Abstract
Defensins are critical components of the innate immune system and play an important role in the integration of innate and adaptive immune responses. Although information on the immunomodulatory properties of peptidoglycan from bacteria is abundant, little is known about the β-defensin induction effect of peptidoglycan from the probiotic Lactobacillus. This study investigated the effect of intact peptidoglycan from L. rhamnosus MLGA on the induction of avian β-defensin 9 in chicken immune cells and intestinal explants. Peptidoglycan from Lactobacillus rhamnosus MLGA dose dependently promoted avian β-defensin 9 mRNA expression in chicken PBMCs, splenocytes, thymocytes, hepatocytes, and chicken embryo jejunum, ileum, and cecum explants and increased the capacity of PBMC or splenocyte lysates to inhibit the growth of Salmonella Enteritidis. In contrast to the effect of L. rhamnosus MLGA-derived peptidoglycan, peptidoglycan derived from pathogenic Staphylococcus aureus reduced avian β-defensin 9 mRNA expression in chicken PBMCs and splenocytes. The inducible effect of peptidoglycan from L. rhamnosus MLGA on avian β-defensin 9 expression in PBMCs and splenocytes was observed without activation of the expression of associated pro-inflammatory cytokines IL-1β, IL-8, and IL-12p40, whereas these cytokine expressions were suppressed by peptidoglycan hydrolysate obtained by lysozyme digestion. The results of the present study show the capability of peptidoglycan derived from L. rhamnosus MLGA to induce the antimicrobial peptide defensin while simultaneously avoiding the deleterious risks of an inflammatory response.
Keywords: Lactobacillus rhamnosus MLGA, peptidoglycan, avian β-defensin 9, pro-inflammatory cytokines
Introduction
Defensins are small cationic antimicrobial peptides with broad-spectrum direct microbicidal activities against bacteria, protozoa, enveloped virus, and fungi.1 Defensins are recognized as important effector molecules of host innate immunity and are widely distributed in various tissues, especially in immune organs, the epithelium of skin, and throughout the digestive, respiratory, and urogenital tracts.1,2 In addition to their direct microbicidal activity, defensins also show multiple biological activities, including anti-tumor and anti-inflammatory properties, neutralizing of endotoxins, wound healing, and chemoattracting lymphocytes, dendritic cells, and monocytes, and thus play an important role in the integration of the innate and adaptive immune responses against infections.3,4 The defensin family of vertebrate animals can be divided into α-, β-, and θ-defensin subgroups based on the spacing pattern of cysteines, which form three conserved disulfide bridges.5 Interestingly, only β-defensins (avian β-defensins, AvBDs) have been reported to exist in avian species so far.2 The chicken genome encodes a total of 14 AvBDs (AvBD1–14), which are densely clustered on chicken chromosome 3q and expressed in a wide range of tissues.6,7
Among the 14 AvBDs, AvBD9 is extensively expressed in various organs and tissues of chicken.6–8 AvBD9 displays broad-spectrum antibacterial activities against Clostridium perfringens, Staphylococcus aureus, Campylobacter jejuni, Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Shigella sonnei, and shows a prominent fungicidal activity against both unicellular and multicellular fungi.8–10 In addition to the potent antimicrobial activity of AvBD9, this peptide exhibits low hemolytic activity and low toxicity against animal cells,8 and has multiple functions in the host defense against infection,11 suggesting that AvBD9 plays an important role in both innate and adaptive immunity. Several studies revealed that some dietary compounds, including vitamin D3 and short-chain fatty acids in particular, potently promote AvBD9 expression in different immune cell types and the intestines of chicken, and thus boost host immunity and disease resistance in chicken.11–13 In contrast, tissue-specific reduction of AvBD9 expression was observed in chickens in response to several microbial infections such as Salmonella enterica, infectious bronchitis virus, and Marek’s disease virus infection,14–16 indicating that suppressing AvBD9 expression may be a major immune evasion strategy used by pathogens. Therefore, dietary modulation of the synthesis of endogenous antimicrobial peptides such as AvBD9 may have the potential to be developed as a cost-effective, antibiotic-alternative approach to disease control and prevention for poultry production.
Peptidoglycan, a major component of Gram-positive bacterial cell walls (accounting for approximately 90% of their dry mass), plays crucial roles in bacterial growth and survival as well as in the modulation of host immune responses.17 Peptidoglycan from probiotic lactobacilli has been shown to possess multiple biological activities including immunomodulatory,18–21 anti-tumor,22–24 and anti-infection,25 among which the immunomodulatory properties of peptidoglycan have been especially well documented. It has been demonstrated that peptidoglycan from lactobacilli can regulate not only innate immunity but also adaptive immune responses.26,27
Several studies have shown that specific probiotic Lactobacillus strains strongly induced mRNA expression and protein production of β-defensin in epithelial cells, thus enhancing host defense against infection.28–31 We previously reported that heat-killed Lactobacillus rhamnosus MLGA that was isolated from health chicken intestine enhances AvBD9 gene expression in primary intestinal epithelial cells of chicken embryos,32 implying that specific heat-stable cellular components from this probiotic bacterium contribute to this beneficial effect. Although the immunoregulatory activity of peptidoglycan from Lactobacillus has been studied extensively, little is known about the β-defensin induction effect of peptidoglycan. Given peptidoglycan is the major component of the cell walls of Lactobacillus and there is similar immunological and biological activity between defensin and peptidoglycan, we hypothesized that peptidoglycan from specific probiotic Lactobacillus strains can stimulate defensin expression and thus improve immune defense against pathogens. Therefore, the objective of the present study was to investigate the effect of intact peptidoglycan derived from L. rhamnosus MLGA on the expression of a representative chicken β-defensin (AvBD9) in jejunum, ileum, and cecum explants as well as immune cells. In addition, pro-inflammatory cytokine expression in immune cells in response to peptidoglycan stimulation was determined to examine whether peptidoglycan derived from L. rhamnosus MLGA had an impact on triggering inflammatory response while stimulating AvBD9 expression.
Material and methods
Bacterial strains
The probiotic L. rhamnosus MLGA strain was isolated from the small intestine of a healthy chicken32 and preserved in a glycerin tube at –80°C. L. rhamnosus MLGA was cultured statically in Mann-Rogosa-Sharpe broth (Solarbio, Beijing, China) overnight at 37°C under anaerobic conditions. The bacteria were grown until log phase and then harvested by centrifugation at 2200 g for 20 min and washed three times with sterile 0.9% saline solution (w/v = 1:10). The collected bacteria were used for peptidoglycan preparation. Salmonella Enteritidis (ATCC 13076) was cultured in Tryptic Soy Broth (TSB) medium (Baisi Biotechnology, Hangzhou, China) at 37°C for 8 h. This was followed by three passages every 8 h into fresh TSB for a total of 24 h, to ensure log phase growth. Bacterial cells were washed three times in sterile PBS, pH 7.4, by centrifugation at 1500 g for 15 min, quantified with a spectrophotometer at 625 nm using an established concentration curve, and diluted in sterile PBS as per required concentrations for the experiment. The concentration of S. Enteritidis was confirmed by plating on BGA plates.
Preparation of intact peptidoglycan from L. rhamnosus MLGA
Intact peptidoglycan was extracted from L. rhamnosus MLGA based on a trichloroacetic acid (TCA) method as described previously33 with minor modifications. Briefly, the bacteria were dissolved in 10% TCA (w/v = 1:10) and incubated in a boiling bath for 1 h. The mixture was immediately cooled and then centrifuged at 13,000 g for 10 min. The sediment was washed with distilled water to remove TCA, then treated with a special solvent composed of acetic acid-sodium acetate buffer (0.5 M acetic acid and 0.2 M sodium acetate, pH 4.5), chloroform and methanol at a ratio of 4:5:10 (v/v/v) for 24 h. After centrifugation at 4000 g for 20 min, the insoluble resides were incubated in Tris-HCl (0.1 M, pH 7.5) containing 3500 U trypsin at 37°C in a shaking bath (140 rpm) for 12 h. The mixture was then centrifuged at 8000 g for 20 min. Finally, the sediment obtained was washed with sterile distilled water 4–5 times, lyophilized, and stored at –80°C for further use. The target product was analyzed and confirmed as intact peptidoglycan by means of transmission electron microscopy, amino acid composition analysis, and lysozyme digestion test (data not shown).
Preparation of L. rhamnosus MLGA-derived peptidoglycan hydrolysate by lysozyme
Lyophilized intact peptidoglycan extracted from L. rhamnosus MLGA was suspended in PBS (pH 6.8) to obtain a 10% (w/v) peptidoglycan solution. Chicken egg white lysozyme (Amresco Inc., Ohio, USA) was then added to the solution at a ratio of 1:100 (enzyme/peptidoglycan), followed by incubation for 48 h at 37°C with gentle shaking. After digestion, the reaction solution was heated in a boiling-water bath for 15 min to inactivate the lysozyme and then lyophilized.
Isolation, culture, and stimulation of immune cells and intestinal tissue explants
PBMCs, splenocytes, thymocytes, and hepatocytes were isolated from the anti-coagulated blood, spleens, thymuses, and livers of 3- to 4-wk-old Arbor Acres broiler chickens. Blood was collected in tubes containing heparin sodium from the wing vein. PBMCs were isolated from anti-coagulated blood through gradient centrifugation using Histopaque® 1077 (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer's procedure. Chickens were killed by cervical dislocation and soaked in 75% ethanol for 3 min. The spleens, livers, and thymuses were harvested aseptically, and rinsed in D-Hank’s balanced salt solution (HBSS) (Solarbio, Beijing, China) supplemented with 200 U/ml of penicillin (Solarbio) and 200 µg/ml of streptomycin (Solarbio). Spleens and thymuses were gently disrupted with the flat end of a 10 ml syringe plunger and passed through a cell strainer to obtain single-cell suspensions in HBSS. Splenocytes and thymocytes were collected by a density gradient centrifugation using Histopaque® 1077. Cells were washed three times with HBSS by centrifugation at 500 g at 4°C for 10 min and then re-suspended in complete RPMI 1640 medium containing 10% FBS, 50 mM of L-glutamine, 100 U/ml of penicillin, and 100 µg/ml of streptomycin. Cells were counted on a hemocytometer using trypan blue dye exclusion. Splenocytes and thymocytes were seeded into six-well flat-bottom plates at a concentration of 1 × 106 cells/ml. Hepatocytes and PBMC were seeded into six-well flat-bottom plates at a concentration of 2 × 105 cells/ml and 1 × 107 cells/ml, respectively. All cells were incubated at 37°C in a humidified atmosphere with 5% CO2. The growth medium was discarded and the cells then treated with peptidoglycan at indicated concentrations (2 ml per well) for 6 h. L. rhamnosus MLGA-derived peptidoglycan, S. aureus-derived peptidoglycan (Sigma-Aldrich, St. Louis, MO, USA), or L. rhamnosus MLGA-derived peptidoglycan hydrolysate by lysozyme was diluted in complete RPMI 1640 medium to the desired concentration for stimulating cells. Sections of jejunum, ileum, and cecum were aseptically removed from the 18 d-old specific pathogen-free chicken embryos. The mesentery was removed carefully, and the gut section was cut lengthwise and washed thoroughly with cold HBSS containing 200 U/ml of penicillin and 200 µg/ml of streptomycin. The gut section was then cut into a series of 0.5 cm-long segments. Thereafter, the segments were placed in six-well flat-bottom culture plates in complete RPMI 1640 medium. Jejunal, ileal, and cecal explants were cultured at 37°C in a humidified atmosphere with 5% CO2 in the presence of different concentrations of peptidoglycan for 24 h. The cells or explants untreated with peptidoglycan (RPMI 1640 medium alone) were defined as the control group. Each treatment was performed in triplicate and all the experiments were repeated at least twice. Cells and tissue explants were harvested after corresponding incubation time for RNA extraction.
All animal procedures reported herein were in line with the Laboratory Animal Welfare and Ethics Censorship and approved by the Laboratory Animal Ethics Committee of Jiangxi Agricultural University (Nanchang, Jiangxi, China).
Real-time quantitative RT-PCR
Total RNA was extracted from cells or gut explants using Trizol Reagent (TransGen Biotech, Beijing, China) following the manufacturer’s instructions. RNA purity was assessed by examining the absorbance ratio at 260 and 280 nm using a NanoDrop ND-100 microvolume spectrophotometer (Thermo Fisher Scientific), whereas integrity was verified by electrophoresis on 1% denaturing agarose gel. Reverse transcription was performed using an M-MLV Reverse Transcriptase Kit (ShineGene Bioetch, Shanghai, China) following the manufacturer’s instructions. Real-time RT-PCR was then conducted on FTC2000 Real-Time PCR Detection System (Funglyn Biotech Inc., Canada) in 50 µl reaction volumes containing Hotstart Fluo-PCR mix (ShineGene), primers, fluorescent probes (TaKaRa Biotechnology, Dalian, China), and cDNA. PCR cycling conditions were 94°C for 4 min, followed by 40 cycles of 94°C for 30 s, and 60°C for 1 min. The specificity of PCR reaction was confirmed by the melt curve analysis. The primers and probes used in quantitative real-time PCR reactions are shown in Table 1. The relative gene expression levels were quantified using the comparative 2–ΔΔCt method with the GAPDH gene as a reference for normalization. Results were presented as fold-expression change relative to the control group (medium alone). All the assays were performed in triplicate and repeated at least two times.
Table 1.
The primer and probe sequences of AvBD9 and cytokines for real-time PCR reactions.
| Gene name | Sequence (5′- 3′) | GenBank accession no. | Product size (bp) |
|---|---|---|---|
| AvBD9 | Forward: CACCTTAGCATGCAGGCAGA Reverse: GCCCATTTGCAGCATTTCA Probe: FAM-TTGCATGCCGTGCTCCTTCAGTTG-BHQ1 |
NM_001001611 | 111 |
| IL-1β | Forward: TGACCCGCTTCATCTTCTACC Reverse: GGTCGGGTTGGTTGGTGA Probe: FAM-CACGCGCTTCGAGTCGGCC-BHQ1 |
AJ245728 | 138 |
| IL-8 | Forward: GCTGGTAAAGATGGGGAATGA Reverse: CGTCAGCTTCACATCTTGAATG Probe: FAM-CATTAGCACTCATTCTAAGTTCATCCACCC-BHQ1 |
AJ009800 | 94 |
| IL-12p40 | Forward: GTAACCTGCAGCAGCCCTG Reverse: GTCGGCTGGTGCTCTTCG Probe: FAM-CTGTGACTGAATACACTGCCCAGTGCC-BHQ1 |
NM_213571.1 | 107 |
| GAPDH | Forward: GGTGCTAAGCGTGTTATCATCTC Reverse: CATGGTTGACACCCATCACAA Probe: FAM-CTCCCTCAGCTGATGCCCCCATG-BHQ1 |
NM_204305 | 70 |
AvBD9: avian β-defensin 9.
Antibacterial capacity of PBMCs and splenocytes treated with peptidoglycan from L. rhamnosus MLGA
The antibacterial activity of cell lysates was measured as previously described with slight modifications.11 Briefly, PBMCs and splenocytes were cultured in antibiotic-free RPMI 1640 medium containing 0, 5, 10, 50, or 100 μg/ml of peptidoglycan from L. rhamnosus MLGA for 6 h at 37°C and 5% CO2. Cell culture supernatants were collected, and cells were then scraped, lysed with 1% Triton X-100, and centrifuged 12,000 g for 10 min at 4°C. Serial two-fold dilutions were then prepared from the cell lysate supernatants. Cell culture supernatants and cell lysate dilutions were incubated with 1 × 104 CFU of S. Enteritidis ATCC13076 in TSB medium for 9 h in a 96-well plate at 37°C. Bacterial turbidity was measured at OD590 nm using a Microplate reader (Multiskan MK3, Thermo Fisher Scientific, Shanghai, China). Different concentrations of peptidoglycan from L. rhamnosus MLGA were also directly incubated with S. Enteritidis in TSB medium and the OD590 nm was recorded after 9 h incubation.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) from two to three independent experiments. Data were analyzed using one-way analysis of variance and unpaired two-tailed Student’s t-test was used to evaluate the statistical significance between treatments using SPSS 17.0 software. Differences were considered statistically significant when the P value was < 0.05.
Results
Induction of AvBD9 gene expression in primary chicken immune cells and intestinal tissue explants by peptidoglycan from L. rhamnosus MLGA
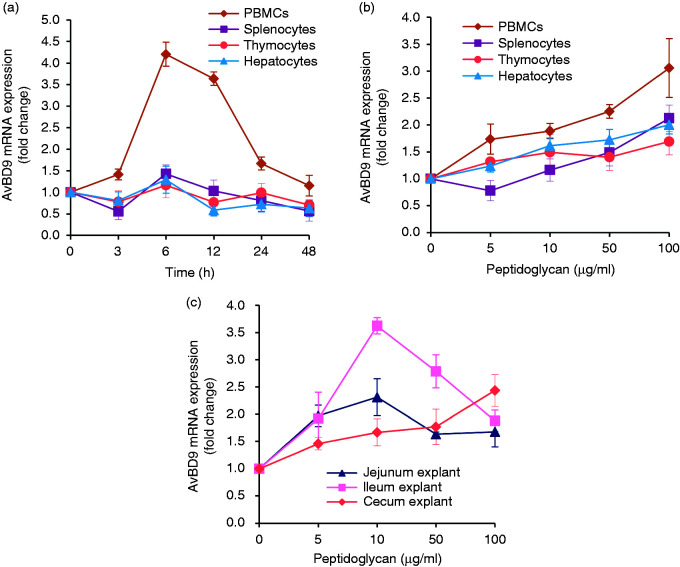
In a previous study we demonstrated that intact peptidoglycan from L. rhamnosus MLGA induces AvBD9 gene expression in primary chicken small intestinal epithelial cells in both time- and dose-dependent manners, with the maximal response occurring at 4 h post-stimulation at a concentration of 50 µg/ml.34 To further examine whether L. rhamnosus MLGA-derived peptidoglycan is capable of stimulating AvBD9 expression in other cell types, especially immune cells, we performed both dose-response and time-course experiments in primary PBMCs, splenocytes, thymocytes, and hepatocytes. An obvious time-dependent increase in AvBD9 expression was elicited in PBMCs in response to treatment of 50 µg/ml peptidoglycan, peaking at 6 h post-stimulation and returning to the basal level after 48 h of incubation (Figure 1a). A maximal response of AvBD9 expression was also observed in splenocytes and hepatocytes following 6 h stimulation, although the magnitude of induction was much less than in PBMCs (Figure 1a). However, no time-dependent induction of AvBD9 was seen in thymocytes on stimulation with 50 µg/ml peptidoglycan (Figure 1a), suggestive of cell-specific regulation of AvBD9 expression by peptidoglycan from L. rhamnosus MLGA. The expression of AvBD9 mRNA was dose-dependently augmented in all tested cells upon peptidoglycan treatment for 6 h, and the induction of AvBD9 expression was more prominent in the PBMCs than in the other three cell types (Figure 1b). Similarly, L. rhamnosus MLGA-derived peptidoglycan treatment exhibited a dose-dependent induction of AvBD9 in jejunum, ileum, and cecum explants after 24 h stimulation, with a maximal effect seen at the concentration of 10 µg/ml in the jejunum and ileum explants, followed by a gradual decline as the peptidoglycan concentration was increased (Figure 1c).
Figure 1.
Intact peptidoglycan from L. rhamnosus MLGA induces avian β-defensin 9 (AvBD9) gene expression in different chicken cell types and intestinal tissue explants. Chicken primary cells were incubated with 50 µg/ml of peptidoglycan for indicated time points (a) or indicated concentrations of peptidoglycan for 6 h (b). (c) Chicken jejunal, ileal, and cecal explants were treated with indicated concentrations of peptidoglycan for 24 h. The AvBD9 mRNA expression was analyzed by real-time RT-PCR, and the relative gene expression was calculated using the comparative 2-ΔΔCt method with the GAPDH gene as a reference for normalization and presented as fold-expression change relative to the control group. Values are given as the mean ± SD of two to three independent experiments.
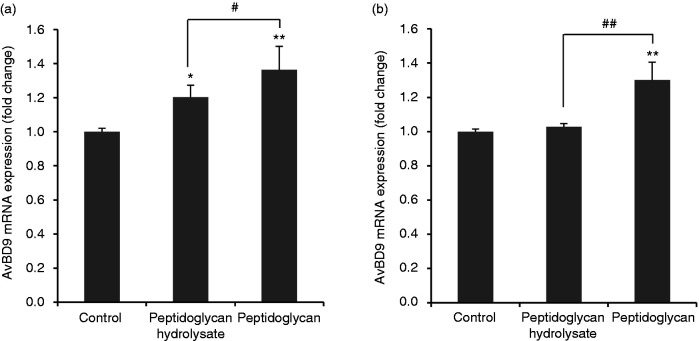
Peptidoglycan can be hydrolyzed by lysozyme (also named N-acetylmuramide glycanohydrolase or muramidase), which is found in monocytes, macrophages, epithelial secretions, and intestinal Paneth cells.35 Furthermore, the beneficial immunomodulatory effects of peptidoglycan from probiotics can be diminished by treatment with N-acetylmuramidase in some cases.23 We next examined the modulatory effect of peptidoglycan fragments released by lysozyme on AvBD9 expression. L. rhamnosus MLGA peptidoglycan hydrolysate at 100 µg/ml increased AvBD9 mRNA expression in PBMCs (Figure 2a) but not in splenocytes (Figure 2b), albeit at a lesser magnitude than intact peptidoglycan.
Figure 2.
Avian β-defensin 9 (AvBD9) mRNA expression in primary chicken PBMCs (a) and splenocytes (b) in response to L. rhamnosus MLGA-derived intact peptidoglycan or its hydrolysate obtained by lysozyme digestion. Primary cells were incubated with 100 µg/ml of L. rhamnosus MLGA-derived intact peptidoglycan or its hydrolysate for 6 h. Real-time RT-PCR was performed to determine the AvBD9 mRNA expression, and the relative gene expression was calculated using the comparative 2-ΔΔCt method with the GAPDH gene as a reference for normalization and presented as fold-expression change relative to the control group. Values are given as the mean ± SD of two to three independent experiments. *P < 0.05, **P < 0.01 compared with control group; #P < 0.05, ##P < 0.01 between the indicated treatments.
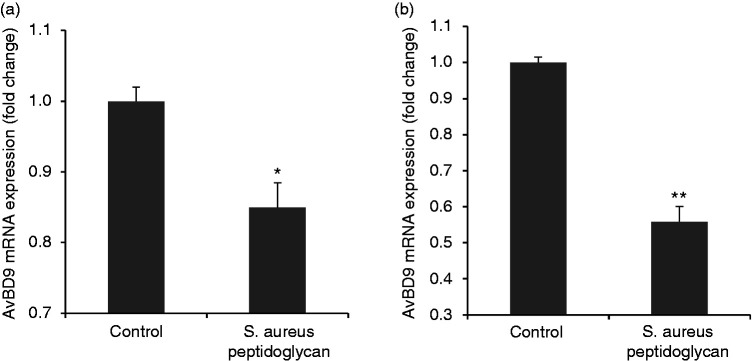
Peptidoglycan from pathogenic bacterium suppresses AvBD9 expression in primary chicken PBMCs and splenocytes
Peptidoglycan is a major cell wall component of Gram-positive bacteria. However, subtle alterations in structural molecules of peptidoglycan in various bacteria cause different interactions between the host cells and microbes, and thus elicit differences in immunobiological effects.27,36 Therefore, we tested the difference in the capacity to modulate AvBD9 expression between peptidoglycans from probiotic L. rhamnosus MLGA and pathogenic S. aureus. In contrast to peptidoglycan from L. rhamnosus MLGA, S. aureus peptidoglycan down-regulated AvBD9 mRNA expression in both PBMCs and splenocytes (Figure 3a and b, respectively).
Figure 3.
Peptidoglycan from pathogenic Staphylococcus aureus suppresses avian β-defensin 9 (AvBD9) expression in primary chicken PBMCs (a) and splenocytes (b). Cells were incubated with S. aureus-derived peptidoglycan at a concentration of 100 µg/ml for 6 h. Real-time RT-PCR was performed to determine the AvBD9 mRNA expression. Values are given as the mean ± SD of two to three independent experiments. *P < 0.05, **P < 0.01 compared with control group.
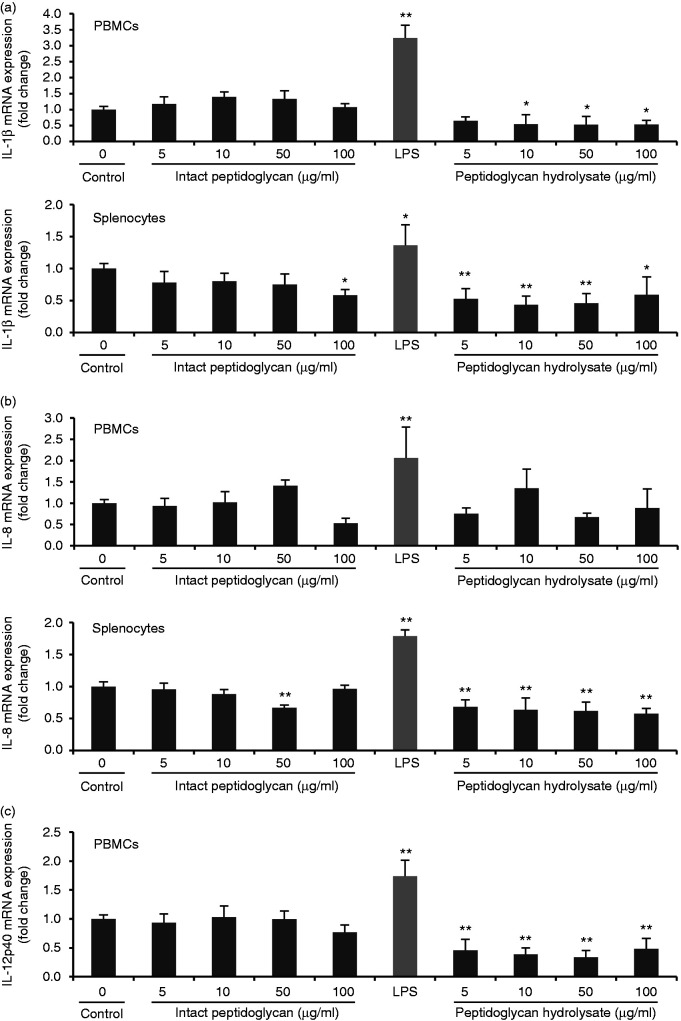
Peptidoglycan from L. rhamnosus MLGA triggers no inflammatory response in PBMCs and splenocytes
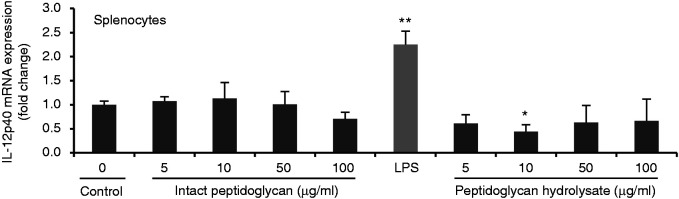
Pro-inflammatory cytokines are known to be potent inducers and up-regulators of defensins in humans37 and chickens.38 To examine whether the up-regulation of AvBD9 expression by L. rhamnosus MLGA-derived peptidoglycan or its hydrolysate by lysozyme was mediated by pro-inflammatory cytokines synthesized in response to peptidoglycan stimulation, we treated PBMCs and splenocytes with and without peptidoglycan or its hydrolysate for 6 h and analyzed the expressions of three representative pro-inflammatory cytokines including IL-1β, IL-8, and IL-12p40. Bacterial LPS from Escherichia coli 055:B5 (Solarbio) at 1 μg/ml was used as a positive control. Intact peptidoglycan showed no induction of all three cytokines both in PBMCs and in splenocytes (Figure 4). Moreover, IL-1β and IL-8 expression was significantly decreased in splenocytes by intact peptidoglycan at 100 and 50 μg/ml, respectively (Figure 4a and b). In contrast, IL-1β, IL-8, and IL-12p40 expressions were markedly induced in response to LPS (Figure 4). Interestingly, L. rhamnosus MLGA peptidoglycan hydrolysate significantly suppressed IL-1β expression in PBMCs and splenocytes (Figure 4a), IL-8 expression in splenocytes (Figure 4b), and IL-12p40 expression in PBMCs (Figure 4c). These results demonstrated the L. rhamnosus MLGA-derived peptidoglycan and its hydrolysate by lysozyme promote AvBD9 expression without being accompanied by inflammatory response, suggesting that AvBD9 expression induction by peptidoglycan from L. rhamnosus MLGA is pro-inflammatory cytokine-independent.
Figure 4.
Peptidoglycan from L. rhamnosus MLGA triggers no inflammatory response in chicken PBMCs and splenocytes. Chicken PBMCs or splenocytes were stimulated with indicated concentrations of peptidoglycan or its hydrolysate by lysozyme, or 1 μg/ml LPS as a positive control for 6 h. Real-time RT-PCR was performed to determine the mRNA expressions of IL-1β (a), IL-8 (b), and IL-12p40 (c), and the relative gene expression was calculated using the comparative 2-ΔΔCt method with the GAPDH gene as a reference for normalization and presented as fold-expression change relative to the control group. Values are given as the mean ± SD of two to three independent experiments. *P < 0.05, **P < 0.01 compared with control group.
Peptidoglycan from L. rhamnosus MLGA increases antibacterial activity of chicken PBMCs and splenocytes against S. Enteritidis
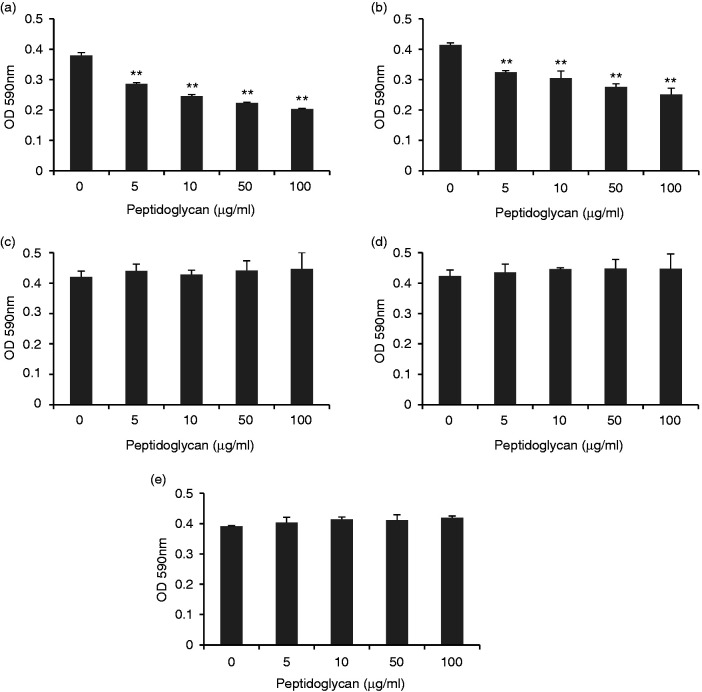
It is known that chicken AvBD9 exerts broad-spectrum antibacterial activities against both Gram-positive and Gram-negative pathogenic strains.8,9 Because L. rhamnosus MLGA-derived peptidoglycan significantly induced AvBD9 expression in chicken PBMCs and splenocytes, we next investigated the functional consequence of AvBD9 induction in PBMCs and splenocytes by peptidoglycan from L. rhamnosus MLGA. As shown in Figure 5, both PBMC (Figure 5a) and splenocyte (Figure 5b) lysates pretreated with peptidoglycan significantly inhibited the growth of S. Enteritidis in a dose-dependent manner. However, the supernatant from the co-culture of PBMCs (Figure 5c) or splenocytes (Figure 5d) with L. rhamnosus MLGA-derived peptidoglycan was not able to inhibit the growth of S. Enteritidis probably due to the low secreted AvBD9 into the extracellular medium. It was worth noting that L. rhamnosus MLGA-derived peptidoglycan itself showed no inhibitory effect on the growth of S. Enteritidis (Figure 5e).
Figure 5.
Peptidoglycan from L. rhamnosus MLGA increases antibacterial activity of chicken PBMCs (a) and splenocytes (b). Chicken PBMCs or splenocytes were treated with or without different concentrations of L. rhamnosus MLGA-derived peptidoglycan for 6 h. Cell lysates were them prepared and incubated with S. Enteritidis (ATCC 13076) for 9 h at 37°C. Bacterial turbidity at OD590 nm was measured as an indication of the bacterial density. S. Enteritidis was also directly incubated with different concentrations of L. rhamnosus MLGA-derived peptidoglycan in cell culture medium alone without chicken primary cells (c) or incubated with supernatants from co-culture of PBMCs or splenocytes with L. rhamnosus MLGA-derived peptidoglycan (d). Values are given as the mean ± SD of two to three independent experiments. **P < 0.01 compared with control group.
Discussion
Peptidoglycan is a dominant cell wall component of Lactobacillus bacteria and is constitutively released from the gut microbiota into the intestinal lumen,36,39 and can be absorbed and then circulate throughout the host.40,41 The immunomodulating effects of peptidoglycans from probiotic Lactobacilli that predominantly colonize the small intestine of the host on the intestinal immune system have been extensively studied. Increasing evidence demonstrates that peptidoglycan from commensal lactic acid bacteria not only affects gut homeostasis but also has substantial effects on systemic immune responses.19,27,42 Nevertheless, the molecular mechanisms of immunomodulation induced by peptidoglycans are not completely understood. Our results showed that intact peptidoglycan from L. rhamnosus MLGA promotes AvBD9 mRNA expression with different potential in chicken embryo jejunum, ileum, and cecum explants, as well as PBMCs, splenocytes, thymocytes, and hepatocytes in a dose-dependent manner. Of note, peptidoglycan hydrolysates (fragments) obtained by lysozyme digestion also has the capacity to induce AvBD9 expression in PBMCs. Furthermore, we observed that L. rhamnosus MLGA-derived peptidoglycan treatment dose dependently enhanced the antibacterial activity of both PBMC and splenocyte lysates against S. Enteritidis, whereas L. rhamnosus MLGA-derived peptidoglycan itself showed no inhibitory effect on the growth of S. Enteritidis, implying that an enhancement in the antibacterial activity of the peptidoglycan-treated cell lysates is, in part at least, due to the augmentation of AvBD9 expression. Based on these results, we propose that the defensin-inducing potential of peptidoglycans from specific probiotic bacteria species in immune cells and gut intestinal tissue of the host is another manner and mechanism of action through which the probiotic strains exert their immunomodulatory and anti-infection effects on the host.
Intact peptidoglycan and peptidoglycan fragments can be recognized by specific receptors termed PRRs, including TLRs, NLRs, peptidoglycan recognition proteins, and C-type lectin-like receptors, which are expressed intracellularly or on the cell surface in a variety of cell types, including various epithelial cells and immune cells of the host.43,44 Upon recognition of peptidoglycan or peptidoglycan-derived fragments, PRRs initiate downstream signaling cascade events that lead to the expression and secretion of a broad range of innate immune effector molecules, including cytokines, chemokines, interferons, host defense peptides such as defensins and cathelicidins, and to the onset of adaptive immune responses.45 The role of TLRs and NLRs in mediating the induction of β-defensin expression by peptidoglycan or its fragments has been previously demonstrated.34,46–48 In the present study, we showed that peptidoglycan hydrolysates (fragments) also promoted AvBD9 expression in PBMCs albeit at a lesser magnitude than intact peptidoglycan from L. rhamnosus MLAG. However, the inducible expression of AvBD9 by peptidoglycan hydrolysates was diminished in splenocytes as compared to intact peptidoglycan. The difference in AvBD9 induction by peptidoglycan hydrolysates between PBMCs and splenocytes may be ascribed to the different expression profile of PRRs between these two kinds of immune cell populations. For example, cytosolic bacterial peptidoglycan sensor NOD1, an NLR family member, is expressed in a variety of different cell types and tissues and is activated by peptidoglycan fragments containing meso-diaminopimelic acid primarily found in Gram-negative bacteria.49 In this sense, the peptidoglycan from Gram-positive L. rhamnosus is unable to be detected by NOD1. Conversely, NOD2 expression is restricted to hematopoietic cells and the epithelium of barrier tissues such as the skin, lungs and gastrointestinal tract.50 NOD2 recognizes N-acetylmuramic acid-containing peptidoglycan fragments with N-acetylmuramyl-L-alanyl-D-isoglutamine or muramyl dipeptide as a shared moiety, ubiquitously present in both Gram-positive and Gram-negative bacteria.51 Therefore, peptidoglycan fragments from L. rhamnosus MLGA can be recognized by NOD2 expressed intracellularly in PBMCs rather than in splenocytes. Another possibility explaining the difference in AvBD9 induction by peptidoglycan hydrolysates is the different composition of immune cell populations between PBMCs and splenocytes. In addition, the destroyed structural integrity of peptidoglycan by lysozyme digestion that causes the inability of corresponding PRR such as TLR2 to recognize the ligand, or the decreased binding affinity with PRRs may contribute to the reduced induction of AvBD9 expression in response to stimulation of lysozyme hydrolysate of peptidoglycan when compared with intact peptidoglycan from L. rhamnosus MLAG.
Studies have shown that peptidoglycans from probiotic lactic acid bacteria stains specifically provoke a pro-inflammatory response,18,52 or exhibit anti-inflammatory activity mainly depending on the subtle difference in the peptidoglycan structure.23,24,36,53 Comparison of the structure of peptidoglycans from various kinds of microorganisms showed the difference in the third amino acid (L-lysine and Meso-A2pm) of the peptide stem may play a critical role in the anti-inflammatory capacity of peptidoglycan.54 Our results showed no significant effects of intact peptidoglycan from L. rhamnosus MLGA on the inflammatory response. Notably, peptidoglycan hydrolysate obtained by in vitro lysozyme digestion, which also occurs in vivo in the intestinal tract, exhibited anti-inflammatory properties as observed by suppressed mRNA expression of pro-inflammatory cytokines IL-1β, IL-8, and IL-12p40 in PBMCs and splenocytes in response to the stimulation of hydrolysate of L. rhamnosus MLGA-derived peptidoglycan. In this regard, it would be proposed that L. rhamnosus MLGA-derived peptidoglycan, when administrated orally, could exert an anti-inflammatory effect on the host. In fact, the NOD2-mediated anti-inflammatory capacity of peptidoglycan from selected lactobacilli, when administrated orally, has been demonstrated in a mouse model of colitis.53 Considering the role of the lysozyme in peptidoglycan degradation, which occurs in the gastrointestinal tract, and the fact that peptidoglycan fragments derived from gut microbiota can be translocated from the intestinal mucosa without gut epithelial disruption into circulation allowing the modulation of distal immune cells,40,55,56 it is likely that L. rhamnosus MLGA or peptidoglycan from L. rhamnosus MLGA, when orally administered, could confer an immune-potentiating effect such as defensin induction not only in the gastrointestinal tract but also in the peripheral tissues to boost host immunity and disease resistance without triggering inflammatory responses. In this regard, the peptidoglycan from L. rhamnosus MLAG may have potential use as an alternative to antibiotic feed additives in poultry production. However, more work is needed to further determine the modulatory effects of L. rhamnosus MLAG or its peptidoglycan on β-defensin such as AvBD9 expression in peripheral tissues especially immune tissues besides gastrointestinal tissues of pathogen-challenged and non-challenged chickens, and to clarify the association between induction of AvBD9 expression and the resistance against pathogen infection in L. rhamnosus MLAG- or peptidoglycan-treated chickens by in vivo experiments.
Although considered a broad-spectrum pattern-recognizing system, innate immunity can detect very subtle differences in Gram-positive walls.57 Subtle structural modifications to peptidoglycan can influence the ability of the innate immune system to detect bacteria and can allow bacteria to evade or alter host defenses.42 In the present study, we found that, in contrast to the effect of L. rhamnosus MLGA-derived peptidoglycan, peptidoglycan from the pathogenic S. aureus suppressed AvBD9 expression, which provides evidence that down-regulated expression of a specific β-defensin such as AvBD9 may be an immune escape strategy used by the specific pathogen in chicken infections.
Conclusion
In summary, this study revealed a new possible immunomodulatory mechanism by which the peptidoglycan from L. rhamnosus MLGA enhances the innate defense response through the up-regulation of β-defensin such as AvBD9 expression in the gut as well as immune cells from peripheral tissues of chicken. More importantly, L. rhamnosus MLGA-derived peptidoglycan triggers no inflammatory response in immune cells while promoting β-defensin gene expression. Moreover, this work points out the possible potential use of peptidoglycan from L. rhamnosus as an alternative to feed antibiotic growth promoters in poultry production. However, further work is needed to explore the potential of L. rhamnosus-derived peptidoglycan to enhance the expression of antimicrobial peptides in pathogen-challenged chickens.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant Numbers: 31360555, 31560641) and Key Project of the Natural Science Foundation of Jiangxi Province ((Grant Number 20181ACB20015).
ORCID iD: Guanhong Li https://orcid.org/0000-0002-3589-9023
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature 2002; 415: 389–395. [DOI] [PubMed] [Google Scholar]
- 2.Cuperus T, Coorens M, van Dijk A, et al. Avian host defense peptides. Dev Comp Immunol 2013; 41: 352–369. [DOI] [PubMed] [Google Scholar]
- 3.Hölzl MA, Hofer J, Steinberger P, et al. Host antimicrobial proteins as endogenous immunomodulators. Immunol Lett 2008; 119: 4–11. [DOI] [PubMed] [Google Scholar]
- 4.Lai Y, Gallo RL. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol 2009; 30: 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avila EE. Functions of antimicrobial peptides in vertebrates. Curr Protein Pept Sci 2017; 18: 1098–1119. [DOI] [PubMed] [Google Scholar]
- 6.Lynn DJ, Higgs R, Gaines S, et al. Bioinformatic discovery and initial characterization of nine novel antimicrobial peptides genes in the chicken. Immunogenetics 2004; 56: 170–177. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y, Hughes AL, Ando J, et al. A genome-wide screen identifies a single β-defensin gene cluster in the chicken: Implications for the origin and evolution of mammalian defensins. BMC Genomics 2004; 5: 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yacoub HA, El-Hamidy SM, Mahmoud MM, et al. Biocidal activity of chicken defensin-9 against microbial pathogens. Biochem Cell Biol 2016; 94: 176–187. [DOI] [PubMed] [Google Scholar]
- 9.Van Dijk A, Veldhuizen EJ, Kalkhove SI, et al. The β-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob Agents Chemother 2007; 51: 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu J, Qi K, Xue T, et al. Construction of recombinant pichia pastoris carrying a constitutive AvBD9 gene and analysis of its activity. J Microbiol Biotechnol 2015; 25: 2082–2089. [DOI] [PubMed] [Google Scholar]
- 11.Sunkara LT, Achanta M, Schreiber NB, et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS ONE 2011; 6: e27225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Lu L, Li SM, et al. 1, 25-dihydroxyvitamin-D3 induces avian β-defensin gene expression in chicken. PloS ONE 2016; 11: e0154546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyu W, Deng Z, Sunkara LT, et al. High throughput screening for natural host defense peptide-inducing compounds as novel alternatives to antibiotics. Front Cell Infect Microbiol 2018; 8: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramasamy KT, Verma P, Reddy MR. Differential gene expression of antimicrobial peptides β-defensins in the gastrointestinal tract of Salmonella serovar Pullorum infected broiler chickens. Vet Res Commun 2012; 36: 57–62. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Zhang T, Xu Q, et al. Differential modulation of avian β-defensin and Toll-like receptor expression in chickens infected with infectious bronchitis virus. Appl Microbiol Biotechnol 2015; 99: 9011–9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu S, Jahejo AR, Jia FJ, et al. Transcripts of antibacterial peptides in chicken erythrocytes infected with Marek’s disease virus. BMC Vet Res 2018; 14: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukhithasri V, Nisha N, Biswas L, et al. Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions. Microbiol Res 2013; 168: 396–496. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Shi YH, Le GW, et al. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J Gastroenterol 2005; 11: 6330–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolling Y, Salva S, Villena J, et al. Non-viable immunobiotic Lactobacillus rhamnosus CRL1505 and its peptidoglycan improve systemic and respiratory innate immune response during recovery of immunocompromised-malnourished mice. Int Immunopharmacol 2015; 25: 474–484. [DOI] [PubMed] [Google Scholar]
- 20.Shida K, Kiyoshima-Shibata J, Kaji R, et al. Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced by Lactobacillus casei through Toll-like receptor 2-dependent and independent mechanisms. Immunol 2009; 128: e858–e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Z, Pan D, Guo Y, et al. Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr Polym 2015; 128: 130–137. [DOI] [PubMed] [Google Scholar]
- 22.Fichera GA, Giese G. Non-immunologically-mediated cytotoxicity of Lactobacillus casei and its derivative peptidoglycan against tumor cell lines. Cancer Lett 1994; 85: 83–103. [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Han X, Zhang Y, et al. Whole peptidoglycan extracts from the Lactobacillus paracasei subsp. Paracasei M5 strain exert anticancer activity in vitro. Biomed Res Int 2018; 2018: 2871710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LS, Zhu HM, Zhou DY, et al. Influence of whole peptideoglycan of bifidobacterium on cytotoxic effectors produced by mouse peritoneal macrophages. World J Gastroenterol 2001; 7: 440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clua P, Kanmani P, Zelaya H, et al. Peptidoglycan from immunobiotic Lactobacillus rhamnosus improves resistance of infant mice to respiratory syncytial viral infection and secondary pneumococcal pneumonia. Front Immunol 2017: 8: 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Sun Q, Wang Y, et al. The regulatory effects of L. plantarum peptidoglycan microspheres on innate and humoral immunity in mouse. J Microencapsul 2017; 34: 635–643. [DOI] [PubMed] [Google Scholar]
- 27.Kolling Y, Salva S, Villena J, et al. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PloS ONE 2018; 13: e0194034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlee M, Harder J, Köten B, et al. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol 2008; 151: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzo A, Losacco A, Romano C, et al. Lactobacillus crispatus modulates epithelial cell defense against Candida albicans through Toll-like receptors 2 and 4, interleukin 8 and human β-defensins 2 and 3. Immunol Lett 2013; 156: 102–109. [DOI] [PubMed] [Google Scholar]
- 30.Kusumaningsih T, Subijanto MS, Indrawati R, et al. The level of beta defensin-2 in saliva and its expression in parotid gland epithelial cells after probiotic (Lactobacillus reuteri) induction to inhibit Streptococcus mutans in caries. Eur J Dent 2016; 10: 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi H, Wang L, Xiong Y, et al. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J Anim Sci 2018; 96: 2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li GH, Hong ZM, Jia YJ, et al. Probiotic Lactobacilli stimulate avian beta-defensin 9 expression in cultured chicken small intestinal epithelial cells. Proc Nutr Soc 2012; 71: E239. [Google Scholar]
- 33.Tian PJ, Li BL, Shan YJ, et al. Extraction of peptidoglycan from L. paracasei subp. Paracasei X12 and its preliminary mechanisms of inducing immunogenic cells death in HT-29 cells. Int J Mol Sci 2015; 16: 20,033–20,049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia Y, Si W, Hong Z, et al. Toll-like receptor 2-mediated induction of avian β-defensin 9 by Lactobacillus rhamnosus and its cellular components in chicken intestinal epithelial cells. Food Agr Immunol 2019; 30: 398–417. [Google Scholar]
- 35.Muller CA, Autenrieth IB, Pesche A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci 2005; 62: 1297–1307. [DOI] [PubMed] [Google Scholar]
- 36.Baik JE, Jang YO, Kang SS, et al. Differential profiles of gastrointestinal proteins interacting with peptidoglycans from Lactobacillus plantarum and Staphylococcus aureus. Mol Immunol 2015; 65: 77–85. [DOI] [PubMed] [Google Scholar]
- 37.McDermott AM, Redfern B, Zhang Y, et al. Defensin expression by the cornea: Multiple signaling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci 2003; 44: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelsalam M, Isobe N, Yoshimura Y. Effects of lipopolysaccharide and interleukins on the expression of avian β-defensins in hen ovarian follicular tissue. Poult Sci 2012; 91: 2877–2884. [DOI] [PubMed] [Google Scholar]
- 39.Kang D, Liu G, Lundstrom A, et al. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA. 1998; 95: 10,078–10,082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke TB, Davis KM, Lysenko ES, et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 2010; 16: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arentsen T, Qian Y, Gkotzis S, et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry 2017; 22: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf AJ, Underhill DM. Peptidoglycan recognition by the innate immune system. Nat Rev Immunol 2018; 18: 243–254. [DOI] [PubMed] [Google Scholar]
- 43.Dziarski R. Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci 2003; 60: 1793–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irazoki O, Hernandez SB, Cava F. Peptidoglycan muropeptides: Release, perception, and functions as signaling molecules. Front Microbiol 2019; 10: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011; 34: 637–650. [DOI] [PubMed] [Google Scholar]
- 46.Vora P, Youdim A, Thomas LS, et al. β-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 2004; 173: 5398–5405. [DOI] [PubMed] [Google Scholar]
- 47.Ferrand A, Nabhanni ZA, Tapias NS, et al. NOD2 expression in intestinal epithelial cells protects toward the development of inflammation and associated carcinogenesis. Cell Mol Gastroenterol Hepatol 2019; 7: 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voss E, Wehkamp J, Wehkamp K, et al. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem 2005; 281: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 49.Girardin SE, Boneca IG, Carneiro LA, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003; 300: 1584–1587. [DOI] [PubMed] [Google Scholar]
- 50.Griffin ME, Hespen. CW, Wang YC, et al. Translation of peptidoglycan metabolites into immunotherapeutics. Clin Transl Immunol 2019; 8: e1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003; 278: 8869–8872. [DOI] [PubMed] [Google Scholar]
- 52.Shida K, Kiyoshima-Shibata J, Nagaoka M, et al. Induction of interleukin-12 by Lactobacillus strains having a rigid cell wall resistant to intracellular digestion. J Dairy Sci 2006; 89: 3306–3317. [DOI] [PubMed] [Google Scholar]
- 53.Macho-Fernandez E, Valenti V, Rockel C, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011; 60: 1050–1059. [DOI] [PubMed] [Google Scholar]
- 54.Wu Z, Pan D, Guo Y, et al. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr Polym 2013; 96: 466–473. [DOI] [PubMed] [Google Scholar]
- 55.Tosoni G, Conti M, Diaz Heijtz R. Bacterial peptidoglycans as novel signaling molecules from microbiota to brain. Curr Opin Pharmacol 2019; 48: 107–113. [DOI] [PubMed] [Google Scholar]
- 56.Hergott CB, Roche AM, Tamashiro E, et al. Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood 2016; 127: 2460–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreillon P, Majcherczyk PA. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand J Infect Dis 2003; 35: 632–641. [DOI] [PubMed] [Google Scholar]