Abstract
Transactive response DNA binding protein 43 (TDP-43) pathologies have been well recognized in various neurodegenerative disorders including frontotemporal lobar degeneration (FTLD), amyotrophic lateral sclerosis (ALS), and Alzheimer’s disease (AD). However, there have been limited studies on whether there are any TDP-43 alterations in normal aging. We investigated TDP-43 distribution in different brain regions in normal aged (n = 3 for 26- or 36-month-old) compared to young (n = 3 for 6- or 12-month-old) mice. In both normal aged and young mice, TDP-43 and phosphorylated TDP-43 (pTDP-43) demonstrated a unique pattern of distribution in neurons in some specific brain regions including the pontine nuclei, thalamus, CA3 region of the hippocampus, and orbital cortex. This pattern was demonstrated on higher magnification of high-resolution double fluorescence images and confocal microscopy as mislocalization of TDP-43 and pTDP-43, characterized by neuronal nuclear depletion and cytoplasmic accumulation in these brain regions, as well as colocalization between TDP-43 or pTDP-43 and mitochondria, similar to what has been described previously in neurodegenerative disorders. All these findings were identical in both normal aged and young mice. In summary, TDP-43 and pTDP-43 mislocalization from nucleus to cytoplasm and their colocalization with mitochondria in the specific brain regions are present not only in aging, but also in young healthy states. Our findings provide a new insight for the role of TDP-43 proteinopathy in health and diseases, and that aging may not be a critical factor for the development of TDP-43 proteinopathy in subpopulations of neurons.
Impact statement
Despite increasing evidence implicating the important role of TDP-43 in the pathogenesis of a wide range of age-related neurodegenerative diseases, there is limited study of TDP-43 proteinopathy and its association with mitochondria during normal aging. Our findings of cytoplasmic accumulation of TDP-43 that is highly colocalized with mitochondria in neurons in selective brain regions in young animals in the absence of neuronal loss provide a novel insight into the development of TDP-43 proteinopathy and its contribution to neuronal loss.
Keywords: TDP-43, normal aging, neurodegeneration, mitochondria
Introduction
Transactive response DNA binding protein 43 (TDP-43) pathologies have been well described in several neurodegenerative disorders including frontotemporal lobar degeneration (FTLD),1,2 amyotrophic lateral sclerosis (ALS),3 and Alzheimer’s disease (AD)4 for over a decade now. In the normal state, TDP-43 is expressed in the nucleus, whereas in the diseased states, mislocalization of TDP-43 characterized by loss of TDP-43 expression in the nucleus along with presence of TDP-43-positive inclusions within the cytoplasm is considered to be a key neuropathological feature. This cytoplasmic mislocalization of TDP-43 has been demonstrated to be neurotoxic.5 Nevertheless, very little is known about TDP-43 alterations in normal aging. Phosphorylation of TDP-43 in multiple different sites such as Serine (Ser)-409/410, Ser-403/404, and Ser-379 has also been reported in neurodegenerative disorders including ALS and FTLD-TDP.6 Nevertheless, there has been limited data on phosphorylated TDP-43 (pTDP-43) in normal aging.
Aging is known to be a risk factor for multiple neurodegenerative disorders including AD7,8 and Parkinson’s disease (PD).9 Neuropathological changes in normal aging are characterized by generalized brain atrophy upon gross examination. Upon microscopic examination, protein depositions have been reported including amyloid, tau, α-synuclein, and TDP-43, among others,10 in addition to well-recognized conventional alterations such as corpora amylacea and lipofuscin pigment accumulation. With regard to TDP-43 in normal aging, there have been very few studies done, which had inconsistent findings.11 Geser et al. found that up to 35.3% of their human subjects in control normal aging group age 65 years and older had positive TDP-43 pathology. Some of these patients were reported to have neuronal cytoplasmic inclusions.12 One subject with normal aging in another study demonstrated mild-to-moderate TDP-43 pathology (inclusions) in the basal forebrain, hypothalamus, ventromedial basal forebrain region, and Islands of Calleja.13 Uchino et al.14 reported dystrophic neurites with TDP-43 positivity in the uncus of the hippocampus in 29 out of 107 normal aging elderly human subjects in their study, whereas neuronal cytoplasmic inclusion was found in the subiculum in only one subject in the normal aging group. Cytoplasmic or intranuclear inclusions of TDP-43 were reported to be more rare when compared to TDP-43 neurites, and they are associated with argyrophilic grain pathologies in cognitively normal elderly human subjects in one study.15
Mitochondrial localization of TDP-43 has been demonstrated to be pathologically enhanced in diseases such as ALS16 and FTLD.17 Furthermore, inhibition of this localization of TDP-43 can ameliorate mitochondrial dysfunction and neuronal loss in rodent models.17 Nevertheless, to our knowledge, there have been no studies investigating whether TDP-43 localizes to mitochondria in normal aging. Whether neurodegeneration displays continuity from the normal aging process remains unclear. Due to lack of consistent evidence, controversy has arisen in this area.18 Here, we investigate whether there are TDP-43 changes in normal aged mice compared to young mice, and if there are, what brain regions are affected. In addition, through the use of an automated high-throughput whole brain microscopy and confocal microscopy, TDP-43 alterations in the nucleus and cytoplasm, as well as colocalization with mitochondria, are studied.
Methods
Tissue
Whole brain tissues of six normal young (three 6- and three 12-month-old), and six normal aged (three 26- and three 36-month-old) mice were obtained from National Institute of Aging (NIA) at National Institutes of Health (NIH). The mice were sacrificed when the determined ages were reached. Some of the tissues were fixed in buffered formalin for immunohistochemistry and immunofluorescence staining. Fixed tissues were dehydrated through graded ethanol followed by xylene and embedded in paraffin. Microtome consecutive sections of 6 μm thickness were prepared as described previously.19 Demographic data of mice used in this study are described in Table 1.
Table 1.
Demographic data of mice used in this study including age, sex, body, and brain weights.
| Subject number | Age (months) | Sex | Body weight (g) | Brain weight (g) |
|---|---|---|---|---|
| 1 | 6 | Male | 36.46 | 0.49 |
| 2 | 6 | Male | 35.20 | 0.51 |
| 3 | 6 | Male | 33.19 | 0.49 |
| 4 | 12 | Male | 33.60 | 0.48 |
| 5 | 12 | Male | 32.47 | 0.42 |
| 6 | 12 | Male | 34.24 | 0.49 |
| 7 | 26 | Male | 33.90 | 0.52 |
| 8 | 26 | Male | 36.90 | 0.54 |
| 9 | 26 | Male | 35.80 | 0.49 |
| 10 | 36 | Male | 31.36 | 0.50 |
| 11 | 36 | Male | 32.63 | 0.51 |
| 12 | 36 | Male | 32.61 | 0.49 |
Note: Subjects 1–6 and 7–12 are young and aged groups, respectively.
Immunofluorescence and confocal microscopy
Double immunofluorescence staining was employed in four young (two 6-month-old and two 12-month-old) and four aged (two 26-month-old and two 36-month-old) mice to investigate TDP-43 expression in different brain regions of the mouse whole sagittal brain section, as well as the colocalization between TDP-43 or pTDP-43, and mitochondria. Taken briefly, paraffin embedded whole brain sagittal sections were deparaffinized in xylene and rehydrated in graded ethanol without H2O2 incubation. Then, the rehydrated brain tissue sections were incubated in phosphate buffer saline (PBS) at room temperature (RT) for 10 min followed by antigen retrieval in 1× Immuno/DNA retriever with citrate (BioSB) under pressure using BioSB’s TintoRetriever pressure cooker. The sections were gradually rinsed with distilled H2O five times, then blocked with 10% NGS in PBS for 45 min at RT. The primary antibodies used included widely used and well-characterized rabbit polyclonal anti-TDP-43 (Proteintech, Cat No.: 10782, 1:100), rabbit polyclonal anti-pTDP-43 (Proteintech, Cat No.: 22309, 1:200; phosphorylation of TDP-43 at Ser-409/410), mouse monoclonal anti-NeuN (Millipore Sigma, Cat No.: MAB377, 1:1000), and mitochondrial oxidative phosphorylation antibody cocktail (Abcam, Cat No.: 110411, 1:1000), all of which have been validated and used in our previous studies.17,20,21 The sections were incubated with primary antibodies in PBS containing 1% NGS in PBS overnight at 4°C. After three quick washes with 1% NGS in PBS, the sections were incubated in 10% NGS in PBS for 10 min and rinsed with 1% NGS in PBS. Then, the sections were incubated with Alexa Fluor 488 or 568 dye-labeled secondary antibodies (Invitrogen, 1:300) for 2 h at RT in the dark, washed three times with PBS, stained with DAPI, washed again with PBS for three times, and finally mounted with Fluoromount-G mounting medium (Southern Biotech). The images of the entire sagittal brain sections were obtained via processing using an automated high-resolution microscope, Celldiscoverer 7 (controlled by Zen software, Zeiss). The whole sagittal brain sections were scanned at a 20×/(0.75) Plan Apochromat dry objective. In order to adjust the alignment and reduce overlaps between individual tiles within each image, the stitching process was then performed using the Zen software.
For confocal microscopic study, fluorescence images of one of the 36-month-old mouse brains were captured at RT with a Leica SP8 gSTED confocal microscope equipped with a motorized super Z galvo stage, 2 PMTs, 3 Hyd SP GaAsP detectors for gated imaging, and the AOBS system lasers including a 405 nm, Argon (458, 476, 488, 496, and 514 nm), a tunable white light (470 to 670 nm), and a 592 nm STED depletion laser. A series of confocal images with optical thickness of 300 nm were collected using the 40× oil objective. Since the findings and quality of high-resolution images taken using the Celldiscoverer 7 were similar to the ones from the confocal microscope, the brain images in the remaining mice were examined using the Celldiscoverer 7 without a confocal study.
Results
Nuclear depletion and cytoplasmic accumulation of TDP-43 in specific brain regions in both normal aged and young mice
All mice in both young (6-month-old and 12-month-old) and aged (24-month-old and 36-month old) groups were confirmed to be normal without evidence of organ-specific pathologies including neurodegenerative or systemic disorders upon extensive autopsy studies. In addition, there was no statistically significant difference in brain weights between young and aged mice (P = 0.09, Table 1).
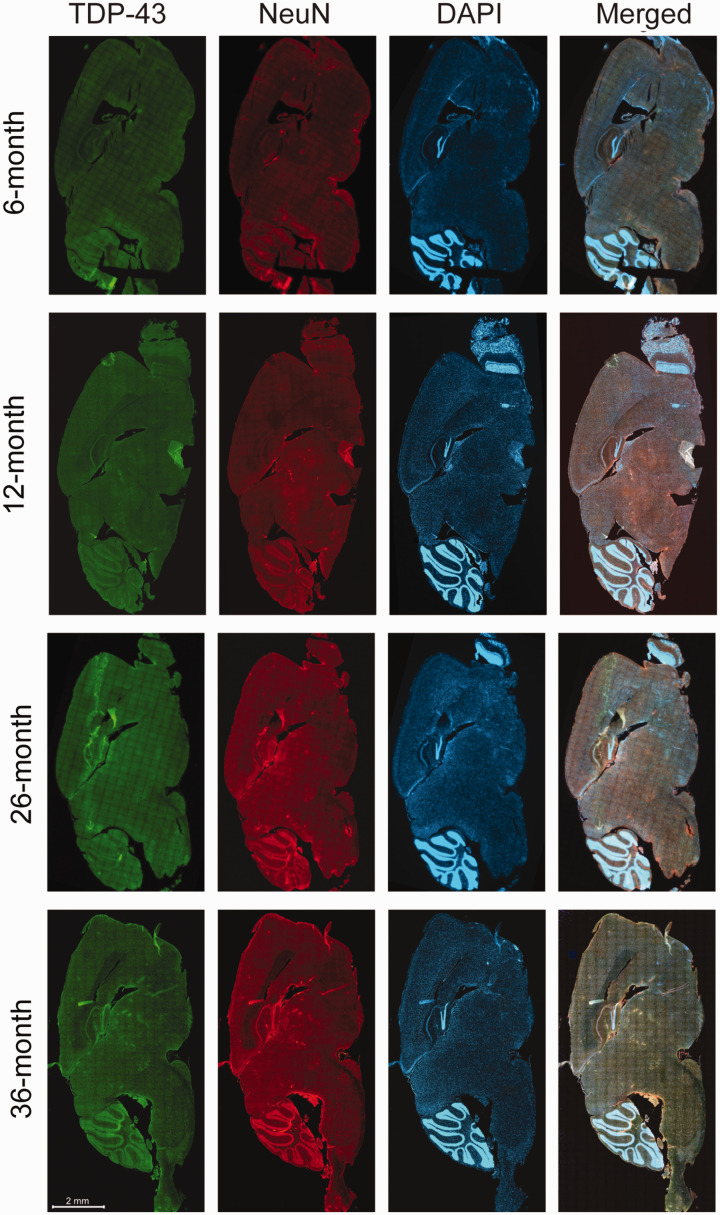
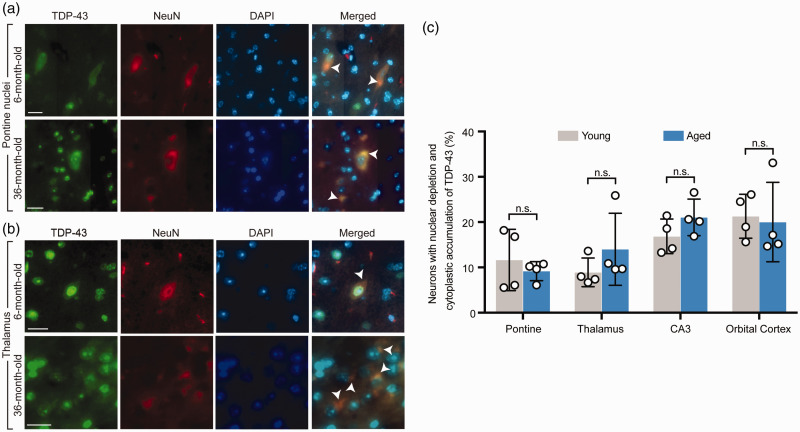
On the whole brain sagittal sections, multiple brain regions with a unique pattern of neuronal TDP-43 expression were identified in both young and aged mice. On low magnification, these brain regions exhibited distinct hyperintense green fluorescent signal of TDP-43 (Figure 1). Upon further examination on higher magnification of the high-resolution images of the whole sagittal brain sections, a unique pattern of nuclear depletion and cytoplasmic accumulation of TDP-43 was demonstrated. Interestingly, TDP-43 that accumulated within the cytoplasm formed aggregates or TDP-43 inclusions. These brain areas with the unique pattern of TDP-43 expression included the pontine nuclei (Figure 2(a)), thalamus (Figure 2(b)), CA3 region of the hippocampus, and orbital cortex (data not shown). There was no statistically significant difference in the number of neurons with unique TDP-43 expression (Figure 2(c)) between the young and aged mice in each brain region involved.
Figure 1.
Distributed neuroanatomical regions with a unique TDP-43 expression were identical between young (6- and 12-month-old) and aged (26- and 36-month-old) mice, upon examination of the whole sagittal brain sections by an automated high-throughput microscope. The unique TDP-43 expression was visualized as distinct hyperintense green fluorescent signals on low magnification (with confirmation of nuclear depletion and cytoplasmic mislocalization on higher magnification in Figure 2), in four specific brain regions including the pontine nuclei, thalamus, CA3 region of hippocampus, and orbital cortex. The scale bar is applied to all brain sections in the figure. (A color version of this figure is available in the online journal.)
Figure 2.
Both aged and young mice demonstrated a unique pattern of nuclear depletion and cytoplasmic mislocalization of TDP-43 in neurons in specific brain regions. (a) Higher magnification of the pontine nuclei from double fluorescence staining of the whole brain sagittal sections demonstrated mislocalization of TDP-43 characterized by nuclear depletion and cytoplasmic accumulation in both representative young (6-month-old) and aged (36-month-old) mice. Arrow heads point to neurons with TDP-43 mislocalization. (b) Higher magnification of the thalamus from double fluorescence staining of the whole brain sagittal sections in representative young (6-month-old) and aged (36-month-old) mice demonstrated findings identical to (a). Arrow heads point to neurons with TDP-43 mislocalization. (c) Quantification of the number of neurons with nuclear depletion and cytoplasmic accumulation of TDP-43 with comparison between young (6- and 12- month-old) and aged (26- and 36-month-old) groups in four brain regions including the pontine nuclei, thalamus, CA3 region of the hippocampus, and orbital cortex. n = 4 mice per group. Data are means ± s.e.m. Student’s t-test. n.s.: not significant. (A color version of this figure is available in the online journal.)
In addition, one of the 36-month-old mice was selected for the study at higher magnification with the confocal microscope. Nuclear depletion and cytoplasmic accumulation of the TDP-43 signals were found in these brain regions including the pontine nuclei, thalamus (Supplemental Figure 1), CA3 region of the hippocampus, and orbital cortex (data not shown), identical to the findings from high-resolution images of the whole brain sagittal sections above.
Nuclear depletion and cytoplasmic accumulation of pTDP-43 in specific brain regions in both normal aged and young mice
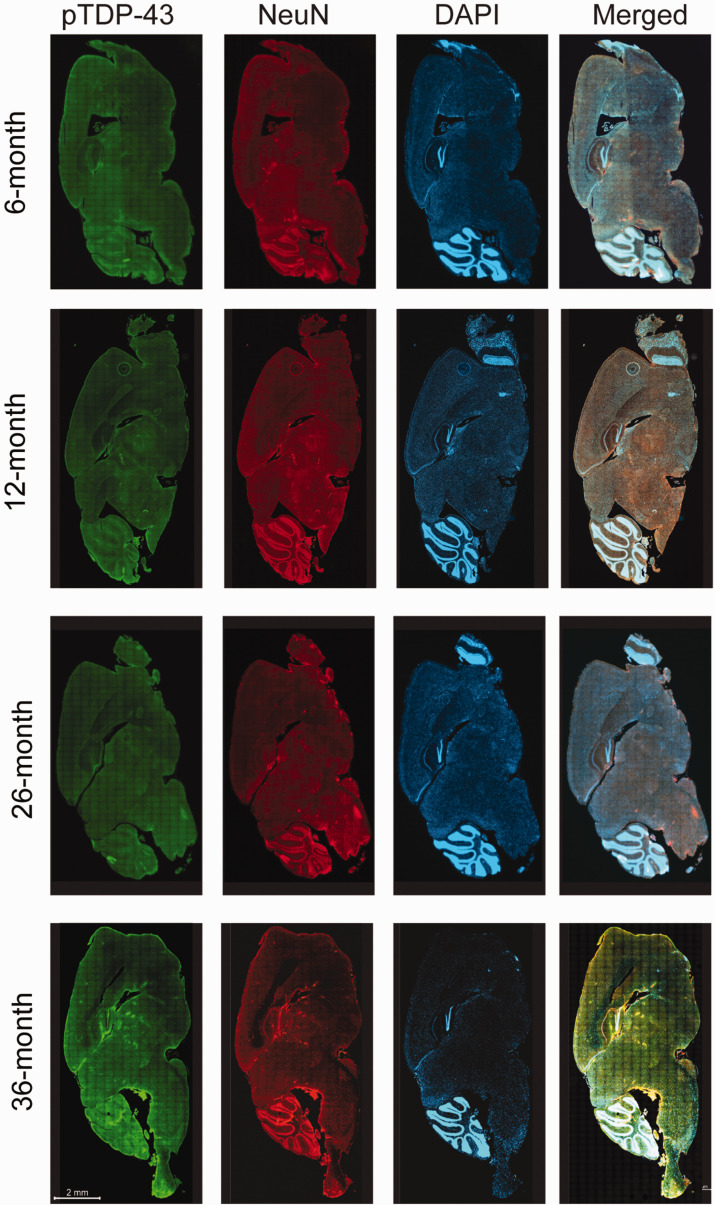
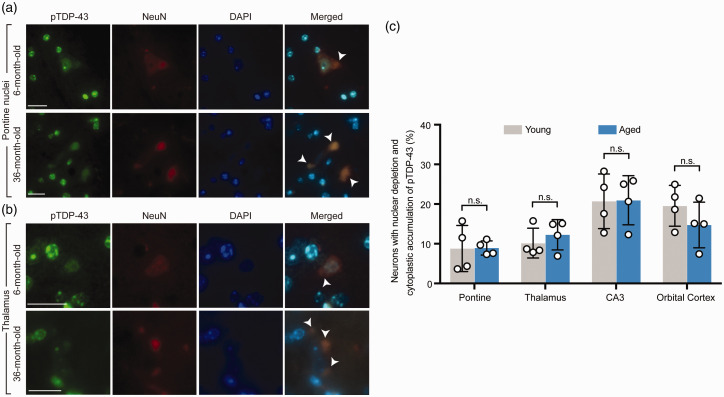
Double fluorescence staining of pTDP-43 demonstrated findings similar to what were found in the TDP-43 staining above. The unique pattern of neuronal pTDP-43 expression, characterized by hyperintense green fluorescent signal of pTDP-43 expression visualized from the whole sagittal brain sections on low magnification was also demonstrated in the same brain regions as seen with TDP-43 staining above (Figure 3). On higher magnification of these high-resolution images, nuclear depletion, cytoplasmic accumulation, and inclusion formation were observed in neurons in these brain regions. These included pontine nuclei (Figure 4(a)), thalamus (Figure 4(b)), CA3 region of the hippocampus, and orbital cortex (data not shown). Similar to TDP-43, pTDP-43 expression showed the same pattern in both young and aged mice (Figure 4(a) and (b)), and there was no statistically significant difference in the number of neurons with nuclear depletion and cytoplasmic accumulation of pTDP-43 (Figure 4(c)) between young and aged mice in each brain region involved.
Figure 3.
Distributed neuroanatomical regions with a unique phosphorylated TDP-43 (pTDP-43) expression were identical between young (6- and 12-month-old) and aged (26- and 36-month-old) mice, upon examination of the whole sagittal brain sections by an automated high-throughput microscope. The unique pTDP-43 expression was visualized as distinct hyperintense green fluorescent signals on low magnification (with confirmation of nuclear depletion and cytoplasmic mislocalization on higher magnification in Figure 4), in four specific brain regions including the pontine nuclei, thalamus, CA3 region of hippocampus, and orbital cortex. The scale bar is applied to all brain sections in the figure. (A color version of this figure is available in the online journal.)
Figure 4.
Phosphorylated TDP-43 (pTDP-43) demonstrated nuclear depletion and cytoplasmic mislocalization in neurons in some specific brain regions, identical to TDP-43. (a) Higher magnification of the pontine nuclei from double fluorescence staining of the whole brain sagittal sections demonstrated mislocalization of pTDP-43 characterized by nuclear depletion and cytoplasmic accumulation in both representative young (6-month-old) and aged (36-month-old) mice. Arrow heads point to neurons with TDP-43 mislocalization. (b) Higher magnification of the thalamus from double fluorescence staining of the whole brain sagittal sections in representative young (6-month-old) and aged (36-month-old) mice demonstrated findings identical to (a). Arrow heads point to neurons with TDP-43 mislocalization. (c) Quantification of the number of neurons with nuclear depletion and cytoplasmic accumulation of pTDP-43 with comparison between young (6- and 12- month-old) and aged (26- and 36-month-old) groups in four brain regions including the pontine nuclei, thalamus, CA3 region of the hippocampus, and orbital cortex. n = 4 mice per group. Data are means ± s.e.m. Student’s t-test. n.s.: not significant. (A color version of this figure is available in the online journal.)
The magnified images obtained by confocal microscopic technique performed only in one of the 36-month-old mice exhibited the nuclear depletion and cytoplasmic accumulation with formation of inclusion of pTDP-43 (Supplemental Figure 2), identical to what were found from high-resolution images of the whole brain sagittal sections above, in these brain regions.
Colocalization between TDP-43 or pTDP-43 and mitochondria in both normal aged and young mice
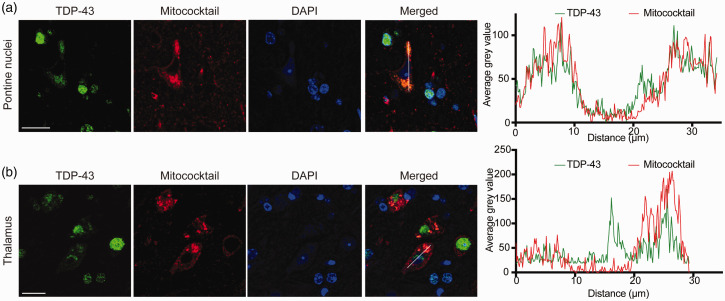
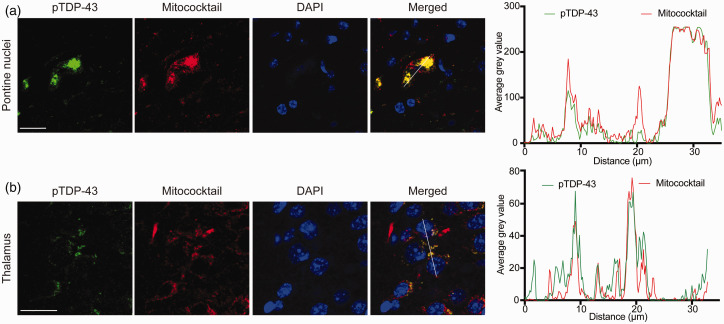
TDP-43 and mitochondria colocalization in neurons was then further investigated by confocal microscopic examination and double fluorescence staining either anti-TDP-43 or anti-pTDP-43 and mitochondrial oxidative phosphorylation complexes antibody cocktail (Mitococktail). Confocal microscopic examination in one of the 36-month-old mice demonstrated the same findings: colocalization between TDP-43 and mitochondria in the cytoplasm in the pontine nuclei (Figure 5(a)), thalamus (Figure 5(b)), CA3 region of the hippocampus, and orbital cortex (data not shown). Similar to TDP-43, colocalization between pTDP-43 and mitochondria in the cytoplasm was also seen in these same brain regions with confocal microscopy (Figure 6(a) and (b)). The colocalization between TDP-43 and mitochondria in these brain regions was confirmed by line-scan analysis (Figure 5(a) and (b)), so is the colocalization between pTDP-43 and mitochondria (Figure 6(a) and (b)).
Figure 5.
Colocalization between cytoplasmic TDP-43 and mitochondria. (a and b) Confocal microscopic images demonstrated colocalization between cytoplasmic TDP-43 accumulation and mitochondria in neurons in two representative specific brain regions: pontine nuclei (a) and thalamus (b). Right, line-scan analysis along the solid white lines drawn in the merged images on the left. Each scale bar = 20 μm. (A color version of this figure is available in the online journal.)
Figure 6.
Colocalization between cytoplasmic phosphorylated TDP-43 (pTDP-43) and mitochondria. (a and b) Confocal microscopic images demonstrated colocalization between cytoplasmic pTDP-43 accumulation and mitochondria in neurons in two representative specific brain regions: pontine nuclei (a) and thalamus (b). Right, line-scan analysis along the solid white lines drawn in the merged images on the left. Each scale bar = 20 μm. (A color version of this figure is available in the online journal.)
By examining double fluorescence staining of the whole brain sagittal sections on low magnification of all mice in both young and aged groups, expression of mitochondrial oxidative phosphorylation protein was demonstrated in the same regions as the ones with the unique expression pattern of TDP-43 and pTDP-43 (data not shown): the pontine nuclei, thalamus, CA3 region of the hippocampus, and orbital cortex. There was no difference in staining patterns between young and aged mice. Similar to the findings seen on confocal microscopic images, colocalization between TDP-43 or pTDP-43 and mitochondria in the cytoplasm was also demonstrated with higher magnification of the double fluorescence images of the whole brain sagittal sections in these brain regions of all mice in both young and aged groups (pTDP-43 and mitochondria colocalization in a representative young mouse shown in Supplemental Figure 3).
Discussion
Our study demonstrated, in both normal aged and young mice, the unique distribution pattern of neuronal TDP-43 and pTDP-43 in some specific brain regions including pontine nuclei, thalamus, CA3 region of hippocampus, and orbital cortex. In addition, nuclear depletion and cytoplasmic accumulation with formation of the inclusion, along with mitochondrial colocalization of TDP-43, were demonstrated in these brain regions. The findings were consistent and identical among all aged and young mice. Given that our mice were healthy without other central nervous system or systemic pathologies, the results from our study represented normal findings in healthy states, not pathological changes.
One great advantage of our study was the technical privilege we had by obtaining high-resolution images from an automated microscope, Celldiscoverer 7, which is more technologically advanced than regular slide scanners. This allowed for examination of the entire sagittal brain sections and allowed us to investigate differential staining patterns (e.g. of TDP-43) among different brain regions without sampling bias. Selective sampling of only some brain regions already known to be vulnerable in neurodegenerative disorders for microscopic examination, as commonly performed in several prior studies in the literature, can lead to bias when investigating which brain regions are affected in normal aging. By using this latter method of study, the positive findings are likely to be in the regions previously described in neurodegenerative disorders. Examination of the fluorescence signal in the entire brain section, as in our study, provides a bird’s-eye view of the distribution of TDP-43 abnormalities in an unbiased way. Furthermore, high magnification for histological details in individual brain regions with increased TDP-43 expression could also be performed in images captured by the Celldiscoverer 7. This allowed for demonstration of the identical findings with comparable quality to what was seen in confocal microscopic images in one mouse, and obviated the need to pursue more time-consuming confocal microscopic studies in other mice.
Intriguingly, the unique pattern of neuronal TDP-43 distribution in some specific brain regions such as the pontine nuclei or thalamus was an unexpected finding in our study, which has never been reported previously in literature. It remains unclear, however, why the unique pattern of TDP-43 distribution was present in these brainstem and diencephalic structures, as well as the CA3 region of the hippocampus and orbital cortex. We speculate that altered TDP-43 distribution may be implicated in normal physiological functions attributed to these anatomical structures. For example, altered TDP-43 distribution found within pontine nuclei may imply its possible role in motor control of facial and bulbar musculature including speech and swallowing functions. Furthermore, thalamic and hippocampal involvement may be attributed to motor slowing, slow reaction time, and memory which can often be seen in normal aging.22,23 However, identical findings seen in normal young mice may argue against the latter speculation.
While spreading and progression patterns of α-synuclein pathology in PD or neurofibrillary tangles in AD have been well recognized, little is known about spreading pattern of TDP-43. In Parkinson’s disease, it has been proposed that the α-synuclein pathology initiates from the dorsal motor nucleus of the vagus nerve in the lower brainstem and subsequently spreads upward to the substantia nigra in the midbrain and widespread cortical regions.24,25 In AD, neurofibrillary tangle pathology is known to begin in the transentorhinal region,26,27 whereas Alzheimer’s disease-related TDP-43 pathology has been reported to initiate in the amygdala.28 In our study, the CA3 region of the hippocampus was found to have the unique pattern of TDP-43 distribution with nuclear depletion and cytoplasmic accumulation in normal aging and young mice. It is possible that TDP-43 pathologies in diseased states may begin in these deep brain regions with higher altered TDP-43 distribution where it is already present in healthy states. Some not-yet-known additional triggers may initiate transition of TDP-43 from its physiologic to pathological states, and subsequently spread to more superficial regions, including cerebral cortices.
Further studies using techniques similar to those provided here could be used to examine the entirely mounted sagittal and coronal brain sections, which could provide insights into the transitional processes of TDP-43 alterations between health and diseased states, as well as the spreading patterns of TDP-43 pathologies in different stages of various neurodegenerative disorders. This technique can be of particular importance in studying TDP-43 distribution and spreading patterns in the brain, as opposed to other proteins such as amyloid or tau where advanced neuroimaging techniques are available to allow in vivo tracking of these proteins.
The nuclear depletion and cytoplasmic accumulation of TDP-43 have been described, and are considered to be one of the most characteristic neuropathological features in FTLD-TDP and ALS,1–3 although not in familial ALS with SOD1 mutations or SOD1-knockin mice.3,29 While extensively studied in various neurodegenerative disease models, histological characterization of TDP-43 in normal aging exists in only few studies.11–15 Our findings of nuclear depletion and cytoplasmic accumulation of TDP-43 in both aged and young mice without any difference, identical to what has been described in neurodegenerative diseases such as FTLD and ALS, provide crucial insights regarding the role of TDP-43 in the pathophysiology of these disorders. Due to the lack of difference between aged and young mice, we speculate that aging may not be a critical factor for TDP-43 pathologies in neurodegenerative diseases such as FTLP-TDP or ALS, and that TDP-43 alterations may be downstream or consequences of neuronal death. Our findings may argue against findings from prior studies where TDP-43 alterations were shown to be a required component in neuronal loss.5,30 Further studies are required to prove the interplay between TDP-43 and neuronal loss.
Colocalization between TDP-43 and mitochondria, although recently described in diseased states,17,31–35 has not been reported in normal young and aged mice or humans. Our study confirms the interaction between TDP-43 and mitochondria, but also provides an important insight regarding the role of mitochondrial localization of TDP-43 in normal healthy states that can imply its normal physiological function. TDP-43 may have normal physiological control of mitochondrial dynamics, including fusion and fission in normal or healthy states, which become impaired in diseased states such as in neurodegenerative disorders.16,36 Consistent with our previous study showing highly phosphorylated TDP-43 within mitochondria,17 pTDP-43 demonstrated higher colocalization with mitochondria than total TDP-43 even though they showed similar brain regional and cellular distributions. TDP-43 pre-inclusions are highly phosphorylated in diseases.37,38 And, TDP-43 phosphorylation has been implicated to increase its oligomerization and fibrillization.6 It is possible that TDP-43 phosphorylation may contribute to its cytoplasmic accumulation under normal physiological conditions. Noteworthily, mitochondria-associated TDP-43 has been reported largely soluble.17 TDP-43 phosphorylation may also be directly involved in its mitochondrial localization to regulate mitochondrial function, worthy further detailed investigation. Mitochondrial structure cannot be well preserved in paraformaldehyde-fixed paraffin-embedded brain tissues. And, it is technically challenging to stain mitochondria in brain neurons by the immunohistochemical approach. On top of these, we were not able to obtain more aged animals to perform biochemical analysis of mitochondria-associated TDP-43 using freshly collected tissues. Therefore, the mitochondrial staining using paraffin-embedded tissues is one limitation of this current study. Nevertheless, we believe that our immunostaining using mitochondrial markers was specific and of sufficient quality to quantify the colocalization between TDP-43 and mitochondria. This study used only male mice since female mice may have estrous cycle-dependent unexpected variations. And, the total mice used for old (older than 26-month-old) and young (younger than 12-month-old) were n = 6 for each group. Although the statistical evaluation of immunohistochemical analysis was possible with these numbers, further investigation with increased sample number including female animals will be necessary to obtain more reliable conclusion.
In summary, our studies demonstrating the unique pattern of altered TDP-43 distribution in selective brain regions, that includes nuclear depletion, cytoplasmic accumulation of TDP-43, as well as colocalization between TDP-43 and mitochondria in healthy mice without any difference between young and old age groups provide a crucial insight for the role of TDP-43 in health and diseases. What triggers TDP-43 alterations in neurodegenerative disorders and the more specific interplay between TDP-43 and neuronal loss, as well as the implications in healthy and diseased states of differentially higher expression of TDP-43 in selective brain regions remain to be further elucidated. It is also of interest to study other species such as humans especially the oldest old to expand these findings.
Supplemental Material
Supplemental material, EBM914253 Supplemental Material for Cytoplasmic mislocalization and mitochondrial colocalization of TDP-43 are common features between normal aged and young mice by Pichet Termsarasab, Thananan Thammongkolchai, Ju Gao, Luwen Wang, Jingjing Liang and Xinglong Wang in Experimental Biology and Medicine
Authors’ contributions
XW conceived and directed the project. PT, TT, and XW wrote the manuscript. PT, TT, JG, LW, and JL contributed to experiments, data analysis, and manuscript preparation.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the US National Institutes of Health (1R01NS089604 to X.W.) and the US Alzheimer’s Association (AARG-17–499682 to X.W.).
ORCID iD
Xinglong Wang https://orcid.org/0000-0001-8657-0159
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006; 351:602–11 [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 2006; 112:539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 2007; 61:427–34 [DOI] [PubMed] [Google Scholar]
- 4.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 2007; 61:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci 2010; 30:639–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 2008; 64:60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fratiglioni L. Epidemiology of Alzheimer’s disease and current possibilities for prevention. Acta Neurol Scand 1996; 165:33–40 [DOI] [PubMed] [Google Scholar]
- 8.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol 2002; 156:445–53 [DOI] [PubMed] [Google Scholar]
- 9.Hindle JV. Ageing, neurodegeneration and Parkinson’s disease. Age Age 2010; 39:156–61 [DOI] [PubMed] [Google Scholar]
- 10.Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered proteins in the aging brain. J Neuropathol Exp Neurol 2016; 75:316–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer’s disease – a review. Int J Clin Exp Pathol 2011; 4:147–55 [PMC free article] [PubMed] [Google Scholar]
- 12.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 2010; 67:1238–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cykowski MD, Takei H, Van Eldik LJ, Schmitt FA, Jicha GA, Powell SZ, Nelson PT. Hippocampal sclerosis but not normal aging or Alzheimer disease is associated with TDP-43 pathology in the basal forebrain of aged persons. J Neuropathol Exp Neurol 2016; 75:397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, Saito Y, Arai T, Nishiyama K, Murayama S. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun 2015; 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 2013; 126:51–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Li L, Lin WL, Dickson DW, Petrucelli L, Zhang T, Wang X. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum Mol Genet 2013; 22:4706–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Wang L, Lu J, Siedlak SL, Fujioka H, Liang J, Jiang S, Ma X, Jiang Z, da Rocha EL, Sheng M, Choi H, Lerou PH, Li H, Wang X. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med 2016; 22:869–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ 2004; 2004:pe26. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol 2000; 59:880–8 [DOI] [PubMed] [Google Scholar]
- 20.Gao J, Wang L, Gao C, Arakawa H, Perry G, Wang X. TDP-43 inhibitory peptide alleviates neurodegeneration and memory loss in an APP transgenic mouse model for Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 2020; 1866:165580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntley ML, Gao J, Termsarasab P, Wang LW, Zeng S, Thammongkolchai T, Liu Y, Cohen ML, Wang XL. Association between TDP-43 and mitochondria in inclusion body myositis. Lab Invest 2019; 99:1041–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 2010; 34:721–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstone A, Mayhew SD, Hale JR, Wilson RS, Bagshaw AP. Thalamic functional connectivity and its association with behavioral performance in older age. Brain Behav 2018; 8:e00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braak H, Del Tredici K. Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis 2017; 7:S73–S87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braak H, Del Tredici K, Rub U, de Vos RAI, Steur E, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003; 24:197–211 [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995; 16:271–8; discussion 78–84 [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006; 112:389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 2014; 127:441–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner BJ, Baumer D, Parkinson NJ, Scaber J, Ansorge O, Talbot K. TDP-43 expression in mouse models of amyotrophic lateral sclerosis and spinal muscular atrophy. BMC Neurosci 2008; 9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousef A, Robinson JL, Irwin DJ, Byrne MD, Kwong LK, Lee EB, Xu Y, Xie SX, Rennert L, Suh E, Van Deerlin VM, Grossman M, Lee VM, Trojanowski JQ. Neuron loss and degeneration in the progression of TDP-43 in frontotemporal lobar degeneration. Acta Neuropathol Commun 2017; 5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Arakawa H, Wang L, Okolo O, Siedlak SL, Jiang Y, Gao J, Xie F, Petersen RB, Wang X. Motor-coordinative and cognitive dysfunction caused by mutant TDP-43 could be reversed by inhibiting its mitochondrial localization. Mol Ther 2017; 25:127–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izumikawa K, Nobe Y, Yoshikawa H, Ishikawa H, Miura Y, Nakayama H, Nonaka T, Hasegawa M, Egawa N, Inoue H, Nishikawa K, Yamano K, Simpson RJ, Taoka M, Yamauchi Y, Isobe T, Takahashi N. TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci Rep 2017; 7:7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan L, Zhou C, Jin E, Kucharavy A, Zhang Y, Wen Z, Florens L, Li R. Cytosolic proteostasis through importing of misfolded proteins into mitochondria. Nature 2017; 543:443–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo JA, Liu T, Trotter C, Fang CC, De Narvaez E, LePochat P, Maslar D, Bukhari A, Zhao X, Deonarine A, Westerheide SD, Kang DE. Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic integrity. Nat Commun 2017; 8:15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvatori I, Ferri A, Scaricamazza S, Giovannelli I, Serrano A, Rossi S, D’Ambrosi N, Cozzolino M, Giulio AD, Moreno S, Valle C, Carri MT. Differential toxicity of TAR DNA-binding protein 43 isoforms depends on their submitochondrial localization in neuronal cells. J Neurochem 2018; 146:585–97 [DOI] [PubMed] [Google Scholar]
- 36.Jiang Z, Wang W, Perry G, Zhu X, Wang X. Mitochondrial dynamic abnormalities in amyotrophic lateral sclerosis. Transl Neurodegener 2015; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mori F, Tanji K, Zhang HX, Nishihira Y, Tan CF, Takahashi H, Wakabayashi K. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol 2008; 116:193–203 [DOI] [PubMed] [Google Scholar]
- 38.Brandmeir NJ, Geser F, Kwong LK, Zimmerman E, Qian J, Lee VM, Trojanowski JQ. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol 2008; 115:123–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM914253 Supplemental Material for Cytoplasmic mislocalization and mitochondrial colocalization of TDP-43 are common features between normal aged and young mice by Pichet Termsarasab, Thananan Thammongkolchai, Ju Gao, Luwen Wang, Jingjing Liang and Xinglong Wang in Experimental Biology and Medicine