Abstract
Insulin signaling is critical for neuroplasticity, cerebral metabolism as well as for systemic energy metabolism. In rodent studies impaired brain insulin signaling with resultant insulin resistance (IR) modulates synaptic plasticity and the corresponding behavioral functions. Despite discoveries of central actions of insulin, in-vivo molecular mechanisms of brain IR until recently has proven difficult to study in the human brain. In the current study, we leveraged recent technological advances in molecular biology and herein report an increased number of exosomes enriched for L1CAM, a marker predominantly expressed in the brain, in subjects with major depressive disorder (MDD) as compared to age- and sex-matched healthy controls (HC). We also report increased concentration of the insulin receptor substrate-1 (IRS-1) in L1CAM+ exosomes in subjects with MDD as compared to age- and sex-matched HC. We found a relationship between expression of IRS-1 in L1CAM+ exosomes and systemic IR as assessed by homeostatic model assessment of IR in HC, but not in subjects with MDD. The increased IRS-1 levels in L1CAM+ exosomes were greater in subjects with MDD and were associated with suicidality and anhedonia. Finally, our data suggested sex differences in serine-312 phosphorylation of IRS-1 in L1CAM+ exosomes in subjects with MDD. These findings provide a starting point for creating mechanistic framework of brain IR in further development of personalized medicine strategies to effectively treat MDD.
Introduction
There is substantial evidence suggesting that insulin regulates neuroplasticity and cerebral metabolism in addition to systemic energy metabolism1–4. Therefore, a precise modulation of the insulin signaling is important for both adaptive brain and systemic functions1–3. A series of studies by our and other groups implicated a systemic metabolic dysfunction known as insulin resistance (IR) in the pathophysiology and treatment of disorders of mood and cognition, including major depressive disorder (MDD)5, 6. A prominent component of IR is hyperinsulemia with subsequent failure of β-cells to respond to the actions of insulin7. The abnormal accumulation of plasma insulin is reflective of both decreased hippocampal volume and aberrant intrinsic connectivity of corticohippocampal circuits in subjects with MDD6, 8–10. Systemic hyperinsulinemia is also associated with childhood trauma in subjects with MDD11, further supporting the role of systemic IR in the pathophysiology of MDD. Interestingly, previous studies also showed that insulin-sensitizing agents improve depressive symptoms12–15. Unlike the role of systemic IR11, 16, in-vivo mechanisms of brain insulin action in the neurobiology of MDD remain to be determined.
Previous studies showed widespread localization of insulin receptor in brain areas important for mood and cognition, including the hypothalamic and hippocampal regions17, 18. Several mechanisms may be involved in regulation of central insulin signaling19, 20. A key molecular marker is the ‘docking protein’ insulin receptor substrate-1 (IRS-1), which plays an important regulatory role in triggering signal transduction pathways to mediate the actions of insulin21. The IRS-1 protein is also positioned to serve as a hub activating negative feedback loop at different steps of the insulin cascade to propagate the actions of insulin on brain functions21. Furthermore, increased phosphorylation of IRS-1 and corresponding desensitization of cells to respond to insulin further propagates brain IR21,22. Studies in rodent models of brain IR showed neuroplasticity deficits, including aberrant hippocampal glutamatergic transmission and impaired long-term potentiation (LTP), as a result of inhibition of brain insulin signaling2. Previous studies also showed cerebral hypometabolism, a postulated proxy of brain IR as assessed by positron emission tomography, in subjects with MDD6. Despite these breakthrough discoveries of central actions of insulin, until recently, in-vivo molecular mechanisms of central IR were difficult to study in the human brain and their assessment has been limited to post mortem brain tissues or proxies of brain IR23, 24.
The recent technological advances in molecular biology to determine the role of exosomes in diseases of the central nervous system (CNS) opened up the possibility to identify in-vivo molecular mediators of insulin signaling in the human brain otherwise inaccessible, thus overcoming the limitations of previous methodologies25, 26. Exosomes are extracellular nanovesicles (20nm to 100nm diameter) secreted from all cells and released to the circulation to mediate brain-body communication27. Recent insight from studies of brain tumor and cognitive disorders showed that circulating brain-enriched exosomes (L1CAM+) carry a constitutive protein cargo reflecting physiological and pathological states, suggesting that brain-enriched exosomes and their molecular fingerprint could serve as non-invasive biomarkers for those diseases26–30. While the new technology of exosomes has been largely used in studies of brain tumor and cognitive disorders26–30, its application to the neurobiology of MDD is novel. By utilizing this innovative technology, we aimed to study in-vivo central insulin signaling in subjects with MDD and age- and sex-matched controls.
Methods
The Rockefeller University Institutional Review Board and the respective Institutional Review Boards of the collaborating Institutions approved the current study.
Primary and secondary study questions
The primary study question was to determine whether i) brain-enriched exosomes and ii) in-vivo brain IR as assessed by expression of both IRS-1 and Serine-312 phosphorylated IRS-1 (pSer-IRS-1) differ between subjects with MDD and respective controls. Subsequent post-hoc analyses aimed at examining potential i) sex-differences in both IRS-1 and pSer-IRS-1 in L1CAM+ exosomes as well as ii) a link between brain insulin signaling and severity of depressive symptoms.
Participants
Briefly, following an initial phone screen, potential participants were evaluated in person in order to determine study eligibility. All participants determined to be eligible to join the study provided written informed consent prior to study enrollment. Study participants, ranging between 20 and 70-years-old, were recruited at the Department of Psychiatry & Behavioral Sciences at Stanford University and the Mood and Anxiety Disorders Program at the Icahn School of Medicine at Mount Sinai. At both study sites, study clinicians or trained coordinators conducted the Structured Clinical Interview for DSM-IV (SCID) or Mini International Neuropsychiatric Interview (MINI) to confirm MDD diagnosis and rule out exclusionary co-morbid conditions. Inclusion criteria included a primary diagnosis of MDD in a current major depressive episode. For this study as for our previous report31, exclusion criteria included a presence of neurologic or other physical illness, such as diabetes, alcohol or substance abuse in the last six months, or an unstable medical illness. A total of 12% of subjects showed psychiatric comorbidities, such as post-traumatic stress disorder, generalized anxiety disorder and bipolar disorder (SI Table 1). Current medication use was assessed at screening for all study participants. Participants were free of current substances of abuse as determined by a urine toxicology test at the time of screening. Healthy controls (HC) were free of lifetime psychiatric illness and significant medical conditions. Participants were free of active infections and systemic illness as confirmed by medical history at the time of study evaluation. Blood samples were obtained via antecubital venous collection using standard techniques and were drawn after a period of fasting (>6 hours). Participants were asked not to exercise for >6 hours before blood draw. Further information for this study cohort was reported in previous papers11, 31, 32.
Clinical and Psychiatric assessment
Clinical assessment consisted of a physical examination, including measures of height, weight and BMI. Other data collected included current medication use and history of failed antidepressant trials. Demographic information, including sex, was also recorded from the participants. The psychiatric examination at screening included SCID or MINI to confirm MDD diagnosis. Trained raters administered the structured depression rating scales: 21-item Hamilton Depression Rating Scale (HDRS-21). With regard to medication use, 27 patients were free of antidepressant medications at the time of study participation and 33 patients were on psychotropic medications. Among the subjects with MDD who were on psychotropic medications, we report antidepressant (51%), mood stabilizers (26%) antipsychotics (26%), other (31%). LAC levels were measured as in our previous publication31.
Assessment of systemic insulin resistance
The status of systemic insulin resistance (IR) was defined accordingly to the following criteria that are in use by the NIH and the World Health Organization (WHO)33, 34: i) Homeostatic Model Assessment of Insulin Resistance (HOMA) greater than 1.9, and ii) BMI greater than 25. The HOMA model is based on measures of fasting plasma glucose (FPG) and insulin (FPI) and is calculated as follows: (fasting glucose * fasting insulin)/405. Previous studies determined the accuracy and precision of the model by comparing it with independent measures of insulin resistance and β-cell function using hyperglycemic and euglycemic clamps and an intravenous glucose tolerance test. The status of IR as assessed by using the HOMA model was highly correlated with measures of IR obtained by use of the euglycemic clamp and the hyperglycemic clamp.
In-vivo assessment of secretion and molecular cargo of L1CAM+ exosomes
Workflow for the isolation, enrichment and analysis of exosomes from human plasma is schematized in Figure 1A. Total circulating exosomes and brain-enriched exosomes were isolated using protocols described in previous papers29, 30 with some modifications. Briefly, 500uL of plasma samples from all experimental conditions were mixed with a protease and phosphatase inhibitor cocktail followed by centrifugation and pre-treatment with thrombin to convert fibrinogen to fibrin and subsequently clear it by centrifugation. Total circulating exosomes were isolated by using a precipitation technology based upon a polymer net for capturing nanovesicles with a diameter <100nm, followed by ultracentrifugation as described in the ExoQuick protocol with minimal modifications (Exoq5tm-1, System Biosciences). The initial step with polymer precipitation was used to rule out the presence of other extracellular vesicles, such as microvesicles, with a diameter greater than 100nm. Specifically, this technique is based on the formation of a mesh-like net with pores of a size up to 100 nm allowing to capture exosomes (vesicles with a size <100nm) while filtering out microvesicles (vesicles with a size from 100nm to 1μm). Isolation of total circulating exosomes was validated by co-presence of several known exosomal markers by using an expression array and following the manufacturer protocol (Exo Check Exosome Antibody Array, System Biosciences, Exoray210a-8). In brief, 50μg of human exosomes were loaded in the array, which has 12 pre‐printed spots including 8 antibodies for known exosome markers (CD63, CD81, ALIX, FLOT1, ICAM1, EpCam, ANXA5 and TSG101). A secondary detection mixture is conjugated to enzyme horseradish peroxidase (HRP). Further validation was performed by the presence in the array of 4 controls (two positive controls, a blank, and the GM130 cis‐Golgi marker to monitor for any cellular contamination). The latter also validated the accuracy of the exosome isolation as showed by the low staining for GM130, thus confirming minimal cellular contamination from cellular vesicles (Fig.1B). We thus isolated a specific subgroup of extracellular vesicles (i.e.: exosomes), and consistently refer to them as exosomes. Subsequently, magnetic beads conjugated with the L1CAM protein (Monoclonal Ab, Thermofisher, Catalog # 13-1719-82) were used for enrichment of L1CAM+ (brain-specific) exosomes. L1CAM is highly expressed in the brain and present on the exosomal surface, allowing the tag and immunoprecipitation of brain-enriched exosomes35. We used the cell marker L1CAM because it is the state-of-the-art used to isolate exosomes (or extracellular vesicles) that are enriched, albeit not selective, for brain origin36 (SI Figure 1). Extant literature supports the assumption of L1CAM being enriched in the brain27–30, 37–42. Available data from three independent datasets37–39 support the enrichment of L1CAM in brain cells as showed by the high protein expression of L1CAM in brain regions (in yellow) as compared to other organs, including immune and endocrine systems (dark red and purple) (SI Figure 1). The enrichment of L1CAM for the brain is also supported by previous rodent studies that showed neuronal origin of L1CAM+ extracellular vesicles isolated from the plasma of transgenic mouse lines expressing the green fluorescent protein on a nestin promoter expressed in brain cells30. In addition, in vitro experiments also showed high L1CAM expression in exosomes derived from cultured neurons40. Western blot experiments confirmed the presence of L1CAM proteins (mAb, Thermofisher, Catalog #13–1719-82, secondary antibody Abcam HRP) in brain-enriched exosomes and the absence of signaling in negative control samples (blank: supernatant material) (Fig.1C).
Assessment of numbers of total and brain-enriched exosomes
To measure the number of total and brain-enriched exosomes, we used ExoCet because it requires low input of starting plasma material, which is a critical factor for clinical studies, and more importantly, the colorimetric assessment of AChE, an enzyme enriched in exosomes, is based upon a number of standards calibrated using NanoSight analysis, thus enabling a highly quantitative measure of the number of exosomes in human samples while at the same time liming the amount of input (Exocet96a-1, System Biosciences). Protein concentration of both IRS-1 and pSerIRS-1 (specifically serine residue 312 of IRS-1) was measured by utilizing ELISA assays (Thermofisher, Catalog # KHO0521; Amsbio, AMS.E-EL-H5554). All groups were evenly divided between the experimental plates to account for any inter-plate variability. A couple of samples in each experiment felt outside the detection range of the respective assays, consistently those values were not used for the analyses. Keeping those values, the results were even stronger. Consistently with previous literature in exosome research43, we report measures of both IRS-1 and pSer-IRS-1 concentrations in L1CAM+ exosomes, thus no normalization was performed. Both measures in each assay were calibrated against at least 5 internal standards.
Statistical analyses
Statistical analyses were conducted using JMP software from SAS (Statistical Analysis System, Institute, Cary, NC, USA). Two-tailed t-test, chi-square test and exact test were used to compare, respectively, continuous and categorical demographic and clinical characteristics between subjects with MDD and controls. To account for the unequal sample size and variance of the MDD and HC groups, we performed statistical analyses, including Welch’s t-Test as needed. Within-group Pearson correlations were conducted to examine the relationship between in-vivo exosomal molecular measures and severity of depressive symptoms. Multiple regression analysis was used to control for other clinical characteristics.
Results
Increased secretion of L1CAM+ exosomes in subjects with MDD as compared to age and sex-matched controls
In a sample of 93 participants, no differences were observed between subjects with MDD (n=64) and healthy controls (n=29) with respect to demographic characteristics, including height, weight and sex (SI Table 1). All MDD subjects were in a depressive episode during study participation. To identify in-vivo molecular mechanisms otherwise inaccessible in the human brain, we isolated exosomes enriched for brain origin from the plasma (Fig.1A) and characterized their molecular cargo with regard to markers of the insulin signaling important for regulation of neuroplasticity among other physiological functions. As fully described in the method section, we first used an initial step with polymer precipitation, which captures exosomes (vesicles with a size <100nm) while filtering out other extracellular vesicles, such as microvesicles, which have a larger diameter in the range of 100nm to 1μm. Subsequently, we confirmed isolation of exosomes by co-presence of several known exosomal markers that include ICAM, Alix, CD81, CD63, EpCAM, ANXA5, TSG101 (Fig.1B). The exclusion of cellular contamination own to cis-Golgi vesicles was confirmed by the low staining for GM130 (Fig.1B). Collectively, these results lend support of the isolation of a specific subgroup of extracellular vesicles (i.e.: exosomes), and consistently refer to them as exosomes. A key feature of brain-enriched exosomes is that they express the membrane surface marker, cell adhesion molecule-1 (L1CAM), which allows their identification and isolation from the plasma of a subject29, 30. Thus, we next used magnetic beads conjugated with the L1CAM protein to enrich for brain origin. Western blot experiments confirmed the isolation of L1CAM+ exosomes (Fig.1C).
Figure 1. Increased number of L1CAM+ exosomes in subjects with MDD as compared to controls.
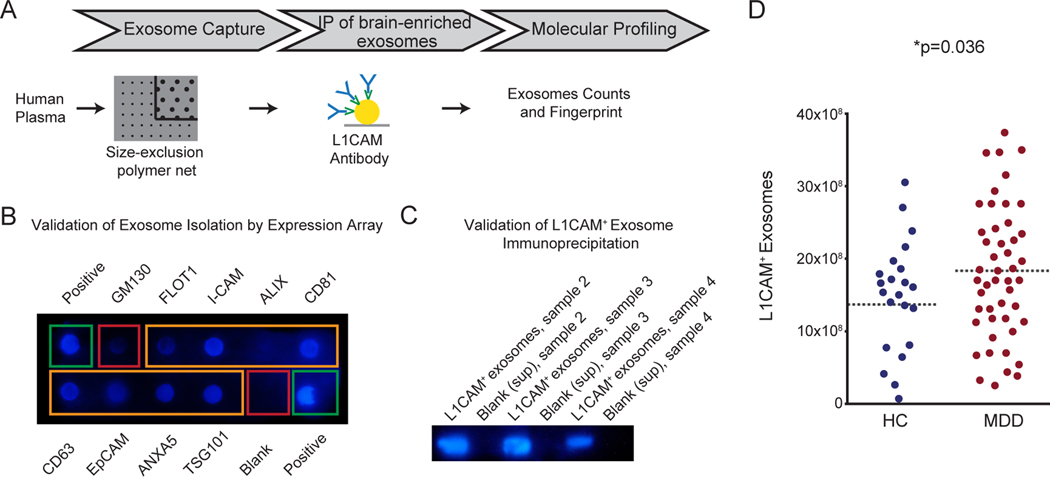
(A) Workflow for the isolation, enrichment and analysis of exosomes from human plasma. Magnetic beads were used for enrichment of L1CAM+ (brain-enriched) exosomes based on the immunoprecipitation of the L1CAM target bound to the exosomal surface. (B) Expression array validated the accuracy of the exosome isolation and confirmed the exclusion of cellular contamination from cellular vesicles as showed by the low staining for GM130, a marker for cis-Golgi vesicles. In addition to positive (green rectangular) and negative (red rectangular) controls, the expression array detected the following known exosome markers (orange rectangular) in our set of samples: Flot-1, ICAM, Alix, CD81, CD63, EpCAM, ANXA5, TSG101. (C) Western blot analysis confirmed isolation of L1CAM+ exosomes. (D) Number of L1CAM+ exosomes from subjects in depressive episode during study participation (n=48) as compared to controls (n=23). * indicates significant comparisons with controls. Dashed bars indicate group mean. See also related Figure SI1.
Next, we quantified the number of L1CAM+ exosomes in subjects with MDD and age- and sex-matched controls. The mean number of L1CAM+ exosomes was higher in the group of subjects with MDD as compared to controls (Fig.1D, p=0.036, HC: 14.1*108±21.8, MDD: 17.7*108±18.02). The relationship between the diagnosis and L1CAM+ exosomes held upon multiple linear regression analysis controlling for age and sex (t=−2.14). No sex-difference is observed in the increased number of L1CAM+ exosomes in subjects with MDD (HC men: 13.8*108±33.6, MDD men: 17.3*108±25.1; HC women: 14.3*108±21, MDD women: 18*108±14). The use of psychotropic medications did not alter the number of brain-enriched exosomes (MDD drug-free: 18.1*108±18, MDD on drug: 17.2*108±18.4).
Furthermore, no significant difference was observed in total circulating exosomes between controls and subjects with MDD (SI Figure 2, p=0.9, HC: 3.4*1010±321.9, MDD: 3.5*1010±358.5). Therefore, we focused our subsequent analyses on characterizing the molecular fingerprint specifically related to L1CAM+ exosomes.
In-vivo IRS-1 expression in L1CAM+ exosomes differs between subjects with MDD and controls
Next, we characterized the in-vivo concentrations of IRS-1 in L1CAM+ exosomes (Fig.2A-B) by using enzyme-linked immunosorbent assays. In-vivo expression of IRS-1 differed significantly between controls and subjects with MDD (Fig.2B) with mean concentrations higher in the group of subjects with MDD (Fig.2B, p=0.003, HC: 5.4*10–7ng/mL ±0.2, MDD: 6.4*10–7ng/mL ±0.2), suggesting an enrichment and elevated turnover of IRS-1 in subjects with MDD. The relationship between MDD and in-vivo IRS-1 expression held upon multiple regression analysis controlling for age and sex (t=−3.10). No sex-difference is observed in the increased concentration of IRS-1 in subjects with MDD (HC men: 13.8*108±33.6, MDD men: 5.3*108±0.3; HC women: 6*108±0.2, MDD women: 6.6*108±0.2). Within the group of subjects with MDD, no difference was observed in IRS-1 expression in L1CAM+ exosomes in relation to use of psychotropic medications (F2,60=1.4, p=0.28).
Figure 2. Increased in-vivo IRS-1 protein expression in L1CAM+ exosomes in subjects with MDD as compared to age- and sex-matched controls.
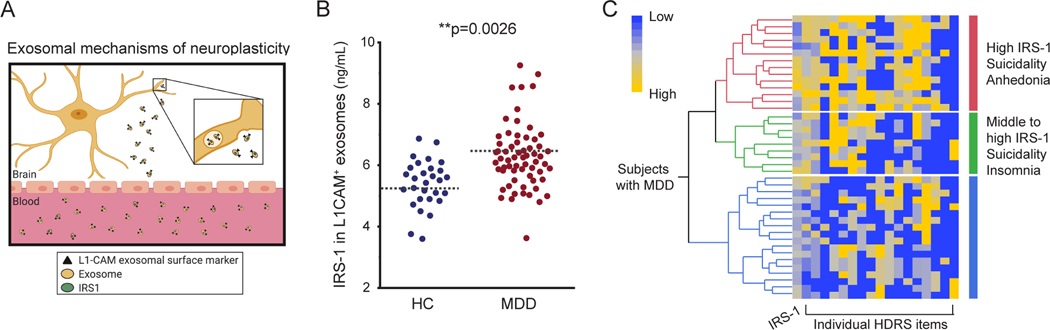
(A) Mechanistic model of the proposed mechanistic framework in disorders of mood and cognition: the ‘docking protein’ insulin receptor substrate-1 (IRS-1) is critical for triggering downstream signal transduction pathways to mediate insulin functions on neuroplasticity. (B) Enzyme-linked immunosorbent assessment of protein expression of the insulin receptor substrate-1 (IRS-1), a modulator of neuroplasticity, in subjects in depressive episode during study participation (n=64) as compared to controls (n=29). See also SI Table 1 for demographic and clinical characteristics of the sample of 93 subjects. * indicates significant comparisons with controls. Dashed bars indicate group mean. (C) The increased level of IRS-1 in L1CAM+ exosomes is greater in subjects with MDD and is associated with suicidality and anhedonia (cluster in red).
Relationship between systemic and brain IR in healthy controls but not in subjects with MDD
First, our data showed that the rate of systemic IR, as assessed by the Homeostatic Model Assessment of Insulin Resistance (HOMA), differed between controls and subjects with MDD with proportion of systemic IR greater in the group of subjects with MDD as compared to the group of HC (53% among subjects with MDD, and 37% among HC, p=0.005). Within the group of controls, the increased expression of IRS-1 in L1CAM+ exosomes correlated with systemic IR (p=0.04, r=0.4). The relationship between systemic IR and the expression of IRS-1 in the group of controls held upon multiple regression analysis controlling for age and sex (t=0.46, t=−1.14, Figure SI 3A). Within the group of subjects with MDD, no relationship was observed between expression of IRS-1 in L1CAM+ exosomes and systemic IR (p=0.8, r=0.03, Figure SI 3B).
In-vivo IRS-1 expression in L1CAM+ exosomes within the MDD group
Within the group of subjects with MDD, no significant relationship was observed between levels of IRS-1 in L1CAM+ exosomes and severity of depressive symptoms as assessed by the total score at the HDRS-21 (p=0.67, r=0.1, SI Figure 4). Next, we tested whether the increased expression of IRS-1 in L1CAM+ exosomes of subjects with MDD was associated with specific symptoms of depression as assessed by the individual items at the HDRS-21. We used hierarchical clustering analyses for a qualitative assessment of this relationship. Our data showed that the highest levels of IRS-1 in L1CAM+ exosomes were associated with depressed mood, feelings of guilt, suicidality and anhedonia, while the association of these symptoms was lower within the group of subjects with MDD and the lowest levels of IRS-1 in L1CAM+ exosomes. Subjects with MDD and middle levels of IRS-1 in L1CAM+ exosomes showed mainly insomnia-related symptoms (Fig. 2C).
Sex-difference in phosphorylated IRS-1 in L1CAM+ exosomes in MDD
Next, we determined in-vivo expression levels of serine-312 phosphorylated IRS-1 (pSer-IRS-1) in L1CAM+ exosomes. We found no between-group difference in pSer-IRS-1 in L1CAM+ exosomes, but our data showed an effect of sex in pSer-IRS-1 in L1CAM+ exosomes. Specifically, we found increased expression levels of pSer-IRS-1 in L1CAM+ exosomes with mean concentrations higher in women with MDD, but not men, as compared to age and sex-matched controls (Fig.3A. Women (pink): p=0.02, HC: 31.5*10–7ng/mL ±1.8, MDD: 37.6*10–7ng/mL ±1.8. Men (blue): p=0.3, HC: 35.1*10–7ng/mL ±1.7, MDD: 38.1*10–7ng/mL ±2.3). Given the above findings of sex-specific increase in phosphorylation of IRS-1 in women, but not men, with MDD, we evaluated the contribution of clinical characteristics such as severity of depressive symptoms on pSer-IRS-1 levels by performing secondary analyses. Our data showed a significant sex-specific positive correlation in that the greater the severity the higher the levels of pSer-IRS-1 in L1CAM+ exosomes of women, but not men, with MDD (Fig.3B, women: p=0.01, r=0.5; SI Figure 5, men: p=0.9, r=0.4). This relationship was significant upon multiple regression analysis controlling for use of psychotropic medications (t=2.58).
Figure 3. Sex-specific differences in phosphorylation of IRS-1 in L1CAM+ exosomes of subjects with MDD.
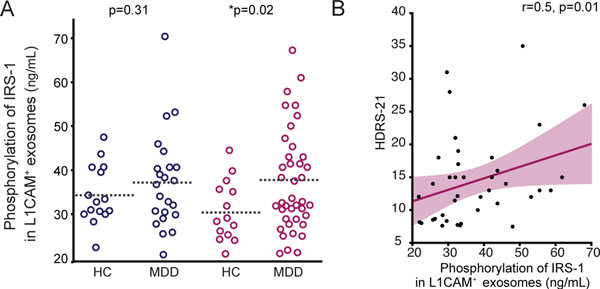
(A) Enzyme-linked immunosorbent assessment of in-vivo levels of phosphorylation of IRS-1 (pSer-IRS-1) in L1CAM+ exosomes in subjects in depressive episode during study participation (n=64) as compared to controls (n=29). These data shows a sex-specific increase of pSer-IRS-1 in L1CAM+ exosomes with mean concentrations higher in women with MDD, but not men, as compared to sex-matched controls (Men (blue): p=0.3, HC: 35.1*10–7ng/mL ±1.7, n=15; MDD: 38.1*10–7ng/mL ±2.3, n=23. Women (pink): p=0.02, HC: 31.5*10–7ng/mL ±1.8, n=14; MDD: 37.6*10–7ng/mL ±1.8, n=40). Sex was not reported for 1 subject. (B) Increased in-vivo pSer-IRS-1 levels in women who exhibit greater severity of depressive symptoms. Regression analysis between pSer-IRS-1 concentrations and depression severity of depressive symptoms as assessed at the HDRS-21. Regression line is indicated in dark pink. * indicates significant comparisons with controls. Dashed bars indicate group mean.
Discussion
Herein, we report in-vivo evidence for brain insulin resistance in subjects with major depressive disorder (MDD) with sex-differences in the relationship with severity of depressive symptoms. The current findings also show the utility of brain-enriched exosomes harvested from the peripheral blood of a patient for the study of the neurobiology of affective diseases, such as depression.
The increased level of brain-enriched exosomes (L1CAM+ exosomes) in subjects with MDD is important because the release of exosomes and their intercellular communication is regulated by glutamate neurotransmission. Previous studies in rodents showed a link between higher secretion of exosomes and increased synaptic glutamatergic activity41, 44. Sustained overflow of synaptic glutamate has been shown to lead to impaired dendritic plasticity of the hippocampus and connected brain areas key to mood regulation45, 46. Moreover, exosomes are endogenous nanovesicles carrying biological material, including proteins critical for physiological functions and key to the pathophysiology of MDD, such as neurogenesis, synaptogenesis and neuroplasticity27. Previous studies showed the presence of subunits of metabotropic glutamate receptor of class-2 (mGluR2), a key marker of neuroplasticity, in L1CAM+ exosomes27. Taken together with the finding of decreased levels of acetyl-L-carnitine (LAC), a modulator of mGluR2 expression and function, the current results suggest that exosomes might represent a novel way for the regulation of glutamate-related neuroplasticity in affective diseases, such as depression.
Available data from three independent datasets37–39 support the enrichment of L1CAM in brain cells as showed by the high protein expression of L1CAM in brain regions (in yellow) as compared to other organs, including immune and endocrine systems (dark red and purple) (SI Figure 1). The enrichment of L1CAM for the brain is also supported by previous rodent studies that showed neuronal origin of L1CAM+ extracellular vesicles isolated from the plasma of transgenic mouse lines expressing the green florescent protein on a nestin promoter expressed in the nervous system30. Early research also demonstrated high L1CAM expression in exosomes derived from cultured neurons40. Our results showed changes in L1CAM+ exosomes but not total circulating exosomes in subjects with MDD. However, yet it is possible that the heterogeneity of the pool of total circulating exosomes (i.e.: exosomes released from all cells, and thus reflecting more systemic physiology) might have masked some other organ-specific relationships. Thus, future studies are warranted to determine the role other cellular types of exosomes and their molecular cargo in MDD.
The findings of accumulation of IRS-1 in L1CAM+ exosomes in subjects with MDD might suggest decreased ability of IRS-1 to mediate insulin physiological actions underlying brain IR own to less IRS-1 in neurons. This observation is in agreement with previous findings of drug-induced increases in IRS-1 levels to enhance insulin sensitivity. However, we cannot rule out that neurons of subjects with MDD might produce more IRS-1. Furthermore, specific symptoms of depression related to depressed mood, feelings of guilt, suicidality and anhedonia were associated with the highest levels of IRS-1 in brain-enriched exosomes. Albeit this was an exploratory analysis, we believe it is important to report as it may help to identify metabolic subtypes of depression characterized by certain neurobiological substrates associated with specific clinical characteristics.
A potential biological underpinning of the observed relationship between IRS-1 in L1CAM+ exosomes and MDD may be that the accumulation of IRS-1 in L1CAM+ exosomes in subjects with MDD leads to decreased binding sensitivity of the insulin receptor with the corresponding deficits in insulin signaling transduction, which is a main molecular underpinning of IR21,22. In turn, deficits in insulin signaling transduction lead to a negative feedback loop with the corresponding desensitization of cells to respond to insulin, further propagating brain IR21,22. While the accumulation of IRS-1 in L1CAM+ exosomes provide the closest available in-vivo molecular signature for brain IR in MDD, these findings generate new hypotheses for future studies aimed at understanding whether accumulation of IRS-1 reflects changes in packaging of the exosomal cargo or synthesis of IRS-1.
Our data also showed a relationship between systemic and brain IR in healthy controls but not in subjects with MDD. Specifically, the higher rate of systemic IR in subjects with MDD as compared to age and sex-matched controls was not associated with increased levels of IRS-1 in agreement with previous studies. These findings suggest a multifactorial etiology in that systemic and brain IR may emerge as separate metabolic changes to promote mood dysfunction, and lend further support to the assumption of IR in L1CAM+ exosomes being of brain origin. The lack of relationship between the increased expression of IRS-1 in L1CAM+ exosomes and systemic IR opens several questions for future research. It is possible that brain IR may not only be a consequence of, but also a starting point for, the development of mood disorders. Although there is no direct evidence, our data may suggest impaired insulin transport across the blood-brain-barrier causing decreased neuronal insulin signaling with the underlying accumulation of IRS-1 in exosomes. Further mechanistic exploration of systemic and brain IR in health and disease by using a dynamic assessment of IR after peripheral/intranasal injection of insulin or a glucose challenge is warranted to develop mechanistic framework of brain IR.
The current findings of increased number of brain-enriched exosomes and their molecular cargo related to insulin signaling in subjects with MDD and a LAC deficiency is of particular importance because exosomes carry a variety of mediators of neuroplasticity relevant to the pathophysiology of MDD. In addition to other molecular markers of the insulin signaling cascade, such as the IGF-1 receptor implicated in the regulation of glucose uptake in neurons, exosome carries key receptors of the glutamatergic system, fibroblastic grow factors (FGFs) and the brain-derived neurotrophic factor (BDNF)27. Further mechanistic exploration of the molecular cargo of brain-enriched exosomes can lead to development of integrative neuroscience-based model of brain plasticity underlying the pathophysiology and treatment of MDD.
In our own and other studies within the translational framework of modulation of insulin resistance with the mitochondrial metabolite acetyl-L-carnitine (LAC), antidepressant-like responses occur after a few days of administration47–49. Notably supplementation of LAC also modulates glutamatergic function in the same rodent phenotypes. In addition to hippocampal glutamatergic dysfunction, rodent phenotypes with impaired brain insulin signaling show aberrant LTP as well as depressive-like behaviors3, 50–52. Given the pleiotropic action of the mitochondrial metabolite LAC on brain and systemic functions, there are multiple pathways by which LAC may regulate insulin signaling. One prominent pathway might involve the acetylating action of LAC on histone acetylation subserving gene expression, which may lead to changes in the expression of the insulin receptor substrate in hippocampal circuits implicated in depression. Another possibility is that modulation of mitochondrial metabolism related LAC may affect brain IR through the β-oxidation of fatty acids for maintaining lipid homeostasis via LAC-related availability acetyl-CoA. If our findings are replicated and the brain IR in subjects with MDD is also characterized by a deficiency of the modulator of glutamatergic function LAC, amelioration of a possible brain IR in subjects with MDD with novel agents such as LAC might provide a mechanistic disease modification for more effective treatment of depression5.
Furthermore, the sex-difference in serine-312 phosphorylation of IRS-1 (pSer-IRS-1) may be akin of an increased vulnerability of women with MDD to brain IR. Because phosphorylation of IRS changes as a function of multiple intracellular serine kinases and pathways53, the increased expression of both IRS-1 and pSerIRS-1 in women with MDD might suggest sex-different neurobiological mechanisms and a more advanced stage of brain IR in women than men with MDD. Consistent with this postulate, the greater increase in pSer-IRS-1 was observed in women with stronger severity of depressive symptoms. Although this was an exploratory analysis, the strength of this correlation remained controlling for use of psychotropic medications. The reported sex-difference in markers of IR in MDD is also supported by clinical and epidemiological studies showing that the prevalence of MDD is twice as higher in women than in men54, 55. Likewise, previous studies reported important sex-differences in numerous IR-related chronic syndromes, such as obesity, diabetes, and cardiovascular disease. It is fascinating to note that the higher vulnerability of women than men to develop mood disorders begins about at the onset of puberty with the beginning of reproductive life. This sex-difference disappears after menopause with the incidence of mood disorders becoming similar in men and women, supporting a key role of estrogen in mood disorders. Previous studies also showed that 17β-estradiol protects neurons from developing IR, and that ovarian hormones also contribute to regulate phosphorylation of IRS-1. Thus multiple biological mechanisms may be involved in the current findings of sex-difference in pSer-IRS-1 in L1CAM+ exosomes of subjects with MDD56. These findings open new basic and translational research areas of sex-specific link between brain IR and regulation of behavior and mood that should be explored for the implementation of precision medicine.
Moreover, while using L1CAM+ exosomes and assessing the substrate of the insulin receptor in those cellular specific exosomes provides the closest available molecular signature for brain IR, our findings cannot completely rule out the involvement of other organs related to the pathophysiology of MDD, such as the immune and endocrine systems given a proportion of L1CAM+ can be secreted by immune and cells and adrenal glands. Dysregulation of the immune system has been implicated in the neurobiology of MDD, and likewise the endocrine system is a key regulator of the responses to stress, a main risk factor for the development of MDD. Thus the findings of accumulation of IRS-1 in L1CAM+ exosomes provide a foundation for future research to determine the relationship between insulin signaling and both immune and endocrine functions as further technological development becomes available.
In conclusion, the findings of in-vivo molecular signatures of brain IR in subjects with MDD provides a starting point for developing mechanistic framework to modulate insulin-signaling pathway with novel agents such as LAC for more effective personalized medicine approach to mechanistic disease modification. Future prospective, placebo-controlled studies will be needed to address some of the limitations inherent in our cross-sectional cohort as well as to test specificity of increased IRS-1 and its phosphorylated form in L1CAM+ exosomes in MDD as compared to other CNS diseases and to determine whether increased number of brain-enriched exosomes and their molecular fingerprint related the insulin pathway might represent trait markers of MDD. Future research is also warranted to study the relationship between increased brain IR and specific phenotypic domains, including cognitive function. Furthermore, while this translational neuroscience study provides comprehensive evidence of in-vivo IR in the human brain, additional development of the technology to study the biology of exosomes will enable further mechanistic brain region specific-studies in MDD among other psychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Robertson Therapeutic Development Foundation to CN, 1R21 MH093948-01A1 (SPO #50260) to NR, a grant from the Hearst Foundation to NR and CN, and, by a grant from the Hope for Depression Foundation (HDRF) to BMC and CN. All data and code will be made available upon request to the corresponding author.
Reference List
- 1.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nature reviews Neurology 2018; 14(3): 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 2015; 16(11): 660–671. [DOI] [PubMed] [Google Scholar]
- 3.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes 2015; 64(11): 3927–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: Anatomical, functional and temporal contexts. (1873–7064 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasca C, Rasgon N, McEwen B. An emerging epigenetic framework of systemic and central mechanisms underlying stress-related disorders. Neuropsychopharmacology 2019; 44(1): 235–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson K, Nasca C, Aasly L, McEwen B, Rasgon N. Insulin resistance, an unmasked culprit in depressive disorders: Promises for interventions. Neuropharmacology 2018; 136(Pt B): 327–334. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev 1995; 75(3): 473–486. [DOI] [PubMed] [Google Scholar]
- 8.Kenna H, Hoeft F, Kelley R, Wroolie T, DeMuth B, Reiss A et al. Fasting plasma insulin and the default mode network in women at risk for Alzheimer’s disease. Neurobiology of aging 2013; 34(3): 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer’s disease. Neurobiology of aging 2011; 32(11): 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wroolie TE, Kenna HA, Singh MK, Rasgon NL. Association between insulin resistance and cognition in patients with depressive disorders: exploratory analyses into age-specific effects. Journal of psychiatric research 2015; 60: 65–72. [DOI] [PubMed] [Google Scholar]
- 11.Nasca C, Watson-Lin K, Bigio B, Robakis TK, Myoraku A, Wroolie TE et al. Childhood trauma and insulin resistance in patients suffering from depressive disorders. Experimental neurology 2019; 315: 15–20. [DOI] [PubMed] [Google Scholar]
- 12.Lin KW, Wroolie TE, Robakis T, Rasgon NL. Adjuvant pioglitazone for unremitted depression: Clinical correlates of treatment response. Psychiatry research 2015; 230(3): 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasgon NL, Kenna HA, Williams KE, Powers B, Wroolie T, Schatzberg AF. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. TheScientificWorldJournal 2010; 10: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntyre RS, Powell AM, Kaidanovich-Beilin O, Soczynska JK, Alsuwaidan M, Woldeyohannes HO et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behavioural brain research 2013; 237: 164–171. [DOI] [PubMed] [Google Scholar]
- 15.Bonato JM, Bassani TB, Milani H, Vital M, de Oliveira RMW. Pioglitazone reduces mortality, prevents depressive-like behavior, and impacts hippocampal neurogenesis in the 6-OHDA model of Parkinson’s disease in rats. Experimental neurology 2018; 300: 188–200. [DOI] [PubMed] [Google Scholar]
- 16.Rasgon NL, McEwen BS. Insulin resistance-a missing link no more. Molecular psychiatry 2016; 21(12): 1648–1652. [DOI] [PubMed] [Google Scholar]
- 17.Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978; 272(5656): 827–829. [DOI] [PubMed] [Google Scholar]
- 18.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res 2012; 2012: 384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012; 148(5): 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2014; 10(1 Suppl): S26–32. [DOI] [PubMed] [Google Scholar]
- 21.Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 1991; 352(6330): 73–77. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. The Journal of biological chemistry 2002; 277(2): 1531–1537. [DOI] [PubMed] [Google Scholar]
- 23.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging 2010; 31(2): 224–243. [DOI] [PubMed] [Google Scholar]
- 24.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L et al. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes 2002; 51(12): 3384–3390. [DOI] [PubMed] [Google Scholar]
- 25.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Developmental cell 2019; 49(3): 347–360. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527(7578): 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Translational psychiatry 2019; 9(1): 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2015; 11(6): 600–607.e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapogiannis D, Mustapic M, Shardell MD, Berkowitz ST, Diehl TC, Spangler RD et al. Association of Extracellular Vesicle Biomarkers With Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA neurology 2019; 76(11): 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mustapic M, Eitan E, Werner JK Jr., Berkowitz ST, Lazaropoulos MP, Tran J et al. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front Neurosci 2017; 11: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasca C, Bigio B, Lee FS, Young SP, Kautz MM, Albright A et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America 2018; 115(34): 8627–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Translational psychiatry 2017; 7(3): e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. WHO Mean Body Mass Index (BMI) 2019. [Google Scholar]
- 34.The Blood Code. HOMA-IR: What it is & why you should know yours. 2019. [Google Scholar]
- 35.Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Current opinion in neurobiology 2008; 18(3): 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapogiannis D, Mustapic M, Shardell MD, Berkowitz ST, Diehl TC, Spangler RD et al. Association of Extracellular Vesicle Biomarkers With Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA neurology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Genotype-Tissue Expression (GTEx) project. Nature genetics 2013; 45(6): 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi H, Lassmann T, Murata M, Carninci P. 5’ end-centered expression profiling using cap-analysis gene expression and next-generation sequencing. Nature protocols 2012; 7(3): 542–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347(6220): 1260419. [DOI] [PubMed] [Google Scholar]
- 40.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B et al. Exosomes are released by cultured cortical neurones. Molecular and cellular neurosciences 2006; 31(4): 642–648. [DOI] [PubMed] [Google Scholar]
- 41.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Molecular and cellular neurosciences 2011; 46(2): 409–418. [DOI] [PubMed] [Google Scholar]
- 42.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta neuropathologica 2014; 128(5): 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018; 560(7718): 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nature reviews Neuroscience 2016; 17(3): 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasca C, Bigio B, Zelli D, de Angelis P, Lau T, Okamoto M et al. Role of the Astroglial Glutamate Exchanger xCT in Ventral Hippocampus in Resilience to Stress. Neuron 2017; 96(2): 402–413.e405. [DOI] [PubMed] [Google Scholar]
- 46.Lau T, Bigio B, Zelli D, McEwen BS, Nasca C. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Molecular psychiatry 2017; 22(2): 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasca C, Xenos D, Barone Y, Caruso A, Scaccianoce S, Matrisciano F et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proceedings of the National Academy of Sciences of the United States of America 2013; 110(12): 4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuccurazzu B, Bortolotto V, Valente MM, Ubezio F, Koverech A, Canonico PL et al. Upregulation of mGlu2 receptors via NF-kappaB p65 acetylation is involved in the Proneurogenic and antidepressant effects of acetyl-L-carnitine. Neuropsychopharmacology 2013; 38(11): 2220–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Lu Y, Xue Z, Li C, Wang C, Zhao X et al. Rapid-acting antidepressant-like effects of acetyl-l-carnitine mediated by PI3K/AKT/BDNF/VGF signaling pathway in mice. Neuroscience 2015; 285: 281–291. [DOI] [PubMed] [Google Scholar]
- 50.Bigio B, Mathe AA, Sousa VC, Zelli D, Svenningsson P, McEwen BS et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: Implications for treatment resistance. Proceedings of the National Academy of Sciences of the United States of America 2016; 113(28): 7906–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grillo CA, Piroli GG, Kaigler KF, Wilson SP, Wilson MA, Reagan LP. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behavioural brain research 2011; 222(1): 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ et al. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiology & behavior 2007; 92(4): 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Draznin B. Molecular Mechanisms of Insulin Resistance: Serine Phosphorylation of Insulin Receptor Substrate-1 and Increased Expression of p85α. Diabetes 2006; 55(8): 2392. [DOI] [PubMed] [Google Scholar]
- 54.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annual review of public health 2013; 34: 119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kessler RC. Epidemiology of women and depression. Journal of affective disorders 2003; 74(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 56.Carvalho CR, Carvalheira JB, Lima MH, Zimmerman SF, Caperuto LC, Amanso A et al. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology 2003; 144(2): 638–647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


