Abstract
Cellular senescence has evolved as a protective mechanism to arrest growth of cells with oncogenic potential but is accompanied by the often pathologically deleterious senescence-associated secretory phenotype (SASP). Here we demonstrate an H2O2-dependent functional disruption controlling senescence-associated Ca2+ homeostasis and the SASP. Senescent cells fail to respond to H2O2-dependent plasma lamellar Ca2+ entry when compared to pre-senescent cells. Limiting exposure to senescence-associated H2O2 restores H2O2-dependent Ca2+ entry as well as transient receptor potential cation channel subfamily C member 6 (TRPC6) function. SA-TRPC6 and SASP expression is blocked by restoring Ca2+ entry with the TRP channel antagonist SKF-96365 or by the mTOR inhibitors rapamycin and Ku0063794. Together, our findings provide compelling evidence that redox and mTOR-mediated regulation of Ca2+ entry through TRPC6 modulates SASP gene expression and approaches which preserve normal Ca2+ homeostasis may prove useful in disrupting SASP activity.
Impact statement
Through its ability to evoke responses from cells in a paracrine fashion, the senescence-associated secretory phenotype (SASP) has been linked to numerous age-associated disease pathologies including tumor invasion, cardiovascular dysfunction, neuroinflammation, osteoarthritis, and renal disease. Strategies which limit the amplitude and duration of SASP serve to delay age-related degenerative decline. Here we demonstrate that the SASP regulation is linked to shifts in intracellular Ca2+ homeostasis and strategies which rescue redox-dependent calcium entry including enzymatic H2O2 scavenging, TRP modulation, or mTOR inhibition block SASP and TRPC6 gene expression. As Ca2+ is indispensable for secretion from both secretory and non-secretory cells, it is exciting to speculate that the expression of plasma lamellar TRP channels critical for the maintenance of intracellular Ca2+ homeostasis may be coordinately regulated with the SASP.
Keywords: Senescence, SASP, calcium, TRPC6, mTOR, hydrogen peroxide
Introduction
Cellular senescence was first described by Hayflick and Moorhead1 in 1961, showing human cells in culture have a finite proliferative capacity, which describe the feature of “senescence” at the cellular level. Cellular senescence is a process of irreversible cell cycle arrest that can be induced by multiple factors, including telomere shortening, oxidative stress, and oncogene activation.2–7 Cellular senescence, a tumor constraining mechanism characterized by irreversible growth arrest, participates in embryonic development, tissue repair, and wound healing.8–12 Cellular senescence is implicated as causal in many age-associated pathologies as a result of the often detrimental senescence-associated secretory phenotype (SASP). The SASP involves secretion of soluble factors (interleukins, chemokines, and growth factors), degradative matrix metalloproteases (MMPs), and insoluble extracellular matrix (ECM) components which alter the tissue microenvironment affecting cell behavior in a paracrine fashion.13 The free radical theory of aging posits that accumulation of macromolecular damage occurs after a lifetime of exposure to oxidants.4 Though often refuted, several tenets of this theory stand true including the two critical traits focusing on species-specific low mitochondrial ROS generation rates at complex I of the electron transport chain (ETC) and lowered levels of fatty acid unsaturation on cellular and mitochondrial membranes in long-lived animals.14 The mechanistic control of senescence and SASP factors by reactive oxygen species (ROS) has also garnered significant focus.15
Calcium (Ca2+) is a ubiquitous intracellular messenger that regulates an extensive repertoire of cellular processes, ranging from contraction, secretion, fertilization, and development, to the control of transcription, proliferation, as well as learning and memory.16 The Ca2+ concentration in cells is sustained by concurrently acting “on” and “off” mechanisms that serve to raise or lower cytosolic Ca2+ levels. Transient receptor potential (TRP) channels comprise a functionally diverse family of 28 channel proteins that mediate the transmembrane flux of cations down their electrochemical gradients, resulting in increased intracellular Ca2+ and Na+ concentrations and cellular depolarization, with cation selectivity depending on pore structure and channel regulation. TRP channels act as cellular biosensors for environmental stimuli and are also responsive to changes in temperature and conditions inside the cell. Certain TRP channels are also being increasingly incriminated as crucial players in detecting cellular redox status with many channels emerging as targets of ROS-mediated regulation either directly through oxidative amino acid modifications or indirectly through second messengers.17 The “calcium overload” hypothesis in aging is supported by several studies that have demonstrated that intracellular Ca2+ levels are typically elevated in aged cells as compared to young cells due to oxidative stress, inefficient Ca2+ handling systems, and loss of Ca2+ signaling homeostasis.18 The link between calcium signaling and senescence has been extensively reviewed.19 Senescence-inducing stresses, including telomere shortening, oncogene activation, and oxidative stress, can trigger activation of phospholipase C (PLC), which cleave PIP2 into DAG and IP3. IP3 then binds to IP3-gated calcium channel, which releases calcium from endoplasmic reticulum (ER), resulting rapid elevation of cytosolic calcium concentration.20–23 In addition to IP3 channel, silencing TRPM7 and TRPM8 can induce replicative senescence in pancreatic adenocarcinoma cells suggesting that these channels may be important for preventing oncogene-induced senescence, and promoting pancreatic tumorigenesis.24,25 The Ca2+ overload can also enhance the deleterious effects of SASP via the activation of calpain and the subsequent processing and functionality of interleukin-1 alpha (IL-1α).26
The mammalian target of rapamycin (mTOR) complexes mTORC1 and mTORC2 play essential roles in regulating growth regulation, nutrient signaling, cytoskeletal organization, cell survival, and aging. mTOR signals also modulate senescence.27 As mitochondria are the primary source of ROS production, it is not surprising that majority of oxidative stress induced senescent-associated changes are mitochondria dependent.28 Mitochondria depleted cells display reduced senescence-associated (SA)-β-gal activity, formation of SA-heterochromatin foci, expression of p16 and p21, and ROS generation.29 Mitochondrial dysfunction can lead to increased mitochondrial ROS, which trigger mTORC1 pathway, resulting in p53-mediated senescence activation.30 In addition, current insight suggests that mTOR modulates SASP by regulating the translation of IL-1α and mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2). IL-1α promotes NF-κB in a cell-autonomous manner to facilitate the transcription of SASP genes. Additionally, MAPKAPK2 phosphorylates and inhibits the RNA-binding protein ZFP36L1, thereby stabilizing SASP gene targets containing AREs (AU-rich elements).31 Shifts in intracellular Ca2+ also play a part in mTOR signaling;32 conversely, mTOR contributes to age-associated shifts in Ca2+ mobilization.33
Our findings illustrate that dysregulated Ca2+ signaling during senescence is affected by desensitization of redox-dependent Ca2+ channels. We found that enzymatic H2O2 removal, treatment with pharmacological Ca2+-modulating agents, or mTOR inhibition can rescue defects in senescence-associated (SA) Ca2+ mobilization and limit SASP. Overall, our studies provide insights into the contributions of Ca2+ in senescence regulation as well as the potential to identify new therapeutic targets limiting SASP and potentially age-associated disease progression.
Materials and methods
Cell culture
Primary human fetal lung IMR-90 fibroblasts were cultured in MEM (Corning Cellgro) with 10% FBS (Biowest) and incubated at 21% or 3% oxygen tension at 37°C with 5% CO2. The cells were lifted in 0.25% Trypsin-2.21 mM EDTA 1X (Corning) and serially passaged 1:4 for pre-senescent passages less than 20 and at 1:2 for passages beyond that. Cells <p15 were considered pre-senescent (young) and >p25 were treated as senescent (old). >p25 cells were confirmed to be senescent by measuring p16 expression and the presence of heterochromatin foci and senescence-associated (SA) β-galactosidase staining (Fig. S3).
SA-β-galactosidase staining
SA-β-galactosidase staining was performed as per manufacturer’s protocol from senescence-galactosidase staining kit (Cell Signaling Technology). Briefly, cells were seeded in 6-well tissue culture plates overnight. On the day of assay, cells were washed once with PBS, and fixed with 1× fixative solution (1 mL per well) at room temperature for 15 min. The plate was then washed twice with PBS and stained with 1 mL of β-gal staining solution for overnight at 37°C in a dry non-CO2 incubator. Images were in nine random fields per condition using light microscopy.
Heterochromatin foci staining
Cells were seeded on coverslips in 6-well tissue culture plates overnight. On the day of staining, cells were washed once with PBS and fixed with cold 4% formaldehyde solution for 15 min at room temperature. The cells were then washed once with cold PBS and mounted on glass slides with Prolong™ Gold Antifade Mount containing DAPI (Thermo Fisher Scientific). There slides were left to dry for overnight in the dark and sealed with clear nail polish. There images were taken using florescent microscope.
Microplate intracellular calcium imaging
Intracellular calcium levels were determined using ratiometric analysis of the calcium binding dye Fura-2-AM with the Molecular Devices FlexStation 3 Multi-mode Microplate Reader. This cell-permeable dye excites at 340 nm when bound to calcium and 380 nm when unbound. Fluorescence intensities were then measured at the emission wavelength of 510 nm. These intensities were represented as a ratio of 340/380 nm.
Cells were seeded on 96-well plates 24 h before performing assay. At the time of assay, media was removed, and cells were incubated with 4 μM Fura-2-AM for 30 min at 37°C. Cells were then washed with 1× HBSS + dextrose three times. Cell culture plate was mounted in a FlexStation plate reader and the treatments were added at specific time points using the Flex mode and fluorescence measurements were obtained.
Microscopic intracellular calcium imaging
Cells were seeded on 6-well plates with cover slips. Cells were incubated with 4 μM Fura-2-AM for 30 min at 37°C in a chamber, washed with 1× HBSS+dextrose without Ca2+ three times, and mounted on the microscope. Fluorescence measurements were obtained at 10 readings per minute and analyzed using a digital fluorescence imaging system (InCyt Im2; Intracellular Imaging, Cincinnati, OH). At indicated times, solutions on coverslip were exchanged.
Quantitative real-time PCR
RNA was isolated from samples using Trizol (Invitrogen) per manufacturer’s instructions. cDNA was synthesized using Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Scientific) with oligo (dT) primers. Real-Time PCR was performed with an Applied Biosystems (AB) 7500 Real-Time Thermocycler with AB SYBR Green. Specific primers were used for each target and the PCR conditions were as follows: 95°C for 10 min, followed by 40 cycles of melting at 95°C for 15 sec, annealing and elongation at 60°C for 1 min. Product specificity was determined by melt curve CT values were normalized to a control housekeeping gene, Actin, and the fold change was found using the ΔΔCT method of analysis. See Table 1 for primer sequences used in the study.
Table 1.
Primer sequences used in the study.
| Species | Target gene |
Specific Primer |
|
|---|---|---|---|
| Sense 5ʹ-3ʹ | Anti-sense 5’-3’ | ||
| Human | β-Actin | AAAGACCTGTACGCCAACACAGTGCTGTCT | CGTCATACTCCTGCTTGCTGATCCACATCTG |
| IL-1α | AACCAGTGCTGCTGAAGGA | TTCTTAGTGCCGTGAGTTTCC | |
| IL-8 | CTCTCTTGGCAGCCTTCCTGATT | AACTTCTCCACAACCCTCTGCAC | |
| Il-6 | CCACACAGACAGCCACTCACC | CTACATTTGCCGAAGAGCCCTC | |
| CDKN2A (p16) | CTTCGGCTGACTGGCTGG | TCATCATGACCTGGATCGGC | |
| CDKN1A (p21) | AGTCAGTTCCTTGTGGAGCC | GACATGGCGCCTCCTCTG | |
| TRPC6 | CTTGTGGTCCTTGCTGTTGC | TCTTCCCCATCTTGCTGCAT | |
Western blot analysis
Cells were lysed in an appropriate volume of RIPA buffer at pH 7.5 (50 mM Tris-HCl, 150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS, 0.1 mM Na3VO4, and protease inhibitors) for 15 min. Cell debris was pelleted by centrifugation at 15,000 r/min at 4°C and the protein-containing supernatant was analyzed for protein concentration using BCA reagents A and B (Thermo Scientific). Equal amounts of protein were then subjected to polyacrylamide gel electrophoresis and subsequently transferred to nitrocellulose membranes followed by overnight incubation with primary antibodies at 4°C. The following day, the membranes were incubated with suitable secondary antibodies at room temperature and the blots were visualized using chemiluminescence.
Statistical analysis
All statistical analyses were performed with the GraphPad Prism 7.0 statistical software package. Statistical significance was assessed using the two-tailed Student’s t test or one-way ANOVA with Tukey–Kramer post-test. P values of <0.05 were considered significant.
Results
Senescence is accompanied by disruption of intracellular Ca2+ homeostasis
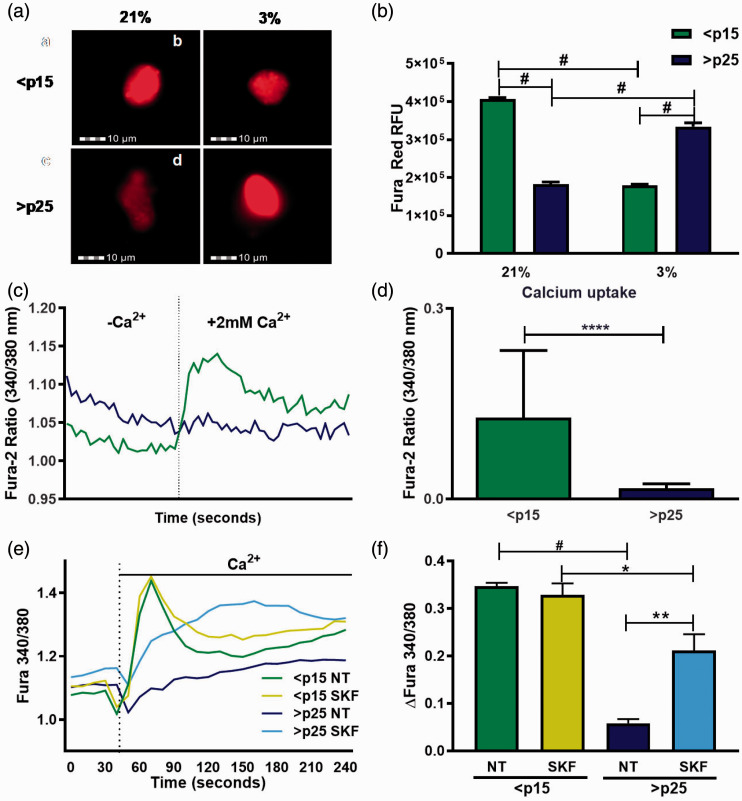
To define basal levels of intracellular Ca2+ in pre-senescent and senescent fibroblasts, we used the fluorescent dye Fura Red/AM whose fluorescence is impeded in the presence of Ca2+.34 Fura Red intensity profiles, monitored by imaging flow cytometry, revealed that baseline intracellular Ca2+ is higher in senescent cells, as observed by reduced fluorescence (Figure 1(a) and (b)) which was reversed in response to low oxygen (3%) (Figure 1(a) and 1(b)). To assess the response of IMR-90 cells to an exogenous stimulus of Ca2+, the relative concentration of intracellular Ca2+ was determined using the ratiometric Ca2+ binding dye Fura-2. While baseline Ca2+ levels remained higher in senescent fibroblasts they failed to respond to addition of extracellular Ca2+ (2 mM) in the bath solution when compared to pre-senescent cells (Figure 1(c) and (d)). We next sought to pharmacologically identify the senescence regulated Ca2+ channels with 2-APB and SKF-96365. 2-APB is a broad-spectrum compound that both inhibits and activates STIM/Orai-mediated store-operated Ca2+ entry (SOCE) channels depending on dose.35 SKF-96365, though a SOCE inhibitor, also modulates Ca2+ entry through certain TRPC channels.36 Pre-treatment with SKF had no effect on extracellular Ca2+-mediated Ca2+ influx in pre-senescent cells while eliciting a sustained Ca2+ response in senescent cells (Figure 1(e) and (f)). Pretreatment with 2-APB enhanced Ca2+ entry into both pre-senescent and senescent cells, with the pre-senescent cells showing an increased sensitivity to this Ca2+ channel modulator (Figure S1). We next performed patch-clamp electrophysiology to determine if SA increases in cytosolic Ca2+ are mediated through depletion of SOCE. Addition of BAPTA in the patch pipette allows for monitoring the biophysical manifestation of SOCE in the form of ICRAC (Ca2+ Release-Activated Ca2+ current).37 BAPTA failed to activate ICRAC in both young and old cell types, while H2O2 activated a small inwardly rectifying ICRAC-like current in both cell types with similar magnitudes. This finding suggests that differences in SOCE do not explain the distinct Ca2+ entry profiles between pre-senescent and senescent cells (Figure S2).
Figure 1.
Senescence is accompanied by dysregulation of intracellular Ca2+ signaling mechanisms. (a) Pre-senescent (<p15) and senescent (>p25) levels of intracellular Ca2+ as measured using the inverse Ca2+ fluorescent dye Fura Red as monitored by imaging flow cytometer under ambient (21%) and low (3%) oxygen conditions. 5000 individual cells (events) were imaged. (b) Quantification of mean Fura Red relative fluorescence intensity representing the RFU ± SEM of multiple independent experiments, n = 5000 events. (c) Representative ratiometric Fura-2 Ca2+ imaging trace of pre-senescent (<p15) and senescent (>p25) fibroblasts treated with an exogenous source of Ca2+ (2 mM). At time 40 s bath media was changed to include 2 mM Ca2+. (d) Total change in intracellular Ca2+ after addition of 2 mM Ca2+ stimulus represented as ΔFura 340/380 ± SEM, n = 3. (e) Ratiometric Ca2+ imaging of pre-senescent and senescent fibroblasts untreated or treated with 10 μM SKF-96365 15 min before imaging. At time 40 s media was changed to include 2 mM Ca2+. (f) Total change in intracellular Ca2+ after addition of 2 mM Ca2+ stimulus represented as ΔFura 340/380 ± SEM, n = 3. *P ≤0.05, **P <0.01, ***P <0.001, #P < 0.0001. (A color version of this figure is available in the online journal.)
SA dysregulation of Ca2+ is redox-regulated
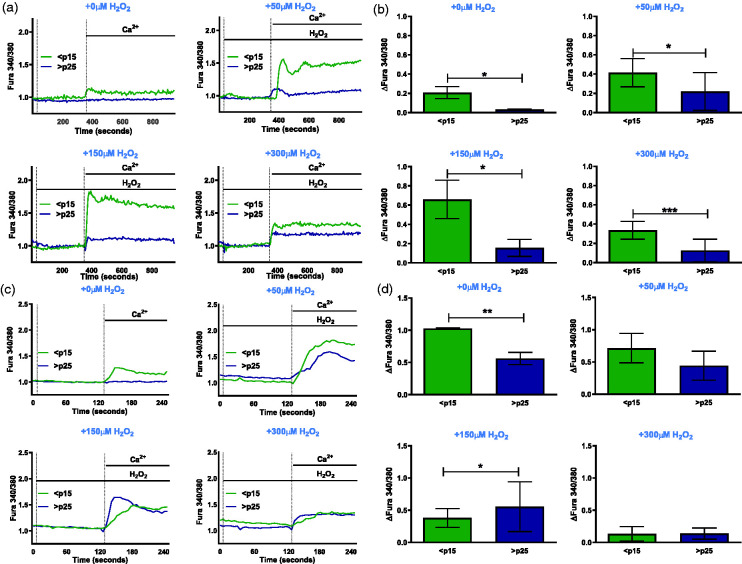
Several redox sensitive Ca2+ channels have been shown to mediate Ca2+ influx.18,38 To demonstrate the redox sensitivity of the Ca2+ channel(s) under study, we used the ratiometric dye Fura-2 to determine whether cells respond to a secondary exogenous H2O2 stimulus, that has been known to elicit a Ca2+ response, in addition to extracellular Ca2+. Exposure to increasing concentration of H2O2 (50, 150, and 300 µM) did not stimulate Ca2+ entry in the absence of extracellular Ca2+ regardless of age (Figure 2(a) and (b)). Upon addition of extracellular Ca2+, pre-senescent cells responded with a robust increase in Ca2+ entry that was severely attenuated in senescent cells.
Figure 2.
Senescence-associated disruption of Ca2+ homeostasis is H2O2-regulated. (a) Ratiometric Ca2+ imaging of pre-senescent (<p15) and senescent (>p25) fibroblasts with 2 mM Ca2+ added at time 330 s and stimulated with increasing H2O2 concentrations of 0 μM, 50 μM, 150 μM, and 300 μM. (b) Quantification of total change in intracellular Ca2+ after addition of 2 mM Ca2+ stimulus with varying H2O2 concentrations represented as ΔFura 340/380 ± SEM, n = 3. (c) Ratiometric Ca2+ imaging of pre-senescent (<p15) and senescent fibroblasts pre-incubated with 200 U/mL recombinant catalase overnight with 2 mM Ca2+ added at time 330 s and stimulated with increasing H2O2 concentrations of 0 μM, 50 μM, 150 μM, and 300 μM. (d) Quantification of total change in intracellular Ca2+ after addition of 2 mM Ca2+ stimulus with varying H2O2 concentrations represented as ΔFura 340/380 as shown in Figure 1. (A color version of this figure is available in the online journal.)
We have previously demonstrated that senescent cells increase steady-state H2O2 levels 3.5-fold from 13.7 to 48.6 pM relative to pre-senescent cells.39 We next determined if the impairment in SA H2O2-mediated Ca2+ entry is attributed to chronic exposure to elevated H2O2. Pre-senescent and senescent cells were treated with 200 U/mL recombinant catalase overnight and the above-mentioned Ca2+ traces were repeated. Catalase pre-incubation rescued the inability of the senescent cells to respond to H2O2-induced entry of Ca2+ (Figure 2(c) and (d)). Together, these results indicate that SA disruption of Ca2+ homeostasis is driven by a H2O2 sensitive regulatory switch.
TRPC6 is overexpressed in senescence but not functional
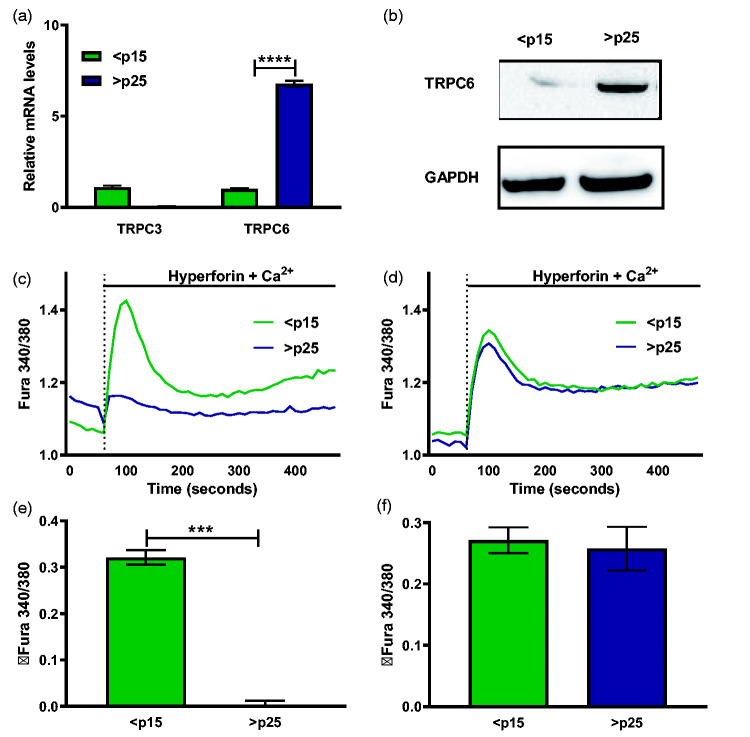
TRP Canonical 6 (TRPC6) stands out as a potential redox-regulated Ca2+ channel40 that has pathophysiological implications in age-associated disorders.41 TRPC3, 6, and 7 channels comprise a subgroup of Ca2+-permeable cation channels within the TRP superfamily and are activated by products of phospholipase C-mediated breakdown of phosphatidylinositol-4,5-bisphosphate (PIP2).42 Relative to pre-senescent cells, senescent fibroblasts display significant increase in both TRPC6 mRNA and protein (Figure 3(a) and (b)). Next, we tested the functionality of TRPC6 in senescent cells and the ability of the channel to respond to a specific agonist. Hyperforin, a polyprenylated acylphloroglucinol phytochemical, is the key active ingredient in clinically used anti-depressants and the compound selectively activates TRPC6 without stimulating other TRPC channel isoforms.43 We observed that senescent cells were insensitive to the action of 10 µM hyperforin (Figure 3(c) and (d)) added along with 2 mM Ca2+ as compared to pre-senescent cells. However, pre-incubation with 200 U/mL recombinant catalase recovers senescent TRPC6 responses to hyperforin-induced Ca2+ entry, further illustrating dysregulation of a redox-sensitive Ca2+ channel accompanying the senescent program.
Figure 3.
TRPC6 is overexpressed during senescence and its agonist unresponsiveness is restored by catalase pretreatment. Relative mRNA levels of TRPC3 and TRPC6 channels in pre-senescent (<p15) and senescent (>p25) cells. (b) TRPC6 and GAPDH protein abundance as measured from cell lysates of pre-senescent and senescent cells. (c) Ratiometric Ca2+ imaging of pre-senescent and senescent fibroblasts with 10 μM hyperforin and 2 mM Ca2+ added at time 60 s. (d) Quantification of total change in intracellular Ca2+ after addition of 10 μM hyperforin and 2 mM Ca2+ stimulus represented as ΔFura 340/380 ± SEM, n = 3. (e) Ratiometric Ca2+ imaging of pre-senescent and senescent fibroblasts pre-incubated with 200 U/mL recombinant catalase overnight with 10 μM hyperforin and 2 mM Ca2+ added at time 60 s (f) Quantification of total change in intracellular Ca2+ after addition of 10 μM hyperforin and 2 mM Ca2+ stimulus represented as shown in Figure 1. (A color version of this figure is available in the online journal.)
SKF reverses Ca2+ dysregulation and suppresses SASP
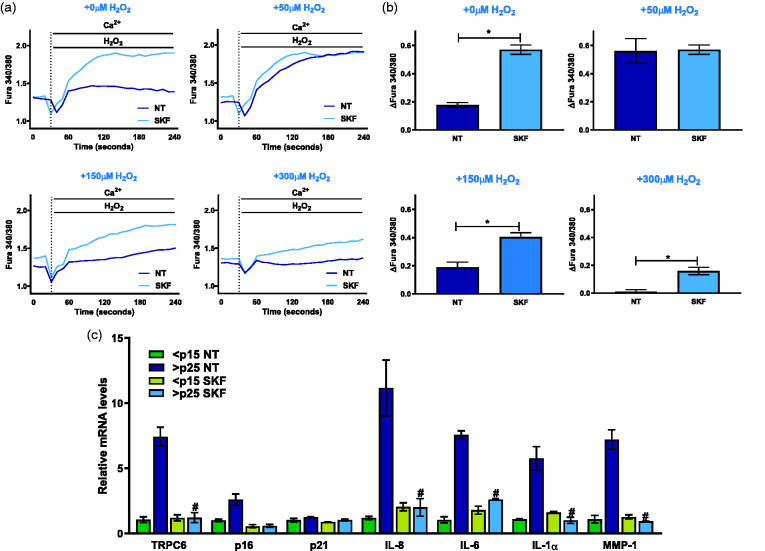
To test the role of the impaired Ca2+ signaling in senescence and SASP, we took advantage of the ability of SKF to restore SA Ca2+ entry (Figure 1(e)) and determine its impact on H2O2-mediated Ca2+ mobilization and on SASP gene expression. Upon pre-treatments with 10 µM SKF for 15 min, we found that senescent fibroblasts retrieved their ability to mobilize Ca2+ in response to H2O2 (Figure 4(a) and (b)). Comparable to the effects of catalase in rescuing the ability of senescent cells to increase cytosolic Ca2+ in response to H2O2 (Figure 2), SKF treatments restored Ca2+ mobilization capacity with different concentrations of H2O2 (50, 150, and 300 µM). Additionally, IMR-90 fibroblasts were treated with 10 µM SKF for 24 h to monitor TRPC6 mRNA levels (Figure 4(c)). We found that SKF inhibited SA TRPC6 expression as well as the mRNA levels of the cell cycle inhibitor p16 and key SASP factors IL-8, IL-6, IL-1α, and MMP-1 (Figure 4(c)). Taken together, these data indicate that restoring Ca2+ entry with SKF limits SASP.
Figure 4.
SKF reverses senescence Ca2+ dysregulation and suppresses SASP. (a) Ratiometric Ca2+ imaging of senescent fibroblasts with 2 mM Ca2+ added at time 40 s following 15 min treatment with or without 10 μM SKF-96365 for and stimulated with increasing H2O2 concentrations of 0 μM, 50 μM, 150 μM, and 300 μM. (b) Total change in intracellular Ca2+ after addition of 2 mM Ca2+ stimulus with varying H2O2 concentrations of 0 μM, 50 μM, 150 μM, and 300 μM as shown in Figure 1. (c) Relative mRNA levels of key senescence and SASP components in pre-senescent and senescent cells as measured by real-time PCR. Results expressed as mean ± SEM and statistically compared against >p25 NT, n = 3. *P ≤0.05, #P <0.0001. (A color version of this figure is available in the online journal.)
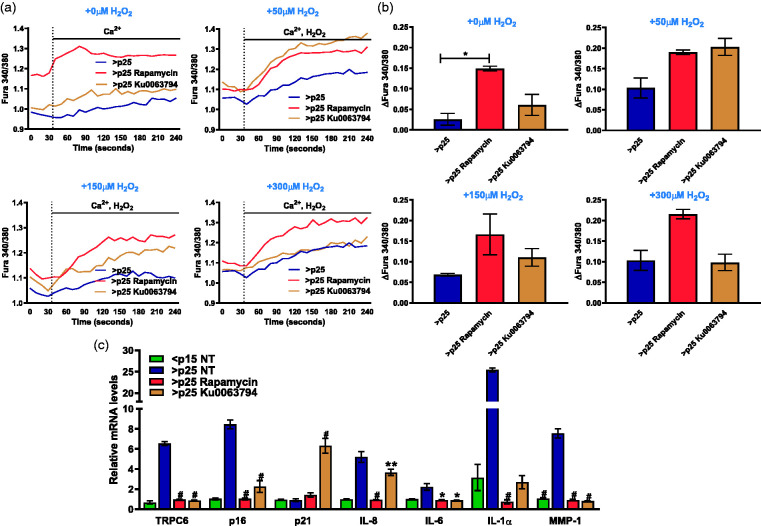
mTOR regulates TRPC6 and SASP
mTOR complex 2 (mTORC2) has been shown to be a regulator of TRPC6 channel expression and activity in podocytes44 and is thought to be essential for maintenance of the senescent state45 Our findings indicate that restoration of SA extracellular Ca2+ mobilization can limit SASP. We next determined whether mTOR inhibition which blocks SASP,46 modulates calcium homeostasis ether basally or in response to H2O2 treatment in senescent IMR-90 fibroblasts. Exposure of senescent cells to rapamycin, an inhibitor of mTORC1, or Ku0063794, a dual inhibitor of mTORC1 and mTORC2, for 24 h augmented the ability of senescent cells to mobilize cytosolic Ca2+ after stimulation with 2 mM Ca2+ (Figure 5(a) and (b)). Rapamycin and Ku0063794 also rescued the ability of the senescent cells to respond to increasing concentrations of H2O2 at 50, 150, and 300 µM (Figure 5(a) and (b)). We further monitored the impact of rapamycin and Ku0063794 on SASP transcript levels and observed reduction in TRPC6, p16, IL-8, IL-6, IL-1α, and MMP-1 (Figure 5(c)). Collectively, these data suggest a critical role for mTOR signaling in regulating SASP and TRPC6 transcripts levels as well as the SA disruption in Ca2+ homeostasis.
Figure 5.
mTOR enhances Ca2+ entry and limits replicative SASP and TRPC6 expression. Ratiometric Ca2+ imaging of senescent fibroblasts with 2 mM Ca2+ added at time 40 s previously untreated or treated with 25 nM rapamycin or 10 μM Ku0063794 and stimulated with increasing H2O2 concentrations of 0 μM, 50 μM, 150 μM, and 300 μM. (b) Total change in intracellular Ca2+ after addition of 2 mM Ca2+ stimulus with varying H2O2 concentrations of 0 μM, 50 μM, 150 μM, and 300 μM as shown in Figure 1. (c) Relative mRNA levels of key senescence and SASP components in untreated fibroblasts and senescent (>p25) fibroblasts pre-incubated with 25 nM rapamycin or 10 μM Ku0063794 for 24 has measured by real-time PCR. Results expressed as mean ± SEM and statistically compared against >p25 NT, n = 3. *P ≤0.05, **P <0.01, #P <0.0001. (A color version of this figure is available in the online journal.)
Discussion
Disturbances in intracellular calcium homeostasis have been well established in physiological senescence-associated processes including cell proliferation, autophagy, apoptosis, and necrosis.47,48 Ca2+ acts as an essential second messenger for cell-to-cell communication and for driving intracellular processes. The role of Ca2+ dysregulation in physiological and organ-specific aging processes has been delineated.26,49,50 Elevated intracellular calcium levels have been observed in response to several forms of senescence-inducing stress (telomere shortening, oncogene activation, or oxidative stress) and are orchestrated through plasma membrane Ca2+ channels or by Ca2+ release from the endoplasmic reticulum.19 For example, Retinoid X receptor alpha (RXRA) is also been shown to regulate Ca2+ signaling through inositol 1, 4, 5-triphosphate receptor type 2 (ITPR2), an endoplasmic reticulum calcium release channel, and mitochondrial calcium uniporter (MCU). Increased ROS production and DNA damage via ITPR2-MCU calcium signaling pathway, triggering senescence via p53 activation are observed in RXRA knocked down human lung fibroblasts, whereas RXRA overexpression can delay replicative senescence.51 Ca2+ entry through non-selective cation channel, transient receptor potential vanilloid 4 (TRPV4) can lead to regulatory volume decrease (RVD) to counteract the increase of cell volume, one of the prominent features of senescent cells. Cells lacking TRPV4 are not able to engage RVD.52 In addition to TRPV4, TRPM7 channel can also be activated by membrane swelling or osmosis swelling.53 TRPC7 is showed to involved in initiation of UVB-induced aging and TRPC7 knocked down can suppress the UVB-induced aging processes.54 Mouse vascular endothelial cells lacking TRPC5 display reduced β-galactosidase staining, implicating in the involvement of TRPC5 in development of senescence.55
Our findings support the possibility that the senescence program is in part controlled by disruptions in normal Ca2+ signaling as evidenced by: (i) alterations in TRPC channel expression; ii) redox- and mTOR-dependent disruption in Ca2+ homeostasis, and (iii) SASP inhibition through restoration of Ca2+ entry. Here, we establish that senescent fibroblasts display a basal increase in intracellular Ca2+ but fail to respond to exogenous Ca2+ addition. Several studies point to persistently elevated intracellular Ca2+ levels in aged cells as compared to young cells, primarily in neuronal cells.56–58 We propose that the observed increase in basal Ca2+ limits further cellular responses to increases in extracellular Ca2+. These and other findings have been taken to justify a prevalent “calcium overload” hypothesis postulating that Ca2+ overload in old cells arising from oxidative stress limits functionality of Ca2+ signaling.18 Increased Ca2+ load has been shown to promote IL-1α processing and functionality, initiating the SASP signaling cascade.26 Here we demonstrate that senescence is accompanied by a defect in extracellular Ca2+ mobilization, that is coupled to increases in TRPC6 and SASP gene expression.
The TRPC channels constitute a family of seven different members, TRPC1-7, of physiological and pathophysiological importance.59,60 TRPC6 expression has been described in selective age-related maladies including vasospastic disorders, hypertension, Alzheimer’s disease, and cardiac hypertrophy.41,61–63 H2O2 has been conclusively proven to activate TRP channels,17,38 including TRPC6, and may govern a novel mechanism for agonist-initiated cellular responses driven by the channel. We provide evidence that hyperforin responsiveness of TRPC6 is lost during senescence and restored by efficient enzymatic H2O2 removal by catalase. Catalase pretreatment also rescues SA loss of H2O2-Ca2+ entry. However, whether rescue of both hyperforin and H2O2-dependent Ca2+ entry occurs through TRPC6 remains to be established. The recovery of H2O2-induced Ca2+ influx by catalase illustrates a dual role for H2O2 in both activating and limiting TRPC6 function. The effect of H2O2 is likely reliant on abundance and duration of exposure to this ubiquitous second messenger. While ROS serves to activate TRPC6 acutely (minutes),64 prolonged exposure, as with the case in several oxidative stress-propagated chronic pathologies, can suppress TRPC6 channel expression.65 TRPC6 activity has been reported to be controlled by diverse modalities including diacylglycerol (DAG), the plant extract hyperforin and H2O2. H2O2 activates TRPC6 by modifying thiol groups promoting its trafficking to the cell surface, conferring DAG sensitivity and channel activation.40 We propose that the TRPC6 channel is overexpressed during senescence and its activity is compromised by increases in steady-state H2O2 production as demonstrated by a lack of senescent cell response to TRPC6-activating hyperforin (Figure 3). Though hyperforin is touted to possess free radical scavenging property,66 hyperforin alone is not sufficient to restore the Ca2+ mobilizing ability of senescent cells. Given the role of TRPC6 in driving disease pathology, identification of the mechanisms dictating chronic regulation of TRPC6 by H2O2 has potential clinical implications.
The age-associated phenotypic shift that that heightens SA Ca2+ levels has been shown to drive the processing of IL-1α and its ability to propagate the SASP.26 We have identified TRPC6 as a potential participant in SA Ca2+ dysregulation and SASP activation. While SKF-96365 is often used to inhibit Store-operated Ca2+ entry and TRPC channel activity, our findings indicate that SKF enhances SA Ca2+ entry and limits the expression of the senescence marker p16 and SASP genes IL-6, IL-8, IL-1α and MMP-1 and TRPC6 itself. How SKF enhances senescence Ca2+ entry is not clear but may involve activation of reverse-mode of Ca2+ entry through the sodium-calcium exchange, NCX-1, as has been reported in cardiac fibroblasts and glioblastoma cells.67,68 Recent studies have also shown the loss of the sodium channel, SCN9a, which regulates plasma membrane depolarization allows cellular escape from oncogene-induced senescence.35 Maintenance of plasma membrane potential is reliant on coordinate regulation of numerous ion channels and together these observations suggest that the dysregulation of ion channel homeostasis impacts downstream signaling which results in the amplification of SA gene expression.mTOR signaling also contributes to regulation of TRPC6 channel expression and activity44,69 as well as SASP gene expression.46,70 Blocking TRPC6 can promote H2O2-mediated autophagy through activation of P13K/Akt/mTOR pathway.71 mTORC2 inhibition has been shown to be responsible for limiting TRPC6 expression in podocytes which are critical for the maintenance of glomerular filtration barrier.44 While mTOR inhibition in models of oncogene or ionizing radiation-induced senescence restricts SASP gene expression by both limiting the translation of the SASP regulator IL-1α70 and through mRNA destabilization of SASP transcripts.46 In our hands, both mTOR inhibitors, rapamycin and ku0063794, serve to restore Ca2+ entry and limit TRPC6 and SASP gene expression resulting from replicative senescence (Figure 5). Inhibition of replicative SASP gene expression by mTOR blockade likely occurs through a similar signaling that restricts IL-1α production70 and promotes SASP transcript destabilization.46 Inhibition of SA TRPC6 expression by mTOR might be attributed to the presence of a number of AUUUA mRNA destabilizing elements in its 3-untranslated region (3ʹ-UTR) which have been shown to play an important role in SASP mRNA stabilization.46 Analysis of the TRPC6 3ʹ-UTR with the ARE score tool72 identified the presence of nine AUUUA pentamers and an ARE score of 11.1, a score which is comparable to many of the labile SASP transcripts. We propose that mTOR elicits regulatory effects on SASP by modulating Ca2+ homeostasis through TRPC6 channel expression. The idea that the mTOR inhibitors also rescue peroxide-induced Ca2+ responses in senescence alludes to an antioxidant-like or antioxidant-promoting nature of these compounds that function to provide defense against cellular oxidative stress. This study furthers our understanding of how SA shifts in steady-state H2O2 levels serve to disrupt Ca2+ homeostasis and SASP. As TRP channels emerge as targets of pharmacologic intervention for numerous disease pathologies, it is exciting to speculate that the effect of TRP interventions may be attributed in part to inhibition of the SASP.
Future Directions
Common age-related metabolic diseases include type-2 diabetes mellitus (T2DM), obesity, atherosclerosis, sarcopenia, and macular degeneration. Since mitochondria are the master regulators of cellular metabolism, it is not surprising that the key underlying factor among these metabolism diseases is mitochondrial dysfunction.73 The quality and quantity of mitochondria are essential to maintain the energetic balance to meet tissue-specific energy demand, as well as to maintain healthy ROS production for signaling purposes. Mitochondria maintain their health through dynamic processes, including mitochondrial fusion, which helps in fusing mitochondria to enhance the energy production, and mitochondrial fission, which helps segregating the damaged mitochondria.74,75 Mitochondrial matrix Ca2+ is an important factor for regulating the production of reducing enzymes essential for electron transport chain (ETC), involved in retaining proton gradient required for ATP production.76 Ca2+ transfer from endoplasmic reticulum to mitochondrial must be tightly regulated as excess Ca2+ can disturb oxidative phosphorylation, increasing the release of ROS, which can lead to cellular damage observed in both aging and neurodegenerative diseases.77 Ca2+ overload due to increased uptake of calcium as a result of enhanced ER-mitochondrial tethering is observed in aged endothelial cells.78 In addition, increased H2O2 as the result of calcium overload is also observed in aged muscle tissues.79 The accumulation of Ca2+ in mitochondrial through mitochondrial calcium uniporter (MCU) from ER can be triggered by ITPR2 during replicative senescence, which can lead to drop in membrane potential and increased ROS.23 With heavy crosstalk between mitochondrial calcium signaling and senescence, it will be interesting to explore the involvement of mitochondrial dynamics in mTOR-dependent regulation of TRPC6 and cellular senescence.
ACKNOWLEDGEMENTS
The authors thank Mohamed Trebak from Penn State for his helpful guidance and insight.
Authors’ contributions
AC collected and analyzed data. AC and ML contributed equally to the preparation of the manuscript. All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript and S.A.T for providing critical reagents.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was generously supported in part by the SUNY Polytechnic Institute Research Seed Grant Program, in part by National Institute of Health R01 [grant number GM125870] to S.A.T.
ORCID iDs
May Y Lee https://orcid.org/0000-0002-3432-4279
J Andrés Melendez https://orcid.org/0000-0001-8021-3097
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25:585–621 [DOI] [PubMed] [Google Scholar]
- 2.Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene 2008; 27:2801–9 [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Ling Ding J, Meng L. Hua Oncogene-induced senescence: a double edged sword in cancer. Acta Pharmacol Sin 2018; 39:1553–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wickens AP. Ageing and the free radical theory. Respir Physiol 2001; 128:379–91 [DOI] [PubMed] [Google Scholar]
- 5.McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 2018; 217:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harley BC, Futcher BA, Greider WC. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345:458–60 [DOI] [PubMed] [Google Scholar]
- 7.de Magalhães JP, Passos JF. Stress, cell senescence and organismal ageing. Mech Age Dev 2018; 170:2–9 [DOI] [PubMed] [Google Scholar]
- 8.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, VanSteeg H, Dollé MET, Hoeijmakers JHJ, DeBruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 2014; 31:722–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yun MH. Cellular senescence in tissue repair: every cloud has a silver lining. Int J Dev Biol 2018; 62:591–604 [DOI] [PubMed] [Google Scholar]
- 10.Jun J, Il Lau LF. Cellular senescence controls fibrosis in wound healing. Aging 2010; 2:627–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñ Oz-Espín D, Cañ Amero M, Maraver A, Gó Mez-Ló Pez G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell 2013; 155:1104–18 [DOI] [PubMed] [Google Scholar]
- 12.Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013; 155:1119–30 [DOI] [PubMed] [Google Scholar]
- 13.Malaquin N, Martinez A, Rodier F. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Exp Gerontol 2016; 82:39–49 [DOI] [PubMed] [Google Scholar]
- 14.Jones DP. Redox theory of aging. Redox Biol 2015; 5:71–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrasekaran A, Idelchik M, del PS, Melendez JA. Redox control of senescence and age-related disease. Redox Biol 2017; 11:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clapham DE. Calcium signaling. Cell 2007; 131:1047–58 [DOI] [PubMed] [Google Scholar]
- 17.Ogawa N, Kurokawa T, Mori Y. Sensing of redox status by TRP channels. Cell Calcium 2016; 60:115–22 [DOI] [PubMed] [Google Scholar]
- 18.Nguyena HT, Sawmillera DR, Markova O, Chen M. Elevated [Ca2+]i levels occur with decreased calpain activity in aged fibroblasts and their reversal by energy-rich compounds: new paradigm for ALZHEIMER’S disease prevention. J Alzheimers Dis 2013; 37:835–48 [DOI] [PubMed] [Google Scholar]
- 19.Martin N, Bernard D. Calcium signaling and cellular senescence. Cell Calcium 2018; 70:16–23 [DOI] [PubMed] [Google Scholar]
- 20.Putney JW, Tomita T. Phospholipase C signaling and calcium influx. Adv Biol Regul 2012; 52:152–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol 2010; 2:a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor CW. Regulation of IP3 receptors by cyclic AMP. Cell Calcium 2017; 63:48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiel C, Lallet-Daher H, Gitenay D, Gras B, Le Calvé B, Augert A, Ferrand M, Prevarskaya N, Simonnet H, Vindrieux D, Bernard D. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat Commun 2014; 5:3792. [DOI] [PubMed] [Google Scholar]
- 24.Yee NS, Brown RD, Lee MS, Zhou W, Jensen C, Gerke H, Yee RK. TRPM8 ion channel is aberrantly expressed and required for preventing replicative senescence in pancreatic adenocarcinoma: potential role of TRPM8 as a biomarker and target. Cancer Biol Ther 2012; 13:592–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee NS, Zhou W, Lee M, Yee RK. Targeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma. Cancer Lett 2012; 318:99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. Redox-control of the senescence regulator interleukin-1 α and the secretory phenotype. J Biol Chem 2013; 288:32149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu S, Cai Y, Wei Y. mTOR signaling from cellular senescence to organismal aging. Aging Dis 2014; 5:263–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler DV, Wiley CD, Velarde MC. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell 2015; 14:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correia‐Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A, Rushton MD, Charles M, Jurk D, Tait SW, Czapiewski R, Greaves L, Nelson G, Bohlooly ‐Y, M, Rodriguez‐Cuenca S, Vidal‐Puig A, Mann D, Saretzki G, Quarato G, Green DR, Adams PD, Zglinicki T, Korolchuk VI, Passos JF. Mitochondria are required for pro‐ageing features of the senescent phenotype. EMBO J 2016; 35:724–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nacarelli T, Azar A, Sell C. Mitochondrial stress induces cellular senescence in an mTORC1-dependent manner. Free Radic Biol Med 2016; 95:133–54 [DOI] [PubMed] [Google Scholar]
- 31.Herranz N, Gallage S, Gil J. TORn about SASP regulation. Cell Cycle 2015; 14:3771–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decuypere JP, Kindt D, Luyten T, Welkenhuyzen K, Missiaen L, De Smedt H, Bultynck G, Parys JB. mTOR-Controlled autophagy requires intracellular Ca2+ signaling. PLoS One 2013; 8:e61020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Cano FE, Camello-Almaraz C, Hernandez D, Pozo MJ, Camello PJ. mTOR pathway and Ca2+ stores mobilization in aged smooth muscle cells. Aging 2013; 5:339–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipp P, Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium 1993; 14:359–72 [DOI] [PubMed] [Google Scholar]
- 35.Warnier M, Flaman J-M, Chouabe C, Wiel C, Gras B, Griveau A, Blanc E, Foy J-P, Mathot P, Saintigny P, Van Coppenolle F, Vindrieux D, Martin N, Bernard D. The SCN9A channel and plasma membrane depolarization promote cellular senescence through Rb pathway. Aging Cell 2018; 17:e12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006; 68:619–47 [DOI] [PubMed] [Google Scholar]
- 37.Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci 2007; 32:235–45 [DOI] [PubMed] [Google Scholar]
- 38.Kozai D, Ogawa N, Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal 2014; 21:971–86 [DOI] [PubMed] [Google Scholar]
- 39.Dasgupta J, Kar S, Remmen H, Van Melendez JA. Age-dependent increases in interstitial collagenase and MAP kinase levels are exacerbated by superoxide dismutase deficiencies. Exp Gerontol 2009; 44:503–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham S, Ding M, Ding Y, Sours-Brothers S, Luchowski R, Gryczynski Z, Yorio T, Ma H, Ma R. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 2010; 285:23466–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erac Y, Selli C, Kosova B, Akcali KC, Tosun M. Expression levels of TRPC1 and TRPC6 ion channels are reciprocally altered in aging rat aorta: implications for age-related vasospastic disorders. Age 2010; 32:223–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trebak M, Vazquez G, Bird GSJ, James WP., Jr. The TRPC3/6/7 subfamily of cation channels. Cell Calcium 2003; 33:451–61 [DOI] [PubMed] [Google Scholar]
- 43.Leuner K, Kazanski V, Muller M, Essin K, Henke B, Gollasch M, Harteneck C, Muller WE. Hyperforin a key constituent of St. John’s Wort specifically activates TRPC6 channels. FASEB J 2007; 21:4101–11 [DOI] [PubMed] [Google Scholar]
- 44.Ding F, Zhang X, Li X, Zhang Y, Li B, Ding J. Mammalian target of rapamycin complex 2 signaling pathway regulates transient receptor potential cation channel 6 in podocytes. PLoS One 2014; 9:e112972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tominaga-Yamanaka K, Abdelmohsen K, Martindale JL, Yang X, Taub DD, Gorospe M. NF90 coordinately represses the senescence-associated secretory phenotype. Aging 2012; 4:695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, Georgilis A, Montoya A, Wolter K, Dharmalingam G, Faull P, Carroll T, Martínez-Barbera JP, Cutillas P, Reisinger F, Heikenwalder M, Miller RA, Withers D, Zender L, Thomas GJ, Gil J. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 2015; 17:1205–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium 2011; 50:211–21 [DOI] [PubMed] [Google Scholar]
- 48.Pinto MCX, Kihara AH, Goulart VAM, Tonelli FMP, Gomes KN, Ulrich H, Resende RR. Calcium signaling and cell proliferation. Cell Signal 2015; 27:2139–49 [DOI] [PubMed] [Google Scholar]
- 49.Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, Smaili SS. Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal 2014; 21:123–37 [DOI] [PubMed] [Google Scholar]
- 50.Borodkina AV, Shatrova AN, Deryabin PI, Griukova AA, Abushik PA, Antonov SM, Nikolsky NN, Burova EB. Calcium alterations signal either to senescence or to autophagy induction in stem cells upon oxidative stress. Aging 2016; 8:3400–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma X, Warnier M, Raynard C, Ferrand M, Kirsh O, Defossez PA, Martin N, Bernard D. The nuclear receptor RXRA controls cellular senescence by regulating calcium signaling. Aging Cell 2018; 17:e12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 2005; 118:2435–40 [DOI] [PubMed] [Google Scholar]
- 53.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem 2007; 19:1–8 [DOI] [PubMed] [Google Scholar]
- 54.Hsu WL, Tsai MH, Wu CY, Liang JL, Lu JH, Kahle JS, Yu HS, Yen CJ, Yen CT, Hsieh YC, Huang YY, Lin LC, Tsai TF, Chen CH, Yoshioka T. Nociceptive transient receptor potential canonical 7 (TRPC7) mediates aging-associated tumorigenesis induced by ultraviolet B. Aging Cell 2020; 19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z, Guo G, Wang H, Si X, Zhou G, Xiong Y, Li S, Dai R, Yang C. TRPC5 channel modulates endothelial cells senescence. Eur J Pharmacol 2017; 802:27–35 [DOI] [PubMed] [Google Scholar]
- 56.Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell 2007; 6:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Övey IS, Naziroğlu M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience 2015; 284:225–33 [DOI] [PubMed] [Google Scholar]
- 58.Ishii T, Takanashi Y, Sugita K, Miyazawa M, Yanagihara R, Yasuda K, Onouchi H, Kawabe N, Nakata M, Yamamoto Y, Hartman PS, Ishii N. Endogenous reactive oxygen species cause astrocyte defects and neuronal dysfunctions in the hippocampus: a new model for aging brain. Aging Cell 2017; 16:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW. The mammalian TRPC cation channels. Biochim Biophys Acta 2004; 1742:21–36 [DOI] [PubMed] [Google Scholar]
- 60.Putney JW. The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol 2004; 14:282–6 [DOI] [PubMed] [Google Scholar]
- 61.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 2013; 33:1732–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding H-Y, Ma H-X. Significant roles of anti-aging protein klotho and fibroblast growth factor23 in cardiovascular disease. J Geriatr Cardiol 2015; 12:439–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Sun S, Wu L, Pchitskaya E, Zakharova O, Fon Tacer K, Bezprozvanny I. Store-operated calcium channel complex in postsynaptic spines: a new therapeutic target for Alzheimer’s disease treatment. J Neurosci 2016; 36:11837–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding Y, Winters A, Ding M, Graham S, Akopova I, Muallem S, Wang Y, Hong JH, Gryczynski Z, Yang S-H, Birnbaumer L, Ma R. Reactive oxygen species-mediated TRPC6 protein activation in vascular myocytes, a mechanism for vasoconstrictor-regulated vascular tone. J Biol Chem 2011; 286:31799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, Ding Y, Ma R. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol 2011; 301:C304–C315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meinke MC, Schanzer S, Haag SF, Casetti F, Müller ML, Wölfle U, Kleemann A, Lademann J, Schempp CM. In vivo photoprotective and anti-inflammatory effect of hyperforin is associated with high antioxidant activity in vitro and ex vivo. Eur J Pharm Biopharm 2012; 81:346–50 [DOI] [PubMed] [Google Scholar]
- 67.Ikeda K, Nakajima T, Yamamoto Y, Takano N, Tanaka T, Kikuchi H, Oguri G, Morita T, Nakamura F, Komuro I. Roles of transient receptor potential canonical (TRPC) channels and reverse-mode Na+/Ca2+ exchanger on cell proliferation in human cardiac fibroblasts: effects of transforming growth factor β1. Cell Calcium 2013; 54:213–25 [DOI] [PubMed] [Google Scholar]
- 68.Song M, Chen D, Yu SP. The TRPC channel blocker SKF 96365 inhibits glioblastoma cell growth by enhancing reverse mode of the Na(+)/Ca(2+) exchanger and increasing intracellular Ca(2+). Br J Pharmacol 2014; 171:3432–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang HT, Wang WW, Ren LH, Zhao XX, Wang ZH, Zhuang DL, Bai YN. The mTORC2/akt/NFKB Pathway-Mediated activation of TRPC6 participates in Adriamycin-Induced podocyte apoptosis. Cell Physiol Biochem 2016; 40:1079–93 [DOI] [PubMed] [Google Scholar]
- 70.Laberge R-M, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez P-Y, Benz CC, Kapahi P, Nelson PS, Campisi J. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 2015; 17:1049–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou X, Xiao H, Zhang Y, Zeng X, Huang M, Chen X, Birnbaumer L, Liao Y. Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis 2018; 9:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spasic M, Friedel CC, Schott J, Kreth J, Leppek K, Hofmann S, Ozgur S, Stoecklin G. Genome-wide assessment of AU-rich elements by the AREScore algorithm. PLoS Genet 2012; 8:e1002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Natarajan V, Chawla R, Mah T, Vivekanandan R, Tan SY, Sato PY, Mallilankaraman K. Mitochondrial dysfunction in age-related metabolic disorders. Proteomics 2020; 20:e1800404. [DOI] [PubMed] [Google Scholar]
- 74.Suárez-Rivero J, Villanueva-Paz M, de la Cruz-Ojeda P, de la Mata M, Cotán D, Oropesa-Ávila M, de Lavera I, Álvarez-Córdoba M, Luzón-Hidalgo R, Sánchez-Alcázar J. Mitochondrial dynamics in mitochondrial diseases. Diseases 2016; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zemirli N, Morel E, Molino D. Mitochondrial dynamics in basal and stressful conditions. Int J Mol Sci 2018; 19:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation Cascade in skeletal muscle mitochondria. Biochemistry 2013; 52:2793–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Müller M, Ahumada-Castro U, Sanhueza M, Gonzalez-Billault C, Court FA, Cárdenas C. Mitochondria and calcium regulation as basis of neurodegeneration associated with aging. Front Neurosci 2018; 12:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madreiter-Sokolowski CT, Waldeck-Weiermair M, Bourguignon M-P, Villeneuve N, Gottschalk B, Klec C, Stryeck S, Radulovic S, Parichatikanond W, Frank S, Madl T, Malli R, Graier WF. Enhanced inter-compartmental Ca 2+ flux modulates mitochondrial metabolism and apoptotic threshold during aging. Redox Biol 2018; 20:458–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Capel F, Demaison L, Maskouri F, Diot A, Buffiere C, Mirand PP, Mosoni L. Calcium overload increases oxidative stress in old rat gastrocnemius muscle. J Physiol Pharmacol 2005; 56:369–80 [PubMed] [Google Scholar]