Abstract
Progressive retinal degeneration manifesting as age-related macular degeneration (AMD) in the elderly affects millions of individuals worldwide. Among various blinding diseases, AMD is the leading cause of central vision impairment in developed countries. Poor understanding of AMD etiology hampers the development of therapeutics against this devastating ocular disease. Currently, daily intravitreal injections of anti-angiogenic drugs, preventing abnormal vessel growth are the only treatment option for wet AMD. However, for dry AMD associated with retinal atrophy, at present there is no cure available. Recent clinical research has demonstrated beneficial effects of plant-derived compounds for various eye disorders. Thus, the ongoing efforts toward discovering efficient treatments preventing or delaying AMD progression focus on implementing a healthy diet rich in vitamins, including vitamin A, E, and C, minerals and carotenoids, in particular lutein and zeaxanthin, to reduce the disease burden. In addition, studies in cell culture and animal models indicated therapeutic potential of dietary polyphenolic compounds present in fruits and vegetables. These natural compounds protect visual function and retinal morphology likely due to their anti-oxidant and anti-inflammatory properties. Although understanding of the exact mechanism of these compounds’ positive effects requires further investigation, they provide non-invasive alternative to battle AMD-like condition. Additionally, studies carried in animal models mimicking AMD-like pathology, examining the pharmacological potential of particular retinoid analogs, demonstrated promising results for their use, and thus they should be considered as an option in developing therapies for AMD. In here, we summarize the most current knowledge regarding developments of therapeutic options to maintain ocular health and prevent vision loss associated with aging.
Impact statement
Age-related macular degeneration (AMD) is a devastating retinal degenerative disease. Epidemiological reports showed an expected increasing prevalence of AMD in the near future. The only one existing FDA-approved pharmacological treatment involves an anti-vascular endothelial growth factor (VEGF) therapy with serious disadvantages. This limitation emphasizes an alarming need to develop new therapeutic approaches to prevent and treat AMD. In this review, we summarize scientific data unraveling the therapeutic potential of the specific retinoid and natural compounds. The experimental results reported by us and other research groups demonstrated that retinoid analogs and compounds with natural product scaffolds could serve as lead compounds for the development of new therapeutic agents with potential to prevent or slow down the pathogenesis of AMD.
Keywords: Degeneration, flavonoids, macula, polyphenols, retina, retinoids
Introduction
Human visual perception is critical to assimilate information from our surrounding. However, genetic background, environmental insults, and changes associated with aging can cause visual deterioration and blindness.1–4 Molecular events of vision are integrated into two pathways, phototransduction, which propagates the signal of light and visual (retinoid) cycle responsible for the retinal chromophore regeneration required to sustain vision.5 Phototransduction occurs in photoreceptors, while retinoid cycle starts in photoreceptors and continues in the retinal pigment epithelium (RPE). The visual receptors, rhodopsin present in rod photoreceptors and cone opsins in cone photoreceptors utilize the same 11-cis-retinal chromophore, which upon light absorption isomerizes to all-trans-retinal, triggering the conformational changes within the protein, resulting in the formation of its active state, Meta II.6 Activated receptor recruits cellular G protein, which transduces the signal into an electrical impulse in the brain. Eventually, all-trans-retinal is released from the receptor and is converted back to 11-cis-retinal in the enzymatic pathway of retinoid cycle.7 High concentration of rhodopsin in the retina can yield up to 5 mM all-trans-retinal under bright light condition. However, even much smaller concentration of this retinoid can be highly toxic if not efficiently cleared.8 The efficiency of the visual system diminishes significantly with age, leading to formation of poisonous photo-products such as retinal dimer, di-retinoid-pyridinium-ethanolamine (A2E) and other condensation products that affect the health of photoreceptors and RPE.9–12 Such accumulated photo-metabolites found in AMD patients are likely contributing to development of retinal degeneration.9,13 Thus, retinoids although critical for maintaining vision, can also trigger specific retinal pathologies when not tightly controlled.13,14 Discovering the molecular targets and elucidating mechanisms involved in this retinal degenerative disorder is mandatory in order to develop efficient therapeutics to combat AMD.15 Studies in animal models featuring anomalies associated with AMD and in cellular models of retinal cells are important first steps before moving to clinical studies.
Prevalence
The first report describing yellowish deposits in the macula, a hallmark of AMD, appeared in the medical literature in 1852.16 However, clearer concept of macular degeneration related to aging emerged around 1970. Currently, about 285 million people worldwide suffer from various severe visual impairments of which 39 million are blind.17,18 Among various ocular disorders, AMD is the leading cause of irreversible blindness.19 According to studies performed in 2014, in 2020 AMD will affect ∼200 million individuals globally, and by 2040 this number will rise to ∼300.17,18 In the United States alone, approximately 11 million people suffer from the progressive deterioration of the macula and this number is predicted to double by 2050.20 Thereby, AMD is a serious global problem causing visual disability in humans over 50 years old in the industrialized world that urgently requires to be addressed.
Classification
AMD is a progressive condition that is broadly classified into early AMD, intermediate AMD, and advanced AMD.21,22 Early AMD features the presence of soft indistinct drusen, pigmentary abnormalities, or increased retinal pigment deposits. Intermediate stage presents with larger drusen and/or non-central geographic atrophy, which may progress overtime to the advanced stage of the disease. While early and intermediate AMD cause only mild impairments of the visual perception, advanced AMD causes blindness. In case of advanced AMD, there are two clinical presentations: (i) dry AMD characterized by the progressive atrophy of the RPE cells related to lipofuscin accumulation in those cells and drusen deposits at the basal laminar membrane with subsequent loss of photoreceptors, resulting in loss of sight, and (ii) wet AMD accompanied by choroidal neovascularization. These abnormal, leaking vessels and lipid deposits disrupt the retinal structure, resulting in focal retinal detachment and rapid, profound loss of central vision, which without treatment could progress to total blindness.23,24
Risk factors
Degeneration of the macular region of the retina is the hallmark of AMD, but the reason for preferential deterioration of this central part of the retina remains unclear. Among recognized risk factors for AMD, advanced age is the most significant. However, various conditions such as genetic predisposition, race, and environment may attribute to a functional decline of vision in AMD.25–27 Smoking, excessive body weight, cardiovascular disease, or high blood pressure increase a risk for the development of AMD.25–29 Long-term exposure to sun without eye protection enhances the probability for developing the disease or accelerating its progression.28,30–32 Specific genetic background, for example, polymorphism in genes encoding complement factor H, complement components 2, and 3 increases the likelihood for AMD in elderly.33–37 In addition, these genetic alterations are potentially epigenetically regulated, but the mechanism of this regulation is at present not well understood.38 People with Caucasian ethnicity are more likely to develop AMD.39 Moreover, females are more inclined to AMD than males, likely because they live longer.27,40 Diets with elevated fat, cholesterol, and high glycemic index foods, as well as low dietary intake of anti-oxidants that are present in green leafy vegetables and fruits are strongly associated with prevalence of AMD.41 Additionally, cigarette smoking has strong implication in the development of AMD.42 However, further studies are necessary to confirm the contribution of all these factors in the AMD pathogenesis.
Pathogenesis
AMD results in the clinical pathophysiology characterized by dysfunction and atrophy of the RPE cells, which further triggers death of photoreceptors. The pathogenesis and progression of the disease have been associated with an enhanced oxidative stress and inflammation.43 Early clinical manifestation of the ocular pathology in AMD patients is the formation of drusen deposits between the RPE cells and Bruch’s membrane, and accumulation of lipid-containing autofluorescent lipofuscin granules in the RPE.44–49 These deposits disrupt the connection between the RPE and the choroidal blood supply, inducing hypoxia. Under hypoxic conditions, the expression of VEGF and other pro-angiogenic factors is induced to promote the formation of new vessels.50,51 Additionally, immune cells, such as macrophages localize at the break-down of Bruch’s membrane, where they induce infiltration of other immune cells from the blood circulation. Soluble factors, including cytokines released by these cells accelerate angiogenesis, and thus progression of the disease.43 However, more research is required to fully understand the exact molecular mechanism underlying the pathogenesis of AMD.
Retinoid imbalance as a cause of AMD
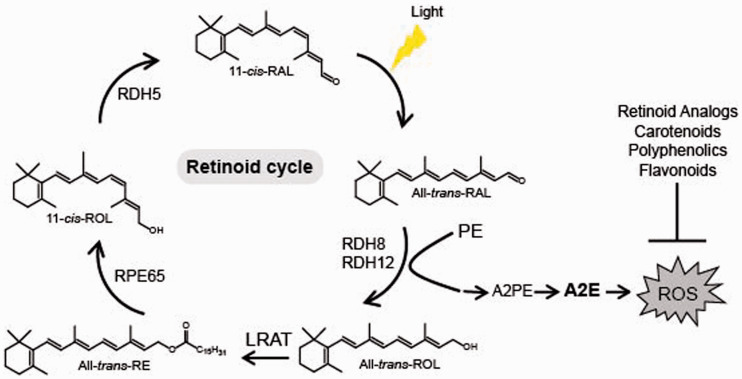
Retinoids are precursors and derivatives of vitamin A with many biological functions throughout the body, thus maintaining balanced retinoid homeostasis is critical. In particular, an efficient supply and metabolism of retinoids in the retina are essential for normal vision. β-carotene present in dietary plants and retinyl esters derived from animal sources are precursors of the visual chromophore 11-cis-retinal to form light sensing receptors, rhodopsin, and cone opsins. Absorption of the light photon triggers isomerization of 11-cis-retinal to its all-trans configuration, which eventually dissociates from the visual receptors, resulting in the formation of unliganded opsin and free all-trans-retinal. Continuous conversion of all-trans-retinal back to 11-cis-retinal is critical to sustain vision and is achieved through the visual cycle (Figure 1), which starts in photoreceptors and continues in the RPE cells. A delay in this process leads to increased local concentrations of all-trans-retinal, and thus excessive accumulation of its byproducts, including all-trans-retinal dimer and A2E in both photoreceptors and in the RPE cells, which continuously phagocytize and degrade membranes originated from rod and cone photoreceptors. Stable, resistant to an enzymatic digest A2E comprises the principle component of lipofuscin granules that are toxic to the RPE11,12,45,47,48,52 and together with drusen deposits at the basal laminar membrane result in the nutritional barrier that contribute to photoreceptors’ death.53,54 The efficiency of the visual cycle in clearing the toxic photo-metabolites and the rate of regeneration of the visual pigments slow down significantly with age, causing a decline in health of photoreceptors and the RPE that lead to development of retinal degeneration.
Figure 1.
Retinoid cycle and synthesis of A2E. Light triggers isomerization of 11-cis-retinal (11-cis-RAL) chromophore to all-trans-retinal (All-trans-RAL). Under normal conditions, all-trans-retinal is reduced to all-trans-retinol (All-trans-ROL) by retinol dehydrogenases 8 and 12 (RDH8 and RDH12, respectively), which then is esterified by lecithin retinol acyltransferase (LRAT) to all-trans-retinyl esters (All-trans-retinyl-RE). These esters can be stored in retinosomes or converted to 11-cis-retinol (11-cis-ROL) by retinal pigment epithelium-specific protein 65 (RPE65) isomerase. 11-cis-Retinol is converted to 11-cis-retinal by retinol dehydrogenase 5 (RDH5), which then can re-associate with opsin forming the visual pigment, rhodopsin. Inefficient reduction of all-trans-retinal to all-trans-retinol results in excess of all-trans-retinal, which can react with phosphatidylethanolamine (PE), resulting in formation of bis-retinoid N-retinyl-N-retinylidene ethanolamine (A2E). Accumulated A2E causes an increase of reactive oxygen species (ROS) detrimental to the retinal cells. Overproduction of ROS can be decreased by specific retinoid analogs, dietary carotenoids and polyphenolics, particularly flavonoids. (A color version of this figure is available in the online journal.)
Retinoids and prevention of AMD
Decreasing the levels of photo-metabolites could have clinical implications decelerating the degenerative processes in the retina during aging. Indeed, introducing the deuterium atom at C20 of vitamin A slowed the rate of all-trans-retinal dimerization in vitro and in rodents due to the kinetic isotope effect.55,56 Consequently, WT mice administered with deuterated vitamin A exhibited 68% less A2E than control mice administered with normal vitamin A. Thus, supplementation with C20-D3-vitamin A that limits the A2E biosynthesis potentially could be a viable approach to slow down the age-related deposition of lipofuscin pigments in the human retina (Table 1). Generation of A2E could also be prevented by slowing the rate of all-trans-retinal formation by treatment with the visual cycle inhibitors. Isotretinoin (13-cis-retinoic acid) decreased levels of 11-cis-retinal by 50% in the eyes of Abca4−/− mice, a model for Stargardt disease, a juvenile AMD, but also in the eyes of WT mice injected daily with 20 mg/kg isotretinoin from two to four-month-old, evidently slowing down the visual cycle.57 Formation of A2E in these mice was also reduced. Thus, isotretinoin potentially could be used as preventive treatment for AMD. However, the dosage regimen would need to be carefully established as patients treated with isotretinoin were experiencing side effects associated with night blindness.58 Reversible suppression of the visual cycle was observed also with other visual cycle inhibitors such as n-(4-hydroxyphenyl) retinamide, retinylamine, and emixustat (Table 1) in mouse models of retinal degeneration.59–63 However, despite their potential beneficial effects delaying degenerative processes in the retina related to excessive light exposure or aging, the use of these visual cycle inhibitors as a therapy for AMD is questionable because prolonged treatment with these chemicals can cause night blindness. Indeed, randomized clinical trial performed to evaluate safety and tolerability of emixustat in AMD patients carried for over two years showed that emixustat not only did not reduce geographic atrophy lesions, but also some patients experienced ocular problems, including delayed dark adaptation, chromatopsia, and erythropsia.64
Table 1.
Potential retinoid therapeutics for AMD-like retinal degeneration.
| Compound name | Chemical structure | Protection mechanism | References |
|---|---|---|---|
| C20-D3-vitamin A |
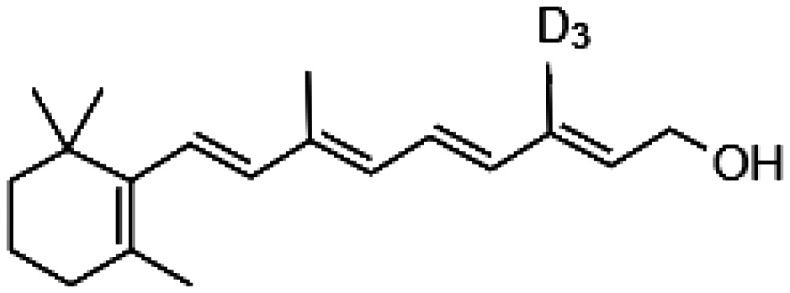
|
• Slowing the rate of all-trans-retinal dimerization • Deceleration of A2E biosynthesis |
55–56 |
| Isotretinoin |
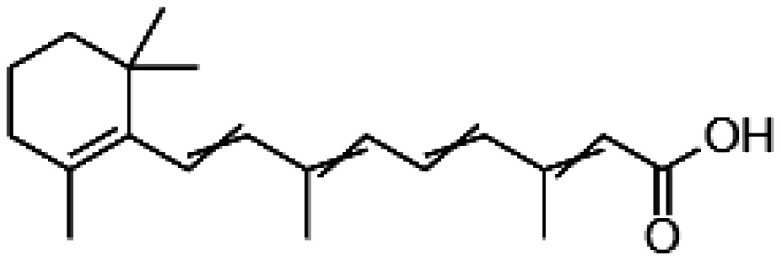
|
• Decrease of 11-cis-retinal levels | 57–58 |
| N-(4-Hydroxyphenyl) retinamide |
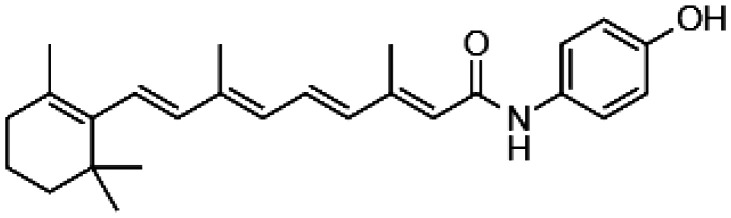
|
• Reversible suppression of the visual cycle | 59 |
| Emixustat |
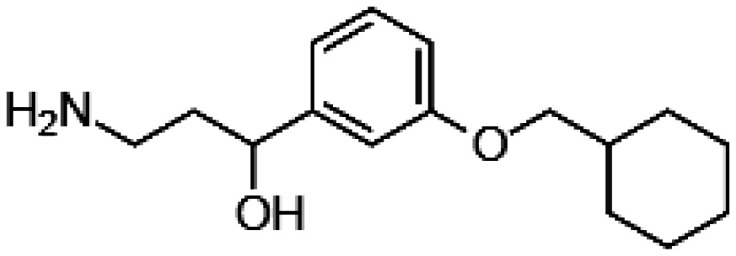
|
• Reversible suppression of the visual cycle | 60–62 |
| Retinylamine |
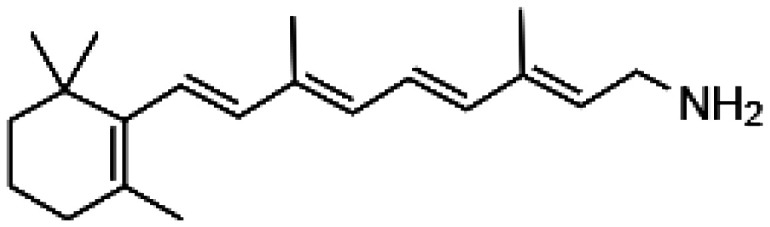
|
• Reversible suppression of the visual cycle | 61 |
| 11-cis-6-member-retinal |
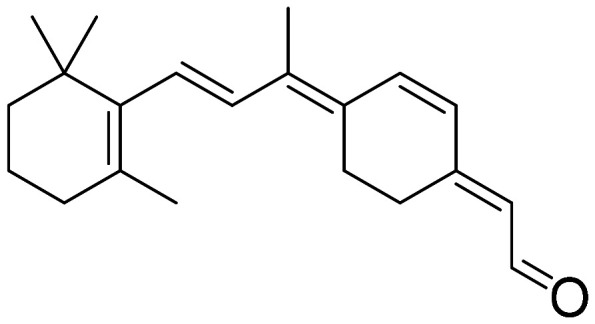
|
• Prevention of the retinal isomerization • Locking the chromophore within the receptor’s ligand-binding pocket |
65 |
Keeping all-trans-retinal in the retina at low level would prevent the aberrant formation of toxic all-trans-retinal byproducts, and thus is critical to prolong the retinal health. Indeed, toxicity of all-trans-retinal was effectively reduced by the FDA-approved primary amine drugs that chemically trapped all-trans-retinal, precluding its binding to membranous phosphatidylethanolamine, and thus reducing the formation of A2E in WT and genetically modified Abca4−/−Rdh8−/− mice, modeling AMD.66
Slowed recovery after photobleaching typifies patients with AMD, and it is likely associated with slower pigment regeneration in photoreceptors. Inefficient regeneration of the visual receptor leads to excessive concentrations of unliganded, constitutively active opsin that accelerates degenerative processes in the retina.67,68 To assure the efficient regeneration of the visual pigment, the visual cycle could be bypassed by the supplementation with an artificial chromophore 9-cis-retinyl acetate as a prodrug. Ingested 9-cis-retinyl acetate is converted to 9-cis-retinyl esters in the liver, followed by their conversion in the RPE to 9-cis-retinal, which then can associate with unliganded opsin forming functional, light sensitive receptor. Unfortunately, this treatment applied to Abca4−/−Rdh8−/− mice, modeling AMD, did not prevent formation of all-trans-retinal upon light illumination and accumulation of A2E, thus it failed to fully protect these mice from the development of retinal degeneration.69 On the other hand, treatment with 11-cis-6-membered-ring-retinal fully protected these mice against light-induced retinal damage65 (Table 1). This retinoid contains a ring between C10 and C13 instead of the double bond between C11=C12, which prevents the light-stimulated conversion from 11-cis to all-trans configuration, locking the chromophore within the receptor’s ligand-binding pocket. Thus, upon binding to opsin 11-cis-6-membered-ring-retinal not only quenches its deleterious constitutive activity, but also prevents an increase of free all-trans-retinal concentration in the retina upon illumination. In such scenario, formation of A2E would also be prevented. Therefore, 11-cis-6-membered-ring-retinal offers potential therapeutic opportunity for AMD-like degenerative disorders.
Natural products as a source of treatment for AMD
Normal physiological processes are compromised during aging. Significant diminution in the control of oxidative stress and tissue regeneration leads to malfunction of many organ systems, including the eye.70 AMD pathogenesis is a complex process and to date, no therapeutic choice can prevent this disease.71 The eye tissue is particularly susceptible to oxidative stress due to continuous photochemical reactions occurring in the retina upon exposure to light.72 High metabolic rate and high oxygen demand in the retina trigger the production of reactive oxygen species (ROS).73 In addition, the outer retina membranes contain high levels of polyunsaturated lipids specifically susceptible to peroxidation. Moreover, the rate of generation of all-trans-retinal during activation of rhodopsin often exceeds the rate of its reduction to all-trans-retinol. The reactive all-trans-retinal conjugates with the membranous phosphatidylethanolamine resulting in formation of A2E, which accumulates in the RPE and contributes to the formation of lipofuscin deposits that enhance oxidative stress in these cells.12 Oxidative stress and associated inflammatory responses lead to activation of apoptotic processes in the retinal cells that culminate in the retinal cell death.74 An imbalance between the levels of pro-oxidative radicals and anti-oxidants contributes to retinal degeneration in AMD. Genetic and environmental factors can further predispose to developing AMD.75 Thus, changes in the exposure to such environmental factors and implementing modifications in daily diet could decrease risk for developing AMD or slow its progression. Treatment with anti-oxidants is one advocated therapy against AMD, to counter the overproduction of ROS in degenerating retinas. Natural products present in plants, fruits, and vegetables are excellent sources of compounds with anti-oxidant properties. The main nutritional anti-oxidants belong to two compound families: carotenoids and polyphenols.76 As reported by the clinical-epidemiological Age-Related Eye Disease Study (AREDS), daily ingestion of anti-oxidant minerals, vitamin, and carotenoids can significantly (up to 28%) lessen the progression of vision loss in patients with moderate macular degeneration.77 Specifically, intake of dietary carotenoids, including zeaxanthin and lutein delayed the progression of the disease in humans.78–80 Thus, enrichment of carotenoids in the diet could have a positive impact on the retinal health.76 Beneficial effects, suppressing oxidative stress and activation of immune response pathways responsible for AMD progression were also found upon ingestion of vitamin A, E, and C.79 Additionally, ocular benefits correlated with diet supplemented with leafy green vegetables and fruits containing polyphenols. Dietary polyphenols possess heterogeneous chemical motives and belong to three main groups: phenolic acids, flavonoids, and polyphenolic amides.81 Their biological activity is associated with anti-oxidant, anti-inflammatory, and anti-apoptotic properties.82–86 In this review, we focus on characterizing specific flavonoids and their therapeutic potential in preventing or delaying the degenerative retinal processes related to AMD.
Flavonoids as AMD preventing agents
Flavonoids are a group of natural chemical compounds found mainly in fruits, vegetables, tea, and herbs. They are widely used in the development of nutraceutical, pharmaceutical, and cosmetic products. Chemical structure of flavonoids in general is composed of two phenyl rings and one heterocyclic ring, which are further modified. Based on the structural differences, flavonoids are subdivided into several subgroups such as flavonols, flavones, isoflavones, flavanols, and anthocyanidines.87 Flavonoids are the most common group of dietary polyphenol compounds with multiple biological activates such as anti-inflammatory, anti-viral, anti-apoptotic, and anti-oxidant, valuable in numerous pathologies.82–84,86,88–90 Flavonoids exhibit health benefits in multiple eye pathologies, including cataract, glaucoma, diabetic retinopathy, retinitis pigmentosa, and AMD.91–95 The positive effects of flavonoids in preventing or slowing down AMD are likely related to their role in decreasing health risk factors, such as obesity, hypercholesterolemia, and hypertension.96 Lifestyle associated with unhealthy diet and smoking, as well as other environmental factors, including exposure to bright light, potentiates ROS production. However, flavonoids either by directly sequestering these oxidative radicals or by activating specific pathways limit cellular concentrations of ROS. Oxidative stress activates host immune and other defense mechanisms eventually leading to retinal cell death. Flavonoids can inhibit the inflammatory reactions and induce the expression of anti-apoptotic genes, halting, or decelerating the retinal degeneration. Additionally, beneficial effects of flavonoids are related to their direct modulatory interaction with the visual receptor, rhodopsin in photoreceptor cells.95,97,98 Collectively, all these positive effects of flavonoids can inhibit the development or progression of AMD (Table 2).
Table 2.
Flavonoids with protective effect against AMD-like symptoms.
| Compound name | Chemical structure | Protection mechanism | References |
|---|---|---|---|
| Quercetin |
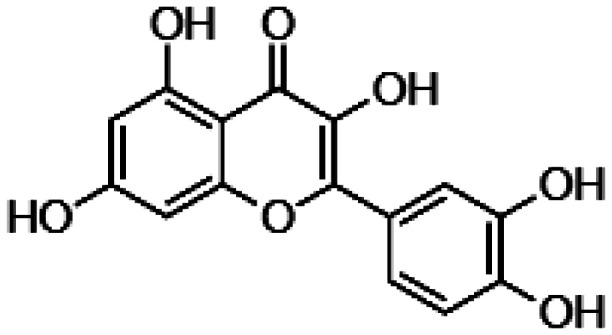
|
• Neutralization of free radical • Decrease levels of pro-inflammatory markers • Negative modulation of the expression levels of apoptotic genes • Enhancement of the expression of anti-apoptotic proteins • Protection against A2E-mediated ROS induction • Blocking the production of adducts of photo-oxidized A2E. |
99–102 |
| Myricetin |
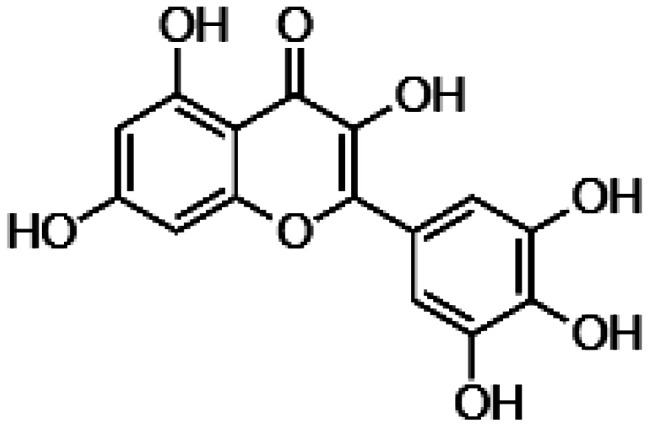
|
• Decrease of ROS levels • Negative modulation of the nitric oxide synthase (NOS2) • Increase of the expression of anti-oxidant enzyme, superoxide dismutase (SOD2) • Increase of the expression of Nrf2 transcription factor • Downregulation of pro-apoptotic factors and inflammatory markers |
103 |
| Kaempferol |
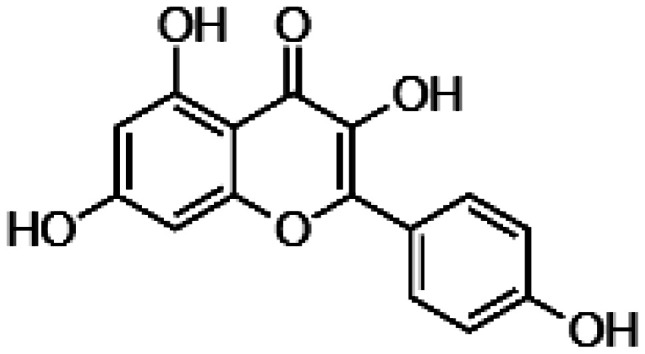
|
• Neutralization of ROS • Decrease of BAX and caspase 3 activation • Upregulation of BCL2 • Downregulation of vascular endothelial growth factor (VEGF) expression |
104,105 |
| Hesperidin |
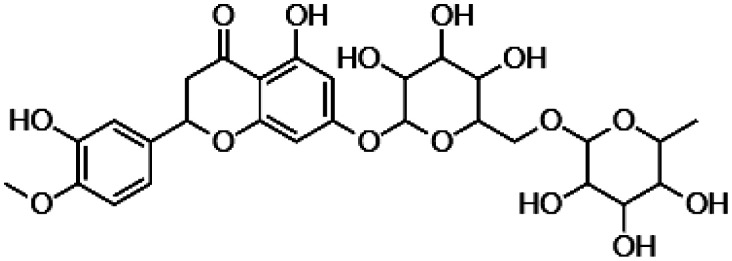
|
• Decrease of the cellular ROS concentration via enhancing levels of SOD2 and glutathione S-transferase (GSH). |
106–109 |
| Fisetin Luteolin |
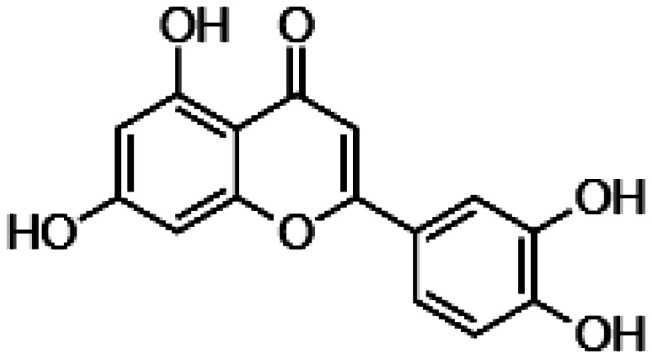
|
• Protection against damage by end-product of lipid peroxidation • Reduction of pro-inflammatory cytokine levels • Decrease of the transcription factor CREB activity • Decrease of the activity of the mitogen activated kinases (MAPKs), including p38 MAPK, JNK and ERK1/2 |
104–111 |
| Nobiletin |
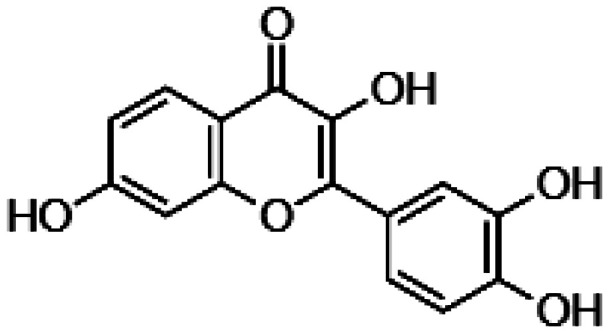
|
• Inhibition of stress-induced activity of caspase‐3/7 • Increase of Akt phosphorylation under ROS insult |
112 |
| Wogonin |
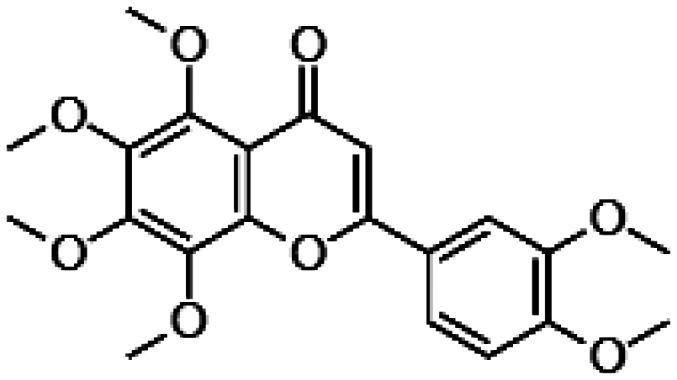
|
• Modulation of the PI3K (phosphatidylinositol-3 kinase) and Akt pathway | 113,114 |
The mechanisms of action of numerous flavonoids have been evaluated in the AMD-related in vitro and in vivo models relevant to vision and this ocular pathology. As the RPE tissue is implicated in the oxidative stress and inflammation associated with AMD, the RPE-derived ARPE-19 cells have been commonly used to evaluate effects of tested flavonoids. To mimic the AMD conditions, these cells could be loaded with A2E, the major pathogenic factor triggering oxidative stress and atrophy of the RPE. Alternatively, these cells have been subjected to oxidative stress generating agent such as H2O2, or toxins activating inflammation such as lipopolysaccharides (LPS). Approaches such as measuring the cell viability or examining levels of cellular markers of oxidative stress, inflammation and apoptosis have been used to assess protective effects of flavonoids against the applied stressor.
Quercetin, flavonoid commonly present in berries, apples, green tea and wine, increased viability of the ARPE-19 cells under H2O2-mediated oxidative stress by direct sequestration of free radicals and through activation of cellular pro-survival mechanisms.99–101 As reported, it also decreased levels of pro-inflammatory markers such as interleukins (IL-6 and IL-8).100 Treatment with quercetin resulted in the negative modulation of the transcription profile of genes encoding proteins implicated in apoptosis such as BAX, FADD (Fas-associated via death domain), and caspases, and enhanced the expression of pro-survival protein BCL-2. Pretreatment of the ARPE-19 cells with quercetin prior to their exposure to A2E reduced levels of ROS and increased viability of these cells.102 Additionally, quercetin showed an inhibitory effect in the production of adducts of methylglyoxal and in the reaction of glutathione with photo-oxidized A2E.102
Myricetin and its glycosylated form myricitrin, naturally present in leaves of S. malaccense, similarly to quercetin, could decrease levels of ROS in the ARPE-19 cells exposed to H2O2 by their direct sequestration.103 In addition, myricetrin downregulated the nitric oxide synthase (NOS2) and increased the expression of anti-oxidant enzyme, superoxide dismutase (SOD2). Myricetin also enhanced the expression of Nrf2, a transcription factor, which regulates the expression of anti-oxidant proteins.103 Moreover, by controlling various pro-apoptotic factors and inflammatory markers at the molecular level, myricetin showed the ability to prevent apoptosis of the retinal cells.
Kaempferol found in variety of plants such as kale, spinach, and broccoli, protected retinal cells by directly neutralizing oxidative stress and by regulating the cellular responses to this stress.104 Its mechanism of protection is also associated with anti-apoptotic effects and downregulation of vascular endothelial growth factor (VEGF) expression.105 These kaempferol’s effects have been validated in a sodium iodate-induced retinal degeneration rat model. Sodium iodate is a toxin that selectively induces damage of the RPE cells by inducing oxidative stress and consequently causes retinal degeneration. In this rat model, pretreatment with kaempferol decreased retinal degeneration by counteracting oxidative stress and decreasing expression of VEGF.105
Hesperidin, a common flavanone glycoside found in the citrus fruits, is widely used in Chinese medicine as anti-inflammatory and anti-oxidant compound.106–109 The main molecular mechanism of protection provided by hesperidin is related to the decrease of the cellular ROS concentration via enhancing levels of SOD2 and glutathione S-transferase (GSH), two main regulators of ROS production in the retinal cells.108
Fisetin and luteolin found in different plants protected the ARPE-19 cells against 4-hydroxynonenal (HNE)-induced cytotoxicity.104,110,111 HNE is an end-product of lipid peroxidation prone to trigger apoptosis. Pretreatment of the ARPE-19 cells with fisetin and luteolin before the exposure to HNE resulted in reduction of the levels of pro-inflammatory cytokines IL-6 and IL-8 in these cells. Additionally, both fisetin and luteolin downregulated the inflammatory reactions by decreasing the activity of the transcription factor CREB and the mitogen-activated kinases (MAPKs) such as p38 MAPK, JNK, and ERK1/2.110
Nobiletin, a polymethoxylated flavonoid present in citrus fruits inhibited stress-induced activity of caspase‐3/7 and significantly increased Akt phosphorylation in the ARPE-19 cells exposed to H2O2.112
Wogonin, a methoxylated flavonoid with anxiolytic properties found in Scutellaria baicalensis, a common herb used in traditional Chinese medicine,115 increased the viability of the ARPE-19 cells challenged with H2O2 via modulation of the PI3K (phosphatidylinositol-3 kinase) and Akt pathway.113,114
Together, all studies examining the effects of different flavonoids demonstrated that they possess the ability to mitigate the oxidative damage, reduce the activation of inflammatory markers, and can inhibit apoptosis, the fundamental processes related to AMD induction and progression. Although the potential benefits of flavonoids in AMD, constitute a relatively novel concept, and the bioactivity of these compounds were mostly characterized in vitro, the promising results provide a rationale to investigate the role of flavonoids in various aspects of vision physiology and pathology.
Effect of flavonoids on the visual pigment regeneration
In addition to anti-oxidant, anti-inflammatory, and anti-apoptotic properties, the positive effects of dietary polyphenols, specifically anthocyanins and flavonoids, on human vision are also related to their direct modulatory effects on the visual receptor, rhodopsin. Enhanced recovery of visual sensitivity after exposure to bright light was noted in patients supplemented with black currant anthocyanins, likely through the modulation of the rod component involved in dark adaptation.116 Eating rich in anthocyanins black berries before the night flights enhanced night vision of British and French war pilots, although this improvement was dependent on the individual. As reported, anthocyanins target directly the visual receptor and upon binding to chromophore-free opsin increase the rate of retinal binding and regeneration of functional rhodopsin.117,118 Similar effect accelerating regeneration of rhodopsin was also noted for flavonoids, specifically quercetin and myricetin.97,98 In AMD, the visual pathology is associated with declined recovery of visual sensitivity after exposure to light, likely due to inadequate pigment regeneration and excess of unliganded opsin. Thus, treatments with polyphenolic compounds could be beneficial for the improvement of the rod cells light sensitivity impaired in AMD patients.
Conclusions
AMD is a multifactorial disease with no remedy available. Understanding the AMD pathogenesis is critical in order to develop effective cures. Potential avenues toward developing the AMD-related therapy should include: (i) an inhibition of toxic photo-metabolites formation; (ii) an effective clearance of photo-products; (iii) silencing of accumulated unliganded, constitutively active opsin by retinoid traps; (iv) pharmacological modulation of opsin by compounds enhancing regeneration of the functional receptor; (v) reducing oxidative stress and inhibition of inflammatory processes activated in the retina; and (vi) discovering novel targets related to AMD pathogenesis that could be modulated pharmacologically. The accumulated knowledge indicates the retinal health benefits of the specific retinal analog, 11-cis-6-membered-ring-retinal, which upon binding to opsin reduces concentration of ligand-free opsin and silences its constitutive activity, which otherwise accelerates retinal degeneration. Binding of 11-cis-6-membered-ring-retinal also prevents the formation of poisonous free all-trans-retinal and its byproducts mediated by light, thus halts the formation of A2E. Furthermore, the rates of rhodopsin regeneration with the native chromophore 11-cis-retinal could be enhanced by dietary polyphenols such as flavonoids and anthocyanidins. The latter also decreases ROS formation and activates multiple pathways with pro-survival outcomes. Thus, treatment with these compounds either alone or in combination should be seriously considered as non-invasive remedy option preventing or delaying AMD pathogenesis and progression.
Authors’ contributions
TP, JTO and BJ wrote and design the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by funding from the National Institutes of Health EY025214 (BJ).
ORCID iD
Beata Jastrzebska https://orcid.org/0000-0001-5209-8685
References
- 1.Monge ZA, Madden DJ. Linking cognitive and visual perceptual decline in healthy aging: the information degradation hypothesis. Neurosci Biobehav Rev 2016; 69:166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizzo M, Sparks J, McEvoy S, Viamonte S, Kellison I, Vecera SP. Change blindness, aging, and cognition. J Clin Exp Neuropsychol 2009; 31:245–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohm MRR, Thanos S. [ The aging retina in the context of cerebral neurodegenerative diseases. Klin Monbl Augenheilkd 2019; 236:682–90 [DOI] [PubMed] [Google Scholar]
- 4.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration – emerging pathogenetic and therapeutic concepts. Ann Med 2006; 38:450–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palczewski K. Chemistry and biology of the initial steps in vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 2014; 55:6651–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jastrzebska B, Tsybovsky Y, Palczewski K. Complexes between photoactivated rhodopsin and transducin: progress and questions. Biochem J 2010; 428:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palczewski K. Chemistry and biology of vision. J Biol Chem 2012; 287:1612–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda T, Golczak M, Maeda A. Retinal photodamage mediated by all-trans-retinal. Photochem Photobiol 2012; 88:1309–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer NP, Higbee D, Currin MB, Blakeley LR, Chen C, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal. J Biol Chem 2012; 287:22276–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci 2010; 31:284–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De S, Sakmar TP. Interaction of A2E with model membranes. Implications to the pathogenesis of age-related macular degeneration. J Gen Physiol 2002; 120:147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J Biol Chem 2009; 284:20155–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparrow JR. Vitamin A-aldehyde adducts: AMD risk and targeted therapeutics. Proc Natl Acad Sci U S A 2016; 113:4564–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiser PD, Palczewski K. Retinoids and retinal diseases. Annu Rev Vis Sci 2016; 2:197–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veleri S, Lazar CH, Chang B, Sieving PA, Banin E, Swaroop A. Biology and therapy of inherited retinal degenerative disease: insights from mouse models. Dis Model Mech 2015; 8:109–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong PT. A historical analysis of the quest for the origins of aging macula disorder, the tissues involved, and its terminology. Ophthalmol Eye Dis 2016; 8:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014; 2:e106–16 [DOI] [PubMed] [Google Scholar]
- 18.Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Resnikoff S, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR. Vision loss expert G. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and Meta-analysis. Lancet Glob Health 2017; 5:e888–e97 [DOI] [PubMed] [Google Scholar]
- 19.Katibeh M, Pakravan M, Yaseri M, Pakbin M, Soleimanizad R. Prevalence and causes of visual impairment and blindness in Central Iran; the yazd eye study. J Ophthalmic Vis Res 2015; 10:279–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis 2016; 3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet 2018; 392:1147–59 [DOI] [PubMed] [Google Scholar]
- 22.Ferris FL, 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, Sadda SR. Beckman initiative for macular research classification C. Clinical classification of age-related macular degeneration. Ophthalmology 2013; 120:844–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, Waheed NK, Chakravarthy U, Rosenfeld PJ, Holz FG, Souied EH, Cohen SY, Querques G, Ohno-Matsui K, Boyer D, Gaudric A, Blodi B, Baumal CR, Li X, Coscas GJ, Brucker A, Singerman L, Luthert P, Schmitz-Valckenberg S, Schmidt-Erfurth U, Grossniklaus HE, Wilson DJ, Guymer R, Yannuzzi LA, Chew EY, Csaky K, Mones JM, Pauleikhoff D, Tadayoni R, Fujimoto J. Consensus nomenclature for reporting neovascular Age-Related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 2019;5:616–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigo-Diaz E, Tahir HJ, Kelly JM, Parry NRA, Aslam T, Murray IJ. The light and the dark of early and intermediate AMD: cone- and rod-mediated changes are linked to fundus photograph and FAF abnormalities. Invest Ophthalmol Vis Sci 2019; 60:5070–9 [DOI] [PubMed] [Google Scholar]
- 25.Heesterbeek TJ, Lores-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt 2020; 40:140–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechanteur YT, van de Ven JP, Smailhodzic D, Boon CJ, Klevering BJ, Fauser S, Groenewoud JM, van der Wilt GJ, den Hollander AI, Hoyng CB. Genetic, behavioral, and sociodemographic risk factors for second eye progression in age-related macular degeneration. Invest Ophthalmol Vis Sci 2012; 53:5846–52 [DOI] [PubMed] [Google Scholar]
- 27.McGuinness MB, Finger RP, Karahalios A, Guymer RH, English DR, Chong EW, Hodge AM, Robman LD, Giles GG, Simpson JA. Age-related macular degeneration and mortality: the Melbourne collaborative cohort study. Eye 2017; 31:1345–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modenese A, Gobba F. Macular degeneration and occupational risk factors: a systematic review. Int Arch Occup Environ Health 2019; 92:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tikellis G, Robman LD, Dimitrov P, Nicolas C, McCarty CA, Guymer RH. Characteristics of progression of early age-related macular degeneration: the cardiovascular health and age-related maculopathy study. Eye 2007; 21:169–76 [DOI] [PubMed] [Google Scholar]
- 30.Oddone E, Taino G, Vita S, Schimd M, Frigerio F, Imbriani M. Macular degeneration: peculiar sunlight exposure in an agricultural worker. Med Lav 2019; 110:241–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong RA, Mousavi M. Overview of risk factors for age-related macular degeneration (AMD). J Stem Cells 2015; 10:171–91 [PubMed] [Google Scholar]
- 32.Schick T, Ersoy L, Lechanteur YT, Saksens NT, Hoyng CB, den Hollander AI, Kirchhof B, Fauser S. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina 2016; 36:787–90 [DOI] [PubMed] [Google Scholar]
- 33.Scholl HP, Fleckenstein M, Fritsche LG, Schmitz-Valckenberg S, Gobel A, Adrion C, Herold C, Keilhauer CN, Mackensen F, Mossner A, Pauleikhoff D, Weinberger AW, Mansmann U, Holz FG, Becker T, Weber BH. CFH, C3 and ARMS2 are significant risk loci for susceptibility but not for disease progression of geographic atrophy due to AMD. PLoS One 2009; 4:e7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cipriani V, Matharu BK, Khan JC, Shahid H, Stanton CM, Hayward C, Wright AF, Bunce C, Clayton DG, Moore AT, Yates JR. Genetic variation in complement regulators and susceptibility to age-related macular degeneration. Immunobiology 2012; 217:158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Natoli R, Fernando N, Jiao H, Racic T, Madigan M, Barnett NL, Chu-Tan JA, Valter K, Provis J, Rutar M. Retinal macrophages synthesize C3 and activate complement in AMD and in models of focal retinal degeneration. Invest Ophthalmol Vis Sci 2017; 58:2977–90 [DOI] [PubMed] [Google Scholar]
- 36.Deangelis MM, Silveira AC, Carr EA, Kim IK. Genetics of age-related macular degeneration: current concepts, future directions. Semin Ophthalmol 2011; 26:77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly E, Rhatigan M, O’Halloran AM, Muldrew KA, Chakravarthy U, Cahill M, Kenny RA, Doyle SL. Prevalence of age-related macular degeneration associated genetic risk factors and 4-year progression data in the Irish population. Br J Ophthalmol 2018; 102:1691–5 [DOI] [PubMed] [Google Scholar]
- 38.Blasiak J, Salminen A, Kaarniranta K. Potential of epigenetic mechanisms in AMD pathology. Front Biosci 2013; 5:412–25 [DOI] [PubMed] [Google Scholar]
- 39.Vanderbeek BL, Zacks DN, Talwar N, Nan B, Musch DC, Stein JD. Racial differences in age-related macular degeneration rates in the United States: a longitudinal analysis of a managed care network. Am J Ophthalmol 2011; 152:273–82 e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joachim N, Mitchell P, Rochtchina E, Tan AG, Wang JJ. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology 2014; 121:917–25 [DOI] [PubMed] [Google Scholar]
- 41.Ersoy L, Ristau T, Lechanteur YT, Hahn M, Hoyng CB, Kirchhof B, den Hollander AI, Fauser S. Nutritional risk factors for age-related macular degeneration. Biomed Res Int 2014; 2014:413150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye 2005; 19:935–44 [DOI] [PubMed] [Google Scholar]
- 43.Rashid K, Akhtar-Schaefer I, Langmann T. Microglia in retinal degeneration. Front Immunol 2019; 10:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei L, Tzekov R, McDowell JH, Smith WC, Tang S, Kaushal S. Formation of lipofuscin-like material in the RPE cell by different components of rod outer segments. Exp Eye Res 2013; 112:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci 1999; 40:2988–95 [PubMed] [Google Scholar]
- 46.Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci U S A 2002; 99:3842–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamb LE, Simon JD. A2E: a component of ocular lipofuscin. Photochem Photobiol 2004; 79:127–36 [DOI] [PubMed] [Google Scholar]
- 48.Crouch RK, Koutalos Y, Kono M, Schey K, Ablonczy Z, A2e L. Prog Mol Biol Transl Sci. Prog Mol Biol Transl Sci 2015; 134:449–63 [DOI] [PubMed] [Google Scholar]
- 49.Pallitto P, Ablonczy Z, Jones EE, Drake RR, Koutalos Y, Crouch RK, Donello J, Herrmann J. A2E and lipofuscin distributions in macaque retinal pigment epithelium are similar to human. Photochem Photobiol Sci 2015; 14:1888–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alizadeh E, Mammadzada P, Andre H. The different facades of retinal and choroidal endothelial cells in response to hypoxia. Int J Mol Sci 2018; 19:3846–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christakis PG, Agron E, Klein ML, Clemons TE, Campbell JP, Ferris FL, Chew EY, Keenan TD; Age-Related Eye Diseases Study Research Group. Incidence of macular atrophy after untreated neovascular age-related macular degeneration: age-related eye disease study report 40. Ophthalmology 2019; S0161-6420(19):32297–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guan Z, Li Y, Jiao S, Yeasmin N, Rosenfeld PJ, Dubovy SR, Lam BL, Wen R. A2E distribution in RPE granules in human eyes. Molecules 2020; 25:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iriyama A, Inoue Y, Takahashi H, Tamaki Y, Jang WD, Yanagi Y. A2E, a component of lipofuscin, is pro-angiogenic in vivo. J Cell Physiol 2009; 220:469–75 [DOI] [PubMed] [Google Scholar]
- 54.Boyer NP, Tang PH, Higbee D, Ablonczy Z, Crouch RK, Koutalos Y. Lipofuscin and A2E accumulate with age in the retinal pigment epithelium of nrl-/- mice. Photochem Photobiol 2012; 88:1373–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman Y, Ma L, Washington I. Deuterium enrichment of vitamin a at the C20 position slows the formation of detrimental vitamin a dimers in wild-type rodents. J Biol Chem 2011; 286:7958–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma L, Kaufman Y, Zhang J, Washington I. C20-D3-vitamin a slows lipofuscin accumulation and electrophysiological retinal degeneration in a mouse model of Stargardt disease. J Biol Chem 2011; 286:7966–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radu RA, Mata NL, Nusinowitz S, Liu X, Sieving PA, Travis GH. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt’s macular degeneration. Proc Natl Acad Sci U S A 2003; 100:4742–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gross EG, Helfgott MA. Retinoids and the eye. Dermatol Clin 1992; 10:521–31 [PubMed] [Google Scholar]
- 59.Puntel A, Maeda A, Golczak M, Gao SQ, Yu G, Palczewski K, Lu ZR. Prolonged prevention of retinal degeneration with retinylamine loaded nanoparticles. Biomaterials 2015; 44:103–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Kiser PD, Badiee M, Palczewska G, Dong Z, Golczak M, Tochtrop GP, Palczewski K. Molecular pharmacodynamics of emixustat in protection against retinal degeneration. J Clin Invest 2015; 125:2781–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin a arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci 2005; 46:4393–401 [DOI] [PubMed] [Google Scholar]
- 62.Kubota R, Calkins DJ, Henry SH, Linsenmeier RA. Emixustat reduces metabolic demand of dark activity in the retina. Invest Ophthalmol Vis Sci 2019; 60:4924–30 [DOI] [PubMed] [Google Scholar]
- 63.Kubota R, Gregory J, Henry S, Mata NL. Pharmacotherapy for metabolic and cellular stress in degenerative retinal diseases. Drug Discov Today 2020; 25:292–304 [DOI] [PubMed] [Google Scholar]
- 64.Rosenfeld PJ, Dugel PU, Holz FG, Heier JS, Pearlman JA, Novack RL, Csaky KG, Koester JM, Gregory JK, Kubota R. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: a randomized clinical trial. Ophthalmology 2018; 125:1556–67 [DOI] [PubMed] [Google Scholar]
- 65.Gao S, Parmar T, Palczewska G, Dong Z, Golczak M, Palczewski K, Jastrzebska B. Protective effect of a locked retinal chromophore analog against Light-Induced retinal degeneration. Mol Pharmacol 2018; 94:1132–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maeda A, Golczak M, Chen Y, Okano K, Kohno H, Shiose S, Ishikawa K, Harte W, Palczewska G, Maeda T, Palczewski K. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat Chem Biol 2011; 8:170–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain GL. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J Physiol 2005; 568:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of leber congenital amaurosis. Nat Genet 2003; 35:158–64 [DOI] [PubMed] [Google Scholar]
- 69.Maeda T, Maeda A, Matosky M, Okano K, Roos S, Tang J, Palczewski K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci 2009; 50:4917–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging 2018; 13:757–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Handa JT, Bowes Rickman C, Dick AD, Gorin MB, Miller JW, Toth CA, Ueffing M, Zarbin M, Farrer LA. A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun 2019; 10:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klein BE, Howard KP, Iyengar SK, Sivakumaran TA, Meyers KJ, Cruickshanks KJ, Klein R. Sunlight exposure, pigmentation, and incident age-related macular degeneration. Invest Ophthalmol Vis Sci 2014; 55:5855–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baksheeva VE, Tiulina VV, Tikhomirova NK, Gancharova OS, Komarov SV, Philippov PP, Zamyatnin AA, Jr., Senin II, Zernii EY. Suppression of light-induced oxidative stress in the retina by mitochondria-targeted antioxidant. Antioxidants 2018; 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the Age-Related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 2016; 2016:3164734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang W, Gawlik K, Lopez J, Wen C, Zhu J, Wu F, Shi W, Scheibler S, Cai H, Vairavan R, Shi A, Haw W, Ferreyra H, Zhang M, Chang S, Zhang K. Genetic and environmental factors strongly influence risk, severity and progression of age-related macular degeneration. Signal Transduct Target Ther 2016; 1:16016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang JJ, Li Hb. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci 2017; 18:96–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. Arch Ophthalmol 2001; 119:1417–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA, Group C. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the carotenoids in age-related eye disease study (CAREDS): ancillary study of the women’s health initiative. Arch Ophthalmol 2006; 124:1151–62 [DOI] [PubMed] [Google Scholar]
- 79.Age-Related Eye Disease Study Research G, SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007; 125:221225–32 [DOI] [PubMed] [Google Scholar]
- 80.Mozaffarieh M, Sacu S, Wedrich A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutr J 2003; 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009; 2:270–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdal Dayem A, Choi HY, Yang GM, Kim K, Saha SK, Cho SG. The anti-cancer effect of polyphenols against breast cancer and cancer stem cells: molecular mechanisms. Nutrients 2016; 8:581–61883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for Us? Oxid Med Cell Longev 2016; 2016:7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-Inflammatory role of polyphenols. Nutrients 2018; 10:1618–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ortega JT, Serrano ML, Suarez AI, Baptista J, Pujol FH, Cavallaro LV, Campos HR, Rangel HR. Antiviral activity of flavonoids present in aerial parts of marcetia taxifolia against hepatitis B virus, poliovirus, and herpes simplex virus in vitro. Excli J 2019; 18:1037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients 2014; 6:6020–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016; 5:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ginwala R, Bhavsar R, Chigbu DI, Jain P, Khan ZK. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019; 8:35–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ortega JT, Suarez AI, Serrano ML, Baptista J, Pujol FH, Rangel HR. The role of the glycosyl moiety of myricetin derivatives in anti-HIV-1 activity in vitro. AIDS Res Ther 2017; 14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Busselberg D. Flavonoids in cancer and apoptosis. Cancers 2019; 11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bungau S, Abdel-Daim MM, Tit DM, Ghanem E, Sato S, Maruyama-Inoue M, Yamane S, Kadonosono K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid Med Cell Longev 2019; 2019:9783429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huynh TP, Mann SN, Mandal NA. Botanical compounds: effects on major eye diseases. Evid Based Complement Alternat Med 2013; 2013:549174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khoo HE, Ng HS, Yap WS, Goh HJH, Yim HS. Nutrients for prevention of macular degeneration and eye-related diseases. Antioxidants 2019; 8:85–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majumdar S, Srirangam R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J Pharm Pharmacol 2010; 62:951–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ortega JT, Jastrzebska B. The retinoid and non-retinoid ligands of the rod visual G protein-coupled receptor. Int J Mol Sci 2019; 20:6218–6236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev 2017; 7:CD000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ortega JT, Parmar T, Jastrzebska B. Flavonoids enhance rod opsin stability, folding, and self-association by directly binding to ligand-free opsin and modulating its conformation. J Biol Chem 2019; 294:8101–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herrera-Hernandez MG, Ramon E, Lupala CS, Tena-Campos M, Perez JJ, Garriga P. Flavonoid allosteric modulation of mutated visual rhodopsin associated with retinitis pigmentosa. Sci Rep 2017; 7:11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao X, Liu M, Tuo J, Shen D, Chan CC. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp Eye Res 2010; 91:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weng S, Mao L, Gong Y, Sun T, Gu Q. Role of quercetin in protecting ARPE19 cells against H2O2induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition. Mol Med Rep 2017; 16:3461–8 [DOI] [PubMed] [Google Scholar]
- 101.Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 2016; 10:84–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Kim HJ, Sparrow JR. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp Eye Res 2017; 160:45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arumugam B, Palanisamy UD, Chua KH, Kuppusamy UR. Protective effect of myricetin derivatives from syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol Vis 2019; 25:47–59 [PMC free article] [PubMed] [Google Scholar]
- 104.Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci 2006; 47:3164–77 [DOI] [PubMed] [Google Scholar]
- 105.Du W, An Y, He X, Zhang D, He W. Protection of kaempferol on oxidative Stress-Induced retinal pigment epithelial cell damage. Oxid Med Cell Longev 2018; 2018:1610751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu SX, Chiou GC. Effects of Chinese herbal products on mammalian retinal functions. J Ocul Pharmacol Ther 1996; 12:377–86 [DOI] [PubMed] [Google Scholar]
- 107.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res 2015; 29:323–31 [DOI] [PubMed] [Google Scholar]
- 108.Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother 2017; 88:124–33 [DOI] [PubMed] [Google Scholar]
- 109.Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R, Jha KA. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res 2013; 87:65–74 [DOI] [PubMed] [Google Scholar]
- 110.Hytti M, Piippo N, Korhonen E, Honkakoski P, Kaarniranta K, Kauppinen A. Fisetin and luteolin protect human retinal pigment epithelial cells from oxidative stress-induced cell death and regulate inflammation. Sci Rep 2015; 5:17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol Vis 2014; 20:242–58 [PMC free article] [PubMed] [Google Scholar]
- 112.Liu L, Wu XW. Nobiletin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage. J Biochem Mol Toxicol 2018; 32:e22052. [DOI] [PubMed] [Google Scholar]
- 113.Yan T, Bi H, Wang Y. Wogonin modulates hydroperoxide-induced apoptosis via PI3K/akt pathway in retinal pigment epithelium cells. Diagn Pathol 2014; 9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nakamura N, Hayasaka S, Zhang XY, Nagaki Y, Matsumoto M, Hayasaka Y, Terasawa K. Effects of baicalin, baicalein, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kappab binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp Eye Res 2003; 77:195–202 [DOI] [PubMed] [Google Scholar]
- 115.Tai MC, Tsang SY, Chang LY, Xue H. Therapeutic potential of wogonin: a naturally occurring flavonoid. CNS Drug Rev 2005; 11:141–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakaishi H, Matsumoto H, Tominaga S, Hirayama M. Effects of black current anthocyanoside intake on dark adaptation and VDT work-induced transient refractive alteration in healthy humans. Altern Med Rev 2000; 5:553–62 [PubMed] [Google Scholar]
- 117.Yanamala N, Tirupula KC, Balem F, Klein-Seetharaman J. pH-dependent interaction of rhodopsin with cyanidin-3-glucoside. 1. Structural aspects. Photochem Photobiol 2009; 85:454–62 [DOI] [PubMed] [Google Scholar]
- 118.Nomi Y, Iwasaki-Kurashige K, Matsumoto H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019; 24. DOI:10.3390/molecules24183311 [DOI] [PMC free article] [PubMed] [Google Scholar]