Abstract
Long-chain n-3 polyunsaturated fatty acids are known to have beneficial effects on intestinal health. However, the underling mechanisms are largely unknown. The present study was conducted to investigate whether docosahexaenoic acid (DHA) attenuates TNF-α-induced intestinal cell injury and barrier dysfunction by modulating necroptosis signalling. Intestinal porcine epithelial cell line 1 was cultured with or without 12.5 µg/ml DHA, followed by exposure to 50 ng/ml TNF-α for indicated time periods. DHA restored cell viability and cell number triggered by TNF-α. DHA also improved barrier function, which was indicated by increased trans-epithelial electrical resistance, decreased FD4 flux and increased membrane localisation of zonula occludins (ZO-1) and claudin-1. Moreover, DHA suppressed cell necrosis in TNF-α-challenged cells, as shown in the IncuCyte ZOOM™ live cell imaging system and transmission electron microscopy. In addition, DHA decreased protein expression of TNF receptor, receptor interacting protein kinase 1, RIP3 and phosphorylation of mixed lineage kinase-like protein, phosphoglycerate mutase family 5, dynamin-related protein 1 and high mobility group box-1 protein. Furthermore, DHA suppressed protein expression of caspase-3 and caspase-8. Collectively, these results indicate that DHA is capable of alleviating TNF-α-induced cell injury and barrier dysfunction by suppressing the necroptosis signalling pathway.
Keywords: DHA, cell injury, barrier function, necroptosis, IPEC-1
Introduction
The intestine is not only important for nutrient digestion and absorption, it also serves to protect against endogenous and exogenous harmful agents. Intestinal epithelial cells are a critical part of the intestinal mucosa, and serve as the first line of defence against noxious Ags and pathogens, maintaining the intestinal structure and barrier function.1 However, epithelial cells are sensitive to various factors such as infection and inflammation, which lead to intestinal epithelial damage, dysfunction and eventually intestinal disorders such as necrotising enterocolitis.2,3 Intestinal epithelial cells are especially susceptible to pro-inflammatory cytokines, which often lead to cell injury and compromise of intestinal integrity.4–6
Necrosis, apoptosis and autophagy are the main forms of cell death associated with intestinal injury in animals and humans.7 Necroptosis combines the features of apoptosis and necrosis, and is mainly mediated by receptor interacting protein kinase 3 (RIP3) and mixed lineage kinase-like protein (MLKL).8–10 Recently, necroptosis has been shown to play an important role in tissue injury or necrosis caused by multiple factors, such as ischaemia reperfusion and inflammation in the heart, brain or intestine.11–13 Therefore, nutritional regulation of necroptosis may exert beneficial effects in alleviating intestinal epithelial cell injury and protecting the intestine function.
Fatty acids are known to be involved in the regulation of intestinal health.14,15 Long-chain n-3 polyunsaturated fatty acids (PUFAs), especially docosahexaenoic acid (DHA, 22:6(n-3)) and eicosapentaenoic acid (EPA, 20:5(n-3)), abundant in deep-sea fish oil, have been reported to sustain intestinal mucosal integrity and barrier function in animal models with colitis and patients with bowel disease.16,17 Therefore, consumption of n-3 PUFA (DHA and EPA) has become officially recommended by health organisations and government agencies.18 Our previous studies have also shown that fish oil (rich in DHA and EPA) prevented LPS-induced intestine injury in a pig model.19,20 However, the precise mechanisms of n-3 PUFAs in modulating intestinal damage and barrier function are still poorly defined.
Therefore, we hypothesised that DHA, a representative long-chain n-3 PUFA in fish oil, could alleviate cell injury and barrier dysfunction by modulating the necroptosis signalling pathway. In the current experiment, TNF-α, a potent pro-inflammatory cytokine, was used to establish a model of cell damage.4 This hypothesis was tested with the use of an intestinal porcine epithelial cell line (IPEC-1), which is highly susceptible to pro-inflammatory cytokine challenge.21
Methods
Materials and Abs
DHA, TNF-α, yoyo-3, fluorescein isothiocyanate–dextran (FD4) and FBS were purchased from Sigma–Aldrich. DMEM-F12 was purchased from HyClone. The Abs against TNF receptor 1 (TNFR1), phosphoglycerate mutase family 5 (PGAM5) and dynamin-related protein 1 (DRP1) were procured from Abcam. Abs against receptor interacting protein kinase 1 (RIP1) and RIP3 were purchased from Santa Cruz Biotechnology. Abs against caspase-3, caspase-8, total MLKL and phospho-MLKL were purchased from Cell Signaling Technology. High mobility group box-1 (HMGB1) Ab was acquired from Abnova. Ab against β-actin was purchased from Sigma–Aldrich. Abs against claudin-1 and zonula occludins (ZO-1) were obtained from Invitrogen and Biorbyt, respectively. HRP-conjugated anti-rabbit and anti-mouse secondary Abs were purchased from Santa Cruz Biotechnology.
Cell culture
The IPEC-1 cell line was derived from the small intestine of a neonatal piglet, which was a gift from Dr Guoyao Wu at Texas A&M University. Cells were isolated from both the jejunum and ileum of newborn pigs immediately after birth (without being nursed by the sow), and the procedures were approved by the Texas A&M University Animal Care and Use Committee. The cells were cultured according to the protocol of previous experiments.22 Cells were cultured in DMEM-F12 medium supplemented with 5% FBS, 1% insulin-transferrin-selenium, 1% penicillin/streptomycin and epidermal growth factor (5 ng/ml). The cells were incubated at 37°C in a humidified atmosphere of 5% CO2, and the culture medium was changed every second day, according to standard culture protocols.
Cell viability assays
IPEC-1 cells were seeded on a 96-well microplate (Corning) and incubated with or without 12.5 μg/ml DHA for 24 h, followed by exposure with or without 50 ng/ml TNF-α for 48 h. The concentrations of DHA and TNF-α were chosen according to Xiao et al.4 and our preliminary research.23 Cell viability was detected with a use of a Cell Counting Kit-8 detection kit (Beyotime Institute of Biotechnology).
Cell number
IPEC-1 cells were seeded onto 12-well plates and incubated with 0 or 12.5 μg/ml DHA for 24 h and then treated with PBS or 50 ng/ml TNF-α for 48 h. Cells were then stained with trypan blue, and total cell count was determined with a haemocytometer.
Lactate dehydrogenase activity measurement
IPEC-1 cells were seeded onto 12-well plates and then incubated with 0 or 12.5 μg/ml DHA for 24 h, followed by the addition of PBS or 50 ng/ml TNF-α for 48 h. Subsequently, cell supernatants were collected for lactate dehydrogenase (LDH) measurement using a commercial kit (Nanjing Jiancheng Institute of Bioengineering), as previously described by Jiao et al.24
Trans-epithelial electrical resistance measurements
IPEC-1 cells were seeded on permeable Transwell™ inserts (membrane area 0.33 cm2; pore size 0.4 mm) placed on 24-well culture plates for 10 d to become confluent and polarised. Cells were then incubated with or without 12.5 μg/ml DHA for 24 h, followed by exposure with or without 50 ng/ml TNF-α for the indicated time points. Trans-epithelial electrical resistance measurements (TEER) were determined with the use of an EVOM volt-ohmmeter (Millipore) connected to a 12-mm EndOhm unit (World Precision Instruments) every 24 h, as described by Xiao et al.4 TEER values were obtained by subtracting the contribution of the filter and bathing solution.
Monolayer paracellular permeability determination
Paracellular permeability was determined by adding 1 mg/ml FD4 to the apical side of the monolayer, as previously described.4 The flux of FITC-dextran was determined by serially sampling the basolateral compartment every 12 h. The concentration of FD4 was measured with a fluorescence microplate reader (Bio-Tek Instruments). The permeability of monolayer cells was defined as the amount of FD4 that was transported to the basolateral chamber from the apical chamber.
IncuCyte ZOOM™ assay
IPEC-1 cells were seeded onto 24-well cell culture plates and incubated with or without 12.5 μg/ml DHA for 24 h. Following incubation, cells were treated with PBS or 50 ng/ml TNF-α in the IncuCyte ZOOM™ live cell imaging system (Essen BioScience) to measure cell necrosis in real time for up to 72 h. The system automatically calculates the relative density of necrotic cells within the initially vacant area at each time point. Yoyo-3 was added to stain nucleic acid. Data were exported and analysed by IncuCyte S3 software (Essen Bioscience).
Transmission electron microscopy
IPEC-1 cells were cultured with 0 or 12.5 μg/ml DHA for 24 h and treated with PBS or 50 ng/ml TNF-α for another 48 h. Cells were then prepared for transmission electron microscopy (TEM; FEI TECNAI G20), as described by He et al.25 For quantitative analysis of necrotic cells, a total of 200 cells were counted, and the number of necrotic cells was recorded. Necrotic cells were defined as cells with morphology characteristics of primary necrosis (plasma membrane ruptures without nuclear condensation).
Western blot analysis
IPEC-1 cells were seeded onto 12-well plates and incubated with 0 or 12.5 μg/ml DHA for 24 h, followed by treatment with PBS or 50 ng/ml TNF-α for another 48 h. Cells were then lysed and subjected to Western blotting, as previously described.19 Blots were incubated with primary Abs against TNFR1 (1:1000), RIP1 (1:1000), RIP3 (1:1000), total MLKL (1:1000), phospho-MLKL (1:1000), PGAM5 (1:1000), DRP1 (1:1000), HMGB1 (1:1000), caspase-3 (1:1000), caspase-8 (1:1000) and β-actin (1:10,000) overnight at 4°C, and then a secondary Ab (1:5000) at 25°C for 2 h. The blots were detected with an enhanced Chemiluminescence Western Blot Kit (Amersham Biosciences) and processed with the Quantity One® software (Bio-Rad Laboratories). The results were expressed as the abundance of each target protein relative to β-actin, except for phosphorylated MLKL, which was normalised with total MLKL.
Confocal immunofluorescence microscopy
IPEC-1 cells were seeded on microscope coverslips on 24-well plates. Following a sequential treatment with DHA and TNF-α, cells were fixed with 4% paraformaldehyde and blotted with 1% BSA. The cells were incubated with primary Abs against claudin-1 (1:500) and ZO-1 (1:50) overnight at 4°C and then incubated with an appropriate secondary Ab (1:5000) at 25°C for 2 h. Nuclei were stained with 4,6-diamidino-2-phenylindole (1 mg/ml). The distribution of tight junction proteins was visualised under a fluorescence confocal laser scanning microscope (Olympus FV101). Five images were taken per slide by the confocal microscope for quantification of fluorescence.
Statistical analysis
All data were analysed with ANOVA using the general linear model procedures of SAS for a 2 × 2 factorial design (SAS Institute). The statistical model included the effects of DHA (control or DHA), TNF-α (PBS or TNF-α) and their interactions. When there was a significant interaction or a trend for interaction, post hoc testing was conducted using Duncan’s multiple comparison tests. P ≤ 0.05 was considered significant, and 0.05 < P ≤ 0.10 was considered a trend.
Results
Cell viability, cell number and LDH activity
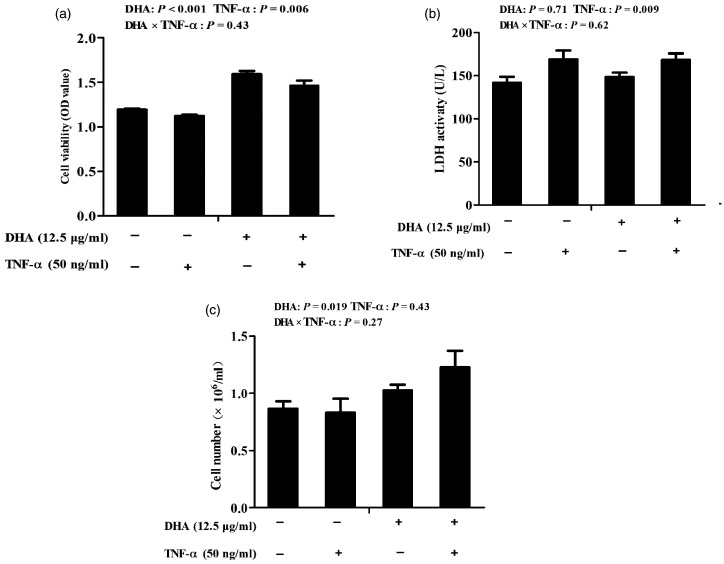
Cells challenged with TNF-α had lower cell viability (P < 0.01) and higher LDH activity (P < 0.01) than control cells (Figure 1). There was no DHA–TNF-α interaction observed for cell viability, cell number or LDH activity. Relative to control cells, cells treated with DHA had higher cell viability (P < 0.001) and cell number (P = 0.05).
Figure 1.
Effect of docosahexaenoic acid (DHA) on the viability (a), cell number (b) and the lactate dehydrogenase (LDH) activity (c) of intestinal porcine epithelial cell 1 (IPEC-1) cells following TNF-α challenge. Cells were incubated with or without 12.5 μg/ml DHA for 24 h, followed by treatment with or without 50 ng/ml TNF-α for 48 h. Values are means ± SEM (n = 8).
Barrier function
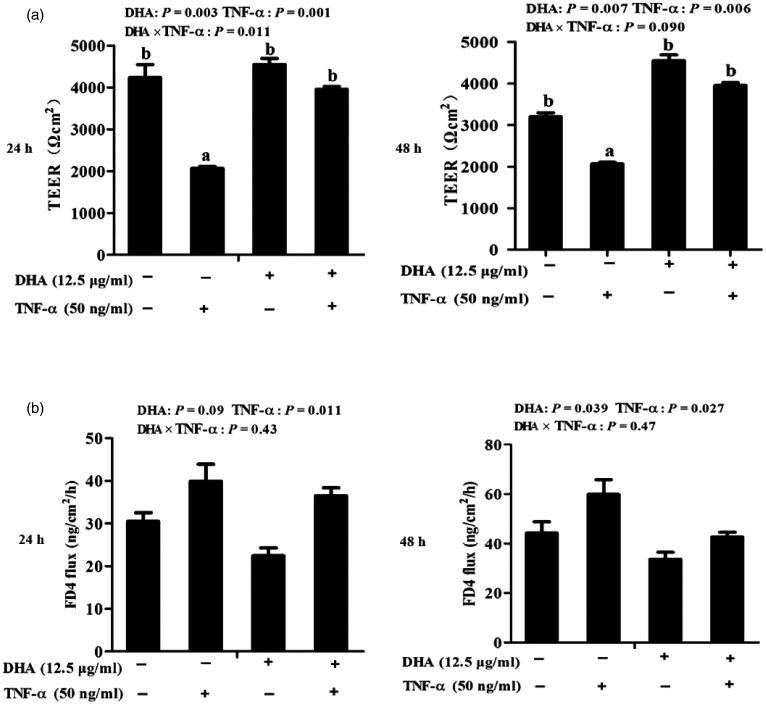
Cells treated with TNF-α had lower TEER than control cells at 24 h (P < 0.01) and 48 h (P < 0.01; Figure 2a). A significant DHA–TNF-α interaction occurred at 24 h (P < 0.05). A trend for a DHA–TNF-α interaction was observed for TEER at 48 h (P < 0.1), in which cells treated with DHA had higher TEER at 24 h (P < 0.01) and 48 h (P < 0.01) than control cells in the presence of TNF-α, whereas TEER did not differ among PBS-treated cells. No DHA–TNF-α interaction was observed for FD4 flux at 24 h or 48 h (Figure 2b). Cells treated with TNF-α had higher FD4 flux than PBS-treated cells at 24 h (P < 0.05) and 48 h (P < 0.05). However, DHA reduced FD4 flux relative to control at 24 h (P < 0.1) and 48 h (P < 0.05).
Figure 2.
Effect of DHA on barrier function of TNF-α-treated IPEC-1 cells. (a) Trans-epithelial electrical resistance (TEER) after TNF-α stimulation for 24 and 48 h. (b) FITC-labelled dextran 4 kDa (FD4) flux after TNF-α stimulation for 24 and 48 h. Values are means ± SEM (n = 6). Means without a common superscript letter differ significantly (P < 0.05).
Tight junction protein distribution
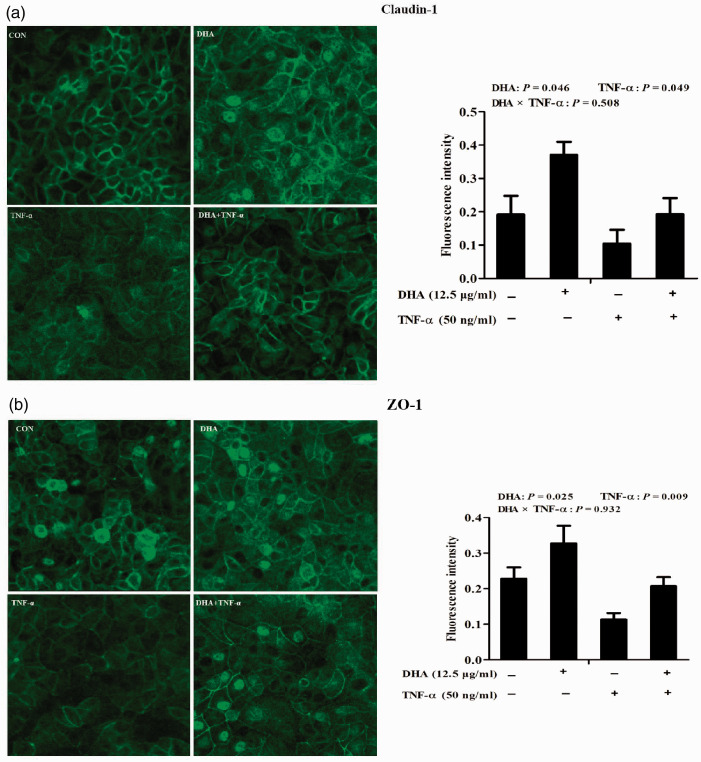
Cellular distribution of tight junction proteins was assessed with laser scanning confocal microscopy. In control cells, tight junction proteins, claudin-1 and ZO-1, were uniformly localised on cell membranes but disrupted by TNF-α. In contrast, DHA restored localisation of both claudin-1 and ZO-1 triggered by TNF-α. Quantification of the fluorescence intensity indicated that, relative to control, TNF-α significantly reduced claudin-1 (P < 0.05; Figure 3a) and ZO-1 (Figure 3b) protein expression (P < 0.01).
Figure 3.
Effect of DHA on membrane distribution of tight junction proteins after TNF-α challenge of IPEC-1 cells. Cells were pre-incubated with 12.5 μg/ml DHA for 24 h and treated with or without 50 ng/ml TNF-α for 48 h. Cellular localisation of claudin-1 (a) and ZO-1 (b) were visualised using a confocal microscope, and the fluorescence intensity was measured. Values are means ± SEM (n = 6).
Cell necrosis
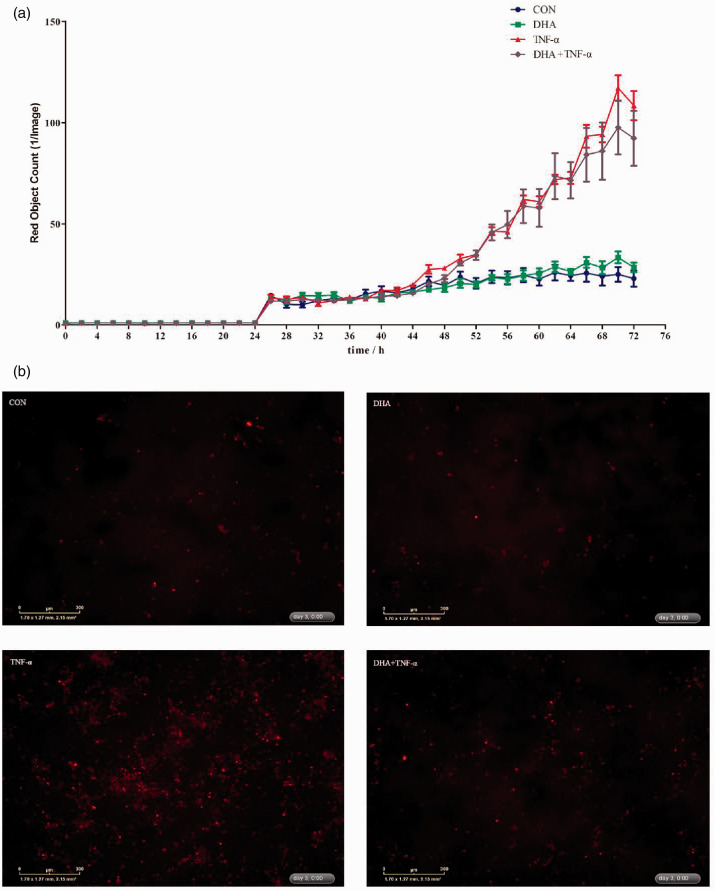
The IncuCyte ZOOM™ live cell imaging system was used to monitor dynamic changes of cell necrosis in response to DHA and TNF-α (Supplemental Videos S1–S4). Relative to the control, TNF-α had a higher number of necrotic cells from 24 to 72 h (Figure 4a). DHA decreased cell necrosis in the presence of TNF-α. There was a DHA–TNF-α interaction observed for necrotic cell density at 48 h after TNF-α treatment in which DHA suppressed cell necrosis in the presence of TNF-α (Supplemental Table S1). The protective effects of DHA on cell necrosis were also verified by images from the system at 24 and 48 h after TNF-α treatment (Figure 4b and Supplemental Table S1).
Figure 4.
Effect of DHA on cell necrosis after TNF-α challenge of IPEC-1 cells. Cells were pre-incubated with 12.5 μg/ml DHA for 24 h and treated with or without 50 ng/ml TNF-α for up to 72 h. (a) Dynamic changes of cell necrosis as revealed by the IncuCyte ZOOM™ live cell imaging system. (b) Representative images of cell necrosis at 48 h after TNF-α stimulation (necrotic cells were red dyed with yoyo-3).
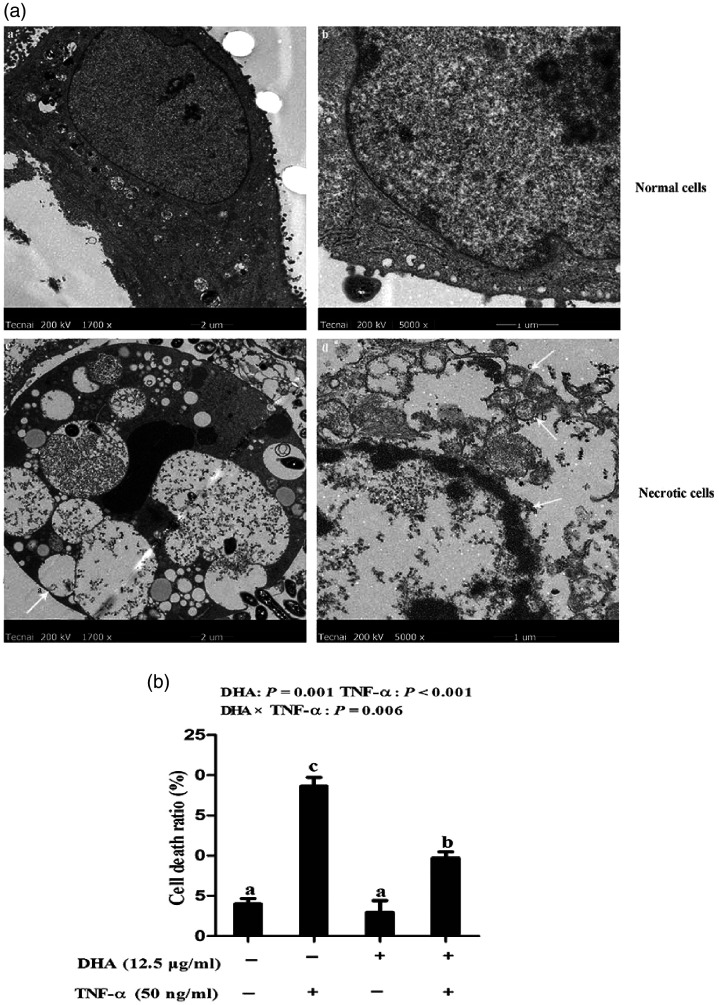
The effect of DHA on the ultrastructure of cell necrosis was further analysed with TEM. Compared to normal cells with intact membranes and normal nuclei, TNF-α caused ruptured cell membranes, overflowed cytoplasm and chromatin, and appearance of apoptotic bodies, vacillated mitochondria and unclear endoplasmic reticulum (Figure 5a). We further measured the necrotic cell number. Consistently, cells treated with TNF-α had a significant higher percentage of necrosis than the control cells (P < 0.001; Figure 5b). A DHA–TNF-α interaction was observed for the percentage of cell necrosis (P < 0.01), in which cells incubated with DHA had lower cell necrosis ratios (P < 0.01) at 48 h among TNF-α treated groups. In contrast, cell necrosis ratios did not differ among PBS-treated cells.
Figure 5.
Effect of DHA on cell necrosis after TNF-α challenge in IPEC-1 cells by TEM. (a) Morphological structure of normal or necrotic cells. The magnification of the two left panels is 1700×, while that of the two right panels is 5000×. (b) Percentage of cell necrosis after 48 h of TNF-α challenge. Values are means ± SEM (n = 6).
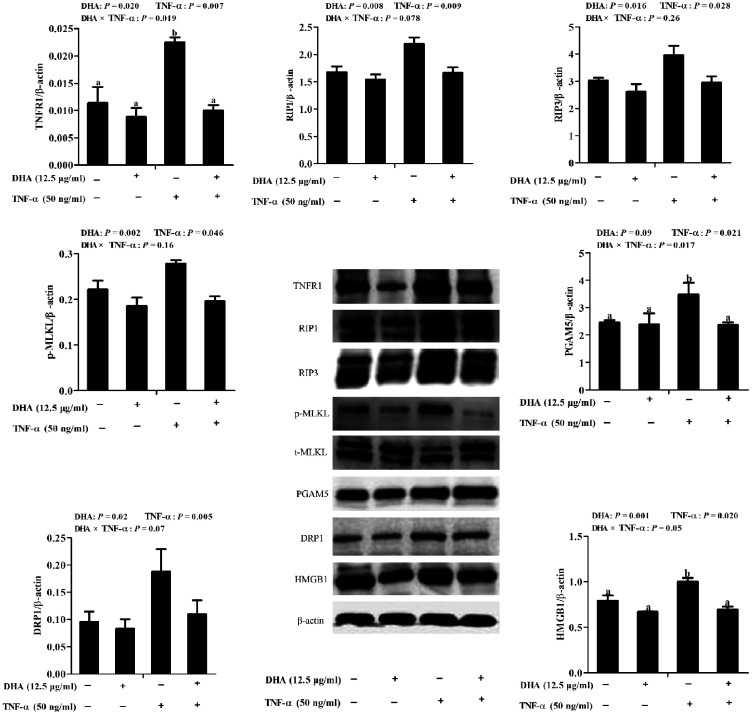
Abundance of necroptosis-related proteins
To explore further the mechanisms responsible for the beneficial effect of DHA against TNF-α-induced IPEC-1 cell injury, key components of the necroptosis signalling pathway were detected by immunoblotting. A trend for a DHA–TNF-α interaction was observed for RIP1 and DRP1, in which DHA decreased the protein expression of RIP1 (P < 0.01) and DRP1 (P < 0.05) in TNF-α-treated cells compared to the control cells. In contrast, RIP1 and DRP1 did not differ among PBS-treated cells (Figure 6). A DHA–TNF-α interaction occurred for TNFR1 (P < 0.05), PGAM5 (P < 0.05) and HMGB1 (P = 0.05) protein expression, in which DHA decreased the protein expression of TNFR1 (P < 0.05), PGAM5 (P < 0.1) and HMGB1 (P < 0.01) compared to the control among TNF-α-treated cells. However, they did not differ among PBS-treated cells. No DHA–TNF-α interaction was observed for RIP3 and phosphorylated MLKL. Cells treated with TNF-α had higher RIP3 (P < 0.05) and MLKL phosphorylation (P < 0.05) than the control cells. DHA decreased the RIP3 (P < 0.05; P = 0.016) and phosphorylated MLKL (P < 0.01) in the presence or absence of TNF-α.
Figure 6.
Effect of DHA on protein abundance of necroptosis-related signalling components in IPEC-1 cells. Cells were first pre-incubated with 12.5 μg/ml DHA for 24 h and then treated with or without 50 ng/ml TNF-α for 48 h. Values are means ± SEM n=6. Means without a common superscript letter differ significantly (P < 0.05). TNFR1: tumour necrosis factor receptor 1; RIP1: receptor interacting protein 1; RIP3: receptor interacting protein 3; MLKL:, mixed-lineage kinase domain like-domain protein; HMGB1: high mobility group box-1 protein; Drp1: dynamin-related protein 1; PGAM5: phosphoglycerate mutase family 5.
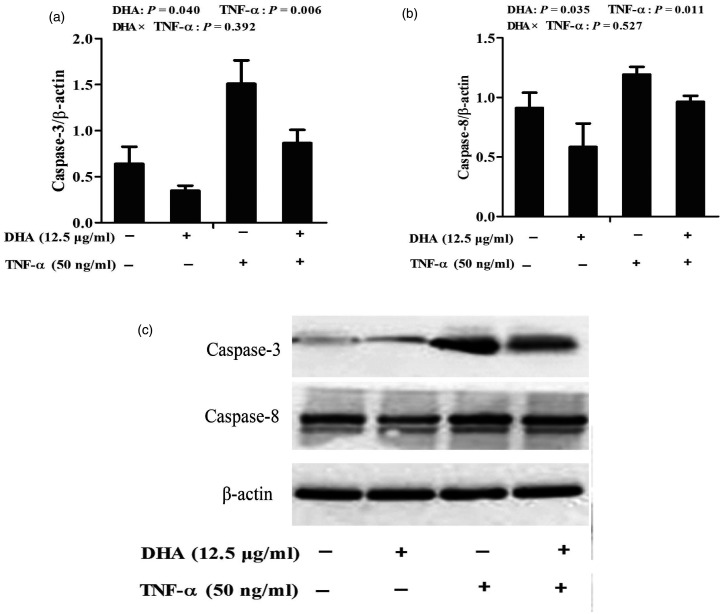
Protein abundance of caspase-3 and caspase-8
TNF-α significantly increased caspase-3 (P < 0.01) and caspase-8 (P < 0.05) protein expression relative to the PBS control (Figure 7). No DHA–TNF-α interaction was observed for caspase-3 and caspase-8 protein expression. DHA decreased protein expression of caspase-3 (P < 0.05) and caspase-8 (P < 0.05) in the presence or absence of TNF-α.
Figure 7.
Effect of DHA on protein expression of caspase-3 (a) and caspase-8 (b) in IPEC-1 cells after TNF-α challenge. Cells were first pre-incubated with 12.5 μg/ml DHA for 24 h and then treated with or without 50 ng/ml TNF-α for 48 h. Values are means ± SEM n=6. (c) A representative blot is shown.
Discussion
The intestinal epithelium is a single layer of cells lining the intestine to serve as a physical defensive barrier against potentially harmful luminal contents, such as noxious Ags and pathogens. Simultaneously, epithelial cells are susceptible to various factors, such as infection and inflammation, which also lead to intestinal impairment and severe gut diseases.3 Substantial evidence has shown that pro-inflammatory cytokine TNF-α is implicated in intestinal damage and barrier dysfunction in various cell types, including intestinal epithelial cells.4,5 In this study, we used a TNF-α-induced IPEC-1 cell injury model to explore the protective effect of DHA on epithelial cell injury. In our preliminary experiments, different concentrations of DHA (0, 6.25, 12.5, 25 and 50 μg/ml) and TNF-α (0, 10, 20, 40 and 50 ng/ml) were used to explore the appropriate doses. According to the results, 12.5 μg/ml DHA and 50 ng/ml TNF-α were chosen to conduct our further study.23
Cell viability and number are common metrics for assessing cell growth. The enzyme LDH is found in virtually all living cells, and is released extracellularly when cells are damaged. Therefore, it is commonly used as a marker of cell injury. In agreement with earlier reports,26,27 we demonstrated that TNF-α decreased cell viability, but such effects were largely reversed by DHA. Our results are similar to the findings of Pacheco et al. who reported that DHA increased cell viability after TNF-α stimulation in L929 cells.26 Han et al. also found that DHA could increase the proliferation of mouse spleen cells and improve the viability of NK cells in vivo.28 Our previous research also found that fish oil (rich in DHA and EPA) increased intra-epithelial lymphocyte numbers in the small intestine of LPS-challenged piglets.20
Intestinal epithelium is a physical barrier against the penetration of luminal bacteria and dietary allergens into the mucosa. Epithelial cell barrier function can be commonly evaluated by TEER and mucosal-to-serosal flux of FD4. In the current study, DHA supplementation increased TEER and reduced FD4 permeability across the IPEC-1 monolayer induced by TNF-α exposure. DHA supplementation prevented the drop in TEER associated with TNF treatment. Similar to our results, Willemsen et al. reported that DHA supported epithelial barrier integrity in T84 cells by improving TEER and reducing IL4-mediated permeability.29 Zhao et al. also found that DHA protected the intestinal barrier function of IL-10-deficient mice by ameliorating intestinal permeability.30
The maintenance of intestinal epithelial barrier function primarily relies on a contiguous layer of intestinal epithelia and tight junctions between epithelial cells.31 Tight junctions consist of many proteins such as claudins, occludins and ZO-1.32 Besides their role of sealing tight junctions, claudin-1 and ZO-1 also selectively regulate paracellular permeability.33 So, any alteration of tight junction proteins directly contributes to barrier function impairment.34 TNF-α has been reported to induce tight junction dysfunction by many signalling pathways.35,36 Thomas et al. found TNF-α-induced intestinal epithelial tight junction impairment requires NF-kappa B activation.35 Feng et al. reported that TNF-α-induced loss of intestinal barrier function required TNFR1 and TNFR2 signalling activation in a mouse model of total parenteral nutrition.36 In this study, we demonstrated that DHA supplementation reversed the disruption of claudin-1 and ZO-1 caused by TNF-α, which is consistent with the results of TEER and FD4. Similarly, Wang et al. reported that DHA attenuated ischaemia–reperfusion-induced intestinal barrier injury by attenuating the damage of tight junction structure and elevating the expression of tight junction proteins.37 In another in vitro study, Beguin et al. also found that DHA prevented the disruption of epithelial barrier function and redistribution of key tight junction proteins, including occludin and ZO-1, induced by pro-inflammatory cytokines.38 Liu et al. also reported dietary addition of fish oil which was rich in n-3 PUFA could enhance intestinal integrity and barrier function indicated by improved intestinal morphology, decreased plasma diamine oxidase activity and increased mucosal diamine oxidase activity, as well as enhanced protein expression of intestinal tight junction proteins, including occludin and claudin-1, in weaned piglets.19 In this current study, DHA improved barrier function by improving tight junction protein expression. It was possible that DHA alleviated the barrier function dysfunction induced by TNF-α which may be associated with cell injury restoration.
Necrosis can cause epithelial cell injury and barrier dysfunction.39 Therefore, we determined cell necrosis using the IncuCyte ZOOM™ live cell imaging system, and observed that TNF-α increased epithelial cell necrosis, while DHA reversed the TNF-α effect. Consistently, we utilised TEM to characterise the ultrastructure of cells. Compared to the control cells, TNF-α challenge resulted in plasma membrane and nuclear membrane rupture, as well as nuclear and cellular swelling, and mitochondrial vacuolisation. DHA supplementation suppressed cellular structure damage caused by TNF-α. Similarly, Kishida et al. reported that DHA reduced L929 cell necrosis induced by TNF-α.27 DHA also effectively attenuated TNF-α-induced necroptosis and autophagy in L929 cells.26 These results suggest that the protective role of DHA on cell injury may be related to the inhibition of cell necrosis.
To elucidate further the molecular mechanisms underlying the beneficial effect of DHA on intestinal cell injury and barrier function impairment, we studied its impact on the necroptosis signalling pathway, which is primarily mediated by RIP1/RIP3/MLKL. Once triggered by TNF-α, Fas, TNF-α-related apoptosis-inducing ligands and TLR agonists, TNFR1 is activated to form a primary membrane complex known as complex I, which in turn leads to sequential formation of cytoplasmic complex II and the RIP1/RIP3/MLKL necrosome and activation of PGAM5 and Drp1 to cause mitochondrial fragmentation and release of intracellular contents such as HMGB1 outside of necrotic cells.9,40,41 Necroptosis occurs when caspase-8 activation fails or is inhibited after complex II formation.42 Several studies have reported that necroptosis is involved in the dysfunction of intestinal epithelial cells.43,44 Therefore, inhibition of necroptosis could ameliorate cell damage.45 In the present study, TNF-α challenge up-regulated protein expression of TNFR1, RIP1, RIP3, phosphorylated MLKL, PGAM5, DRP1 and HMGB1, indicative of epithelial cell necroptosis. However, DHA reduced the protein abundance of TNFR1, RIP1, RIP3, phosphorylated MLKL, PGAM5, DRP1 and HMGB1, implying a protective effect. To our knowledge, the current study revealed for the first time that DHA is capable of alleviating cell injury and protecting barrier function by suppressing necroptosis signalling. Consistently, DHA was reported earlier to inhibit TNF-α-induced necroptosis by reducing oxidative stress, ceramide production and autophagy in L929 cells.26 Zhu et al. also found that the dietary addition of flaxseed oil could enhance intestinal integrity and barrier function in weaned piglets, which was involved in modulating necroptosis and TLR4/NOD signalling pathways.46 These results showed that DHA suppressed the necroptosis signalling pathway, which may finally lead to reduced cell injury and better barrier function.
Necroptosis occurs when apoptosis is inhibited and apoptosis is caspase-dependent regulated cell death.47 Interestingly, in our study, TNF-α challenge up-regulated the protein expression of caspase-3 and caspase-8, indicating TNF-α induced intestinal epithelial cell apoptosis. However, DHA supplementation down-regulated the protein expression of caspase-3 and caspase-8, which suggests that DHA alleviated cell injury and protected barrier function via inhibition of apoptosis. Similarly, n-3 fatty acids (DHA and ALA) could prevent oxidative stress-induced apoptosis by inhibiting apoptotic gene expression and DNA fragmentation of gastric epithelial cells.48 So, in the current study, DHA inhibited not only the necroptosis signalling pathway induced by TNF-α, but also the apoptosis signalling pathway.
In summary, we provide evidence suggesting that DHA alleviates intestinal epithelial cell damage, improves cell barrier function and decreases cell necrosis and apoptosis in intestinal epithelial cells following TNF-α exposure. These beneficial effects of DHA are mainly mediated by suppressing the cell necroptosis signal in IPEC-1 cells. These findings have provided important leads to develop nutritional interventions to treat or prevent human or animal intestinal diseases.
Supplemental Material
Supplemental material, sj-pdf-5-ini-10.1177_1753425920966686 for Docosahexaenoic acid alleviates cell injury and improves barrier function by suppressing necroptosis signalling in TNF-α-challenged porcine intestinal epithelial cells by Kan Xiao, Qiao Xu, Congcong Liu, Pengwei He, Qin Qin, Huiling Zhu, Jing Zhang, Ashley Gin, Guolong Zhang and Yulan Liu in Innate Immunity
Supplemental material, sj-pdf-6-ini-10.1177_1753425920966686 for Docosahexaenoic acid alleviates cell injury and improves barrier function by suppressing necroptosis signalling in TNF-α-challenged porcine intestinal epithelial cells by Kan Xiao, Qiao Xu, Congcong Liu, Pengwei He, Qin Qin, Huiling Zhu, Jing Zhang, Ashley Gin, Guolong Zhang and Yulan Liu in Innate Immunity
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the Projects of Wuhan Science and Technology Bureau (2018020401011304), Innovative Research Groups of the Natural Science Foundation of Hubei Province (2019CFA015) and National Natural Science Foundation of China (31772615 and 31802070).
ORCID iD: Yulan Liu https://orcid.org/0000-0001-9617-9305
Supplemental material: Supplemental material for this article is available online.
References
- 1.Blikslager AT, Moeser AJ, Gookin JL, et al. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 2007; 87: 545–564. [DOI] [PubMed] [Google Scholar]
- 2.Turner J. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9: 799–809. [DOI] [PubMed] [Google Scholar]
- 3.Peterson L, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014; 14: 141–153. [DOI] [PubMed] [Google Scholar]
- 4.Xiao K, Cao ST, Jiao LF, et al. TGF-β1 protects intestinal integrity and influences smads and MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate Immun 2017; 23: 276–284. [DOI] [PubMed] [Google Scholar]
- 5.Khan MR, Uwada J, Yazawa IT, et al. Activation of muscarinic cholinoceptor ameliorates tumor necrosis factor-α-induced barrier dysfunction in intestinal epithelial cells. FEBS Lett 2015; 589: 3640–3647. [DOI] [PubMed] [Google Scholar]
- 6.Pinton P, Braicu C, Nougayrede J, et al. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J Nutr 2010; 140: 1956–1962. [DOI] [PubMed] [Google Scholar]
- 7.Nunes T, Bernardazzi C, De Souza H. Cell death and inflammatory bowel diseases: apoptosis, necrosis, and autophagy in the intestinal epithelium. Biomed Res Int 2014; 2014: 218493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silke J, Rickard J, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol 2015; 16: 689. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Sun L, Su L, et al. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 2014; 54: 133–146. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Yousefi S, Simon H. Necroptosis and neutrophil-associated disorders. Cell Death Dis 2018; 9: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adameova A, Goncalvesova E, Szobi A, et al. Necroptotic cell death in failing heart: Relevance and proposed mechanisms. Heart Failure Rev 2016; 21: 213–221. [DOI] [PubMed] [Google Scholar]
- 12.Su X, Wang H, Kang D, et al. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem Res 2015; 40: 643–650. [DOI] [PubMed] [Google Scholar]
- 13.Pierdomenico M, Negroni A, Stronati L, et al. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol 2014; 109: 279–287. [DOI] [PubMed] [Google Scholar]
- 14.Liu YL. Fatty acids, inflammation and intestinal health in pigs. J Anim Sci Biotechnol 2015; 6: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao G, Tang L, Yuan F, et al. Eicosapentaenoic acid enhances heat stress-impaired intestinal epithelial barrier function in Caco-2 cells. PLoS One 2013; 8: e73571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoch B, McNabb WC, Roy N. Influence of polyunsaturated fatty acids on intestinal barrier function during colitis. Agro Food Industry Hi Tech 2010; 21: 29–32. [Google Scholar]
- 17.Ungaro F, Rubbino F, Danese S, et al. Actors and factors in the resolution of intestinal inflammation: lipid mediators as a new approach to therapy in inflammatory bowel diseases. Front Immunol 2017; 8: 1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris WS. International recommendations for consumption of long-chain omega-3 fatty acids. J Cardiovasc Med 2007; 8: 50–52. [DOI] [PubMed] [Google Scholar]
- 19.Liu YL, Chen F, Odle J, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr 2012; 142: 2017–2024. [DOI] [PubMed] [Google Scholar]
- 20.Zhu HL, Liu YL, Chen SK, et al. Fish oil enhances intestinal barrier function and inhibits corticotropin-releasing hormone/corticotropin-releasing hormone receptor 1 signalling pathway in weaned pigs after lipopolysaccharide challenge. Br J Nutr 2016; 115: 1947–1957. [DOI] [PubMed] [Google Scholar]
- 21.Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 2004; 286: G367–376. [DOI] [PubMed] [Google Scholar]
- 22.Liu N, Ma XS, Luo X, et al. L-glutamine attenuates apoptosis in porcine enterocytes by regulating glutathione-related redox homeostasis. J Nutr 2018; 148: 526–534. [DOI] [PubMed] [Google Scholar]
- 23.Wang SH. Study on the establishment of intestinal cell injury model and the protective effects of n-3 PUFA. Wuhan, PR China: Wuhan Polytechnic University, 2017. [Google Scholar]
- 24.Jiao N, Wu Z, Ji Y, et al. L-glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J Nutr 2015; 145: 2258–2264. [DOI] [PubMed] [Google Scholar]
- 25.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 2009; 137: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 26.Pacheco F, Almaguel F, Evans W, et al. Docosahexanoic acid antagonizes TNF-α- induced necroptosis by attenuating oxidative stress, ceramide production, lysosomal dysfunction, and autophagic features. Inflamm Res 2014; 63: 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishida E, Tajiri M, Masuzawa Y. Docosahexaenoic acid enrichment can reduce L929 cell necrosis induced by tumor necrosis factor. Biochim Biophys Acta 2006; 1761: 454–462. [DOI] [PubMed] [Google Scholar]
- 28.Han L, Lei H, Tian Z, et al. The immunomodulatory activity and mechanism of docosahexaenoic acid (DHA) on immunosuppressive mice models. Food Funct 2018; 9: 3254–3263. [DOI] [PubMed] [Google Scholar]
- 29.Willemsen L, Koetsier M, Balvers M, et al. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur J Nutr 2008; 47: 183–191. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Shi P, Sun Y, et al. DHA protects against experimental colitis in IL-10-deficient mice associated with the modulation of intestinal epithelial barrier function. Br J Nutr 2015; 114: 181–188. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Akhtar S, Choudhry M. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochim Biophys Acta 2012; 1822: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci 2013; 70: 631–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest Res 2015; 13: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrer R, Moreno J. Role of eicosanoids on intestinal epithelial homeostasis. Biochem Pharmacol 2010; 80: 431–438. [DOI] [PubMed] [Google Scholar]
- 35.Thomas YM, Gary KI, Neil TH, et al. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol 2004; 286: G367–376. [DOI] [PubMed] [Google Scholar]
- 36.Feng YJ, Teitelbaum DH. Tumour necrosis factor-α-induced loss of intestinal barrier function requires TNFR1 and TNFR2 signalling in a mouse model of total parenteral nutrition. J Physiol 2013; 15: 3709–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Pan L, Lu J, et al. N-3 PUFAs attenuate ischemia/reperfusion induced intestinal barrier injury by activating I-FABP–PPARγ pathway. Clinic Nutr 2012; 31: 951–957. [DOI] [PubMed] [Google Scholar]
- 38.Beguin P, Errachid A, Larondelle Y, et al. Effect of polyunsaturated fatty acids on tight junctions in a model of the human intestinal epithelium under normal and inflammatory conditions. Food Funct 2013; 4: 923–931. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Ling Y, Cao Z, et al. Targeting intestinal epithelial cell-programmed necrosis alleviates tissue injury after intestinal ischemia/reperfusion in rats. J Surg Res 2018; 225: 108–117. [DOI] [PubMed] [Google Scholar]
- 40.Hildebrand JM, Tanzer MC, Lucet IS, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A 2014; 111: 15072–15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Jiang H, Chen S, et al. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 2012; 148: 228–243. [DOI] [PubMed] [Google Scholar]
- 42.Dondelinger Y, Darding M, Bertrand MJ, et al. Poly-ubiquitination in TNFR1- mediated necroptosis. Cell Mol Life Sci 2016; 73: 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welz P, Wullaert A, Vlantis K, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011; 477: 330–334. [DOI] [PubMed] [Google Scholar]
- 44.Günther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 2011; 477: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Almagro M, Vucic D. Necroptosis: pathway diversity and characteristics. Semin Cell Dev Biol 2015; 39: 56–62. [DOI] [PubMed] [Google Scholar]
- 46.Zhu HL, Wang HB, Wang SH, et al. Flaxseed oil attenuates intestinal damage and inflammation by regulating necroptosis and TLR4/NOD signaling pathways following lipopolysaccharide challenge in a piglet model. Mol Nutr Food Res 2018; 62: 1700814. [DOI] [PubMed] [Google Scholar]
- 47.Broker L, Kruyt F, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res 2005; 11: 3155–3162. [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Kang S, Jung U, et al. Effects of Omega-3 fatty acids on apoptosis of human gastric epithelial cells exposed to silica-immobilized glucose oxidase. Ann N Y Acad Sci 2009; 1171: 359–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-5-ini-10.1177_1753425920966686 for Docosahexaenoic acid alleviates cell injury and improves barrier function by suppressing necroptosis signalling in TNF-α-challenged porcine intestinal epithelial cells by Kan Xiao, Qiao Xu, Congcong Liu, Pengwei He, Qin Qin, Huiling Zhu, Jing Zhang, Ashley Gin, Guolong Zhang and Yulan Liu in Innate Immunity
Supplemental material, sj-pdf-6-ini-10.1177_1753425920966686 for Docosahexaenoic acid alleviates cell injury and improves barrier function by suppressing necroptosis signalling in TNF-α-challenged porcine intestinal epithelial cells by Kan Xiao, Qiao Xu, Congcong Liu, Pengwei He, Qin Qin, Huiling Zhu, Jing Zhang, Ashley Gin, Guolong Zhang and Yulan Liu in Innate Immunity