Abstract
Objectives
Identify splatter/aerosol distribution from dental procedures in an open plan clinic and explore aerosol settling time after dental procedures.
Methods
In two experimental designs using simulated dental procedures on a mannequin, fluorescein dye was introduced: (1) into the irrigation system of an air-turbine handpiece; (2) into the mannequin's mouth. Filter papers were placed in an open plan clinic to collect fluorescein. An 8-metre diameter rig was used to investigate aerosol settling time. Analysis was by fluorescence photography and spectrofluorometry.
Results
Contamination distribution varied across the clinic depending on conditions. Unmitigated procedures have the potential to deposit contamination at large distances. Medium volume dental suction (159 L/min air) reduced contamination in the procedural bay by 53%, and in other areas by 81-83%. Low volume suction (40 L/min air) was similar. Cross-ventilation reduced contamination in adjacent and distant areas by 80-89%. In the most realistic model (fluorescein in mouth, medium volume suction), samples in distant bays (≥5 m head-to-head chair distance) gave very low or zero readings (< 0.0016% of the fluorescein used during the procedure). Almost all (99.99%) of the splatter detected was retained within the procedural bay/walkway. After 10 min, very little additional aerosol settled.
Conclusions
Cross-infection risk from dental procedures in an open plan clinic appears small when bays are ≥ 5 m apart. Dilution effects from instrument water spray were observed, and dental suction is of benefit. Most settled aerosol is detected within 10 min indicating environmental cleaning may be appropriate after this. Clinical Significance: Aerosols produced by dental procedures have the potential to contaminate distant sites and the majority of settled aerosol is detectable after 10 min. Dental suction and ventilation have a substantial beneficial effect. Contamination is likely to be minimal in open plan clinics at distances of 5 m or more.
Keywords: Aerosols, Dental equipment, Dental fallow time, Dental high-speed equipment, Dental infection control, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) has had a detrimental impact on the ability to provide dental care in the primary and secondary care environment. Routine dental care in the UK ceased at the beginning of the pandemic, with Aerosol Generating Procedures (AGPs) advised only for emergency treatment [[1], [2], [3], [4]] in designated urgent dental care centres [5]. Whilst there are limited data available informing a safe return to practice in primary care [6,7], very little is known about the risk of disease transmission in secondary care environments, particularly with respect to open plan clinics (i.e. multiple chairs in one clinical area with only modest dividing walls that do not reach the ceiling). The resultant negative impact on specialist dental services and dental education continues to be highly problematic [8]. This has led to recommendations such as the construction of self-contained “pods” with adequate ventilation [9]. Although such recommendations acknowledge the lack of evidence for these proposed measures, the logistical, financial, and possible safety challenges they present to large teaching institutions cannot be overlooked. These challenges are largely dependent on the environmental and ventilation parameters within individual institutions.
Dental procedures produce both aerosol and splatter contaminated with saliva and/or blood [10,11]. Transmission of pathogens including viruses such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is therefore possible. The salivary gland has been identified as a possible reservoir of SARS-CoV-2 infection [12,13], with viral RNA isolated from the saliva of infected individuals [14,15], many of whom may be asymptomatic [16]. Early data suggest SARS-CoV-2 may remain viable and infectious in aerosol for several hours, and on surfaces for several days [17]. The potential viral load of aerosol and splatter created during dental procedures is not known, and nor is any estimate of the dilution effect produced by the water irrigation from the instruments available. Similarly, the effect of mitigating measures such as suction and ventilation is not well understood or explored [18].
Several studies have investigated splatter and aerosol contamination using a tracer dye [[19], [20], [21], [22], [23], [24], [25]] or microbiological analyses [10,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. Previous tracer dye studies have not investigated contamination over large distances, and previous microbiological studies have detected culturable bacteria as markers of aerosol and splatter distribution. However, bacteria are much larger than viruses, such as SARS-CoV-2, and have fundamentally different aerosol dynamics. Importantly, most of these studies have only been conducted in a single surgery environment, with very few investigating the potential for transmission in the open plan clinic environment. The few studies examining open plan clinics [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36]] have assessed culturable bacterial contamination and the findings are hard to interpret due to a lack of: procedure standardisation/reporting, information of the clinic set up, and accurate reporting of ventilation and suction rates. One of these studies [34] placed culture plates 2–3 feet (0.6–0.9 m) from the patient’s mouth and therefore did not examine distant contamination. We recently reported findings of contamination following dental procedures up to 4 m from the source, albeit at very low levels [37]. This work used a tracer dye methodology and was the first study to apply a combination of digital image analysis and spectrofluorometric analysis to dental aerosol research. Our previously reported findings may represent a problem for open plan clinic environments such as those often found in secondary care and teaching institutions, because there is a theoretical potential for contamination of adjacent bays, with exposure of the operator and patient to aerosols containing pathogens such as SARS-CoV-2. This potential problem was the motivation for the present study, to provide further evidence to inform a safe return to dental education and specialist dental services.
Equally challenging to both dental care provision and dental education is the requirement for a post-operative fallow time following AGPs—that is, the time following an AGP after which the environment is safe to enter due to an adequate reduction in possible airborne pathogens. A fallow-time of 60 min was initially recommended for all settings in the UK [6], however, this has not been universally adopted internationally due to concerns over a limited evidence base [38]. More recently the recommended fallow time in England was updated to 20 min for settings that can demonstrate ventilation rates of 10–12 air changes per hour (ACH), and one hour for those with 6 ACH [39]. Similarly, fallow times have been suggested in the range of 10 min to 2 h and 27 min depending on ACH [9]. Our recent tracer dye work in a setting with 6.5 ACH demonstrated that contamination from settled aerosol on samples exposed to the air 30–40 minutes after an AGP was 0.02 % of that from samples exposed from the start of the 10-minute procedure until 10 min after [37]; this suggests that a 60-minute fallow time may be too long. It has been suggested that the fallow time should not be reduced below 10-minutes to allow larger droplets to settle onto surfaces [9]. On the same basis, it has been advised that environmental cleaning can happen 10 min after an AGP, even within the fallow period [9]. Although this advice is pragmatic, there is very little evidence to support this practice in open plan or closed clinical environments.
The aim of this paper was to develop our previously reported methodology to investigate the distribution of aerosol and splatter, from a single simulated infected source, following dental procedures in the open plan clinic environment and the effect of mitigating factors. A secondary aim was to examine the time course of aerosol deposition following an AGP to inform fallow time recommendations (i.e. to determine the minimum time before environmental cleaning can occur). Such evidence is essential to expediate a safe and economically viable return to specialist and primary care dental services and to dental education.
2. Materials and methods
A total of nine experiments were conducted in this study: six experiments were conducted in the open plan clinic to investigate aerosol and splatter distribution in this setting (open plan clinic experiments); three experiments were conducted in a clinical teaching laboratory to investigate the persistence of dental aerosols (time course experiments). The same single clinical procedure was conducted in all of the experiments and each of the nine experiments were carried out on three separate occasions. Each procedure was conducted on dental models (AG-3, Frasaco GmbH; Tettnang, Germany) and consisted of a 10-minute crown preparation of the right maxillary central incisor tooth using a high-speed air-turbine handpiece (irrigation rate: 14 mL/min in open plan clinic experiments and 29.3 mL/min in time course experiments).
2.1. Open plan clinic experiments
The methods used have previously been described in detail [37,40], however, for this study modifications were made to allow modelling in an open plan clinic environment. A 1 m diameter rig was constructed with four rods arranged at 90° intervals around a dental chair with a dental training mannequin attached (P-6/3 TSE, Frasaco GmbH; Tettnang, Germany). The position of the mannequin’s mouth was 73 cm above the floor. A linear 12 m rig was constructed in the adjacent walkway which had collection platforms at 0.5 m intervals for the 2 m either side of the centre, and then subsequently at 1 m intervals out to 6 m either side of the centre; the centre of this linear rig was adjacent to the mannequin. Both rigs supported 30 mm diameter grade 1 qualitative cotton-cellulose filter papers (Whatman, Cytiva; MA, USA) and were positioned 86 cm from the floor. Filter papers were also placed in adjacent and distant bays: four on benchtops (86 cm height); one on the bracket table situated directly over the dental chair; and two on top of separating divides to the other half of the clinic where appropriate (123 cm height). Fluorescein dye was used as a tracer as a 2.65 mM solution. Two fluorescein models were used in this investigation to model aerosol and splatter and both have been previously described. In model 1 fluorescein solution was introduced into the irrigation reservoirs of the dental unit, representing a worst-case scenario for splatter and aerosol distribution [37]. In model 2, fluorescein solution was introduced into the mouth of the dental mannequin via 1 mm internal diameter tubing, mimicking high normal stimulated salivary flow (1.5 mL/min) and representing a more clinically relevant model [40]. Dental models were soaked in fluorescein for 5 min before experiments and primed with 5 mL of fluorescein immediately prior to starting each experiment. Four, 10 mm diameter cotton rolls were secured to the left and right, upper and lower buccal surfaces of the models to provide a reservoir of fluorescein.
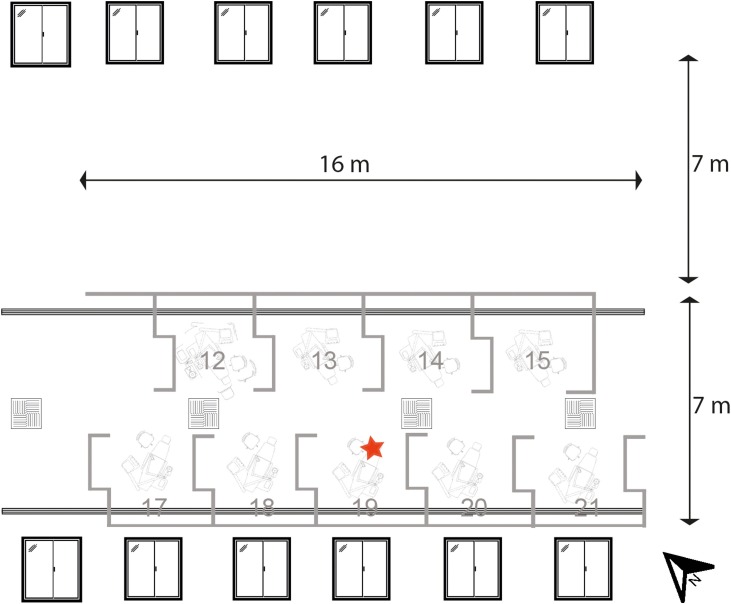
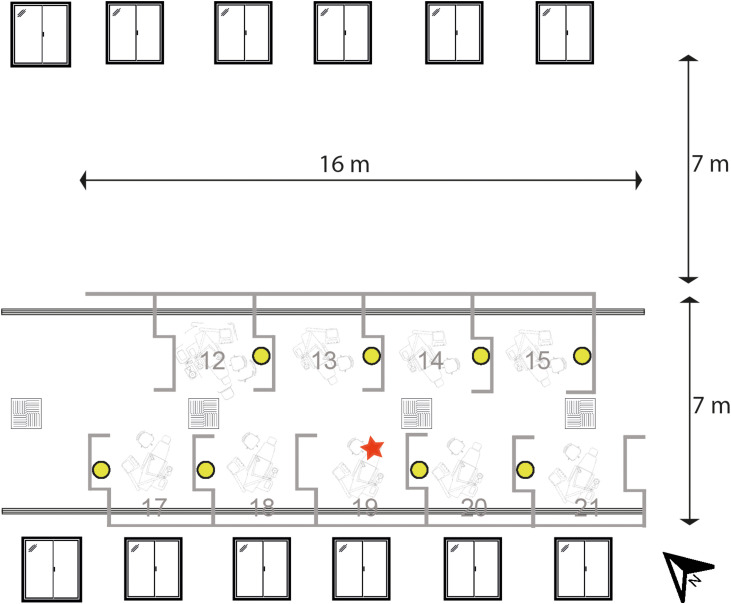
Fig. 1 illustrates both models. The open plan clinic used in the present study was a 21-chair clinic; half of the clinic was used for the experiments, and the layout of the open plan clinic is shown in Fig. 2 . Six (1.2 m × 1.36 m) windows were present on two sides of the clinic which opened to half of their width. Standard hospital ventilation provided 3.45 ACH (this was assessed by an external engineering contractor). Each bay was 2.86 m wide by 2.75 m deep, separated by walls 1.44–1.54 m high. A series of six experiments were conducted to test different dental mitigation factors and environmental conditions as described in Table 1 (open plan clinic experiments: 1–6). Dental suction was used with an 8.2 mm aspirator tip in two experiments at two flow rates: low volume suction, 40 L/min of air; medium volume suction, 159 L/min of air. The same single clinical procedure was conducted in all the experiments as described. One of three similarly experienced operators (RH, height: 170 cm; CB, height: 167 cm; DE, height: 187 cm) performed the procedure. Following each procedure, the filter papers were left in situ for 30 min before collection as our previous work demonstrated that very little, if any, contamination was detected after this time [37]. During all experiments, only the operator and assistant were present within the experimental area, leaving immediately after the procedure. Fluorescein contamination was also collected from a 400 cm2 area on a benchtop in each of the eight bays surrounding the bay in which experiments were conducted; this method is described in detail in the Supporting Information (available online).
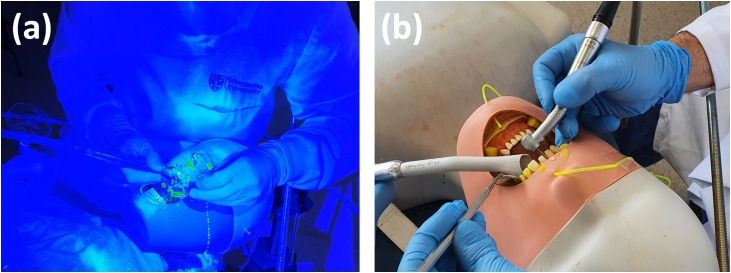
Fig. 1.
Experimental models used in this study. Fluorescein dye was either introduced into the irrigation systems of the dental instruments (a) or the mouth of the mannequin (b).
Fig. 2.
Plan view of the open plan clinic area. Red star indicates the location of the aerosol generating procedure. Air intake (square vents) and air output (long vents) are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 1.
Experiment details. AGP: Aerosol Generating Procedure. Model 1: dye in the instrument irrigation system. Model 2: dye in the mannequin mouth only. CSU: Clinical Simulation Unit. *Wind direction, speed, and gust speed were: repetition 1–W, 7 mph, 14 mph; repetition 2–E, 8 mph, 14 mph; repetition 3–NW 5 mph, 7 mph. #Wind direction, speed, and gust speed were: repetition 1–W, 11 mph, 19 mph; repetition 2–W, 11 mph, 19 mph; repetition 3–W 11 mph, 20 mph.
| Experiment number | Location | Model | Assistant | Operator(s) (repetition 1, 2, 3) | Suction flowrate, L/min air | Cross-ventilation | Sample exposure time (end of AGP = time zero), minutes |
|---|---|---|---|---|---|---|---|
| 1 | Open clinic | 1 | No | RH, RH, RH | No suction | No | −10 to 30 |
| 2 | Open clinic | 1 | Yes | DE, RH, CB | 40 | No | −10 to 30 |
| 3 | Open clinic | 1 | Yes | RH, RH, CB | 159 | No | −10 to 30 |
| 4 | Open clinic | 1 | Yes | RH, RH, CB | 159 | Yes* | −10 to 30 |
| 5 | Open clinic | 2 | Yes | RH, RH, RH | 159 | No | −10 to 30 |
| 6 | Open clinic | 2 | Yes | RH, RH, RH | 159 | Yes# | −10 to 30 |
| 7 | CSU | 1 | No | RH, RH, RH | 105 | No | 0 to 5 |
| 8 | CSU | 1 | No | RH, RH, RH | 105 | No | 10 to 15 |
| 9 | CSU | 1 | No | RH, RH, RH | 105 | No | 20 to 25 |
2.2. Time course experiments
Three experiments (experiments 7–9 in Table 1) were conducted in a clinical simulation unit (CSU) with 6.5 ACH (this was assessed by an external engineering contractor). A rig with eight 4 m rods spaced at 45° intervals was constructed around a dental training unit (Model 4820, A-dec; OR, USA) with a dental mannequin containing model teeth (Frasaco GmbH; Tettnang, Germany). The rig supported filter papers spaced at 0.5 m intervals as previously described [37]. In our previous work using the same procedure (10-minute anterior crown preparation; fluorescein model 1) we reported data collected at three timepoints: start of procedure to 10 min post-procedure, 30–40 min post procedure, and 60–70 min post procedure. In the present study we examined three additional time points: 0–5 min post procedure by uncovering filter papers immediately at the end of the procedure; 10–15 min and 20–25 min post procedure, by placing new filter papers on clean platforms at both timepoints. A single operator (RH) completed these experiments. During the procedure only the operator was present within the experimental area. During the collection period there were 4–5 investigators present within the experimental area in order to collect and place new filter papers (estimated total time within the experimental area =9 min). Each procedure was repeated on three separate occasions.
2.3. Sample analysis
Contamination of filter papers was assessed as previously described [37], using both image and spectrofluorometric analysis. Filter papers were first assessed with fluorescence photography by illuminating samples using a 400–500 nm wavelength halogen lamp. Images were then analysed using ImageJ (v1.48 NIH; MD, USA) to calculate a surface area measurement (mm2) of fluorescein contamination. Spectrofluorometric analysis was then performed by eluting fluorescein from samples in distilled water, and then using a microplate reader (Synergy HT, BioTek; VT, USA) to calculate relative fluorescence units (RFU). Examiners for both measurements were blind to the experimental conditions.
For model 1 (dye in irrigation systems) mean measurements of blank samples + 3 standard deviations (180 RFU) was used as the lower limit of detection; hence a zero reading was assigned to values below 180 RFU. For model 2 (dye in mouth only) mean measurements of blank samples + 3 standard deviations (89 RFU) was used as the lower limit of detection; hence a zero reading was assigned to values below 89 RFU. Between experiments using models 1 and 2 the experimental area was thoroughly cleaned and left for 5 days; new dental unit tubing was used between models to exclude contamination. For time course experiments in the CSU, we used a previously reported lower detection threshold of 164 RFU, calculated using the same methodology, to allow comparison between previous data [37]. For readings above the detection limit of the instrument (>100,000 RFU), a value of 100,000 RFU was assigned.
Data were collected using Excel (2016, Microsoft; WA, USA) and analysed using SPSS (version24, IBM Corp.; NY, USA) using basic descriptive statistics. Heatmaps were created to show distribution of contamination using Python 3 [41]. For each coordinate on the heatmap, the maximum value recorded from three repetitions of each clinical procedure was used. Logarithmic transformation was performed on the data (Log10). When total contamination was calculated, if data were missing, a mean value was imputed from the other two repetitions to ensure comparisons across conditions were representative.
3. Results
In total 1881 samples were collected (excluding blank negative controls). 12 samples were removed from the analysis due to processing errors during the experiments themselves and 26 samples were removed due to laboratory processing errors. In total, image analysis data were available was recorded for 1869 samples and spectrofluorometric analysis data for 1843 samples. Data from at least two replicates were available for each condition/location.
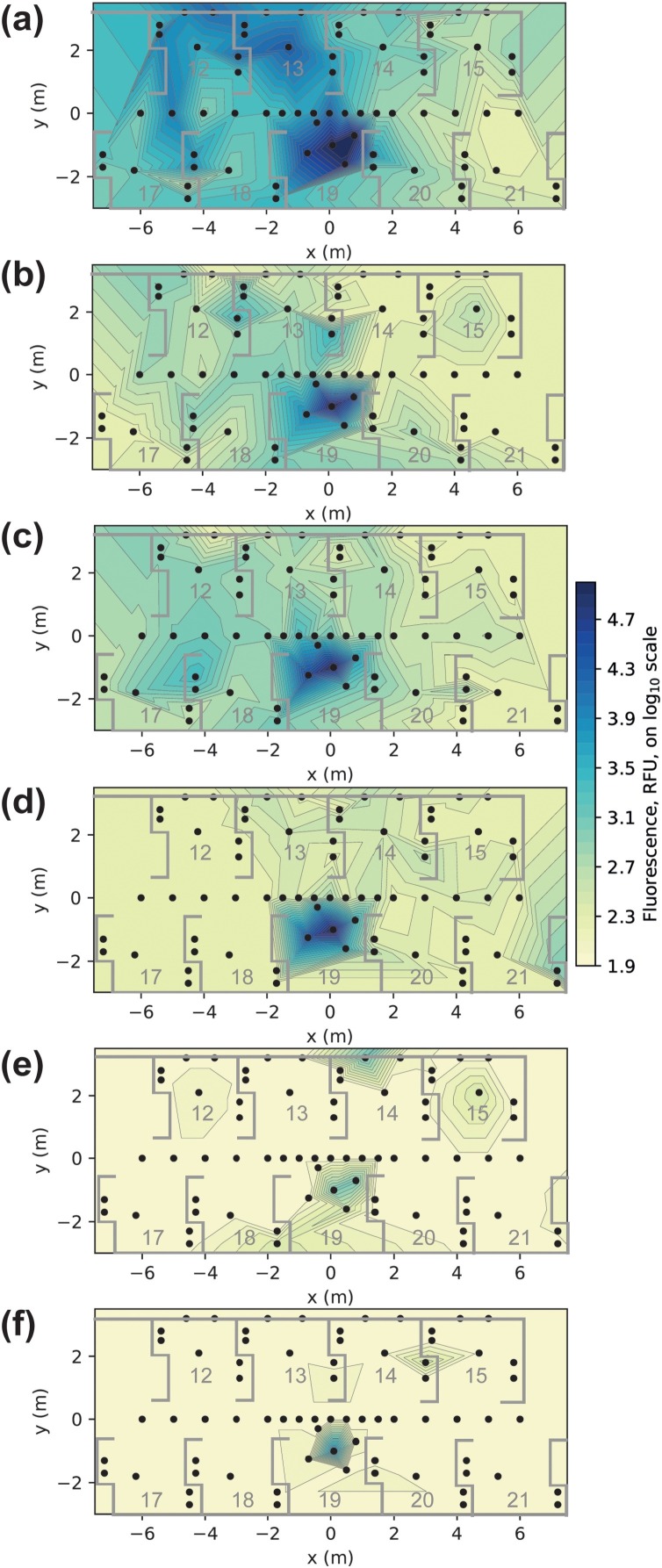
A series of heatmaps are shown in Fig. 3 which present fluorescein contamination across the open plan clinic from the six experiments (see Table 1). Panels A–D, which show model 1, demonstrate where the water spray from the high-speed air-turbine travels, and the relative impact of dental suction and cross-ventilation on contamination patterns. Panels E and F, which show model 2, demonstrate where contamination from the mouth may be transported. In panel E, there are positive readings in distant bays (bay 12 and 15). Using a standard curve [37] we can estimate the mass of fluorescein contained within the 30 mm diameter (706.9 mm2) filter paper samples using RFU values. The contaminated sample in bay 12 contained 0.28 μg of fluorescein, whist the sample in bay 15 had 0.32 μg. During the 10 min of the crown preparation procedure, a total of 20 mg of fluorescein was introduced into the mouth. Hence, the fluorescein (which models saliva) detected on the samples in bays 12 and 15 represented 1/71,400 (0.0014 %) and 1/63,500 (0.0016 %) of the fluorescein introduced into the system during the procedure, respectively. The data used to create the heatmaps is available in Table S1 (available online).
Fig. 3.
Heatmaps presenting spectrofluorometric analysis data from a 10-minute high-speed air-turbine anterior crown preparation. Panels represent different experimental conditions. (a): no dental suction, model 1 (dye in handpiece). (b): low volume dental suction (40 L/min), model 1. (c): medium volume dental suction (159 L/min), model 1. (d): medium volume dental suction with cross-ventilation (windows open on both sides of clinic), model 1. (e): medium volume dental suction, model 2 (dye in mouth). (f): medium volume dental suction with cross-ventilation, model 2. RFU: relative fluorescence units.
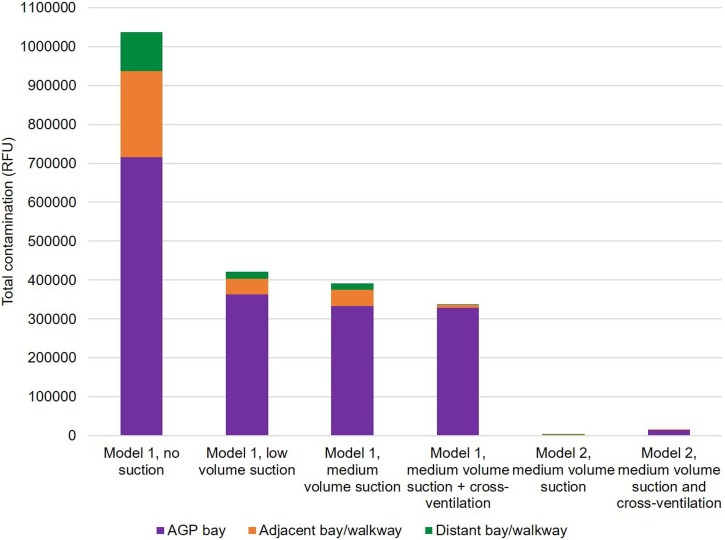
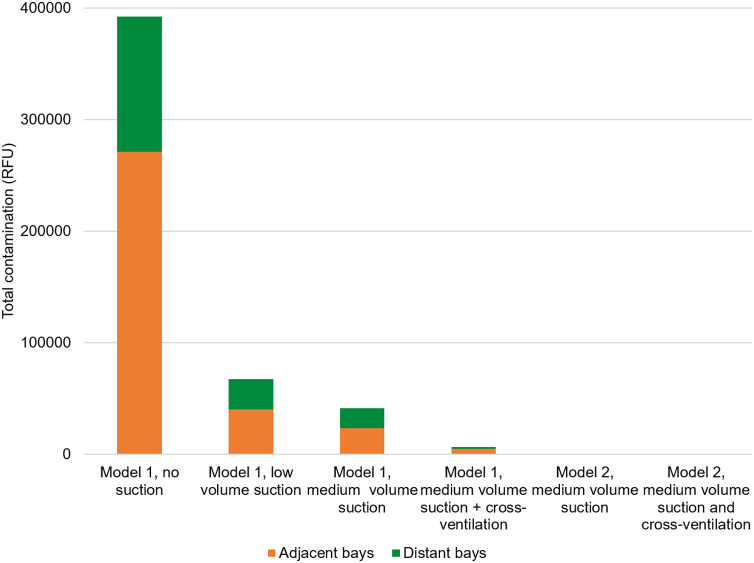
Fig. 4 shows the total contamination (sum across 3 repetitions) by experimental condition, based on the spectrofluorometric analysis. Where data were missing, a mean value was imputed from the other two repetitions to ensure comparisons across conditions were representative. Data are presented for the bay where the procedure was performed (AGP bay), and for adjacent and distant bays including their respective, associated walkways; the head-to-head distances for distant bays were ≥ 5 m. Medium volume dental suction reduced contamination within the AGP bay by 53 %, in adjacent bays/walkway by 81 %, and in distant bays/walkway by 83 %. Low volume dental suction reduced contamination within the AGP bay by 49 %, in adjacent bays/walkway by 82 %, and in distant bays/walkway by 82 %. Cross ventilation reduced contamination in the bay by 2%, in adjacent bays by 80 % and in distant bays by 89 % compared with medium volume suction alone. Table S2 (available online) provides detailed descriptive statistics for this analysis. Spectrofluorometric analysis of contamination collected from a 400 cm2 area on benches in each of the bays, other than the AGP bay, showed a similar pattern; these data are reported in the Supporting Information (available online).
Fig. 4.
Stacked bar chart presenting the impact of two levels of dental suction, cross ventilation and experimental model on total contamination across the experimental rig and mannequin in experiments 1–6 (open plan clinic experiments). The y-axis represents the sum of samples from the three repetitions of experimental condition. AGP: Aerosol Generating Procedure.
When considering the combined data from the four experiments in model 1, the vast majority of the contamination (99.99 %) detected on image analysis (likely indicating mainly splatter) was located to within the AGP bay (99.2 %) or the walkway associated with this bay (0.7 %) (total of 8664 mm2; range 0–717 mm2; 63 positive samples of 155 samples). Outside this area, there were 14 samples with positive readings on image analysis (total of 0.6 mm2; range 0–0.2 mm2; 14 positive samples of 676 samples in total), 6 of these were in the bays opposite (bays 13 and 14), 6 in the walkway and 2 in distant bays (readings of 0.06 and 0.246 mm2 respectively).
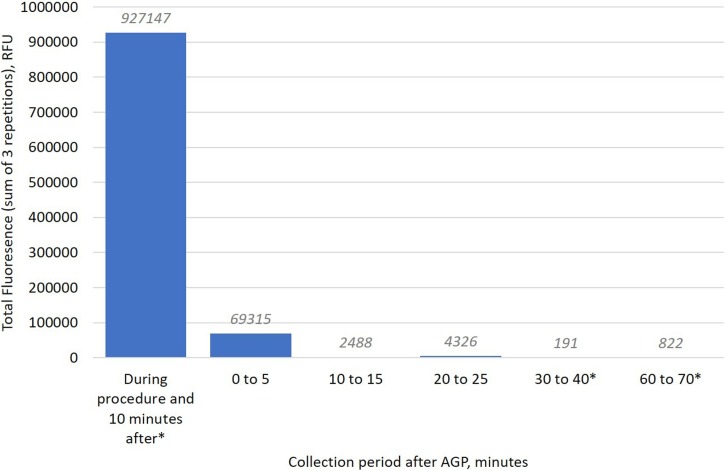
Table 2 shows the data from the detailed time course experiments in the CSU (6.5 ACH). The vast majority of the post-AGP contamination was detected within the 0–5-minute period, with little detected in sampling at longer time periods. We measured up to 25 min in this study and 60 min in a previous comparable study (same rig, operator and analysis technique) [37]. Fig. 5 presents the spectrofluorometric analysis data from these time course experiments. This demonstrates that the samples collected between 0–5 min represent 7 % of the baseline total (samples exposed during the 10-minute procedure and 10 min after). The samples collected at 10–15 min represented 0.3 % of the baseline total and for those at 20–25 min this was 0.5 %.
Table 2.
Total contamination across the experimental rig (including mannequin samples) from time course experiments (experiments 7–9). Both contaminated surface area using image analysis and fluorescence by spectrofluorometric analysis are presented. The experimental procedure was a 10-minute anterior crown preparation on dental models with a high-speed air-turbine handpiece without dental suction. AGP: Aerosol Generating Procedure. RFU: relative fluorescence units.
| Sample exposure period from end of AGP, minutes | Surface area, mm2 | Fluorescence, RFU |
|---|---|---|
| 0 to 5 | 2.2 | 69,315 |
| 10 to 15 | 0.2 | 2,488 |
| 20 to 25 | 0.0 | 4,326 |
Fig. 5.
Bar chart presenting the total contamination across the experimental rig and mannequin as measured by spectrofluorometric analysis and presented by collection time in experiments 7–9 (time course experiments). The experimental procedure was a 10-minute anterior crown preparation with a high-speed air-turbine handpiece without dental suction. The y-axis represents the sum of all samples collected at each time point. Three data points were missing; a mean value was imputed from the other two repetitions of each of these data points to ensure comparability (n per time point = 207). RFU: relative fluorescence units. AGP: aerosol generating procedure. *Comparative data are presented from a previous study [37].
4. Discussion
Our findings from model 1 (dye in irrigation system) demonstrate that splatter contamination (indicated by our image analysis outcome data) was mainly contained within the AGP bay and associated walkway area; this corroborates our previous findings that the majority of contamination is limited to the first 1.5 m from the procedure [37]. Outside the bay a small number of small splatter droplets were detected; most of these were in the two bays opposite the AGP bay or in the walkway. The asymmetrical design of this clinic means each bay opens onto two other bays, with no physical barrier or wall. Hence, our data show that with 1.5 m high lateral bay partition with open fronts, at least 99.99 % of splatter following an AGP is contained within the bay. This is also likely to be an underestimate as proportionally fewer samples were collected from within the AGP bay. It suggests that a 1.5 m high bay partition with a patient positioned 73 cm above the floor (operator heights 1.67 m–1.87 m) may be adequate to prevent distant splatter contamination. Previous recommendations that bay partitions should have a height of 2 m above the patient are therefore likely to be unnecessary [9]. These findings are also in keeping with a recent study reporting splatter analysis from visual analysis of litmus paper when citric acid was added to the irrigation system [42]. The authors reported a similar distribution pattern of splatter up to 1.33 m. This study, however, only analysed splatter in single repetitions, and did not report the level of suction used.
Aerosol, as demonstrated by the spectrofluorometric data, has the potential to travel several metres, supporting our previous findings using model 1 [37]. However, this represents the ‘water spray’ from a high-speed air-turbine handpiece and does not necessarily represent where pathogens from the mouth may go. This technique (model 1, dye in irrigation system) is useful for modelling air flow, identifying ‘hot spots’, and to assess the impact of different mitigation factors such as suction and cross ventilation. Model 2 (dye in mouth) would not have the sensitivity to allow these to be assessed in detail.
We assessed the impact of two levels of dental suction within this study: medium volume suction (159 L/min air) and low volume suction (40 L/min air) as defined by current standards [43]. It is worth noting many previous studies using ‘high volume dental suction’ did not report their suction level [20,28,42] or used suction that fell below the 250 L/min criteria for high volume aspiration [31]. Reassuringly, we observed little difference in the reductions of close and distant contamination between low and medium suction, perhaps indicating that there is quite a low threshold for suction exerting substantial benefit. The effect of dental suction in the AGP bay itself was moderate, most likely because the majority of local contamination was comprised of large droplets or high velocity small droplets that would not be removed by dental suction. However, dental suction had a marked effect on contamination in adjacent and distant bays. This may be explained by the fact that suction removes smaller lighter droplets (aerosol) more easily, and it is these that likely cause distant contamination. Hence, dental suction with a wide bore aspiration tip should be an essential component of dental treatment, especially in an open plan clinic environment. Further research should investigate the role of higher levels of suction beyond those evaluated in the present study.
The setup of our open plan clinic allowed us to assess the impact of cross-ventilation. Six windows on each side of the clinic were fully opened to allow cross-ventilation. Our findings show a reduction in settled aerosol detected outside the AGP bay (i.e. in adjacent and distant bays). However, this will likely be affected by wind speed and direction and could be unpredictable. Opening windows will reduce the post-operative fallow time and this has been reported as equivalent to one additional air change per hour [9]. Previous work from our group has shown the benefit of opening windows to improve ventilation by measuring carbon dioxide levels [44].
Data from our most realistic model (model 2, dye in mouth) produced reassuring results for distant contamination in open plan clinics. Several authors have previously discussed the dilution effect of the dental water spray on saliva [8,18]. Moderate contamination was detected within the AGP bay using model 2 and more modest contamination was seen in bays opposite where there is no barrier. In distant bays (i.e. those ≥ 5 m away) we either detected very low levels or no fluorescein contamination. These positive readings in distant bays represent less than 0.0016 % of the fluorescein introduced into the system during the procedure. This is equivalent to a half a teaspoon of ‘saliva’ in a bathtub full of water (2.5 mL in 150 L). An important consideration is the homogeneity of any dilution effect; the dilution of ‘saliva’ transported from the mouth in the water spray of dental instruments is unlikely to be completely even. Our data would support this assumption as samples in distant bays showed varying levels of contamination from zero readings (below our lower level of detection) to positive readings (with dilution factors of 60,000–70,000 times).
There are some early data on the salivary viral load of SARS-CoV-2 in infected but asymptomatic individuals [15]. From this is it possible to estimate the number of viral copies in the distant bay samples if the patient was positive but asymptomatic. This would equate to an estimated 31.5 copies over 707 mm2 (or 4.5 copies/cm2) for the positive sample in bay 15 and 8 copies over 707 mm2 (or 4.0 copies/cm2) for the positive sample in bay 12. The infective dose of SARS-CoV-2 is still largely unknown, but it is likely that a viral load of several hundred viral copies in a susceptible individual may be required [45].
In our previous work, we demonstrated that very little additional settled aerosol contamination can be detected between 30 and 60 min after the procedure [37] (the current study evaluated time intervals under 30 min). Hence, our data provide strong evidence that the majority of larger droplets settle within 10 min, and support the pragmatic recommendations of the Short Life Working Group lead by National Services Scotland [9] and the Scottish Dental Clinical Effectiveness Programme [46] which state a minimum time of 10 min before environmental cleaning may begin. It is important to note that the time after which environmental cleaning is appropriate is not necessarily the same as the recommended post-operative fallow time between patients, because suspended aerosol particles may remain in the air within the dental clinic. Within the post-operative fallow time, respiratory protection should be used for all entering the clinical area to protect against this suspended aerosol. Further work should be conducted to explore this important factor.
The present study has several limitations. We rely on aerosol and splatter passively settling onto filter papers. Active sampling techniques (e.g. air samplers) are available and should be used in future research to complement this work. The benefits of our approach in this experiment are that it enables sampling of many locations simultaneously (70 in the present study) in an open plan clinic and mapping of the distribution of contamination. This would be difficult to achieve using active sampling. Additionally, our findings about cross-ventilation should be interpreted with this in mind—increased air turbulence may have caused less settling of aerosols onto our samples. We also only operated in one bay (to model an infective patient and examine where contamination might travel) and future research may evaluate more complex scenarios with procedures being conducted in multiple bays. This future research would require different tracer dyes for each bay and likely require complex computational modelling. Our study is designed to evaluate aerosol and splatter, and therefore shows potential for contamination of surfaces from saliva and blood which may harbour micro-organisms. However, it is not a biological model and further work to validate these findings with a biological model is an important next step. Our understanding of the infective dose of SARS-CoV-2 is evolving, and therefore our results should be interpreted in this evolving context. Finally, our study design was based on several worst-case scenario assumptions to give the most conservative results with a safe margin of error. For example, the salivary flow rate used was at the high end of the spectrum of stimulated salivary flow rates; for the heatmaps we used the maximum readings obtained over the three repetitions of each condition (not the mean); and the clinical procedure we conducted was at the front of the mouth and without rubber dam.
5. Conclusions
The cross-infection risk from conducting AGPs in an open plan clinic environment appears to be small, particularly when bays are 5 m apart in a setting with 3.45 ACH. Very little splatter was detected outside of the AGP bay and more distant contamination from settled aerosol was at very low levels. There is a major dilution effect from the water spray of dental instruments. Dental suction has a substantial positive effect, particularly on more distant contamination. Comparison of different suction flow rates indicates that even low volume suction (40 L/min air, with a wide-bore aspirating tip) confers a substantial benefit. Time course experiments show that the majority of dental aerosol contamination that is destined to settle onto surfaces within the first 60 min, does so within the first 10 min post-procedure, meaning environmental cleaning may be appropriate after this period.
CRediT authorship contribution statement
Richard Holliday: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. James R. Allison: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration, Funding acquisition. Charlotte C. Currie: Methodology, Investigation, Writing - original draft, Writing - review & editing, Funding acquisition. David C. Edwards: Methodology, Investigation, Writing - original draft, Writing - review & editing, Funding acquisition. Charlotte Bowes: Methodology, Investigation, Writing - original draft, Writing - review & editing, Funding acquisition. Kimberley Pickering: Investigation, Writing - review & editing. Sarah Reay: Investigation, Writing - review & editing. Justin Durham: Conceptualization, Methodology, Writing - review & editing, Funding acquisition. Joanna Lumb: Methodology, Writing - review & editing. Nadia Rostami: Methodology, Investigation, Writing - review & editing, Funding acquisition. Jamie Coulter: Investigation, Writing - review & editing. Christopher Nile: Methodology, Writing - review & editing, Funding acquisition. Nicholas Jakubovics: Methodology, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was supported by research grants from the British Endodontic Society (JA principal grant holder), Royal College of Surgeons of Edinburgh (DE principal grant holder) and the School of Dental Sciences, Newcastle University. Richard Holliday is funded by a National Institute for Health Research (NIHR) Clinical Lectureship. Charlotte Currie is funded by an NIHR Doctoral Research Fellowship. Jamie Coulter is funded by a NIHR BRC Doctoral Research Fellowship. David Edwards is funded by an NIHR Academic Clinical Fellowship. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR or Department of Health and Social care. Nadia Rostami was funded during this period by the Dunhill Medical Trust (RPGF1810/101) who kindly extended her funding to support this urgent COVID-19 related research. Newcastle upon Tyne Hospitals NHS Foundation Trust provided a staff member to support this project (Sarah Reay). We would like to thank Ekaterina Kozhevnikova for her wider support for this work. We would also like to thank Nisha Patel, Ciara Docherty, Ben Walker, Pei Xian Chen and Thomas Bradshaw for their help with data collection. The authors declare that there are no conflicts of interest and agree to be accountable for the work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jdent.2020.103565.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bridgman C. 2020. Letter to All Primary Care Dental Teams in Wales, 23rd March.https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/2020.03.23%20CDO%20Wales%20COVID-19%20advice%20letter.pdf (Accessed 23/06/2020) [Google Scholar]
- 2.Donaldson M. 2020. COVID-19: Outline Strategic Plan for General Dental Services and Updated Guidance for General Dental Practice, 23rd March.http://www.hscbusiness.hscni.net/pdf/HSCB_COVID-19_GDS-Strategic-Plan.pdf (Accessed 23/06/2020) [Google Scholar]
- 3.Ferris T. 2020. Letter to NHS Dental Services (ref: POL/33888), 23rd March.https://www.fgdp.org.uk/sites/fgdp.org.uk/files/editors/2020.03.23%20CDO%20Scotland%20COVID-19%20advice%20letter.pdf (Accessed 23/06/2020) [Google Scholar]
- 4.Hurley S., Neligan M. 2020. Letter to General Dental Practices and Community Dental Services (ref: 001559), 25th March.https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/issue-3-preparedness-letter-for-primary-dental-care-25-march-2020.pdf (Accessed 06/06/2020) [Google Scholar]
- 5.Carter E., Currie C.C., Asuni A., Goldsmith R., Toon G., Horridge C., Simpson S., Donnell C., Greenwood M., Walton G., Cole B., Durham J., Holliday R. The first six weeks - setting up a UK urgent dental care centre during the COVID-19 pandemic. Br. Dent. J. 2020;228(11):842–848. doi: 10.1038/s41415-020-1708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Office of the Chief Dental Officer England . Office of The Chief Dental Officer England; London: 2020. Standard Operating Procedure Transition to Recovery, Version 4. [Google Scholar]
- 7.Innes N., Johnson I., Al-Yaseen W., Harris R., Jones R., Kc S., McGregor S., Robertson M., Wade W., Gallagher J. A systematic review of droplet and aerosol generation in dentistry. J Dent. 2021;105 doi: 10.1016/j.jdent.2020.103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durham J., Bagg J., Bain S., Bissell V., Burgden D., Chadwick B., Chapple I., Coulthard P., Curtis M., Deery C., Edwards M., Glenny A., Hector M., Ibbetson R., McCreary C., Mighell A., O’Connell B., Porter S., Tredwin C., Walls A. 2020. COVID-19 – Returning to Student-Led Dental Clinical Treatments.https://www.dentalschoolscouncil.ac.uk/wp-content/uploads/2020/06/COVID-19-report-on-returning-to-student-leddental-clinical-treatments.pdf (Accessed 22/06/2020) [Google Scholar]
- 9.National Services Scotland . National Services Scotland; Edinburgh: 2020. Ventilation, Water and Environmental Cleaning in Dental Surgeries Relating to COVID-19. [Google Scholar]
- 10.Bennett A.M., Fulford M.R., Walker J.T., Bradshaw D.J., Martin M.V., Marsh P.D. Microbial aerosols in general dental practice. Br. Dent. J. 2000;189(12):664–667. doi: 10.1038/sj.bdj.4800859. [DOI] [PubMed] [Google Scholar]
- 11.Miller R.L. Characteristics of blood-containing aerosols generated by common powered dental instruments. Am. Ind. Hyg. Assoc. J. 1995;56(7):670–676. doi: 10.1080/15428119591016683. [DOI] [PubMed] [Google Scholar]
- 12.Pascolo L., Zupin L., Melato M., Tricarico P.M., Crovella S. TMPRSS2 and ACE2 coexpression in SARS-CoV-2 salivary glands infection. J. Dent. Res. 2020 doi: 10.1177/0022034520933589. [DOI] [PubMed] [Google Scholar]
- 13.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020 doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 14.To K.K.-W., Tsang O.T.-Y., Yip C.C.-Y., Chan K.-H., Wu T.-C., Chan J.M.-C., Leung W.-S., Chik T.S.-H., Choi C.Y.-C., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W.-S., Fung A.Y.-F., Hung I.F.-N., Cheng V.C.-C., Chan J.F.-W., Yuen K.-Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P., Geng B., Muenker M.C., Moore A.J., Vogels C.B.F., Petrone M.E., Ott I.M., Lu P., Lu-Culligan A., Klein J., Venkataraman A., Earnest R., Simonov M., Datta R., Handoko R., Naushad N., Sewanan L.R., Valdez J., White E.B., Lapidus S., Kalinich C.C., Jiang X., Kim D.J., Kudo E., Linehan M., Mao T., Moriyama M., Oh J.E., Park A., Silva J., Song E., Takahashi T., Taura M., Weizman O.-E., Wong P., Yang Y., Bermejo S., Odio C., Omer S.B., Dela Cruz C.S., Farhadian S., Martinello R.A., Iwasaki A., Grubaugh N.D., Ko A.I. Saliva or nasopharyngeal swab specimens for detection of sars-cov-2. N Engl J Med. 2020;383(13):1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P., Norddahl G.L., Saemundsdottir J., Sigurdsson A., Sulem P., Agustsdottir A.B., Eiriksdottir B., Fridriksdottir R., Gardarsdottir E.E., Georgsson G., Gretarsdottir O.S., Gudmundsson K.R., Gunnarsdottir T.R., Gylfason A., Holm H., Jensson B.O., Jonasdottir A., Jonsson F., Josefsdottir K.S., Kristjansson T., Magnusdottir D.N., le Roux L., Sigmundsdottir G., Sveinbjornsson G., Sveinsdottir K.E., Sveinsdottir M., Thorarensen E.A., Thorbjornsson B., Löve A., Masson G., Jonsdottir I., Möller A.D., Gudnason T., Kristinsson K.G., Thorsteinsdottir U., Stefansson K. Spread of SARS-CoV-2 in the icelandic population. N. Engl. J. Med. 2020;382(24):2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein J.B., Chow K., Mathias R. Dental procedure aerosols and COVID-19. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentley C.D., Burkhart N.W., Crawford J.J. Evaluating spatter and aerosol contamination during dental procedures. J. Am. Dent. Assoc. 1994;125(5):579–584. doi: 10.14219/jada.archive.1994.0093. [DOI] [PubMed] [Google Scholar]
- 20.Chanpong B., Tang M., Rosenczweig A., Lok P., Tang R. Aerosol-generating procedures and simulated cough in dental anesthesia. Anesth. Prog. 2020 doi: 10.2344/anpr-67-03-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiramana S., Bindu O S.H., Kadiyala K., Prakash M., Prasad T., Chaitanya S. Evaluation of minimum required safe distance between two consecutive dental chairs for optimal asepsis. J. Orofac. Res. 2013;3:12–15. [Google Scholar]
- 22.Dahlke W.O., Cottam M.R., Herring M.C., Leavitt J.M., Ditmyer M.M., Walker R.S. Evaluation of the spatter-reduction effectiveness of two dry-field isolation techniques. J. Am. Dent. Assoc. 2012;143(11):1199–1204. doi: 10.14219/jada.archive.2012.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrel S.K., Barnes J.B., Rivera-Hidalgo F. Reduction of aerosols produced by ultrasonic sealers. J. Periodontol. 1996;67(1):28–32. doi: 10.1902/jop.1996.67.1.28. [DOI] [PubMed] [Google Scholar]
- 24.Harrel S.K., Barnes J.B., Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. J. Am. Dent. Assoc. 1998;129(9):1241–1249. doi: 10.14219/jada.archive.1998.0421. [DOI] [PubMed] [Google Scholar]
- 25.Veena H.R., Mahantesha S., Joseph P.A., Patil S.R., Patil S.H. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J. Infect. Public Health. 2015;8(3):260–265. doi: 10.1016/j.jiph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Al-Amad S.H., Awad M.A., Edher F.M., Shahramian K., Omran T.A. The effect of rubber dam on atmospheric bacterial aerosols during restorative dentistry. J. Infect. Public Health. 2017;10(2):195–200. doi: 10.1016/j.jiph.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Dutil S., Meriaux A., de Latremoille M.C., Lazure L., Barbeau J., Duchaine C. Measurement of airborne bacteria and endotoxin generated during dental cleaning. J. Occup. Environ. Hyg. 2009;6(2):121–130. doi: 10.1080/15459620802633957. [DOI] [PubMed] [Google Scholar]
- 28.Holloman J.L., Mauriello S.M., Pimenta L., Arnold R.R. Comparison of suction device with saliva ejector for aerosol and spatter reduction during ultrasonic scaling. J. Am. Dent. Assoc. 2015;146(1):27–33. doi: 10.1016/j.adaj.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Miller R.L., Micik R.E., Abel C., Ryge G. Studies on dental aerobiology: II. Microbial splatter discharged from the oral cavity of dental patients. J. Dent. Res. 1971;50(3):621–625. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 30.Rautemaa R., Nordberg A., Wuolijoki-Saaristo K., Meurman J.H. Bacterial aerosols in dental practice - a potential hospital infection problem? J. Hosp. Infect. 2006;64(1):76–81. doi: 10.1016/j.jhin.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmerman M.F., Menso L., Steinfort J., van Winkelhoff A.J., van der Weijden G.A. Atmospheric contamination during ultrasonic scaling. J. Clin. Periodontol. 2004;31(6):458–462. doi: 10.1111/j.1600-051X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe A., Tamaki N., Yokota K., Matsuyama M., Kokeguchi S. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J. Hosp. Infect. 2018;99(3):303–305. doi: 10.1016/j.jhin.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Zemouri C., Volgenant C.M.C., Buijs M.J., Crielaard W., Rosema N.A.M., Brandt B.W., Laheij A., De Soet J.J. Dental aerosols: microbial composition and spatial distribution. J. Oral Microbiol. 2020;12(1) doi: 10.1080/20002297.2020.1762040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Maghlouth A., Al Yousef Y., Al Bagieh N. Qualitative and quantitative analysis of bacterial aerosols. J. Contemp. Dent. Pract. 2004;5(4):91–100. [PubMed] [Google Scholar]
- 35.Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl. Environ. Microbiol. 1995;61(8):3165–3168. doi: 10.1128/aem.61.8.3165-3168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobza J., Pastuszka J.S., Bragoszewska E. Do exposures to aerosols pose a risk to dental professionals? Occup. Med. 2018;68(7):454–458. doi: 10.1093/occmed/kqy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allison J.R., Currie C.C., Edwards D.C., Bowes C., Coulter J., Pickering K., Kozhevnikova E., Durham J., Nile C.J., Jakubovics N., Rostami N., Holliday R. Evaluating aerosol and splatter following dental procedures: addressing new challenges for oral healthcare and rehabilitation. J. Oral Rehab. 2021;48:61–72. doi: 10.1111/joor.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CoDER Working Group . COVID-19 Dental Services Evidence Review (CoDER) Working Group; 2020. Recommendations for the Re-opening of Dental Services: A Rapid Review of International Sources. [Google Scholar]
- 39.Hurley S. 2020. Issue 6., Preparedness Letter for Primary Dental Care - 28 August 2020.https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/C0690-dental-update-letter-from-sara-hurley-28-aug-2020.pdf (Accessed 07/09/2020) [Google Scholar]
- 40.Llandro H., Allison J., Currie C., Edwards D., Bowes C., Durham J., Jakubovics N., Rostami N., Holliday R. Evaluating aerosol and splatter during orthodontic debonding: implications for the COVID-19 pandemic. Br. Dent. J. 2021;203(1):28. doi: 10.1038/s41415-020-2503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rossum G., Drake F.L. CreateSpace; Scotts Valley, CA: 2009. Python 3 Reference Manual. [Google Scholar]
- 42.Shahdad S., Patel T., Hindocha A., Cagney N., Mueller J.-D., Seoudi N., Morgan C., Din A. The efficacy of an extraoral scavenging device on reduction of splatter contamination during dental aerosol generating procedures: an exploratory study. Br. Dent. J. 2020 doi: 10.1038/s41415-020-2112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NHS Estates . The Stationary Office; London: 2003. HTM 2022 - Supplement 1: Dental Compressed Air and Vacuum Systems. [Google Scholar]
- 44.Patel N., Docherty C., Allison J., Walton G., Cole B., Jakubovics N., Durham J., Holliday R. OSF Preprints; 2020. Letter to the Editor: Ventilation Is Key, Open a Window. [DOI] [Google Scholar]
- 45.Beggs C.B. Is there an airborne component to the transmission of COVID-19? : a quantitative analysis study. medRxiv. 2020 doi: 10.1101/2020.05.22.20109991. [DOI] [Google Scholar]
- 46.Scottish Dental Clinical Effectiveness Programme . 2020. Mitigation of Aerosol Generating Procedures in Dentistry A Rapid Review.https://www.sdcep.org.uk/wp-content/uploads/2020/09/SDCEP-Mitigation-of-AGPS-in-Dentistry-Rapid-Review.pdf (Accessed 13/10/2020) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.