Abstract
Age-related mobility decline is often associated with negative physical and psychological outcomes, such as frailty, in the elderly population. In C. elegans, during the early stage of the aging process, a progressive deficit of synaptic exocytosis in the motor neurons results in a functional decline at the neuromuscular junctions, which eventually leads to degeneration of both neurons and muscles. This age-dependent functional decline can be ameliorated by pharmacological interventions, such as arecoline, a muscarinic AChR agonist known to promote synaptic exocytosis at the neuromuscular junctions. In this study, we found that a short-term treatment of arecoline during the early stage of aging, when the NMJ functional decline begins, not only slows muscle tissue aging, but also extends lifespan in C. elegans. We have also demonstrated that arecoline acts on the GAR-2/PLCβ pathway in the motor neurons to increases longevity. Together, our findings suggest that synaptic transmission in aging motor neurons may serve as a potential target for pharmacological interventions to promote both health span and lifespan, when applied at the early stage aging.
Impact statement
The functional decline of motor activity is a common feature in almost all aging animals that leads to frailty, loss of independence, injury, and even death in the elderly population. Thus, understanding the molecular mechanism that drives the initial stage of this functional decline and developing strategies to increase human healthspan and even lifespan by targeting this process would be of great interests to the field. In this study, we found that by precisely targeting the motor neurons to potentiate its synaptic releases either genetically or pharmacologically, we can not only delay the functional aging at NMJs but also slow the rate of aging at the organismal level. Most importantly, we have demonstrated that a critical window of time, that is the early stage of NMJs functional decline, is required for the beneficial effects. A short-term treatment within this time period is sufficient to extend the animals’ lifespan.
Keywords: Longevity, aging, C. elegans, synaptic exocytosis, motor neuron, neuromuscular junction, arecoline, tomosyn
Introduction
As most animals and humans age, they will inevitably experience physiological declines in many areas that ultimately lead to decreased vitality and eventually death.1 Deterioration in motor function represents one of the most prominent physiological declines in aging animals and humans.2,3 In humans, the decline in motor function begins in middle age.3–7 Subsequently, this age-related reduction in motor activity eventually leads to physiological deterioration in elderly8,9 and appears to be a major risk factor for loss of independence and falling that leads to injury and mortality.10,11 Thus, the development of therapeutic interventions that can delay or slow the age-associated motor activity decline would have a great impact in the healthcare system.
As a widely utilized model organism for aging studies, the age-associated decline of motor activity has also been characterized in the nematode Caenorhabditis elegans.12–16 In C. elegans, the decline of motor activity begins at Days 4–5 of adulthood, which is near the end of their reproductive period, and then rapidly deteriorates.14 Recent studies have suggested that deficits in neuronal functions may be the primary contributor of this age-associated decline. For example, it has been reported by others that subsets of neurons in old animals exhibit mild morphological abnormalities (e.g. extra neuronal branches) and deteriorations at synapses in C. elegans.17,18 We have also previously reported that motor neurons at NMJs undergo a progressive functional decline that begins at Day 5 of adulthood, which parallels the progressive decline of motor activity.12 Furthermore, we found that motor neurons first develop a deficit in synaptic vesicle fusion followed by that in quantal size and vesicle docking/priming, suggesting that specific functional deteriorations in synaptic transmission at the NMJ may be a causative factor for the age-associated motor activity decline. Subsequently in mid-life, muscle cells begin to develop functional deficits, which lead to sarcopenia in late-life.12,19
As the rate of the age-associated decline in motor activity is found to be inversely correlated with longevity,14 pharmacological interventions that can stimulate synaptic transmission at NMJs may be able to restore motor health, slow the rate of aging, and ultimately increase longevity. Previously, our study has shown that arecoline, a muscarinic AChR agonist known to specifically potentiate neurotransmitter release at NMJs, could enhance motor activity in aged worms. Here, we examined the longevity effect of arecoline treatment in mid-life and the temporal requirement of arecoline on lifespan extension in C. elegans. Furthermore, the mechanism of action of arecoline on aging and longevity was investigated.
Materials and methods
C. elegans strains
All strains were maintained at 20˚C on NGM plates seeded with E. coli OP50 strain using the standard method, unless otherwise stated. Animals were cultured for at least three generations in optimum conditions before they were used for experiments. The following alleles and strains were used in the study: Wild-type Bristol N2; RB896: gar-1(ok755); RB756: gar-2(ok520); VC223: tom-1(ok285); VC6576: gar-3(gk305); EQ405: gar-2(ok520); iqEx126 [pAH564(myo-3p::gar-2::SL2::mCherry) + rol-6(su1006)]; EQ407: gar-2(ok520); iqEx127 [pAH558(acr-2p::gar-2::SL2::CFP) + pAH560(unc-25p::gar-2::SL2::YFP) + rol-6(su1006)]; EQ1023: gar-2(ok520); out-crossed 6X to Hsu lab N2; EQ1025: gar-3(gk305); out-crossed 6X to Hsu lab N2; MT1083: egl-8(n488); CF1038: daf-16(mu86); TJ356: zIs356 [daf-16p::daf-16a/b::GFP + rol-6(su1006)].
Wild-type (N2), RB9896, RB756, VC6567, MT1083, CF1038, and TJ356 strains were obtained from Caenorhabditis Genetics Center (Saint Paul, MN). For generation of EQ405 strain, a plasmid DNA mix consisting of 80 ng/µL of pRF4(rol-6p::rol-6(su1006)) and 20 ng/µL of pAH564(myo-3p::gar-2::SL2::mCherry) was microinjected into the gonad of young adult N2 hermaphrodite animals. For generation of EQ407 strain, a plasmid DNA mix consisting of 80 ng/µL of pRF4(rol-6p::rol-6(su1006)), 10 ng/µL of pAH558(acr-2p::gar-2::SL2::CFP), and 10 ng/µL of pAH560(unc-25p::gar-2::SL2::YFP) was microinjected into the gonad of young adult N2 hermaphrodite animals. F1 progeny was then selected on the basis of the roller phenotype. Individual F2 progenies were isolated to establish independent line. Microinjection of N2 worms with pRF4(rol-6p::rol-6(su1006)) alone did not affect the mean lifespan of wild-type animals when grown on OP50 or HT115(DE3) bacteria (data not shown).
RNA interference analysis
The RNAi clones used in this study were picked from Julie Ahringer’s library and were confirmed by sequencing using M13 forward primer (5′-TGTAAAACGACGGCCAGT-3′). HT115(DE3) E. coli bacteria transformed with either empty vector (L4440) or plasmid expressing double-stranded RNA for desired gene were grown in LB supplemented with 100 µg/mL Carbenicillin at 37˚C overnight. Bacterial cultures were seeded on nematode growth medium (NGM) or high-growth (HG) plates containing 100 µg/mL Carbenicillin. IPTG was added to the plates to a final concentration of 1 mM. Animals were subjected to RNAi by transferring unhatched eggs to RNAi plates at 25˚C, unless otherwise stated. For RNAi only during adulthood, worms were transferred from L4440 plates to RNAi plates at late L4 to young adult stage.
Lifespan analysis
Synchronized eggs were transferred by picking to NGM plates seeded with OP50 or HT115(DE3) bacteria (for RNAi conditions) at 20˚C, unless otherwise stated. On Day 1 of adulthood, worms were transferred to fresh plates with bacteria at a density of 12–15 worms per plate. Worms were subsequently transferred to new plates every other day until egg-laying ceased. Viability of worms was scored every one to two days. Animals that were immobile and did not respond to gentle touch of a platinum pick were scored as dead. Worms that bagged, exploded, crawled off plates, or were accidentally killed during the experiment were censored.
DAF-16 nuclear localization assay
For quantification of DAF-16::GFP localization, synchronized eggs from TJ356 animals (i.e. transgenic animals expressing DAF-16::GFP) were seeded onto either vehicle control or drug plates. The GFP expression of these animals was then analyzed using an Olympus BX61 (Olympus America Inc., Center Valley, PA, USA) fluorescent microscope. Worms were blindly scored without knowing the identity of the treatments for the presence or absence of GFP accumulation within the intestinal nuclei as one-day-old adult (n = 60 or greater for all treatments). An animal was scored as having nuclear localized DAF-16::GFP when at least one intestinal cell shows nuclear accumulation of DAF-16::GFP.
Quantification of locomotion activity
Locomotion behavior was assayed on NGM plates using an automated worm tracking system described previously.20,21 In short, NGM plates were spread with a thin layer of freshly grown OP50 bacteria 5 min prior to tracking. We performed tracking at 20°C and at a relative humidity of ∼40% with the lid off. The Worm Tracker consists of a stereomicroscope (Zeiss Stemi 2000 C) mounted with a digital camera (Cohu 7800) and a digital motion system (Parker Automation) that follows worm movement. A customer-developed software package was used to control the system. Worm images were recorded at 2 Hz for 5 min, and the mean centroid speed of each worm was quantified and displayed in real time.
Statistical analysis
For lifespan experiments, Mantel–Cox log-rank test was performed using STATA (Stata Corp, College Station, TX, USA). Details of statistical tests used, number of biological replicates and P values for each experiment are included in figure legends.
Results
Genetic activation of synaptic transmission at NMJs during early stage of aging extends lifespan in C. elegans
Synaptic exocytosis at NMJs was found to undergo a progressive functional decline in C. elegans, beginning at Days 4–5 of adulthood.12 It is believed that this deterioration of synaptic exocytosis with age contributes to the progressive decline of motor activity.12 Thus, we first examined whether boosting synaptic exocytosis via genetic interventions could restore motor health and consequently increase longevity, and if yes, then what is the temporal requirements for such interventions.
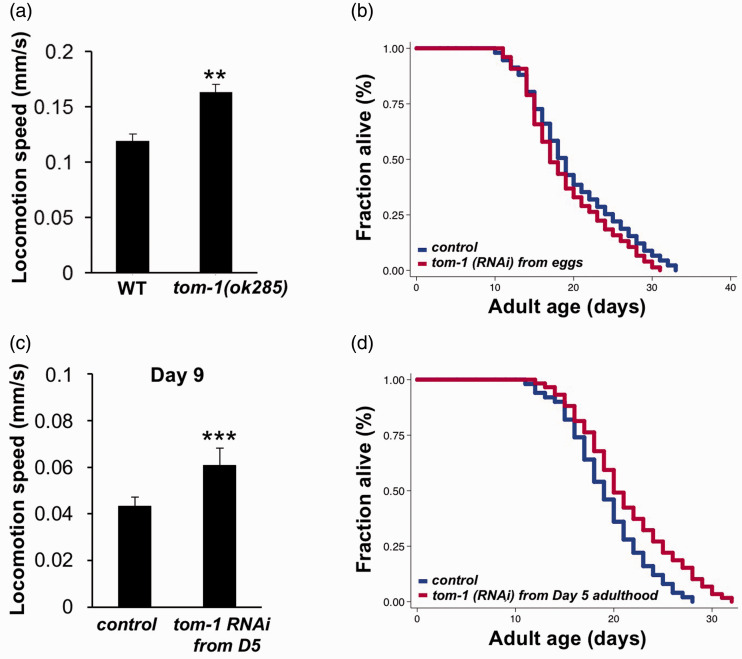
tom-1 encodes the C. elegans ortholog of tomosyn, a syntaxin-interacting protein, which is known to suppress synaptic exocytosis by inhibiting the priming of synaptic vesicles.15,22 Previous studies have reported that cholinergic neurotransmission at NMJs is increased and, consequently, the forward/backward reversals and incidence of pauses are increased in tom-1 hypomophic mutants.15,22 Here, we found that tom-1(ok285) mutants exhibit a 30% increase in locomotion speed compared to wild types (Figure 1(a)), suggesting that synaptic transmission at NMJ could be boosted by genetically inactivating TOM-1.
Figure 1.
Genetic inactivation of tom-1 increases both motor activity and longevity. (a) tom-1(ok285) worms show higher locomotion speed than wild-type animals at Day 1 of adulthood (unpaired t-test; **P<0.01). (b) Lifespan analysis of animals grown on empty vector control (blue) or tom-1 RNAi (red) bacteria from hatching. (c) Locomotion speed of Day 9 of adulthood treated with empty vector control or tom-1 RNAi initiated from Day 5 of adulthood (unpaired t-test; ***P<0.001). (d) Survival curves of animals fed with empty vector control (blue) or tom-1 RNAi (red) bacteria from Day 5 of adulthood (log-rank test; P < 0.0001). The graphs are representative of two independent experiments. Detail statistical data are included in Table S1.
We then asked whether genetic inactivation of TOM-1 slows aging and extends lifespan. We found that RNAi knock-down of tom-1 from hatching did not affect lifespan in wild type N2 animals (Figure 1(b)). However, when the RNAi knock-down of tom-1 was initiated at Day 5 of adulthood, both the locomotion speed and the longevity of tom-1 RNAi animals are significantly increased (Figure 1(c) and (d)). As functional decline at NMJs normally begins at Days 4–5 of adulthood in C. elegans, our findings suggest that potentiating synaptic transmission after, but not before, the functional decline of NMJs begins might delay aging and increase longevity.
Short-term arecoline treatment during early stage of aging extends lifespan in C. elegans
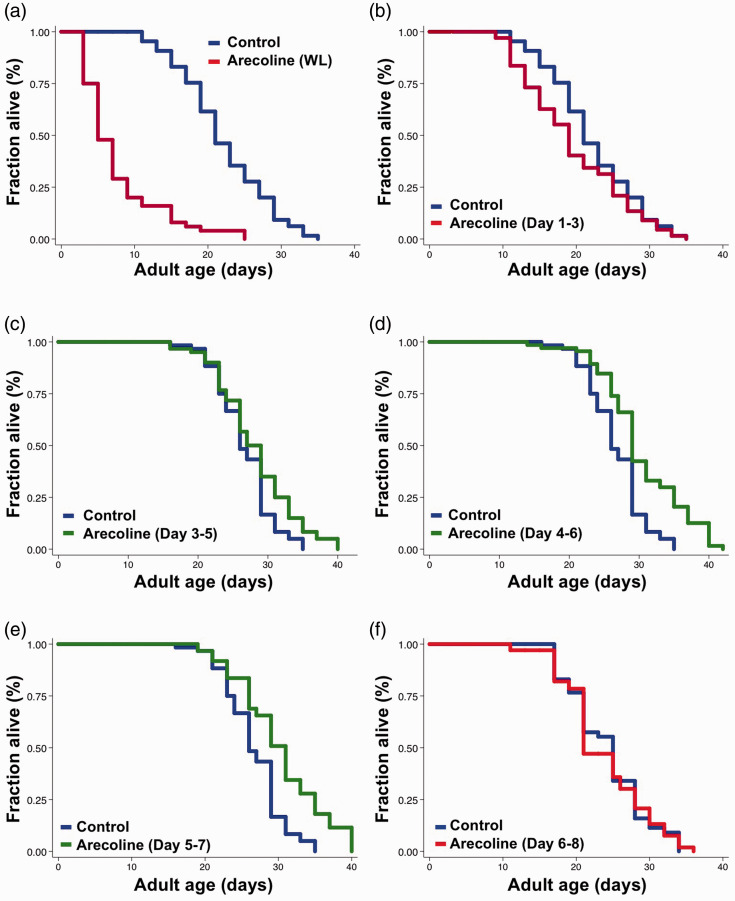
Our genetic studies have indicated that potentiate NMJ functions at early stage of aging could extend lifespan. Thus, we set out to examine whether similar effects can be reproduced using pharmacological interventions. We have previously demonstrated that arecoline treatments in both Day 9 and Day 15 adult worms could significantly increase the neurotransmitter release at NMJs and the locomotion speed,12 suggesting that arecoline treatment might be able to ameliorate the functional decline of NMJs in aging animals. Therefore, we first examined the effect of arecoline treatment on worms’ lifespan when applied for their entire life. We found that whole-life treatment of arecoline significantly shortened the lifespan of animals (Figure 2(a)), indicating that long-term arecoline treatments may be detrimental to worms.
Figure 2.
Arecoline fed from mid-life stages extends lifespan in C. elegans. Survival curves of control (blue) and 0.2 mM arecoline-treated (red) worms from (a) whole adulthood, P < 0.0001, and (b) early adulthood from Day 1 to Day 3, P = 0.0002. Survival curves of animals treated with control vehicle (blue) and arecoline (green) from mid-life stages: (c) Day 3 to Day 5, P = 0.0002, (d) Day 4 to Day 6, and (e) Day 5 to Day 7. (f) Survival curves of control (blue) and arecoline-treated (red) worms from adult Day 6 to Day 8. The arecoline groups of animals were maintained on NGM plates with 0.2 mM arecoline for the above-mentioned periods. P-values represent results of log-rank calculation. The graphs are representative of two or three independent experiments. Detail statistical data are included in Table S1.
Thus, we then limited the duration of the arecoline treatment to three days. Based on our results with tom-1, potentiating synaptic releases at NMJs during the early stage of its functional decline could significantly extend lifespan. Therefore, we performed lifespan assays with short-term (i.e. three days) arecoline treatments starting at different stages of adulthood. We found that arecoline treatment from Days 1 to 3 slightly shortened the lifespan of wild type animals, whereas Days 3 to 5 treatment produced no effect on worm lifespan (Figure 2(b) and (c)). However, when we applied arecoline to adult worms during Days 4 to 6 or Days 5 to 7, the lifespans were significantly extended (Figure 2(d) and (e), 13.3% and 13.4%, respectively), similar to what we observed in tom-1 RNAi worms. Intriguingly, Days 4 to 7 is in the exact period when animals demonstrate the most dramatic drop in motor activity and locomotion speed during aging.14 Indeed, the animals treated with arecoline from Days 4 to 6 or Days 5 to 7 had better performance in locomotion compared to the control group (data not shown). Furthermore, no significant lifespan extension was observed in animals treated with arecoline from Days 6 to 8 (Figure 2(f)). These results together suggested that deceleration of functional decline at NMJs by pharmacological interventions might extend lifespan. However, it has to be done during the early stage of functional aging.
To determine the optimal concentration of arecoline to extend lifespan, we subsequently performed lifespan analysis in the presence of 0.004, 0.02, 0.1, or 0.2 mM arecoline during Day 4 to Day 6 adulthood. We found that lifespan extensions were consistently observed when animals were treated with 0.1 mM and 0.2 mM of arecoline (Table S1). Therefore, in the following experiments, the regimen of arecoline treatment for lifespan analysis was set at 0.1 mM arecoline from Day 4 to Day 6.
The lifespan extension induced by arecoline requires GAR-2 muscarinic acetylcholine receptor in motor neurons
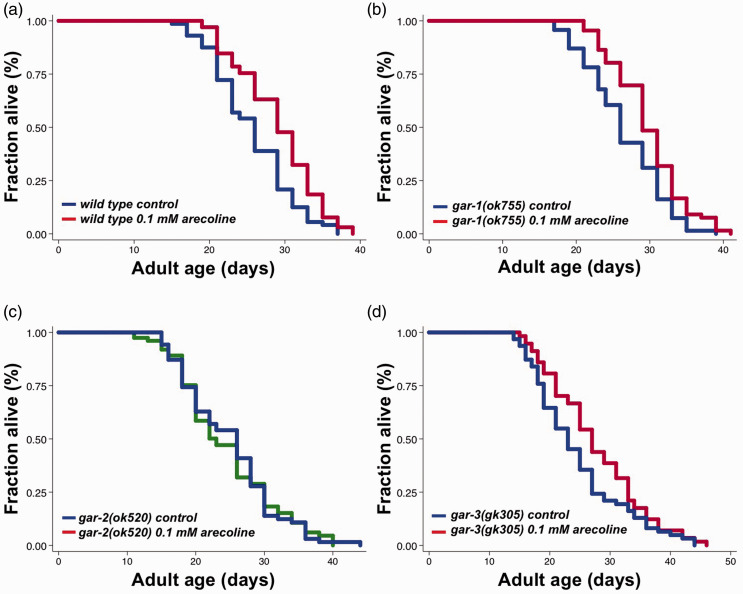
Arecoline is a known agonist of muscarinic acetylcholine receptors (mAChRs). In C. elegans, there are three G protein-linked muscarinic acetylcholine receptor genes: gar-1, gar-2, and gar-3. gar-1 and gar-3 encode mAChRs homologous to M1, M3, and M5 classes of mammalian muscarinic acetylcholine receptors and gar-2 belongs to the M2/M4 class of mAChRs.23 To identify which mAChR mediates the longevity effects of arecoline, we first performed lifespan analysis on gar-1, gar-2, and gar-3 loss-of-function mutants treated with 0.1 mM arecoline from adult Days 4 to 6. We found that gar-1(ok755) and gar-3(gk305) mutants lived 12.17% and 12.51% longer, respectively, upon arecoline treatment, similar to those observed in WT animals (Figure 3(a), (b) and (d)). However, arecoline-induced lifespan extension was completely abolished in gar-2(ok520) mutants (Figure 3(c)). Together, our results indicated that gar-2 might be the mAChR that mediates the longevity effect of short-term arecoline treatment in C. elegans.
Figure 3.
gar-2 is required in lifespan extension induced by arecoline. Survival curves of (a) wild type animals subjected to control (blue) and 0.1 mM arecoline (red) treatment from adult Day 4 to Day 6, P = 0.0005. (b) gar-1(ok755) mutants subjected to control (blue) and 0.1 mM arecoline (red) treatment from adult Day 4 to Day 6, P = 0.0009. (c) gar-2(ok520) mutants subjected to control (blue) and 0.1 mM arecoline (green) treatment from adult Day 4 to Day 6, P = 0.9372. (d) gar-3(gk305) mutants subjected to control (blue) and 0.1 mM arecoline (red) treatment from adult Day 4 to Day 6, P = 0.0227. P-values represent results of log-rank. The graphs are representative of two or three independent experiments. See Table S1 for statistical analysis and additional repeats.
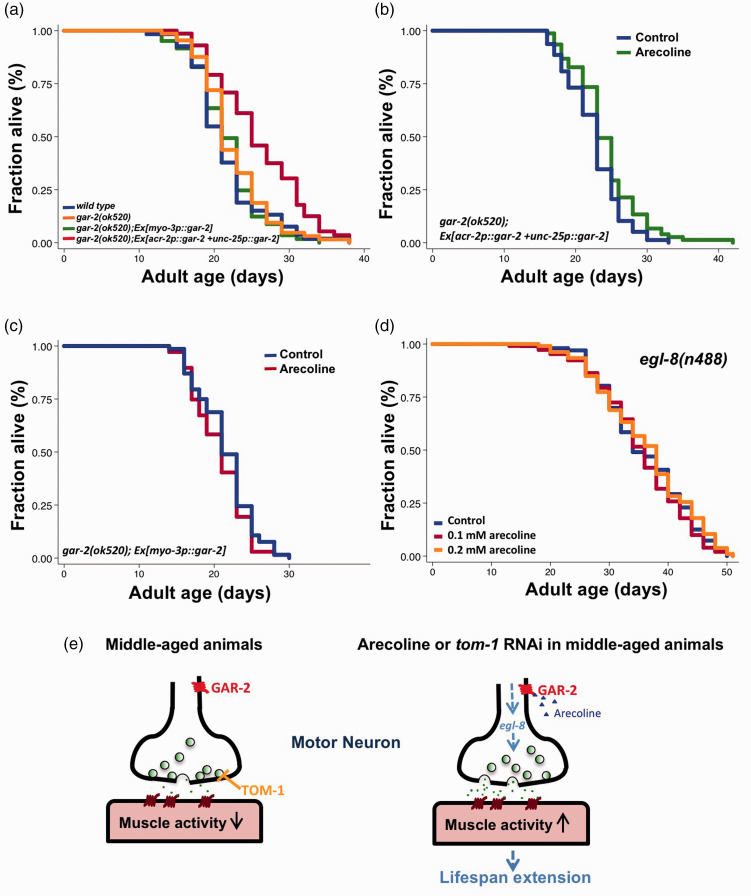
Based on these and our previous findings, we hypothesized that arecoline may act through GAR-2 receptor in the motor neurons to potentiate synaptic exocytosis at NMJ and consequently to slow functional and organismal aging. GAR-2 has been shown to express mainly in the motor neurons, but also in other types of neurons.24 To clarify whether the longevity effect mediated by arecoline is indeed dependent on GAR-2 in the motor neurons, we generated transgenic animals where GAR-2 is driven by the cholinergic motor neuron promoter, acr-2,25 and the GABAergic motor neuron promoter, unc-25,26 in gar-2(ok520) mutants. Surprisingly, we found that gar-2(ok520) mutants expressing GAR-2 in motor neurons lived 15.7% longer than wild-type animals even without any arecoline treatment, while similar gar-2 transgenic animals driven by the muscle myo-3 promoter did not show any lifespan phenotypes (Figure 4(a)). It is possible that overexpression of GAR-2 alone might lead to moderate agonist-independent activation of mAChR. Thus, animals with neuronal overexpression of GAR-2 had an increased basal NMJ function, which leads to the increased longevity.
Figure 4.
Arecoline acts on GAR-2/EGL-8 axis in motor neurons to extend lifespan. (a) Survival plots of wild type N2 (blue), gar-2(ok520) (orange), muscle-specific (green), and motor neuron-specific (red) GAR-2-rescued gar-2(ok520) mutants. (b) Survival curves of motor neuron-specific GAR-2-rescued gar-2(ok520) mutants with (green) or without (blue) 0.1 mM arecoline from adult Day 4 to Day 6. P = 0.0302. (c) Survival curves of muscle-specific GAR-2-rescued gar-2(ok520) mutants with (red) or without (blue) 0.1 mM arecoline from adult Day 4 to Day 6. P = 0.2625. (d) Survival curves of egl-8(n488) mutants with vehicle (blue), 0.1 mM arecoline (red, P = 0.5142 against control group) or 0.2 mM arecoline (orange, P = 0.5676 against control group) from adult Day 4 to Day 6. P-values are derived from log-rank test. The graphs are representative of two or three independent experiments. See Table S1 for statistical analysis and additional repeats. (e) A working model for arecoline-induced longevity in aged worms.
We next tested the longevity effects of short-term arecoline treatment on neuronal or muscular GAR-2-rescued mutants. Our results indicated that arecoline treatment from adult Days 4 to 6 could further extend the lifespan by 6% in gar-2(ok520);acr-2::gar-2;unc-25::gar-2 mutants (Figure 4(b)). In contrast, arecoline did not affect lifespan in gar-2(ok520) mutants expressing GAR-2 in the muscle (Figure 4(c)). Together, our data indicate that pharmacological activation of GAR-2 in the motor neurons, but not in muscle cells, is sufficient to extend lifespan.
Arecoline-induced lifespan extension requires egl-8/PLCβ, a downstream effector of Gq signals
DAF-16/FOXO has been known to be an essential transcription factor in several longevity regulatory signaling pathways in worms, such as the Insulin/IGF-1 signaling (IIS) and signaling from the reproductive system. Thus, we wanted to investigate whether DAF-16 plays any role in regulating the arecoline-induced longevity. To address this question, we first treated animals expressing daf-16::gfp reporter with arecoline for 1 and 24 h and analyzed the nuclear translocation of DAF-16. However, arecoline failed to induce nuclear translocation of DAF-16 in all conditions (Figure S1(a)). We then performed lifespan analysis on daf-16(mu86) null mutants treated with 0.1 mM arecoline from Days 4 to 6. The results indicated that arecoline treatment slightly extends lifespan in daf-16 null mutants (Figure S1(b)), suggesting that DAF-16/FOXO might be partially required for the arecoline-induced longevity.
Previous studies have shown that egl-8/phospholipase Cβ (PLCβ) is required to mediate the effects of arecoline in stimulating acetylcholine release at NMJs in C. elegans.27,28 Arecoline activates egl-8/phospholipase Cβ (PLCβ) via Gq protein to produce more diacylglycerol (DAG), which facilitates neurotransmitter release.27 Since we have demonstrated that arecoline acts through GAR-2 in the motor neurons to extend lifespan, we then examined whether the PLCβ signaling pathway is responsible for the arecoline-induced longevity. Hence, egl-8(n488) lost-of-function mutants were treated with 0.1 mM and 0.2 mM arecoline from Days 4 to 6. No lifespan extension was observed in arecoline-treated egl-8 mutants (Figure 4(d)), suggesting that the arecoline-induced longevity requires EGL-8/PLCβ activity. Taken together, our findings suggest that arecoline might act on GAR-2 to trigger the activation of the egl-8/PLCβ signaling pathway in the motor neurons, resulting in an enhancement of synaptic transmission at NMJ and subsequently a delay in NMJ functional aging as well as organismal aging (Figure 4(e)).
Discussion
We have previously reported that the progressive decline in synaptic exocytosis in the motor neurons might contribute significantly to the age-associated motor activity declines, which is a major predictor for animals’ lifespan.12,14 In this study, we have further demonstrated that we could slow both functional aging in the neuromuscular system and increase longevity by targeting the synaptic exocytosis directly or indirectly via genetic or pharmacological interventions. By targeting the negative regulators, TOM-1, we have shown that activation of synaptic vesicles release from the presynaptic motor neurons at the early stage of NMJ aging can significantly extend worm lifespan. By pharmacologically targeting the GAR-2 receptor to potentiate synaptic releases in the motor neurons through arecoline, we can also extend animals’ lifespan. Most significantly, we found that these interventions have to be applied at the right time, when the motor activity and NMJs function just begin to deteriorate but remain mostly intact (i.e. Days 4–7 of adulthood), in order to be beneficial for longevity (Figures 1(c) and 2(d) to (e)). This also suggest that decelerating the age-associated functional decline of NMJs, rather than boosting basal NMJs activity, is more beneficial in the context of health span and lifespan extension.
Therefore, we proposed that a temporally optimized short-term intervention targeting the early stage of the functional aging may be more beneficial than long-term treatments when it comes to anti-aging strategies. Consistent with this idea, recent evidence suggests that high-intensity long-term exercise reduces the beneficial effects of exercise.29,30 Moreover, transgenic overexpression of the vesicular acetylcholine transporter (VAChT) in mice increases synaptic acetylcholine and accelerates NMJ degeneration,31 suggesting that elevating basal levels of synaptic acetylcholine throughout the entire life may not be optimal. Thus, whole-life arecoline treatment, which constantly enhances synaptic acetylcholine release, may also be harmful to animals as we have observed.
In this study, we have also elucidated the molecular mechanism of action for arecoline in longevity regulation. We have found that arecoline acts on to the muscarinic acetylcholine receptor, GAR-2, in the motor neurons to potentiate neurotransmitter release at the NMJs and consequently increases longevity in C. elegans (Figures 3 and 4). We have also shown that egl-8/PLC is the downstream effector of arecoline to regulate longevity (Figure 4(d)). Therefore, mammalian mAChR isoforms expressed in the motor neurons may serve as potential therapeutic targets for future development of interventions combating age-related motor activity declines, such as sarcopenia and frailty.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220950639 for Short-term enhancement of motor neuron synaptic exocytosis during early aging extends lifespan in Caenorhabditis elegans by Tsui-Ting Ching, Yen-Chieh Chen, Guang Li, Jianfeng Liu, XZ Shawn Xu and Ao-Lin Hsu in Experimental Biology and Medicine
ACKNOWLEDGMENTS
We thank Caenorhabditis Genetics Center (University of Minnesota), which is supported by the NIH Office of Research Infrastructure Programs (P40 OD010440) for providing some of the C. elegans strains used in the studies. We also thank Travis Mazer for the initial optimization of the arecoline treatment conditions.
Authors’ contributions
TTC, XZSX, and ALH participated in the conceptual design of the studies interpretation of the studies and analysis of the data; TTC, YCC, and GL conducted the experiments; TTC and ALH wrote the manuscript; JL and XZSX help edited the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research: This work was supported by the National Institute on Aging [R01 AG051439 to ALH and XZSX] and Ministry of Science and Technology of Taiwan [MOST-1032311B010007 to TTC].
ORCID iDs
Guang Li https://orcid.org/0000-0003-4277-9844
Ao-Lin Hsu https://orcid.org/0000-0002-2864-3134
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Kenyon CJ. The genetics of ageing. Nature 2010; 464:504–12 [DOI] [PubMed] [Google Scholar]
- 2.Wolkow CA. Identifying factors that promote functional aging in Caenorhabditis elegans. Exp Gerontol 2006; 41:1001–6 [DOI] [PubMed] [Google Scholar]
- 3.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 2007; 34:1091–6 [DOI] [PubMed] [Google Scholar]
- 4.Keller IM, Kalache A. Promoting healthy aging in cities: the healthy cities project in Europe. J Cross Cult Gerontol 1997; 12:287–98 [DOI] [PubMed] [Google Scholar]
- 5.Macintosh BR, Jones D, Devrome AN, Rassier DE. Prediction of summation in incompletely fused tetanic contractions of rat muscle. J Biomech 2007; 40:1066–72 [DOI] [PubMed] [Google Scholar]
- 6.Leversen JS, Haga M, Sigmundsson H. From children to adults: motor performance across the life-span. PLoS One 2012; 7:e38830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-Related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci 2016; 71:1184–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rantanen T, Guralnik JM, Ferrucci L, Penninx BW, Leveille S, Sipila S, Fried LP. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc 2001; 49:21–7 [DOI] [PubMed] [Google Scholar]
- 9.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002; 50:889–96 [DOI] [PubMed] [Google Scholar]
- 10.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 2002; 57:B359–65 [DOI] [PubMed] [Google Scholar]
- 11.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc 2003; 51:636–41 [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu AL, Xu XZ. Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metab 2013; 18:392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science 2002; 298:2398–401 [DOI] [PubMed] [Google Scholar]
- 14.Hsu AL, Feng Z, Hsieh MY, Xu XZ. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging 2009; 30:1498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen JM, Madison JM, Dybbs M, Kaplan JM. Antagonistic regulation of synaptic vesicle priming by tomosyn and UNC-13. Neuron 2006; 51:303–15 [DOI] [PubMed] [Google Scholar]
- 16.Hosono R. Age dependent changes in the behavior of Caenorhabditis elegans on attraction to Escherichia coli. Exp Gerontol 1978; 13:31–6 [DOI] [PubMed] [Google Scholar]
- 17.Pan CL, Peng CY, Chen CH, McIntire S. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proc Natl Acad Sci U S A 2011; 108:9274–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tank EM, Rodgers KE, Kenyon C. Spontaneous age-related neurite branching in Caenorhabditis elegans. J Neurosci 2011; 31:9279–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002; 419:808–14 [DOI] [PubMed] [Google Scholar]
- 20.Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 2006; 127:621–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Feng Z, Sternberg PW, Xu XZ. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 2006; 440:684–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracheva EO, Burdina AO, Holgado AM, Berthelot-Grosjean M, Ackley BD, Hadwiger G, Nonet ML, Weimer RM, Richmond JE. Tomosyn inhibits synaptic vesicle priming in Caenorhabditis elegans. PLoS Biol 2006; 4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YS, Park YS, Chang DJ, Hwang JM, Min CK, Kaang BK, Cho NJ. Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem 1999; 72:58–65 [DOI] [PubMed] [Google Scholar]
- 24.Lee YS, Park YS, Nam S, Suh SJ, Lee J, Kaang BK, Cho NJ. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem 2000; 75:1800–9 [DOI] [PubMed] [Google Scholar]
- 25.Barbagallo B, Prescott HA, Boyle P, Climer J, Francis MM. A dominant mutation in a neuronal acetylcholine receptor subunit leads to motor neuron degeneration in Caenorhabditis elegans. J Neurosci 2010; 30:13932–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci 1999; 19:539–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 1999; 24:335–46 [DOI] [PubMed] [Google Scholar]
- 28.Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the gqalpha pathway in C. elegans. Neuron 1999; 24:323–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin BA, Thompson PD, Al-Zaiti SS, Albert CM, Hivert MF, Levine BD, Lobelo F, Madan K, Sharrief AZ, Eijsvogels TMH, American Heart Association Physical Activity Committee of the Council on L, Cardiometabolic H, Council on C, Stroke N, Council on Clinical C, Stroke C. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective – an update: a scientific statement from the American Heart Association. Circulation 2020; 141:e705–e36 [DOI] [PubMed] [Google Scholar]
- 30.Clarke PM, Walter SJ, Hayen A, Mallon WJ, Heijmans J, Studdert DM. Survival of the fittest: retrospective cohort study of the longevity of Olympic medallists in the modern era. Br J Sports Med 2015; 49:898–902 [DOI] [PubMed] [Google Scholar]
- 31.Sugita S, Fleming LL, Wood C, Vaughan SK, Gomes MP, Camargo W, Naves LA, Prado VF, Prado MA, Guatimosim C, Valdez G. VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions. Skelet Muscle 2016; 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220950639 for Short-term enhancement of motor neuron synaptic exocytosis during early aging extends lifespan in Caenorhabditis elegans by Tsui-Ting Ching, Yen-Chieh Chen, Guang Li, Jianfeng Liu, XZ Shawn Xu and Ao-Lin Hsu in Experimental Biology and Medicine