Abstract
Objective:
Platelet-rich plasma (PRP) is an emerging therapeutic strategy for treatment of osteoarthritis (OA); however, there is a lack of preclinical and clinical evidence for its efficacy and its mechanism of action is unclear. In the current study, we utilized leukocyte poor-PRP (LP-PRP) and leukocyte rich-PRP (LR-PRP) to mimic clinical point of care formulations and assessed their potential to alter disease progression in a mouse model of post-traumatic OA.
Method:
Three-month-old wild-type male FVB/N mice received destabilization of the medial meniscus (DMM) surgery to induce OA. To assess the efficacy of LP-PRP and LR-PRP, mice were given intraarticular injections at 2-, 7- and 28-days post-surgery. Mice were then assessed at 5-, 9-, and 13-weeks post-surgery for changes in chronic pain using the hot plate nociceptive assay. At 14-weeks, OA pathogenesis was evaluated using histology and phase-contrast μCT.
Results:
Treatment with LP-PRP and to a lesser extent LR-PRP preserved cartilage volume and surface area compared to phosphate-buffered saline (PBS) as measured by phase-contrast μCT. However, both treatments had higher Osteoarthritis Research Society International (OARSI) and synovitis scores compared to sham, and neither substantially improved scores compared to PBS controls. With respect to thermal hyperalgesia, PBS-treated mice displayed reduced latency to response compared to sham, and LR-PRP but not LP-PRP improved latency to response at 5-, 9- and 13-weeks post-surgery compared to PBS.
Conclusion:
The results of this study suggest that effects of PRP therapy on OA progression and disease-induced hyperalgesia may be leukocyte-dependent. And while LP-PRP and to a lesser extent LR-PRP protect from volume and surface loss, significant pathology is still seen within OA joints. Future work is needed to understand how the different components of PRP effect OA pathogenesis and pain, and how these could be modified to achieve greater therapeutic efficacy.
Keywords: platelet-rich plasma, PRP, leukocyte, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is a disease of synovial joints marked by progressive loss of articular cartilage, subchondral bone remodeling, and synovial hyperplasia that leads to the development of chronic pain and motor impairments1, 2. In the US alone, it is estimated that the number of adults with OA will increase by 49% by 2040, resulting in both direct and indirect healthcare costs totaling $6.4 billion3, 4. Despite its health and socioeconomic impacts, there are no disease-modifying therapeutics available for OA. As such, the current standard of care is limited to short-term symptomatic relief of pain achieved through use of oral non-steroidal anti-inflammatories and intra-articular steroid injections5, 6. As such, there is a significant need to develop therapies that can alter OA progression and promote joint tissue repair. Among potential candidates that are currently being used in clinical practice is platelet-rich plasma (PRP).
PRP is an autologous blood-derived therapy in which red blood cells are removed from whole blood through centrifugation, and platelets are reconstituted at supraphysiological concentrations in plasma7. While much of the beneficial effects of PRP are attributed to platelets, other cell types are present in its composition, namely white blood cells (or leukocytes)7. In this regard, PRP formulations have been broadly classified into two main categories – leukocyte rich-PRP (LR-PRP) and leukocyte poor-PRP (LP-PRP). Leukocytes consist of granulocytes including neutrophils and lymphoblast-derived natural killer cells and B- and T- lymphocytes8. LR-PRP is defined as having a greater number of leukocytes when compared to whole blood, whereas the concentration of leukocytes in LP-PRP is equivalent or reduced when compared to baseline. While LP-PRP has been favored anecdotally in clinical applications due to concerns of potential post-injection inflammation caused by leukocytes, there have been no well-controlled studies that have compared LR-PRP to LP-PRP in the context of OA7, 9. Pre-clinical support for LP-PRP has mainly been derived from in-vitro experiments10, and in-vivo studies showing beneficial effects of PRP in osteochondral lesion models; however, they have not compared the two formulations directly11. And while a recent meta-analysis reported relief of OA pain in patients up to 1 year following treatment with PRP12, the lack of strong evidence has lead most professional societies to state that they cannot recommend either for or against the use of PRP until further, higher quality studies become available13.
To address the therapeutic efficacy of different PRP formulations, we first adapted protocols used in clinical care to generate and characterize the components of LP-PRP and LR-PRP from mice. Next, we tested whether PRP therapy could prevent cartilage loss and protect from disease-induced pain in the destabilization of the medial meniscus (DMM) model of post-traumatic OA. We showed that three sequential injections of LP-PRP and to a lesser extent LR-PRP protected from cartilage loss compared to phosphate-buffered saline (PBS)-injected mice when assessed by phase-contrast μCT; however, neither therapy reduced histopathological scores for cartilage degeneration or synovitis compared to PBS. For assessments of disease-induced thermal hyperalgesia, LR-PRP but not LP-PRP increased the latency to response at all time points post-surgery compared to PBS-treated mice. Taken together, PRP therapy may alter OA progression and improve disease-induced pain in a leukocyte-dependent manner.
MATERIALS AND METHODS
Animals
Three-month-old male FVB/N mice were obtained from Baylor College of Medicine’s Center for Comparative Medicine (Houston, TX) mouse colony. All studies were performed with approval from the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). Mice were housed 4 to 5 mice to a cage in a pathogen-free environment with ad libitum access to food and water and under a 14h light/10h dark cycle. Male mice were used to minimize variability between weight and activity levels and subchondral bone differences.
Preparation of PRP
For each round of injections, fresh PRP was prepared on the day of injection from 9 separate non-surgical adult male FVB/N mice at 12 weeks-of-age each weighing approximately 30 grams. Only fresh, unactivated PRP was used. In brief, under isoflurane anesthesia, 1–1.2 mL of whole blood was drawn from each non-surgical mouse by cardiac puncture through a 22-gauge needle into a 1 mL syringe preloaded with 100 μL of anticoagulant citrate dextrose solution (ACD). The ACD formulation consisted of 85 mM sodium citrate, 69 mM of citric acid, and 20 g/L of glucose. Whole blood was then transferred into a 15 mL tube with 60 μL of ACD solution.
PRP was prepared using a double spin protocol to mimic clinical formulations that are currently being used7. Specifically, each tube of whole blood was centrifuged using a Beckman Coulter Allegra X-22 Centrifuge at 1500 RPM for 6 min. This effectively sedimented red blood cells from the platelets and buffy coat contained within the supernatant. This supernatant served as our leukocyte poor-PRP (LP-PRP) formulation. We next generated our leukocyte rich-PRP (LR-PRP) formulation by taking the supernatant (LP-PRP) from the first spin and spinning it a second time at 3400 RPM for 15 min to effectively sequester platelets from the buffy coat. The resulting platelet-poor plasma supernatant was aspirated leaving only 175 μL remaining in the tube. This volume was used to resuspend the pelleted platelets yielding our LR-PRP formulation. The resulting LP-PRP and LR-PRP formulations obtained from these non-surgical mice were then administered by intraarticular injection to surgical mice of comparable age at 2-, 7-, or 28-days post-surgery (see below).
Cell count analysis
Platelet, red blood cell and white blood cell counts were established from 175 μL each of whole blood, LP-PRP and LR-PRP formulations using scatter cytogram and platelet integrated analysis (ADVIA 120 Hematology Analyzer, Siemens).
Destabilization of the medial meniscus model of post-traumatic OA
Destabilization of medial meniscus (DMM) surgery was performed on both left and right knee joints of 12-week-old male FVB/N mice as described by Glasson et al14. Sham surgery was identical in all steps except for the medial meniscotibial ligament transection. Sham mice did not receive any intraarticular injections post-surgery. A terminal timepoint of 3.5 months post-surgery was selected to model moderate disease as our group has demonstrated previously15.
Intraarticular injections
Surgical animals were randomly assigned to one of three treatment groups (PBS, LP-PRP and LR-PRP) by a researcher not blinded to treatment, while sham mice did not receive intraarticular injections. Intraarticular injections were performed at 2-, 7-, and 28-days post-DMM surgery. The early time point of 2-days post-surgery was chosen in an effort to intervene during the acute inflammatory phase of disease onset7. To allow for a pulsatile delivery given the rapid clearance of injectables from the knee16, injections were then repeated on days 7 and 28 post-surgery. A total of 5 μl of LP-PRP, LR-PRP or sterile 1×PBS was injected per knee joint (both right and left knees received injections) using a 30-gauge needle (Hamilton). The total number of mice per group at the start of this study was as follows: 10 sham, 10 PBS, 11 LP-PRP, and 12 LR-PRP.
Histology
At 3.5 months post-surgery, mice were euthanized, and the left hind limb was dissected and fixed for 48 h on a shaker at room temperature in 4% paraformaldehyde (PFA) (the right hindlimb was processed for phase-contrast μCT – see below). Samples were decalcified at 4°C in 10% EDTA with 1×PBS for 10 days (with one change out at 5 days) prior to paraffin embedding using a standard protocol. Samples were sectioned at 6 μm and stained with Safranin-O/ Fast Green to visualize joint structures and articular cartilage proteoglycan content. Sections from the medial knee joint were scored for pathological changes and articular cartilage loss using the Osteoarthritis Research Society International (OARSI) histological grading system17. The evaluator was blinded to the treatment and procedure.
To assess for the presence of synovitis, sections from the medial knee joint were scored using the following 3-point scale: score of 0 if synovium was two cell layers thick with only mild edema; score of 1 if synovium exhibited increased thickness and mild inflammatory cell infiltration; score of 2 if synovium was multiple cell layers thick with moderate inflammatory cell infiltration and some papillary excrescence; and score of 3 if synovium had lots of papillary excrescence and severe inflammatory cell infiltration18. One sample from each of the sham, PBS, and LR-PRP groups was not processed or evaluated due to technical difficulties.
Phase-contrast μCT imaging and analysis
At 3.5 months post-surgery, the right hind limb of each mouse was dissected, stained with contrast agents, scanned by phase-contrast μCT, and subsequently analyzed using TriBON software (RATOC, Tokyo, Japan) as previously described15, 18–20. Cartilage volume and surface area of bone covered by cartilage (cartilage surface) within the medial compartment of the knee joint were determined by a blinded evaluator. One sample from each of the sham, PBS, and LP-PRP groups was not processed or evaluated due to technical difficulties.
Hotplate nociceptive assay
Experimental mice were evaluated at 5-, 9-, and 13-weeks post-surgery for thermal hyperalgesia using the hotplate nociceptive assay as previously described15, 18. In brief, on the day of assessment, mice were transferred to the room of analysis and allowed to acclimate for at least 30 minutes at 50 Lux and 60 dB. Each mouse was then placed on the hotplate (Columbus Instruments, Columbus, OH) at 55°C and observed for a maximum of 45 seconds. The latency period to hind limb flicking or licking in either affected limb was recorded.
Statistical analysis
Sample size was based on previous publications. Data are shown in the text as mean ± S.D., and differences between groups are presented as either the mean difference or mean rank difference together with p-values and the 95% CI for the difference between the means or mean ranks (95CIdiff) where applicable. For graphs, data are shown as either min-to-max box and whisker plots (median with interquartile range) together with individual points (Figures 1, 2, 3) or as a scatter plot depicting the median with interquartile range for each group (Fig. 4). For data in Figs. 1-3 whose residuals passed the Shapiro-Wilk test for normality, groups were compared using one-way ANOVA followed by Tukey’s post-hoc tests. For data where residuals did not have a normal distribution, groups were compared using Kruskal-Wallis followed by Dunn’s post-hoc tests. For the hot plate data in Fig. 4, groups were compared using two-way repeated measures ANOVA followed by Tukey’s post-hoc tests to account for data collected from the same animal at 5-, 9-, and 13-weeks. For most correlation analyses (except those including synovitis scores – see below), Pearson correlations were performed. For the statistical analyses described above, we used Prism 8.3.1 (GraphPad Software, La Jolla, CA). As Prism does not provide 95% CI of differences between mean ranks within the Kruskal-Wallis test, these were calculated using the pairw.kw function from the asbio R package (R version 3.6.0). Given the limited range of possible values, Kendall correlations (instead of Pearson) were computed for comparisons using synovitis data using the R Kendall package, and the kendall.ci function within the NSM package was used to produce the confidence interval for Kendall’s tau. For all tests, the exact p-value is reported in both the figures and text, and a p-value of < 0.05 was considered statistically significant.
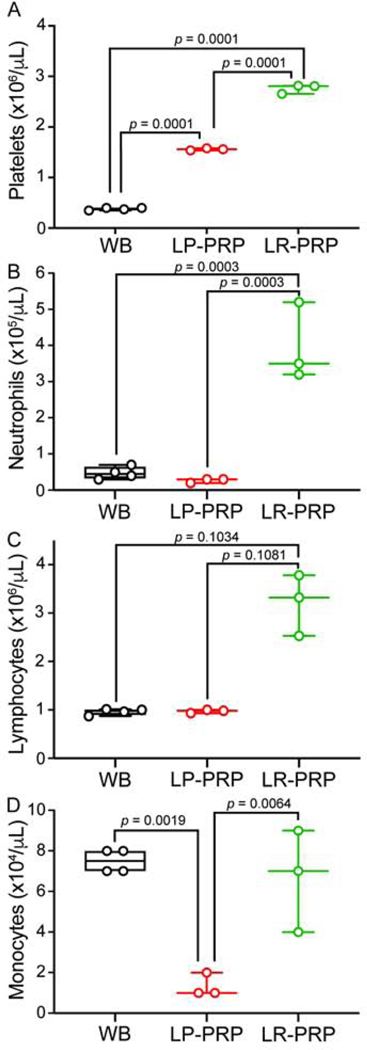
Figure 1. LR-PRP exhibits a higher leukocytic profile and higher platelet count compared to LP-PRP.
Twelve-week-old male mice underwent cardiac puncture for whole blood collection and PRP preparation. Platelet counts were measured by platelet integrated analysis. A, LP-PRP and LR-PRP platelet counts were more concentrated compared to whole-blood (WB). B, LR-PRP contains more neutrophils than both WB and LP-PRP. C, LR-PRP contains more lymphocytes than WB and LP-PRP. D, LR-PRP and WB have more monocytes than LP-PRP. Data are plotted as min-to-max box and whisker plots with individual data points. Data in A, B, and D were analyzed by one-way ANOVA followed by Tukey’s post-hoc tests; in contrast, data in C was compared using Kruskal-Wallis followed by Dunn’s post-hoc tests. n = 3–4 mice from 3 independent collections. WB, whole blood; PRP, platelet-rich plasma; LR, leukocyte-rich, LP, leukocyte-poor.
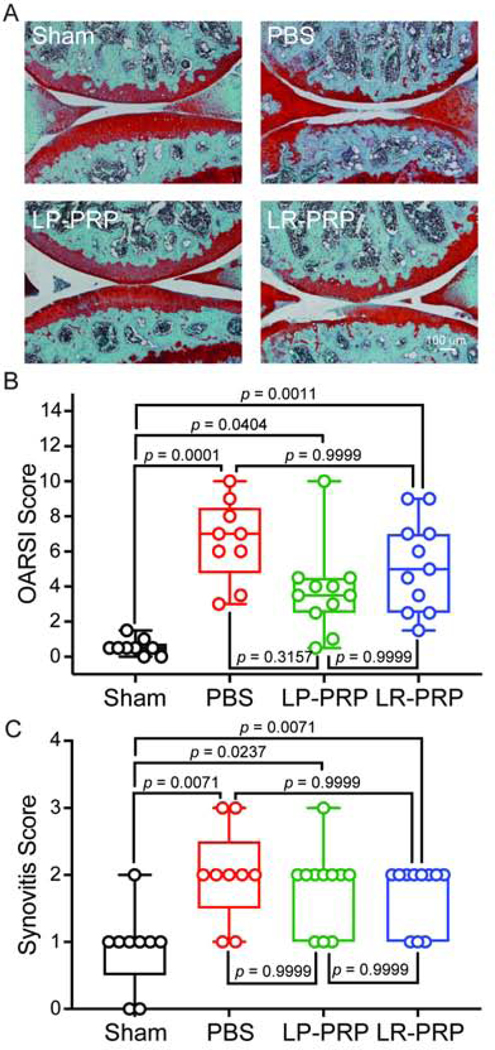
Figure 2. PRP therapy only modestly protects from OA pathology but not synovitis in mice with post-traumatic OA.
Twelve-week-old male mice were injected intraarticularly with LP-PRP, LR-PRP or PBS at 2-, 7-, and 28-days post-DMM surgery. Fourteen weeks later, joints were collected, sectioned and processed for Safranin-O/ Fast Green histochemical staining. A, representative histological images from the medial compartment of knees joints at 3.5-months post-surgery from each experimental group. B, OARSI scoring of histological sections. PBS-treated mice had greater OARSI scores compared to sham controls indicating development of post-traumatic OA. While the LP-PRP group had higher OARSI scores compared to sham, there was a modest but insignificant decrease compared with PBS-treated mice. LR-PRP displayed an intermediate effect between PBS- and LP-PRP-treated groups. C, synovitis scores were performed on the same sections used in B. Mice treated with PBS, LP-PRP or LR-PRP post-surgery all had higher synovitis scores compared to sham. Data are plotted as min-to-max box and whisker plots with individual data points. Differences between groups were analyzed using Kruskal-Wallis followed by Dunn’s post-hoc tests. n = 9 mice for sham, 9 mice for PBS, 11 mice for LP-PRP and 11 mice for LR-PRP.
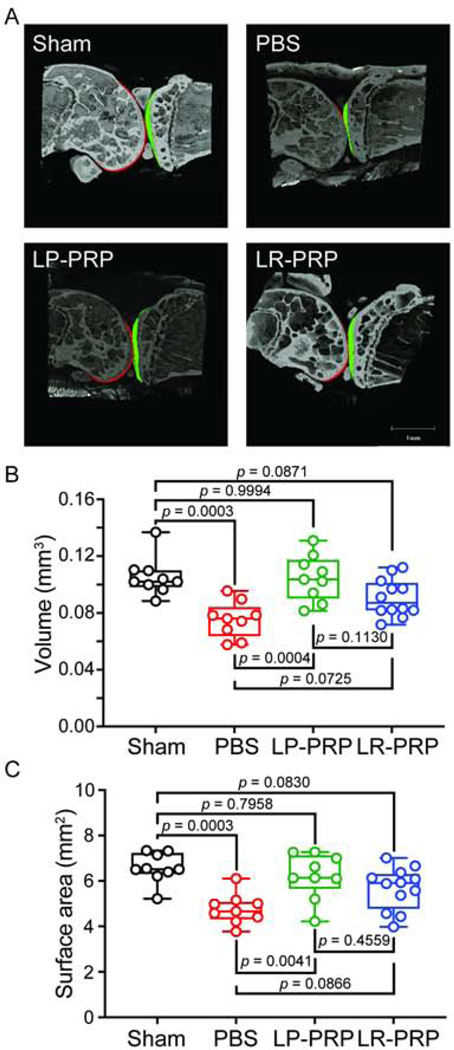
Figure 3. PRP therapy preserves cartilage volume and surface in mice with post-traumatic OA.
DMM surgery was performed on 12-week-old male mice to induce OA followed by intraarticular delivery of LP-PRP, LR-PRP or PBS at 2-, 7-, and 28- days post-surgery. Fourteen weeks post-surgery, joints were collected and processed for phase-contrast μCT analysis. A, Representative μCT images of the medial compartment of surgical joints from each experimental group. Femoral articular cartilage is indicated in red, and tibial articular cartilage is indicated in green. B, PBS-treated mice had less cartilage volume compared to sham. LP-PRP protected from cartilage volume loss, while only a modest increase in cartilage volume was observed for the LR-PRP group. C, PBS-treated mice exhibited a loss of cartilage surface that was protected by LP-PRP and to a lesser extent by LR-PRP. Data are plotted as min-to-max box and whisker plots with individual data points. Differences between groups were analyzed by one-way ANOVA followed by Tukey’s post-hoc tests. n = 9 mice for sham, 9 mice for PBS, 11 mice for LP-PRP and 11 mice for LR-PRP.
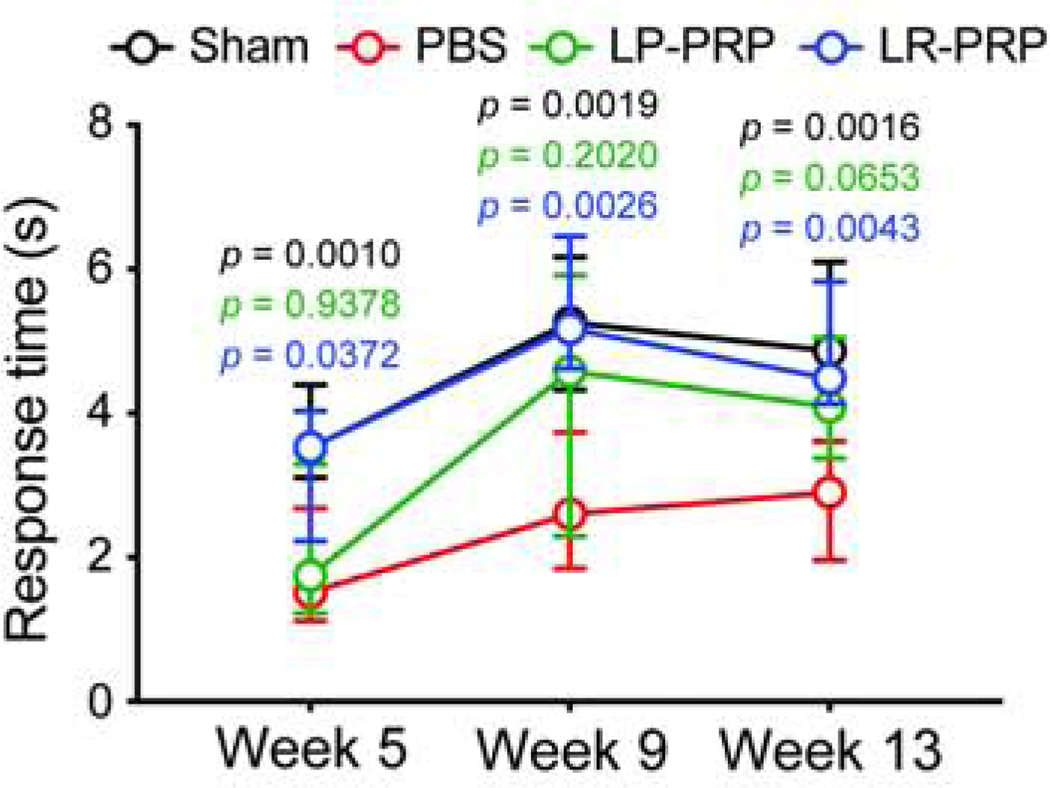
Figure 4. PRP therapy protects from OA-induced thermal hyperalgesia.
DMM surgery was performed on 12-week-old male mice to induce OA followed by intraarticular delivery of LPPRP, LR-PRP or PBS at 2-, 7-, and 28-days post-surgery. To assess time-dependent effects of PRP therapy on OA-induced pain, mice were evaluated using the hot plate nociceptive assay. PBS-treated mice had reduced response times at all three time points compared to sham (p-values in black). LR-PRP at increased response times at all time points compared to PBS-treated mice (p-values in blue), and was comparable to sham controls. In contrast, LP-PRP at 5-weeks post-surgery had a comparable response time to PBS, that was modestly increased at later time-points (p-values in green). Data are plotted as median with interquartile range. Differences between groups were analyzed by repeated measures two-way ANOVA followed by Tukey’s post-hoc tests. p-values displayed on the graph represent sham (black), LP-PRP (green), and LR-PRP (blue) compared to the PBS group. n = 10 mice for sham, 10 mice for PBS, 11 mice for LP-PRP and 11 mice for LR-PRP.
RESULTS
LR-PRP contains more lymphocytes and neutrophils compared to WB and LP-PRP
It is understood that PRP formulations vary in their contents, namely platelet and leukocyte concentrations; however, few pre-clinical and clinical studies report their platelet counts and even fewer report their leukocyte profiles. In this regard, we confirmed that our double spin protocol generated LP-PRP (1.556×106 ± 0.0216×106) with a mean increase in platelets of +1.178×106 (p = 0.0001, 95CIdiff [+1.061×106, +1.294×106]) and LR-PRP (2.760×106 ± 0.0898×106) with a mean increase of +2.381×106 (p = 0.0001, 95CIdiff [+2.264×106, +2.498×106]) compared to whole-blood (0.3788×106 ± 0.0245×106) (Figure 1A). LR-PRP also had an increased number of platelets compared to LP-PRP (+1.203×106, p = 0.0001, 95CIdiff [+1.078×106, +1.328×106]). In accordance with recent definitions that describe LR-PRP as being neutrophil-rich21, we confirmed that LR-PRP (0.3967×106 ± 0.1079×106) had higher levels of neutrophils compared with whole blood (0.0475×106 ± 0.0171×106) (+0.3492×106, p = 0.0003, 95CIdiff [+0.2169×106, +0.4814×106]) and LP-PRP (0.0267×106 ± 0.0058×106) (+0.37×106, p = 0.0003, 95CIdiff [+0.2286×106, +0.5114×106]) (Figure 1B). Leukocytes consist of natural killer cells and B- and T- lymphocytes in addition to neutrophils8. In this regard, our LR-PRP (3.210×106 ± 0.6322×106) had a mean rank increase in leukocytes of +4.875 (p = 0.1034, 95CIdiff [−0.6440, +10.39]) and +5.167 (p = 0.1081, 95CIdiff [−0.7334, +11.07]) compared to whole blood (0.960×106 ± 0.0638×106) and LP-PRP (0.970×106 ± 0.0361×106), respectively (Figure 1C). Finally, when evaluating monocytes across the three preparations, we observed that whole blood (0.075×106 ± 0.0058×106) and LR-PRP (0.0667×106 ± 0.0252×106) had comparable amounts (−0.0083×106, p = 0.7362, 95CIdiff [−0.0405×106, +0.0238×106]), whereas LP-PRP had a lower monocyte concentration with a mean decrease of −0.0617×106 (p = 0.0019, 95CIdiff [−0.0938×106, −0.0295×106]) compared to whole-blood and −0.0533×106 (p = 0.0064, 95CIdiff [−0.0877×106, −0.0189×106]) compared to LR-PRP (Figure 1D).
PRP slows the progression of post-traumatic OA
PRP therapies vary in leukocyte concentration and currently no in-vivo data have compared the therapeutic efficacy of LP-PRP and LR-PRP in pre-clinical models of post-traumatic OA. To test the therapeutic efficacy of PRP using the DMM model of post-traumatic OA, LP-PRP, LR-PRP or PBS were injected into knee joints of FVB/N male mice at 2-, 7- and 28-days post-surgery and compared to uninjected sham controls. At 3.5 months post-surgery, joints were collected from each group, sectioned and stained using Safranin-O/ Fast Green to assess OA development and proteoglycan loss (Figure 2A). Histopathological scoring revealed that PBS-treated mice had higher OARSI scores (6.611 ± 2.315) with a mean rank increase of +24.33 (p = 0.0001, 95CIdiff [+9.847, +38.82]) compared to sham controls (0.5556 ± 0.4640) (Figure 2B). Mice treated with LP-PRP (3.727 ± 2.463) or LR-PRP (5.227 ± 2.602) also had higher OARSI scores with respective mean rank increases of +14.19 (p = 0.0404, 95CIdiff [+0.3744, +28.00]) and +19.64 (p = 0.0011, 95CIdiff [+5.829, +33.45]) compared to sham (Figure 2B), indicating that neither PRP preparation prevented OA onset. Compared to PBS controls, LP-PRP showed a slight decrease in OARSI scores (−10.15, p = 0.3157, 95CIdiff [−23.96, +3.666]) whereas LR-PRP had no effect (−4.692, p > 0.9999, 95CIdiff [−18.50, +9.120]) (Figure 2B). Consistent with the OARSI data, there were no therapeutic effects of either PRP formulation with respect to synovitis when compared to sham or PBS-treated mice (Figure 2C). Note that a positive correlation between OARSI scores and synovitis scores was also observed (r = +0.38, p = 0.0057, 95CI [+0.153, +0.604]) (Supplemental Figure 1A). Taken together, while LP-PRP treatment led to a slight decrease in OARSI scores, neither PRP preparation effectively prevented OA pathology or synovial inflammation in the DMM-model of post-traumatic disease.
To examine the extent of articular cartilage degeneration throughout the knee, we next examined joints by phase-contrast μCT (Figure 3). Representative images showed joint space narrowing in the medial compartment of PBS-treated mice compared to sham controls (Figure 3A). Quantification revealed that cartilage volume (0.0752 ± 0.0126) and surface (4.765 ± 0.6619) in mice treated with PBS exhibited respective mean decreases of −0.0303 (p = 0.0003, 95CIdiff [−0.0478, −0.0127]; Figure 3B) and −1.812 (p = 0.0003, 95CIdiff [−2.874, −0.7491]; Figure 3C) compared to sham. In contrast, cartilage volume (0.1048 ± 0.016) and surface (6.216 ± 1.001) in the LP-PRP group was greater compared to PBS-treated mice with mean increases of +0.0295 (p = 0.0004, 95CIdiff [+0.012, +0.0471]) and +1.45 (p = 0.0041, 95CIdiff [+0.3876, +2.513]), respectively (Figure 3B, C). Importantly, cartilage volume and surface in the LP-PRP group was comparable to sham. On the other hand, cartilage volume (0.09065 ± 0.01312) and surface (5.668 ± 0.9271) of LR-PRP-treated mice showed only modest respective mean increases of +0.0154 (p = 0.0725, 95CIdiff [−0.001, +0.0318]) and +0.9022 (p = 0.0866, 95CIdiff [−0.0918, +1.896]) compared to PBS (Figure 3B, C). And unlike LP-PRP, mice treated with LR-PRP also had modest reductions in cartilage volume of −0.0149 (p = 0.0871, 95CIdiff [−0.0313, +0.0015]) and surface of −0.9096 (p = 0.0830, 95CIdiff [−1.904, +0.0845]) compared to sham controls. Note that cartilage volume (r = −0.46, p = 0.0053, 95CI [−0.682, −0.149]) and surface (r = −0.44, p = 0.007, 95CI [−0.672, −0.132]) negatively correlated with increasing OARSI scores indicative of consistency between methods (Supplemental Figure 1B–C). Together with the histopathological assessments, these results indicate that while there is significant OA pathology in joints treated with either PRP formulation, LP-PRP reduces the extent of damage to greater degree compared to LR-PRP in the context of the whole-joint.
PRP therapy protects from thermal hyperalgesia in a leukocyte concentration-dependent manner
OA pathogenesis and the accompanying alterations to joint structures are thought to contribute to the development of chronic pain and functional impairments22. To measure hypersensitivity to a noxious stimulus, we examined latency to a hind limb response using the hot plate nociception assay across three time points. At 5-weeks post-surgery, PBS-treated mice (1.852 ± 0.992) displayed a mean decrease in response time of −1.825 (p = 0.001, 95CIdiff [−2.903, −0.7465]) compared to sham (3.677 ± 0.654) indicative of disease-induced thermal hyperalgesia (Figure 4). Like the PBS group, LP-PRP mice (2.114 ± 1.085) also displayed a mean decrease of −1.563 (p = 0.0044, 95CIdiff [−2.666, −0.4604]) compared to sham. In contrast, LR-PRP (3.389 ± 1.364) increased mean response time relative to PBS (+1.537, p = 0.0372, 95CIdiff [+0.0763, +2.998]) and was comparable to sham (−0.2879, p = 0.9223, 95CIdiff [−1.619, +1.043]) indicating that LR-PRP but not LP-PRP provides some protection from thermal hyperalgesia at 5-weeks post-surgery (Figure 4). While the increased latency to response was maintained in the LR-PRP group at 9-weeks, LP-PRP showed only a modest mean increase in response time of +1.728 (p = 0.202, 95CIdiff [−0.6626, +4.118]) compared to PBS-treated mice and a modest mean decrease of −0.7062 (p = 0.8381, 95CIdiff [−3.140, +1.727]) compared to sham suggestive of a potential intermediate effect though the data were variable (Figure 4).
At the final time-point, PBS-treated mice (2.909 ± 1.805) continued to display a mean reduction in response time of −2.061 (p = 0.0016, 95CIdiff [−3.369, −0.7525]) compared to sham (4.970 ± 0.98) (Figure 4). In contrast, LR-PRP-treated mice (4.680 ± 0.93) maintained a mean increase in latency to response of +1.771 (p = 0.0043, 95CIdiff [+0.5177, +3.024]) compared to the PBS group, and were comparable to sham (−0.29, p = 0.8981, 95CIdiff [−1.468, +0.4266]). Similar to 9-weeks, LP-PRP (4.156 ± 1.040) showed a modest increase in mean response time of +1.247 (p = 0.0653, 95CIdiff [−0.0625, +2.557]) and a modest decrease of −0.8136 (p = 0.2842, 95CIdiff [−2.054, +0.4266]) compared to PBS and sham, respectively (Figure 4). In this regard, OARSI scores alone showed a negative correlation with the response time to noxious heat (i.e., thermal hyperalgesia) (r = −0.35, p = 0.0345, 95CI [−0.617, −0.018]), whereas no significant correlation was observed for synovitis scores (r = −0.15, p = 0.2592, 95CI [−0.39, +0.089]), cartilage volume (r = +0.27, p = 0.1139, 95CI [−0.066. +0.548]) or cartilage surface (r = +0.30, p = 0.0787, 95CI [−0.035, +0.570]) (Supplemental Figure 2). Taken together, these results indicate that LR-PRP and to a lesser extent LP-PRP provide some protection from disease-induced thermal hyperalgesia in the DMM mouse model of post-traumatic OA.
DISCUSSION
In recent years, the lack of disease-modifying therapeutics for OA has led to the adoption of autologous therapies such as PRP into clinical practice without clear mechanistic rationale for their use13. Indeed, few pre-clinical studies have examined the effects of intra-articular PRP therapy on OA pathogenesis in animal models of disease23, 24. To this end, this study examined the therapeutic efficacy of two clinically relevant PRP formulations – LP-PRP and LR-PRP – using the DMM mouse model of disease. We demonstrated that repeated intraarticular delivery of LP-PRP but not LR-PRP only modestly reduced OARSI and synovitis scores at 3.5 months post-surgery though the effect was not significant. In contrast, phase-contrast μCT revealed that LP-PRP treatment protected from cartilage volume and surface loss compared to PBS controls, with values comparable to those of sham mice. Interestingly, though LP-PRP was more protective against cartilage pathology and loss, LR-PRP provided the better protection from the development of thermal hyperalgesia compared to the PBS-treated mice at 5-, 9- and 13-weeks post-surgery. Taken together, these data indicate that while PRP therapy may have a modest protective effect when examining OA pathology, cartilage loss and associated pain, leukocyte concentration may differentially affect radiographic and functional aspects of disease pathogenesis. In this regard, typical leukocyte profiling for PRP is often limited to neutrophils21; however, we demonstrated that lymphocytes are also modestly (though not significantly) increased and represent the predominant lymphocytic cell-type in LR-PRP based on concentration. And while mouse and human leukocyte profiles differ at baseline, the LR-PRP used in this study is similar to human PRP preparations25.
To the best of our knowledge this is the first study to utilize the DMM model of post-traumatic OA to evaluate clinically relevant fresh (not frozen) PRP formulations with reported white blood cell profiles. Using this model, we demonstrated that while neither LP-PRP nor LR-PRP provided robust protection from OA pathology at 14-weeks post-surgery, LP-PRP did reduce the extent of disease burden within the joint as quantified using phase-contrast μCT. In a study by Chiou and colleagues, PRP provided protection from cartilage loss in the anterior cruciate ligament transection (ACLT) model of post-traumatic OA only when combined with hyaluronic acid (HA)23. Given that the ACLT model causes more severe disease compared to DMM15, this may explain the loss of effectiveness with PRP alone in comparison to our findings. This study also employed thawed PRP (stored frozen) whereas we used multiple injections of freshly isolated PRP. The distinction between fresh and frozen PRP is important as freeze-thaw effectively lyses platelets thereby altering the delivery kinetics of growth factors and other molecules resulting in more rapid and less sustained treatment. In a second mouse study utilizing the tibial loading model of post-traumatic OA, it was reported the multiple intraarticular injections of PRP had no significant effect on OARSI scoring or subchondral bone volume compared to either HA or PBS24, indicating a lack of therapeutic efficacy for PRP in this model. While we too did not observe a strong therapeutic effect of either LP-PRP or LR-PRP on OA histopathology, we demonstrated that LP-PRP did protect from the extent of cartilage loss as quantified by phase-contrast μCT – a technique that the authors of these two papers did not employ. It is important to note that we have observed similar differences in sensitivity between OARSI and μCT in our previous publications examining an interleukin-1 receptor antagonist gene therapy for OA18. The authors from this second study also prepared their PRP formulations differently and administered them over longer intervals compared to our own – a fact that may have prevented the early exposure of joint tissues to chondroprotective mediators as achieved in our study. Ultimately, as with any therapeutic, the severity of the animal model and the techniques used to evaluate disease are likely to have important impacts as to the efficacy that is observed. The use of activated compared to unactivated PRP as used in our study would also greatly impact the kinetics of delivery of the various growth factors and cytokines present in PRP. To address these issues, researchers should characterize their PRP preparations. In this regard, a limitation of the current study was that we did not characterize our own PRP preparations prior to each injection. That being said, we did analyze three independent preparations and demonstrated that we could consistently reproduce the platelet and leukocyte profiles for whole-blood, LP-PRP and LR-PRP.
In evaluating the protective effects of PRP on OA-associated pain, we found that LR-PRP provided some protection from thermal hyperalgesia compared to PBS at all time points post-surgery, whereas LP-PRP had only a modest effect at the 13-week time point. These findings are in keeping with a study that found that multiple injections of PRP releasate reduced pain in mice with collagenase-induced disease26. Similarly, using male Sprague Dawley rats, Yan and colleagues reported protective effects of repeated PRP releasate injections with respect to mechanical allodynia and thermal hyperalgesia at 28 days following intra-articular collagenase delivery27. Taken together, data from our study and that of others suggests that PRP therapy can protect from chronic pain phenotypes in pre-clinical models of arthritis.
In our study, we found that intraarticular injections of LP-PRP provided better protection against radiographic progression of OA whereas LR-PRP was more effective at blocking thermal hyperalgesia at post-surgery. The dichotomy in our results suggests that the presence of leukocytes may differentially impact joint tissue degeneration and OA-associated pain. In this regard, Xu and colleagues reported that LP-PRP and to a lesser extent LR-PRP when combined with bone marrow stromal cells promoted cartilage repair in a rabbit articular cartilage defect model. With respect to leukocyte-dependent differences, the authors attributed the greater pro-inflammatory profile of LR-PRP to its poorer therapeutic efficacy10. However, as we did not characterize the molecular makeup of our PRP preparations, the pro-inflammatory differences between them and how this relates to their protective effects remains unclear. In this regard, it is important to note clinical data in human patients does support that radiographic evidence of disease does not always associate with clinical presentations such as pain and disability28.
To date there are approximately 15 randomized control trials evaluating PRP therapy for knee OA, with the majority of these studies favoring PRP based on patient-reported pain and functional outcomes29. Despite these encouraging findings, there is a lack of consensus in both the clinical and preclinical arena on optimal PRP formulation for the treatment of OA, particularly regarding leukocyte concentration. This is due in part to a lack of detailed characterization of PRP both in pre-clinical and clinical studies. We limited our study to two distinct PRP therapies that reflect current clinical formulations to assess for therapeutic efficacy using the DMM mouse model of post-traumatic OA. While the mechanism is still unclear, we have provided well-controlled evidence that repeated treatment with PRP in the early stages of OA pathogenesis can alter or delay disease progression.
In conclusion, the results of our study support the safety profile of PRP, and highlight some potential beneficial effects of PRP therapy for treatment of post-traumatic OA. At the same time, differences we observed between LP-PRP and LR-PRP in terms of their chondroprotective and analgesic properties point towards compositional and mechanistic complexities that remain unknown. As such, more in-vivo studies are needed to identify the ideal PRP formulation and therapeutic windows to intervene before clinical practice outpaces sound scientific data. Taken together, LP-PRP and LR-PRP therapies are viable options for the treatment of structural and functional effects respectively in a DMM induced post-traumatic osteoarthritis mouse model.
Supplementary Material
Relationship between OARSI scores and synovial and radiographic measures of OA progression. Pearson or Kendall correlation analyses were performed to examine the relationship between OARSI score compared to A, synovitis score, B, cartilage volume and C, cartilage surface. A significant positive correlation was observed for OARSI score compared with synovitis score, whereas significant negative correlations were detected compared with cartilage volume as well as cartilages surface.
Relationship between thermal hyperalgesia and histological and radiographic measures of OA progression. Pearson or Kendall correlation analyses were performed to examine the relationship between thermal hyperalgesia (graphed as “Latency to response (s)”) at 13-weeks post-surgery compared to A, OARSI score, B, synovitis score, C, cartilage volume and D, cartilage surface. A significant negative correlation was observed for OARSI scores compared with latency to response (i.e., thermal hyperalgesia) in end-stage disease. No other significant correlations were detected.
Funding
The study was supported by the BCM Intellectual and Developmental Disabilities Research Center (HD024064) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the Rolanette and Berdon Lawrence Bone Disease Program of Texas, the BCM Center for Skeletal Medicine and Biology, and the Pamela and David Ott Center for Heritable Disorders of Connective Tissue. P. Jayaram was supported by a PMR Foundation: ERF Matterson Grant. S. Ketkar was supported by a U01 grant from the NIH.
Footnotes
Conflicts
No conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage 2019; 27: 359–364. [DOI] [PubMed] [Google Scholar]
- 2.Sherwood J. Osteoarthritis year in review 2018: biology. Osteoarthritis Cartilage 2019; 27: 365–370. [DOI] [PubMed] [Google Scholar]
- 3.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol 2016; 68: 1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie F, Kovic B, Jin X, He X, Wang M, Silvestre C. Economic and humanistic burden of osteoarthritis: a systematic review of large sample studies. Pharmacoeconomics 2016; 34: 1087–1100. [DOI] [PubMed] [Google Scholar]
- 5.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 6.McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: A Randomized Clinical Trial. JAMA 2017; 317: 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol 2013; 9: 721–730. [DOI] [PubMed] [Google Scholar]
- 8.Ross MH, Kaye GI, Pawlina W. Chapter 9 - Blood. In: Histology: A Text and Atlas: Lippincott Williams & Wilkins 2003:214–245. [Google Scholar]
- 9.Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016; 44: 792–800. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z, Yin W, Zhang Y, Qi X, Chen Y, Xie X, et al. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci Rep 2017; 7: 43301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fice MP, Miller JC, Christian R, Hannon CP, Smyth N, Murawski CD, et al. The role of platelet-rich plasma in cartilage pathology: an updated systematic review of the basic science evidence. Arthroscopy 2019; 35: 961–976.e3. [DOI] [PubMed] [Google Scholar]
- 12.Johal H, Khan M, Yung SP, Dhillon MS, Fu FH, Bedi A, et al. Impact of platelet-rich plasma use on pain in orthopaedic surgery: a systematic review and meta-analysis. Sports Health 2019; 11: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthroscopy Association of Canada, Kopka M, Sheehan B, Degen R, Wong I, Hiemstra L, Ayeni O, et al. Arthroscopy Association of Canada position statement on intra-articular injections for knee osteoarthritis. Orthop J Sports Med 2019; 7: 2325967119860110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 2007; 15: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 15.Stone A, Grol MW, Ruan MZC, Dawson B, Chen Y, Jiang MM, et al. Combinatorial Prg4 and Il-1ra gene therapy protects against hyperalgesia and cartilage degeneration in post-traumatic osteoarthritis. Hum Gene Ther 2019; 30: 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther 2018; 29: 2–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010; 18 Suppl 3: S17–23. [DOI] [PubMed] [Google Scholar]
- 18.Nixon AJ, Grol MW, Lang HM, Ruan MZC, Stone A, Begum L, et al. Disease-modifying osteoarthritis treatment with interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis Rheumatol 2018; 70: 1757–1768. [DOI] [PubMed] [Google Scholar]
- 19.Ruan MZ, Dawson B, Jiang MM, Gannon F, Heggeness M, Lee BH. Quantitative imaging of murine osteoarthritic cartilage by phase-contrast micro-computed tomography. Arthritis Rheum 2013; 65: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med 2013; 5: 176ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med 2018; 11: 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller RE, Malfait AM. Osteoarthritis pain: What are we learning from animal models? Best Pract Res Clin Rheumatol 2017; 31: 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiou CS, Wu CM, Dubey NK, Lo WC, Tsai FC, Tung TDX, et al. Mechanistic insight into hyaluronic acid and platelet-rich plasma-mediated anti-inflammatory and anti-apoptotic activities in osteoarthritic mice. Aging (Albany NY) 2018; 10: 4152–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan X, Sandell LJ, Chinzei N, Holguin N, Silva MJ, Schiavinato A, et al. Therapeutic efficacy of intra-articular hyaluronan derivative and platelet-rich plasma in mice following axial tibial loading. PLoS One 2017; 12: e0175682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayaram P, Yeh P, Patel SJ, Cela R, Shybut TB, Grol MW, et al. Effects of Aspirin on growth factor release from freshly isolated leukocyte-rich platelet-rich plasma in healthy men: a prospective fixed-sequence controlled laboratory study. Am J Sports Med 2019; 47: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 26.Khatab S, van Buul GM, Kops N, Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA, et al. Intra-articular injections of platelet-rich plasma releasate reduce pain and synovial inflammation in a mouse model of osteoarthritis. Am J Sports Med 2018; 46: 977–986. [DOI] [PubMed] [Google Scholar]
- 27.Yan L, Zhou L, Xie D, Du W, Chen F, Yuan Q, et al. Chondroprotective effects of platelet lysate towards monoiodoacetate-induced arthritis by suppression of TNF-α-induced activation of NF-κB pathway in chondrocytes. Aging (Albany NY) 2019; 11: 2797–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 2008; 9: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennell KL, Hunter DJ, Paterson KL. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr Rheumatol Rep 2017; 19: 24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between OARSI scores and synovial and radiographic measures of OA progression. Pearson or Kendall correlation analyses were performed to examine the relationship between OARSI score compared to A, synovitis score, B, cartilage volume and C, cartilage surface. A significant positive correlation was observed for OARSI score compared with synovitis score, whereas significant negative correlations were detected compared with cartilage volume as well as cartilages surface.
Relationship between thermal hyperalgesia and histological and radiographic measures of OA progression. Pearson or Kendall correlation analyses were performed to examine the relationship between thermal hyperalgesia (graphed as “Latency to response (s)”) at 13-weeks post-surgery compared to A, OARSI score, B, synovitis score, C, cartilage volume and D, cartilage surface. A significant negative correlation was observed for OARSI scores compared with latency to response (i.e., thermal hyperalgesia) in end-stage disease. No other significant correlations were detected.