joely Kaufman-Janette has served on advisory boards for Allergan plc (acquired by AbbVie Inc.), Bonti, Inc. (acquired by Allergan plc), Endo Pharmaceuticals Inc., Evolus, Inc., Galderma, Johnson & Johnson Consumer Inc., and Lutronic; has received payment for research from Allergan plc, Endo Pharmaceuticals Inc., Galderma, Revance Therapeutics, Inc., Evolus, Inc., Merz North America, Teoxane Laboratories, and Croma-Pharma GmbH; and has served as a clinical investigator and consultant for Endo Pharmaceuticals Inc. Lawrence Bass reports being a consultant for Galderma Laboratories, L.P., and LG Chem; a consultant and investigator for Cynosure, A Hologic Company, and Merz North America; and has served on the advisory board and been a clinical investigator for Endo Pharmaceuticals Inc. M Todd Kirby is a former employee of Endo Pharmaceuticals Inc. He is currently an employee of Jazz Pharmaceuticals plc and an adjunct faculty member of the Department of Psychiatry and Health Behavior, Medical College of Georgia, Augusta University. Qinfang Xiang, Michael P McLane, and Saji Vijayan are employees of Endo Pharmaceuticals Inc.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cosmet Investig Dermatol. 2017;10:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avram MM. Cellulite: a review of its physiology and treatment. J Cosmet Laser Ther. 2005;6:181–185. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph C, Hladik C, Hamade H, et al. Structural gender-dimorphism and the biomechanics of the gluteal subcutaneous tissue – implications for the pathophysiology of cellulite. Plast Reconstr Surg. 2019;143:1077–1086. [DOI] [PubMed] [Google Scholar]
- 4.Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221–229. [DOI] [PubMed] [Google Scholar]
- 5.Pereira de Godoy JM, Guerreiro de Godoy MF. Physiopathological hypothesis of cellulite. Open Cardiovasc Med J. 2009;3:96–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251–262. [DOI] [PubMed] [Google Scholar]
- 7.Luebberding S, Krueger N, Sadick NS. Cellulite: an evidence-based review. Am J Clin Dermatol. 2015;16:243–256. [DOI] [PubMed] [Google Scholar]
- 8.Davis DS, Boen M, Fabi SG. Cellulite: patient selection and combination treatments for optimal results—a review and our experience. Dermatol Surg. 2019;45:1171–1184. [DOI] [PubMed] [Google Scholar]
- 9.French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry. 1987;26:681–687. [DOI] [PubMed] [Google Scholar]
- 10.QWO (collagenase clostridium histolyticum-aaes) for injection, for subcutaneous use [package insert]. 2020Malvern, PA: Endo Aesthetics LLC; [Google Scholar]
- 11.Xiaflex (collagenase clostridium histolyticum) for injection, for intralesional use [package insert]. 2019Malvern, PA: Endo Pharmaceuticals Inc; [Google Scholar]
- 12.Sadick NS, Goldman MP, Liu G, et al. Collagenase Clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (cellulite): a randomized trial. Dermatol Surg. 2019;45:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hexsel DM, Dal’forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2009;23:523–528. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg AS, Sauna ZE. Immunogenicity assessment during the development of protein therapeutics. J Pharm Pharmacol. 2018;70:584–594. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Available at https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed December 3, 2020. [DOI] [PMC free article] [PubMed]
- 16.Young VL, Sadick N, Liu G, et al. Comparisons of clinician-reported and patient-reported cellulite severity scales with existing scales for measurement of cellulite severity. Paper presented at: Northeastern Society of Plastic Surgeons 34th Annual Meeting; September 8–10, 2017; Newport, RI. [Google Scholar]


