Abstract
Research has shown that autistic adults have poor health outcomes. We conducted a systematic review to identify existing interventions to address health outcomes for autistic adults and to determine whether these interventions address the priorities of the autistic community. We searched PubMed for articles that included an intervention, a primary health outcome measured at the individual (not system) level, and a sample population of at least 50% autistic adults. Studies were excluded if they were not peer-reviewed, had a focus on caregivers, were expert opinions on specific interventions, untested protocols, or interventions without a primary health outcome. Out of the 778 articles reviewed, 19 were found to meet the stated criteria. Based on the evidence gathered, two were considered emerging evidence-based approaches: cognitive behavioral approaches and mindfulness. The remaining interventions included in the review did not have sufficient evidence to support current use with this population. The majority of the studies included samples of young autistic adults, primarily male, without an intellectual disability. Anxiety, quality of life, depression, and behavioral issues were among the health outcomes measured in the final included articles. More research on preferred interventions with prioritized health outcomes of the autistic adult population is needed.
Lay abstract
Autistic adults have more health problems then their same-aged peers. Yet little research has been conducted that focuses on addressing these health problems. In order to guide future research, it is important to know what intervention studies have been done to improve health outcomes among autistic adults. The project team and student assistants read studies that were published between 2007 and 2018 in the online research database, PubMed. We looked for studies published in English, which were peer-reviewed and included (1) an intervention, (2) an outcome that was related to health, and (3) a study group that included autistic adults. We did not include studies that had outcomes about employment (unless there was a health outcome), studies about caregivers or caregiving, or expert opinions about interventions. Of 778 reviewed articles, 19 studies met all of the criteria above. Within these studies, two approaches were found to have emerging evidence for their use in autistic adults: cognitive behavioral interventions and mindfulness-based approaches for improved mental health outcomes. The remaining intervention approaches did not have enough articles to support their use. Many of the outcomes were about reduced symptoms of co-occurring mental health diagnoses (e.g. reduced anxiety, depression). Most of the participants in these studies were male and did not have intellectual disability. Most study participants were adults younger than 40. There are not many intervention studies that address health outcomes among autistic adults. More research is needed on interventions which are desired by the adult autism community and address preferred health outcomes such as increased quality of life or well-being.
Keywords: adult, autism spectrum disorder, intervention, systematic review
Introduction
Much of what is known about the health and healthcare needs of autistic adults has emerged from health services research documenting the frequency of co-occurring conditions, types of healthcare received, and costs of care. Health services research suggests that autistic adults have different healthcare needs than same-aged peers without autism spectrum disorder (ASD; Croen et al., 2015; Zerbo et al., 2019). Within the literature, poor healthcare outcomes have been identified among autistic adults, such as early death/mortality (e.g. Bilder et al., 2013), increased rates of psychiatric emergency department utilization (e.g. Vohra et al., 2016), and less use of preventive care visits for cancer screenings (Nicolaidis et al., 2014, 2015). Utilization and costs are also higher among autistic adults in a privately insured large healthcare group (e.g. Zerbo et al., 2019). It is reported that autistic persons face multiple challenges which are specific to autism, as it applies to accessing healthcare (Burke & Stoddart, 2014).
Although poor health outcomes have been frequently documented, few studies have evaluated the efficacy of interventions to address individual health outcomes in the autistic population. The majority of spending on autism research continues to fund genetic and other research aimed at targeting brain mechanisms, risk factors focusing on prevention of ASD and causes of ASD, and interventions that primarily target children (Interagency Autism Coordinating Committee (IACC), 2016). According to the IACC (2016), research on autistic adults comprises approximately 2% of the national funding from both public and private sectors. There is an urgent need to understand the available literature on effective interventions to improve health outcomes for children aging into adulthood.
The purpose of this systematic review was to comprehensively identify interventions used with autistic adults to address health outcomes and to evaluate the quality of available interventions. The specific systematic review question asked by our project team was “What interventions (I-intervention) implemented for individuals are currently documented in the literature that evaluate the impact on health-related outcomes (O-outcome) for autistic adults (P-population)?” The results of this systematic review were reviewed by autistic research partners to identify which interventions were important to the autistic adult community in order to further contribute to the recommendations based on the available literature.
Methods
Our systematic review protocol was developed using the Preferred Reporting Items of Systematic Reviews and Meta-Analyses document (PRISMA; Appendix 1) (Moher et al., 2009). In addition to implementing a systematic search of the literature, we also ensured applicability of our results through using participatory-action research approaches to check our results with 18 paid community research partners (Community Council) to make recommendations based on available literature and community priorities. Community partners held multiple roles, including professional roles as researchers and medical/mental health professionals, authors, and advocates; most identified as an autistic adults and/or a parent of an autistic adult. Based on their involvement in the study activities, all Community Council members were offered the opportunity to be authors, acknowledged contributors, or if preferred, not acknowledged by name.
Included study characteristics
We used clear definitions to ensure that inclusion and exclusion criteria were applied systematically during the review process to select relevant studies (Table 1). Inclusion criteria for relevant studies were defined as follows: population inclusion required having a sample in which at least 50% of the study sample was 18 years or older and having a sample in which the primary study population were individuals identified as having an “autism spectrum disorder.” Due to the changes in diagnostic criteria when changing from the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM IV) to Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association, 2013), we ensured that studies which referred to study populations with outdated terms (e.g. “high-functioning autism,” “Asperger’s syndrome,” and/or “Pervasive-Developmental Disorder not otherwise specified”) were also included. Studies were included even if they did not contain a confirmatory diagnostic assessment; participant characteristics from included studies can be found in Appendix 4.
Table 1.
Definitions guiding selection of included studies.
|
Population: Studies were included if 50% of the
study population was greater than 18 years of age (the
sample must explicitly examine an adult group separately, if
there is a range of ages). The majority of the sample must
include autistic adults, which includes those with older
recognized diagnoses such as Asperger’s syndrome,
high-functioning autism, autistic disorder, and pervasive
developmental disorder—not otherwise specified.
Health: “a state of complete physical, mental, and social well-being, and not merely the absence of disease or infirmity” (World Health Organization (WHO), 1948). Intervention: Interventions in our study must address an individual’s health needs and be aimed at intervening at the individual level. We excluded interventions aimed at addressing organizational or system interventions (e.g. procedures and policies that impact many individuals). We did not include interventions aimed at caregivers of autistic adults. Health and health outcomes: Physical and mental health outcomes aimed at addressing an individual’s personal health or well-being, including but not limited to ameliorating specific chronic or physical health conditions (e.g. cardiovascular outcomes; management of weight), mental health outcomes (e.g. depression, anxiety) or mental well-being, mortality and morbidity, or quality of life. We excluded outcomes that were measured at the family, community, or health-system level (e.g. annual checkups, access to healthcare, insurance payment and coverage, or system quality indicators (e.g. coordinated care, timely physician communication, indicators of patient-centered medical homes). |
We included Interventions that addressed at least one physical, mental, and/or social health outcome. Due to the focus at the time of the review on individual-level (as opposed to system-level) interventions, our search incorporated interventions where the autistic adult was the target of intervention. We included studies with Outcomes which addressed ‘health’ (defined in Table 1). Study type was not specified as an inclusion/exclusion criterion; therefore, we included all qualitative and quantitative evidence of any level (I–V) as long as it met the above criteria. We limited studies to those published in the English language which were conducted with human participants (no animal studies) and were published in the past 10 years (in year 2007 or later) at the time of the last search.
We specifically excluded studies that were not published in peer-reviewed journals (e.g. newspaper or magazine articles), articles that discussed caregiving or families of autistic adults (e.g. focused on interventions for caregivers of adults), expert opinions regarding interventions, protocols of interventions which had not been tested, and vocational or educational interventions which did not have a physical or mental health outcome as a primary outcome of the study. Educational and vocational interventions were defined as those conducted in post-secondary education settings or work/vocational settings (simulated, volunteer, or paid).
Information sources and article management
For this review, we searched the largest existing medical database, PubMed, which contains both self-archived and peer-reviewed journal archived records. We trialed search terms in early 2017 with the assistance of a medical librarian, and the final search string was implemented on October 14, 2017 (Appendix 2). The use of Medical Search Headings (MeSH) and Boolean operators was used to ensure search terms were combined to meet our research question as posed above.
Screening and selection process
Following implementation of the PubMed search described above, all relevant article records were downloaded by title and abstract into the reference manager software EndNote® Version X7.7.1. The process for screening studies included dividing of relevant records among a team of six graduate research students, use of folders in EndNote to track included and excluded articles, and regular meetings with the principal investigator to review questionable titles or abstracts. All authors involved in article selection underwent training with practice screening using study definitions. In addition, a random selection of 10% of originally identified records was checked by the principal investigator (T. B.) to ensure reliability in screening to include/exclude articles. During the title/abstract screen, articles that clearly met our inclusion criteria were retained. Articles which were questionable based on title or abstract were also retained for full-text review. Articles identified for full-text screen were obtained in full-text PDF and stored offline. We evaluated all PDFs using the same inclusion/exclusion criteria and excluded articles were documented with reasons (available upon request).
Following the first review of available studies, the Community Council recommended targeted searches of the literature for specific desired interventions identified during priority-setting activities (Benevides et al., 2020). A second search of PubMed was conducted for specific interventions including cognitive behavioral therapy (CBT), use of medical marijuana, animal-assisted therapy, and trauma-informed interventions. Using the same terms for the Population, but adding specific keywords and MeSH terms for interventions, resulted in additional articles for screening (Appendix 3). The last search was conducted 8 August 2018. Once limits for “adults 18+ years” were applied, the titles and abstracts were screened to ensure they met inclusion criteria using the same process as the previous search. Figure 1 illustrates the flow of articles in our study (Moher et al., 2009).
Figure 1.
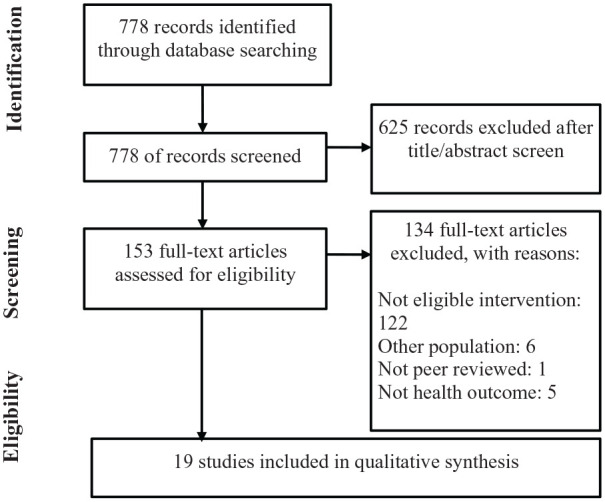
PRISMA flow diagram of included and excluded studies from both searches.
Evaluation of risk of bias in included studies
Included studies were evaluated using risk of bias criteria proposed by the Cochrane group (Higgins et al., 2011). Because we did not find many randomized controlled trials (RCTs), we anticipated that the overall risk of bias in studies was high. The risk of bias was taken into consideration when evaluating the certainty of the available evidence as a whole.
Data extraction
We extracted the following data from included studies: Title, author, year; study design; characteristics of the interventions, and characteristics of included participants. We extracted characteristics of the interventions in included studies such as intervention type (using categories defined by Young and colleagues (2010), duration/frequency/intensity of intervention(s), health outcome measured, and location where intervention was conducted. Characteristics of study participants were extracted across the articles and included mean age of all study participants, gender distribution (percent males), diagnosis type, and percentage of those in study sample with intellectual disability (Appendix 4). Qualitative synthesis of results across the articles were planned to identify themes of available interventions, whether the existing studies aligned with early priorities identified by the autistic adult community and whether specific gaps existed in available evidence.
Certainty of available evidence
We used guidelines proposed by the National Professional Development Center on Autism Spectrum Disorder (n.d.) and Centers for Medicare and Medicaid to define evidence-based practices for children with autism (Young et al., 2010). Interventions were considered to be “Evidence based” if there were two or more high-quality RCTs to support that intervention or if there were five high-quality single-subject designs conducted by at least three different research groups and having at least 20 research participants across all single-subject designs. “Emerging evidence” was evidence in which there were two or more studies which existed that were of lower quality or less-rigorous study design, and the available evidence showed some or no effect, and did not produce negative effects on participants (e.g. poor outcomes). “Unestablished interventions” was evidence in which studies showed negative effects on outcomes of the participants, or all the available studies on that intervention showed no effect, or there was only one study with that intervention which was available for review.
Results
Among the 778 articles obtained from our PubMed searches, we identified 19 studies which met our inclusion/exclusion criteria. Among the included studies, the overall risk of bias across all available intervention studies was high (Table 2). The majority of included studies used a case report or case series design (n = 7; 37%), with very little description of the methods used to confirm autism diagnosis, implementation methods, and measure outcomes (Enticott et al., 2011; Hsieh et al., 2014; Nilsson & Ekselius, 2009; Roser et al., 2009; Sajith et al., 2017; Wachtel et al., 2010; Weiss & Lunsky, 2010). Three studies (16%) used a single-subject design (Brundage et al., 2013; Campillo et al., 2014; Tiger et al., 2009); however, only two of those included a multiple baseline design as a control. Three studies (16%) used a pre-test–post-test single group design; these had no control and thus were unable to control for most threats to internal validity (Ekman & Hiltunen, 2015; Gal et al., 2015; Siew et al., 2017). Two studies (10%) used a pre-test–post-test non-equivalent control group design, and both were considered high-quality quasi-experimental designs (McGillivray & Evert, 2014; Sizoo & Kuiper, 2017). Four studies (21%) used a randomized controlled design to examine the effect of an intervention for adults (Hesselmark et al., 2014; McVey et al., 2016; Russell et al., 2013; Spek et al., 2013).
Table 2.
Risk of bias in included studies.
| Author(s) (year) | Study design | Controla | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessors | Incomplete outcome data (attrition) | Selective reporting |
|---|---|---|---|---|---|---|---|---|
| Brundage et al. (2013) | Single-subject ABAB design | + | − | − | − | − | + | + |
| Campillo et al. (2014) | Single-subject AB design | − | − | − | − | − | + | + |
| Ekman & Hiltunen (2015) | Pre-test–post-test single group | − | − | − | − | − | ? | + |
| Enticott et al. (2011) | Case study | − | − | − | − | − | − | − |
| Gal et al. (2015) | Pre-test–post-test single group | − | − | − | − | − | − | + |
| Hesselmark et al. (2014) | Randomized controlled trial | + | + | + | − | − | + | + |
| Hsieh et al. (2014) | Case study | − | − | − | − | − | − | − |
| McGillivray& Evert (2014) | Pre-test–post-test non-equivalent control group | + | − | − | − | − | + | + |
| McVey et al. (2016) | Randomized controlled trial | + | ? | ? | − | − | + | + |
| Nilsson & Ekselius (2009) | Case study | − | − | − | − | − | − | − |
| Roser et al. (2009) | Case study | − | − | − | − | − | − | − |
| Russell et al. (2013) | Randomized controlled trial | + | + | + | − | + | + | + |
| Siew et al. (2017) | Mixed methods with pre-test–post-test single group | − | − | − | − | − | + | + |
| Sajith et al. (2017) | Case study | − | − | − | − | − | + | − |
| Sizoo & Kuiper (2017) | Pre-test–post-test non-equivalent control group | + | − | − | − | − | − | + |
| Spek et al. (2013) | Randomized controlled trial | + | + | + | − | − | + | + |
| Tiger et al. (2009) | Single-subject ABABC | + | − | − | + | − | + | + |
| Wachtel et al. (2010) | Case study | − | − | − | − | − | + | + |
| Weiss & Lunsky (2010) | Case series | − | − | − | − | − | + | + |
+ = Low risk of bias; — = High risk of bias; ? = Unclear risk of bias.
For the purposes of single-subject designs, the use of a stable baseline and a stable withdrawal phase was considered an adequate control. For other study designs, control was achieved with a comparison group that was equivalent to the treatment group upon pre-testing.
Synthesis of available interventions
Table 3 presents characteristics of available interventions and extracted data from each study. Cognitive behavioral interventions are considered an emerging evidence-based approach for improving self-reported mood and anxiety symptoms among autistic adults, based on the availability of six studies (32% of identified literature): two RCTs (Hesselmark et al., 2014; Russell et al., 2013), two quasi-experimental designs (McGillivray & Evert, 2014; Sizoo & Kuiper, 2017), one pre-test–post-test single-group design (Ekman & Hiltunen, 2015), and one case series (Weiss & Lunsky, 2010). The available evidence from RCTs suggests, however, that CBT is not significantly better than other alternative interventions such as “anxiety management” or “recreational groups,” but that CBT does result in small clinical effects on self-reported outcomes (Cohen’s d range: 0.31–0.33) and large effect for blinded assessor ratings of obsessive-compulsive behavior outcomes (d = 1.01). All of the available CBT studies were conducted with autistic individuals who were verbal and who had no apparent intellectual disability. The most commonly occurring CBT approach used was group CBT, ranging from a total exposure of 12–108 h of therapy, with most studies providing a median of 18.75 h of total therapy. All group CBT studies delivered the intervention weekly, using a manualized approach with adaptations for adults on the spectrum. Of the two RCTs investigating this approach, they differed greatly in the format (one used a group CBT format and the other used individual sessions). Neither RCT reported negative outcomes of their CBT that would suggest they were detrimental to the health outcomes. This approach requires additional investigation in comparison with alternative interventions, and to determine the point at which clinical effects are observed. Although both RCT studies examined long-term outcomes to determine whether effects were maintained after discontinuation of the CBT, high rates of missing data substantially impact interpretation. Future research of this approach should include long-term follow-up with participants.
Table 3.
Study characteristics of included studies.
| Author | Intervention type | Intervention (frequency, intensity, duration) | Comparison (frequency, intensity, duration) | Health outcome measured | Country conducted |
|---|---|---|---|---|---|
| Brundage et al. (2013) | Language (behavioral) | Fluency rules program plus social pragmatic treatment (two/week for 50 min, for 13 weeks) | Social pragmatic treatment using Garcia Winner’s Think Social! Model (two/week for 50 min, for 9 weeks) | Stuttering rates (audio recorded) | USA |
| Campillo et al. (2014) | Assistive technology (software) | Tic-Tac software to visually display waiting times between 3 and 10 min each session | No software used, intervals of 3–10 min waiting periods | % waiting intervals with anxiety-related behavior (video observation) | Spain |
| Ekman & Hiltunen (2015) | Cognitive behavioral intervention | Individual CBT modified with visualization for anxiety and communication style (not manualized) (one/every 2 weeks, 45–60 min/session for 15 sessions) | None | Individualized goals based on self-reported estimated level of anxiety and desired target behaviors | Sweden |
| Enticott et al. (2011) | Medical | Deep transcranial magnetic stimulation (5 Hz, nine treatments over 11 days, each treatment including thirty 10-s rTMS with 20-s interval) | None | Self-reported social-relating and interpersonal understanding | Unclear (Australia or Israel) |
| Gal et al. (2015) | Vocational | Vocational training course (simulation) (6 h/day, 5 days/week, for 3 months) followed by actual work environment (6 h/day, 5 days/week, for 3 months) | None | Self-reported quality of life, subjective well-being | Israel |
| Hesselmark et al. (2014) | Cognitive behavioral intervention | Manualized group CBT (one/week for 3 h, for 36 weeks) | Recreational group activity (one/week for 3 h, for 36 weeks) | Self-reported quality of life, self-esteem, psychiatric symptoms | Sweden |
| Hsieh et al. (2014) | Medical | Medical treatment in intensive care unit following respiratory emergency | None | Medical, behavioral outcomes (observed) while ventilated | USA |
| McGillivray & Evert (2014) | Cognitive behavioral intervention | Group intervention using CBT and established literature. Outline of 9-week program available (one/week for 2 h, for 9 weeks) | Waitlist | Self-reported depression, anxiety, and stress | Australia |
| McVey et al. (2016) | Social skills intervention | Manualized PEERS® for Young Adults (one/week for 90 min, for 16 weeks) | Waitlist | Parent and self-reported social phobia, social anxiety, loneliness | USA |
| Nilsson & Ekselius (2009) | Medical | Electroconvulsive therapy (5.6–7.0 s stimulations on right hemisphere, three/week, for 4 weeks) | None | Unclear measurement of obsessive-compulsive behaviors, catatonia-like behaviors, panic attack, and hypochondria behaviors | Sweden |
| Roser et al. (2009) | Medical | Paliperidone (6 mg/day during 1 week hospital stay, followed by 4 months outpatient treatment) | None | Unclear measurement of anxiety, “distress with psychotic features” | Germany |
| Russell et al. (2013) | Cognitive behavioral intervention | CBT for OCD using exposure and response prevention, adapted for autism (manualized) (1 h/session, for 7–20 sessions) | Anxiety management (manualized) (1 h/session, for 7–20 sessions) | Interview assessment of co-occurring obsessive-compulsive symptoms | United Kingdom |
| Siew et al. (2017) | Peer mentoring | Peer mentoring with one-on-one support for individual needs (weekly meetings for 5 months) | None | Self-reported well-being, social anxiety, communication anxiety | Australia |
| Sajith et al. (2017) | Medical | Electroconvulsive therapy (varied intensity, duration, and frequency) | None | Aggression and catatonia (observed), “aberrant behavior” (unclear—possibly parent reported) | Singapore |
| Sizoo & Kuiper (2017) | Complementary/integrative medicine intervention
Cognitive behavioral intervention |
Manualized group cognitive behavioral therapy adapted for autism (one/week for 90 min, for 13 weeks) | Group mindfulness-based stress reduction (one/week for 90 min, for 13 weeks) | Self-reported anxiety and depression scores, mood, rumination | Netherlands |
| Spek et al. (2013) | Complementary/integrative medicine intervention | Mindfulness-based therapy, adapted for adults with ASD (one/week for 2.5 h for 9 weeks) | Waitlist | Self-reported depression, anxiety, rumination symptoms | Netherlands |
| Tiger et al. (2009) | Behavioral intervention | Differential reinforcement of other behavior | None | Skin picking (observed) | USA |
| Wachtel et al. (2010) | Medical | Electroconvulsive therapy | None | Self-injurious behavior, aggression, disruptive behavior (observed) | USA |
| Weiss & Lunsky (2010) | Cognitive behavioral intervention | Group CBT using manualized “Mind over Mood” (one/week for 1 h for 12 weeks) | None | Self-reported depression and anxiety symptoms | Canada |
Mindfulness approaches are also considered an emerging evidence-based approach for autistic adults based on two high-quality studies comprising a RCT and a pre-test–post-test non-equivalent control group (quasi-experimental) design. These studies used manualized group mindfulness approaches to address self-reported health outcomes of depression and anxiety among autistic adults without intellectual disability (Sizoo & Kuiper, 2017; Spek et al., 2013), one of which was discussed above because the mindfulness was compared to group CBT. Neither study reported negative outcomes, and both studies had effect sizes at the end of treatment ranging from d = 0.07–0.78 for self-reported depression symptoms and effects between d = 0.37–0.76 for self-reported anxiety symptoms. Mindfulness approaches were implemented once a week for 1.5–2.5 h a session, for 9–12 weeks, resulting in a total number of hours of exposure to treatment between 19.5 and 22.5 h. These studies were conducted with autistic adults with no apparent intellectual disability.
Among the included studies, 32% (n = 6) implemented medical interventions to address health outcomes among autistic adults (Table 3), with the most frequently occurring medical intervention being “electroconvulsive therapy” (ECT; n = 3 studies; Nilsson & Ekselius, 2009; Sajith et al., 2017; Wachtel et al., 2010), one study describing the use of a pharmacological intervention (Roser et al., 2009), one study describing a multi-component intervention in the intensive care unit for respiratory distress (Hsieh et al., 2014), and one study describing the use of deep transcutaneous magnetic stimulation to the brain (Enticott et al., 2011). None of the medical interventions used strong study designs, and a high risk of bias is present for these interventions; thus, all medical interventions are considered to be unestablished evidence for intervention.
For ECT specifically, only four cases of adults ages 19–38 years, primarily with co-occurring intellectual disability, have been reported in the peer-reviewed literature to use this intervention (Nilsson & Ekselius, 2009; Sajith et al., 2017; Wachtel et al., 2010). None of the participants provided their own consent to participate (parental consent was obtained in all cases). ECT is an invasive medical procedure in which electrical waves are transmitted to the brain. All of the studies which implemented this type of intervention described the effects on social behaviors, psychological symptoms such as obsessive-compulsive behaviors, and self-injurious behaviors. One ECT study reported a negative response (Sajith et al., 2017) with worsening symptoms prompting discontinuation of ECT treatment. Due to the high risk of bias in these studies, and negative response in one of four cases, electroconvulsive therapy is an unestablished intervention.
The remaining identified studies used a variety of intervention approaches to address different health outcomes, but due to the types of study designs and limited available evidence, all are considered to be unestablished interventions.
Participant characteristics from included studies are provided in Appendix 4. The majority of included studies examined interventions in autistic young adults, with mean ages of samples in the mid-20-year-old range. Very few studies discussed interventions with autistic adults over the age of 40 years, with only two studies having an approximate mean sample age over 40 years (Spek et al., 2013; Weiss & Lunsky, 2010). A large number of studies (42%) included only male samples. Finally, the majority of participants in included CBT and mindfulness studies were considered to be “high-functioning ASD” or “Asperger’s syndrome.” Medical interventions were primarily used with autistic adults with corresponding intellectual disability.
Discussion
Our systematic review of the literature revealed few available intervention approaches aimed at addressing health outcomes among autistic adults. The two primary intervention approaches considered to have emerging evidence were cognitive behavioral interventions and complementary/integrative mindfulness interventions, both aimed at reducing psychiatric symptoms (e.g. depression, anxiety, obsessive-compulsive disorder) in autistic adults without intellectual disability. Others have found that cognitive behavioral interventions are of benefit to autistic children (e.g. Weston et al., 2016), as are mindfulness interventions (e.g. Cachia et al., 2016) and our study suggests there is evidence for these approaches in adults 18 years and older.
Many of the interventions reviewed in this study did not have sufficient evidence supporting their use in autistic adults. Future research is needed to better understand the effect of social skill interventions to address social anxiety, vocational interventions to address quality of life, and technology applications for reduced anxiety. In our review, ECT (n = 3 studies), transcranial magnetic stimulation (TMS; n = 1), pharmacological (n = 1), and a behavioral paradigm (n = 1) were used primarily on autistic adults with intellectual disability. These interventions were not identified as interventions that were desired by the autistic community (Benevides et al., 2020), and significant distrust of medical treatments such as these should be recognized by the research community. Whether an intervention is viewed positively by autistic people is an important consideration, in addition to its evaluated effectiveness, given autistic people will be participants in these interventions. In addition, none of the included studies in our review reported using community–stakeholder partnership in evaluating the interventions.
No studies were found that addressed integrative health approaches through yoga, animal-assisted therapy, or tai-chi for this population, despite autistic community reported interest in research for these topics. In addition, the adult autistic community desires evidence-based information about medical marijuana for anxiety and other mental health symptoms but no studies were identified that examined this intervention for this population. Although these interventions are being reported as useful anecdotally in the adult autistic community, well-designed comparative effectiveness research has not yet been developed.
Community council review of findings
The purpose of involving the Community Council was to provide an informed opinion to these findings that reflects autistic people’s shared ownership of the research narrative, as related to the presented evidence. The importance of including community members in patient-centered outcomes research is necessary for scientific communities to address what is deemed to be important and to understand perceptions of existing or available approaches. All 18 Community Council members (78% of whom identify as autistic) were provided with both the long report and lay summary of the preliminary review of the literature and were asked to respond to several questions related to the results in June 2018. Community Council members were asked the following questions: “Would you recommend any of the existing interventions that we found? If yes, which? If no, why not?” and “What other interventions or approaches were not in the list, but should be considered for autistic adults?” In all, 13 Community Council contributors provided emailed responses (72%); responses were collated across many individual contributors. Once the systematic review was completed, all Community Council members were again invited to be authors if desired and were asked to provide written input to the discussion and conclusions of this report.
CBT for improving mental health
In the general population, CBT is a well-established, evidence-based intervention for anxiety, with over 50 years of research and successful application documented (e.g. Higa-McMillan et al., 2015). Some people with autism spectrum conditions find CBT very helpful in alleviating their anxiety or depression symptoms. The goal of CBT is to enable people to take control of how they interpret and deal with things in their environment. Modifications to CBT for young autistic individuals in order to tailor approaches for coping skills and exposure appear to be an important component of evaluated studies. The implementation of these protocols always incorporates modifications to standard CBT and should be considered as one approach to assist the individual in self-management of their anxiety. Additional evaluation of these approaches for older autistic adults and the long-term impact of such interventions is necessary. It should be noted that some autistic people report that CBT is unhelpful for them. There is not a “one-size-fits-all” approach to therapy. However, others find the practical and action-based nature of it helpful (Purkis et al., 2016).
Mindfulness-based interventions for improving mental health
Contrary to CBT, mindfulness-based interventions focus on modifying an individual’s thoughts and emotions, by separating themselves from these thoughts and emotions (Conner & White, 2017), with the goal of improved emotional regulation and self-awareness. In everyday settings, mindfulness approaches are being used by autistic adults because of the ease and availability of apps and other online tools. When used outside of the context of research, the implementation of mindfulness techniques varies in duration, frequency, and intensity; they are not likely implemented in the same manner as those that were researched in this review. Mindfulness is often seen by autistic adults as being helpful for decreasing stress, anxiety, ruminating thoughts, anger, and aggression because it enables people to safely observe their world and themselves. It can be a positive and self-caring strategy that both centers a person to reality but grounds them in a less volatile or stressful way of being at the same time. Future research should aim to examine the ease of use, cost, and long-term benefits of mindfulness approaches, including those that are accessed through apps by individuals on their own, as compared to other available approaches.
Social skills interventions for improving health
The use of PEERS® for Young Adults intervention (McVey et al., 2016), which is a group-based intervention targeting social skills, has preliminarily been shown to address social anxiety in a moderately sized sample. Community Council member contributors reflected on the importance of social skills for ensuring relationships with others in work and personal lives are healthy. Some autistics who ascribe to a social model of disability see some social skills interventions as teaching camouflaging. Camouflaging has been identified as one of the predictors of suicidality (e.g. Cassidy et al., 2018). In addition, some social skills interventions present specific behaviors as negative or wrong, and therefore might promote feelings of shame as related to features of autism that are part of one’s identity. It is recommended that all interventions which involve and aim to support autistic adults, including social skills interventions, include their feedback and input during the development and evaluation of the content.
Vocational interventions and health
Remarkably, while there are many vocational and employment interventions that exist within the field of autism research, only one was identified as measuring a health outcome, quality of life (Gal et al., 2015). Community Council members reflected on the importance of measuring quality of life outcomes, no matter the intervention, as quality of life is an essential indicator of health.
Prescription medication and dosing
Few studies examined the impact of pharmacological or medical interventions, despite the high rate of use by medical practitioners. The autistic community has anecdotally reported that medication side effects and dosing are not well evaluated, and use of medications is felt to be poorly tolerated. Evaluation of medication use and side effects among autistics is needed and should involve the autistic community in their development of research.
Along with prescription drugs, other Community Council members described the need for studies on cannabidiol drugs (CBDs) and other forms of medical marijuana which has possible anxiety-reduction effects. The careful study of this for the autistic adult population has not been addressed in the peer-reviewed literature, despite increasing numbers of states that consider autism a qualifying condition for its use.
ECT
It is important to note that none of the Community Council felt that ECT was an appropriate intervention for autistic individuals. Major concerns from the Community Council were raised related to this intervention approach, including possible damage to the brain, memory, and the extensive risks associated with this approach.
TMS
Community Council members did emphasize the importance of distinguishing ECT from TMS. The majority of Community Council members asked for more information about this approach, as very little is known about its use.
Gaps in the literature
Many Community Council members identified the lack of studies on aging and interventions to address the health of aging autistic adults. Palliative and end-of-life care, dementia care, and addressing chronic health conditions that occur during the course of normal aging are unaddressed topics, especially given the preponderance of studies on young adults in the found literature. Addressing the sensory, social, and physical environmental changes that occur as someone ages in place is an un-researched area in autism; moreover, there is a need to understand proprioceptive and vestibular differences in autism across the lifespan which impact health and well-being.
There were other gaps identified within this review that deserve mention. Interventions that were identified as future areas for research included homeopathic medicine, animal- and equine-assisted interventions, and other evidence-based interventions identified in the general population as improving depression and anxiety (e.g. exercise, nutrition, wellness interventions), none of which have been comprehensively evaluated for the autistic adult population.
Limitations and conclusion
This review was conducted over multiple years, with the last date of search in 2018. Due to the rapid growth in research for addressing autistic adult needs, it is possible that some literature has been missed that was either in press or not indexed at the time of searching, or not captured by the specific search criteria used. Future reviews are important, given the expected growth in this literature. In addition, this review was limited by significant heterogeneity in outcome measures, interventions, and population characteristics. Given the state of the literature, it was difficult to make firm conclusions regarding available evidence. Future work to conduct meta-analyses will depend on literature that both include similar outcomes and interventions.
Our systematic review specifically aimed to identify available interventions conducted with individuals, to better understand the evidence that supports health outcomes for autistic adults. Our study, however, excluded available interventions which aimed to improve system-level outcomes which impact access to care, availability of care, and quality of care. These are important interventions which are also prioritized by the autistic community, but were not included in our review.
A large number of studies were excluded which described educational or vocational interventions; however, the studies addressed post-secondary education outcomes or work/vocational outcomes (e.g. work-related behaviors) only. Although work outcomes are meaningful for improved quality of life, one recommendation is that researchers who examine work-related interventions also include measures of health, quality of life, and well-being.
In order to address chronic health conditions, we recommend testing evidence-based interventions in collaboration with autistic adult research partners to determine whether they are as effective and accepted in this population. None of the included studies in our review reported using community–stakeholder partnership in evaluating the interventions.
Our study is the first, to our knowledge, to comprehensively review available interventions that address health outcomes for autistic adults. Emerging evidence supports the use of cognitive behavioral interventions adapted for autistic individuals without intellectual disability for improving depression, anxiety, and obsessive-compulsive behaviors. In addition, mindfulness-based approaches are an emerging evidence-based practice to improve mental health outcomes for autistic adults without intellectual disability. Further work to define and measure quality of life outcomes as the result of these interventions, as well as investigation into other intervention approaches currently being used by this population to address health and well-being, is needed.
Acknowledgments
We wish to gratefully acknowledge the contributions of our Autistic Adults and other Stakeholders Engage Together (AASET) Community Council (CC) members. The CC members who participated in our project and who provided input throughout the project period are (in alphabetical order): Daria Blinova Tyrina, Amy Gravino, Becca Lory, Liane Holliday-Wiley, Jamie Marshall, Lindsey Nebeker, Kate Palmer, Bill Peters, and Cyndi Taylor. We also acknowledge the project team members who helped organize and manage some AASET project activities: Alex Plank and Patricia Duncan.
Appendix
Appendix 1.
PRISMA checklist.
| Section/topic | No. | Checklist item | Reported on page no. |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | Title page |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | Abstract |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number. | 2 |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2, 3 Table 1 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Appendix 2, 3 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 4 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made. | 3, 4 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 3, 4 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means). | N/A—Not a MA |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis. | N/A—Not a MA |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies). | N/A—Not a MA |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | N/A—Not a MA |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations. | 6–10 Table 3 Appendix 4 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome-level assessment (see Item 12). | Table 2 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot. | N/A—Not a MA |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | N/A—Not a MA |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | N/A—Not a MA |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)). | N/A—Not a MA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health care providers, users, and policy makers). | 7–10 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review level (e.g. incomplete retrieval of identified research, reporting bias). | 9–10 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 9–10 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review. | 10 |
Appendix 2.
PubMed search.
| Search number | Terms | Number of articles located |
|---|---|---|
| 1 | (((((Autism Spectrum Disorder[MeSH Major Topic]) OR Autistic Disorder[MeSH Major Topic])) OR asperger syndrome[MeSH Terms])) NOT ((((autistic disorder/diagnosis[MeSH Terms]) OR autism spectrum disorder/diagnosis[MeSH Terms])) OR Asperger Syndrome/diagnosis[MeSH Terms]) | 14,089 |
| 2 | (((((((((((((((((((Health[MeSH Terms]) OR Health Information Exchange[MeSH Terms]) OR Health Services for Persons with Disabilities[MeSH Terms]) OR quality of life) OR quality of life[MeSH Terms]) OR mental health[MeSH Terms]) OR depression) OR depression[MeSH Terms]) OR depressive disorder[MeSH Terms]) OR (health care quality, access, and evaluation[MeSH Terms])) OR healthcare disparities[MeSH Terms]) OR health services[MeSH Terms]) OR access to healthcare) OR health services accessibility[MeSH Terms]) OR Quality Indicators, Health Care[MeSH Terms]) OR (health services needs and demand[MeSH Terms])) OR anxiety) OR anxiety disorders[MeSH Terms]) OR phobia, social[MeSH Terms]) OR cost of illness[MeSH Terms] | 7,721,874 |
| 3 |
2 NOT:
((((((((((((caregivers[MeSH Terms]) OR mothers/psychology[MeSH Terms]) OR adolescent[MeSH Terms]) OR infant[MeSH Terms]) OR child[MeSH Terms]) OR pregnancy[MeSH Terms]) OR parents[MeSH Terms]) OR child of impaired parents[MeSH Terms]) OR pediatrics))) OR children |
4,255,981 |
| 4 | 1 AND 3 | 1052 |
| 5 | Limits applied to 4: within 10 years, English language, Humans | 597 |
Appendix 3.
PubMed search of specific intervention terms when combined with population terms.
| Intervention Terms combined with (“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autism”[All Fields]) | Number of articles located |
|---|---|
| (“mindfulness”[MeSH Terms] OR “mindfulness”[All Fields]) | 4 |
| “cognitive behaviour therapy”[All Fields] OR “cognitive therapy”[MeSH Terms] OR (“cognitive”[All Fields] AND “therapy”[All Fields]) OR “cognitive therapy”[All Fields] OR (“cognitive”[All Fields] AND “behavior”[All Fields] AND “therapy”[All Fields]) OR “cognitive behavior therapy”[All Fields] | 163 |
| (trauma-informed[All Fields] AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields])) | 0 |
| animal-assisted[All Fields] | 5 |
| ((“cannabis”[MeSH Terms] OR “cannabis”[All Fields] OR “marijuana”[All Fields]) | 9 |
Appendix 4.
Participant characteristics from included studies.
| Author | Study design | Total sample | Diagnosis included (confirming assessment) | Mean age (years) | Gender (% male) | Intellectual disability (%) |
|---|---|---|---|---|---|---|
| Brundage et al. (2013) | Single-subject ABAB design | 1 | Autistic disorder (ADOS) | 21 | 100 | 100 |
| Campillo et al. (2014) | Single-subject AB design | 3 | Autistic disorder (DSM IV criteria) | 25 | 66 | 100 |
| Ekman & Hiltunen (2015) | Pre-test–post-test single group | 18 (11 adults) | ASD diagnosis (verified with psychiatric provider) | 29.8 (adult sample) | 65 (adult sample) | Not reported |
| Enticott et al. (2011) | Case report | 1 | High-functioning ASD (not confirmed) | 20 | 0 | 0 |
| Gal et al. (2015) | Pre-test–post-test single group | 25 | 36% PDD-NOS, 8% ASD, 14% Asperger’s syndrome (psychiatric records) | 19 | 96 | Not reported |
| Hesselmark et al. (2014) | Randomized controlled trial | 75 | Autism spectrum disorder (medical records, ADOS, and clinical interview) | 32 | 55 | 0 |
| Hsieh et al. (2014) | Case report | 1 | Autism spectrum disorder (unclear diagnostic verification) | 22 | 100 | 100 |
| McGillivray & Evert (2014) | Pre-test–post-test non-equivalent control group | 42 | Asperger syndrome or high-functioning autism (face-to-face interview after chart review) | 21 | 76 | Unclear. Excluded participants with “obvious signs of cognitive impairment that might limit their ability to fully participate” (p. 2042) |
| McVey et al. (2016) | Randomized controlled trial | 53 | High-functioning autism, autism syndrome, or pervasive developmental disorder—NOS (ADOS-G) | 20 | 81 | Unclear. Inclusion was verbal IQ 70. Mean IQ was 92 |
| Nilsson & Ekselius (2009) | Case study | 1 | Asperger’s syndrome, obsessive-compulsive disorder, hypochondriasis (unclear diagnostic verification) | 38 | 100 | Not reported |
| Roser et al. (2009) | Case study | 1 | Asperger’s syndrome (Marburg Rating Scale) | 25 | 100 | 0 |
| Russell et al. (2013) | Randomized controlled trial | 46 | Autism spectrum disorder with co-occurring OCD (Autism Diagnostic Interview) | 27 | 76 | Unclear. Inclusion was verbal IQ 70. Mean Verbal IQ 100 |
| Siew et al. (2017) | Mixed methods with pre-test–post-test single group | 10 | Autism spectrum disorder (self-identified) | 18 | 70 | Unclear |
| Sajith et al. (2017) | Case study | 2 | Autism spectrum disorder (unclear diagnostic verification) | 22 | 100 | 100 |
| Sizoo & Kuiper (2017) | Pre-test–post-test non-equivalent control group | 59 | Autism spectrum disorder (chart review) | 37 | 64 | 0 |
| Spek et al. (2013) | Randomized controlled trial | 42 | Autism spectrum disorder (ADI-R, DSM-IV-TR) | 42 | 66 | 0 |
| Tiger et al. (2009) | Case study | 1 | Asperger’s syndrome (unclear diagnostic verification) | 19 y | 100 | 0 |
| Wachtel et al. (2010) | Case study | 1 | Autism, intellectual disability, severe depression with suicidal attempts, catatonia (unclear diagnostic verification) | 19 | 100 | 100 |
| Weiss & Lunsky (2010) | Case series | 3 | Asperger’s syndrome (chart review of previous diagnosis with confirmation from Adult Asperger Assessment) | 40 (estimate based on range) | 66 | 0 |
ADI-R: Autism Diagnostic Interview–Revised; ADOS: Autism Diagnostic Observation Schedule; ASD: autism spectrum disorder; DSM: Diagnostic and Statistical Manual of Mental Disorders (4th ed; American Psychiatric Association, 2003); IQ: intelligence quotient; OCD: obsessive-compulsive disorder; PDD-NOS: Pervasive Developmental Disorder—Not Otherwise Specified.
Footnotes
Author contributions: All authors have reviewed and approved the manuscript prior to submission, and this manuscript has not been submitted to any other journal for publication. The manuscript was provided to the funder as a draft and is not available publicly. T.W.B. conceptualized the need for study, contributed to data analysis, data interpretation, and drafting the manuscript for submission. S.M.S. also conceptualized the need for study. He edited and contributed to significant revisions of the manuscript as submitted. M-L.A., R.C., B.C., D.L.G., L.M., Y.(Jeanette).P., B.R., and K.W. fully read the manuscript, provided significant edits, and provided new content based in their experience as autistic individuals. These authors assisted in the design of autistic friendly language and interpretation of the results. J.M.E., M.C.K., T.M.H., L.E.M., S.M.R. and S.P.W. contributed to data acquisition, data analysis, data interpretation, drafting the manuscript for submission, and critically revised the content of the draft.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded through a Patient-Centered Outcomes Research Institute (PCORI) Eugene Washington PCORI Engagement Award (EAIN# 4208). The views presented in this document are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Ethical approval: This research did not involve human subjects, as it included an analysis of published research. No institutional review board review was conducted.
ORCID iD: Teal W Benevides  https://orcid.org/0000-0003-1395-2628
https://orcid.org/0000-0003-1395-2628
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Author. [Google Scholar]
- Benevides T. W., Shore S. M., Palmer K., Duncan P., Plank A., Andersen M. L., Caplan R., Cook B., Gassner D., Hector B. L., Morgan L., Nebecker L., Purkis Y., Rankonski B., Wittig K., Coughlin S. S. (2020). Listening to the autistic voice: Mental health priorities to guide research and practice from a stakeholder-driven project. Autism. 10.1177/1362361320908410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., Botts E. L., Smith K. R., Pimentel R., Farley M., Viskochil J., . . . Coon H. (2013). Excess mortality and causes of death in autism spectrum disorders: A follow up of the 1980s Utah/UCLA autism epidemiologic study. Journal of Autism and Developmental Disorders, 43(5), 1196–1204. 10.1007/s10803-012-1664-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage S. B., Whelan C. J., Burgess C. M. (2013). Brief report: Treating stuttering in an adult with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(2), 483–489. 10.1007/s10803-012-1596-7 [DOI] [PubMed] [Google Scholar]
- Burke L., Stoddart K. P. (2014). Medical and health problems in adults with high-functioning autism and Asperger syndrome. In Volkmar F. R., Reichow B., McPartland J. C. (Eds.), Adolescents and adults with autism spectrum disorders (pp. 239–267). Springer. [Google Scholar]
- Cachia R. L., Andersen A., Moore D. W. (2016). Mindfulness in individuals with autism spectum disorder: A systematic review and narrative analysis. Review Journal of Autism and Developmental Disorders, 3, 145–178. 10.1177/s40489-016-0074-0 [DOI] [Google Scholar]
- Campillo C., Herrera G., Remirez de, Ganuza C., Cuesta J. L., Abellan R., Campos A., . . . Amati F. (2014). Using Tic-Tac software to reduce anxiety-related behaviour in adults with autism and learning difficulties during waiting periods: A pilot study. Autism, 18(3), 264–271. 10.1177/1362361312472067 [DOI] [PubMed] [Google Scholar]
- Cassidy S., Bradley L., Shaw R., Baron-Cohen S. (2018). Risk markers for suicidality in autistic adults. Molecular Autism, 9(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner C., White S. (2017). Brief report: Feasibility and preliminary efficacy of individual mindfulness therapy for adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(1), 290–300. 10.1007/s10803-017-3312-0 [DOI] [PubMed] [Google Scholar]
- Croen L. A., Zerbo O., Qian Y., Massolo M. L., Rich S., Sidney S., Kripke C. (2015). The health status of adults on the autism spectrum. Autism, 19(7), 814–823. 10.1177/1362361315577517 [DOI] [PubMed] [Google Scholar]
- Ekman E., Hiltunen A. J. (2015). Modified CBT using visualization for autism spectrum disorder (ASD), anxiety and avoidance behavior–a quasi-experimental open pilot study. Scandinavian Journal of Psychology, 56(6), 641–648. 10.1111/sjop.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott P. G., Kennedy H. A., Zangen A., Fitzgerald P. B. (2011). Deep repetitive transcranial magnetic stimulation associated with improved social functioning in a young woman with an autism spectrum disorder. The Journal of ECT, 27(1), 41–43. 10.1097/YCT.0b013e3181f07948 [DOI] [PubMed] [Google Scholar]
- Gal E., Selanikyo E., Erez A. B., Katz N. (2015). Integration in the vocational world: How does it affect quality of life and subjective well-being of young adults with ASD. International Journal of Environmental Research and Public Health, 12(9), 10820–10832. 10.3390/ijerph120910820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmark E., Plenty S., Bejerot S. (2014). Group cognitive behavioural therapy and group recreational activity for adults with autism spectrum disorders: A preliminary randomized controlled trial. Autism, 18(6), 672–683. 10.1177/1362361313493681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa-McMillan C., Francis S., Rith-Najarian L., Chorpita B. (2015). Evidence base update: 50 years of research on treatment for child and adolescent anxiety. Journal of Clinical Child & Adolescent Psychology, 45(2), 91–113. 10.1080/15374416.2015.1046177 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., Altman D. G., Sterne J. A. C. (2011). Assessing risk of bias in included studies. In: Higgins J. P. T., Green S. (Eds.), Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collection; www.handbook.cochrane.org [Google Scholar]
- Hsieh E., Oh S. S., Chellappa P., Szeftel R., Jones H. D. (2014). Management of autism in the adult intensive care unit. Journal of Intensive Care Medicine, 29(1), 47–52. 10.1177/0885066612470236 [DOI] [PubMed] [Google Scholar]
- Interagency Autism Coordinating Committee. (2016). Strategic plan questions 2016. https://iacc.hhs.gov/publications/portfolio-analysis/2016/
- McGillivray J. A., Evert H. T. (2014). Group cognitive behavioural therapy program shows potential in reducing symptoms of depression and stress among young people with ASD. Journal of Autism and Developmental Disorders, 44(8), 2041–2051. 10.1007/s10803-014-2087-9 [DOI] [PubMed] [Google Scholar]
- McVey A. J., Dolan B. K., Willar K. S., Pleiss S., Karst J. S., Casnar C. L., . . . Van Hecke A. V. (2016). A replication and extension of the PEERS® for young adults social skills intervention: Examining effects on social skills and social anxiety in young adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(12), 3739–3754. 10.1007/s10803-016-2911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLOS MEDICINE, 6(7), Article e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Professional Development Center on Autism Spectrum Disorder. (n.d.). Evidence-based practices: What criteria determined if an intervention was effective? FPG Child Development Center, University of North Carolina; https://autismpdc.fpg.unc.edu/what-criteria-determined-if-intervention-was-effective [Google Scholar]
- Nicolaidis C., Kripke C. C., Raymaker D. (2014). Primary care for adults on the autism spectrum. Medical Clinics of North America, 98(5), 1169–1191. 10.1016/j.mcna.2014.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaidis C., Raymaker D. M., Ashkenazy E., McDonald K. E., Dern S., Baggs A. E., . . . Boisclair W. C. (2015). “Respect the way I need to communicate with you”: Healthcare experiences of adults on the autism spectrum. Autism, 19(7), 824–831. 10.1177/1362361315576221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. M., Ekselius L. (2009). Acute and maintenance electroconvulsive therapy for treatment of severely disabling obsessive-compulsive symptoms in a patient with Asperger syndrome. The Journal of ECT, 25(3), 205–207. 10.1097/YCT.0b013e31819746bc [DOI] [PubMed] [Google Scholar]
- Purkis J., Goodall E., Nugent J. (2016). The guide to good mental health on the autism spectrum. Jessica Kingsley Publishers. [Google Scholar]
- Roser P. H. I. S., Juckel G., Brune J. (2009). Paliperidone in an adult patient with Asperger syndrome: Case report. Pharmacopsychiatry, 42(2), 78–79. 10.1055/s-0028-1102913 [DOI] [PubMed] [Google Scholar]
- Russell A. J., Jassi A., Fullana M. A., Mack H., Johnston K., Heyman I., . . . Mataix-Cols D. (2013). Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: A randomized controlled trial. Depression & Anxiety, 30(8), 697–708. 10.1002/da.22053 [DOI] [PubMed] [Google Scholar]
- Sajith S. G., Liew S. F., Tor P. C. (2017). Response to electroconvulsive therapy in patients with autism spectrum disorder and intractable challenging behaviors associated with symptoms of catatonia. The Journal of ECT, 33(1), 63–67. 10.1097/YCT.0000000000000338 [DOI] [PubMed] [Google Scholar]
- Siew C. T., Mazzucchelli T. G., Rooney R., Girdler S. (2017). A specialist peer mentoring program for university students on the autism spectrum: A pilot study. PLOS ONE, 12(7), Article e0180854. 10.1371/journal.pone.0180854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizoo B. B., Kuiper E. (2017). Cognitive behavioural therapy and mindfulness based stress reduction may be equally effective in reducing anxiety and depression in adults with autism spectrum disorders. Research in Developmental Disabilities, 64, 47–55. 10.1016/j.ridd.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Spek A. A., van Ham N. C., Nyklicek I. (2013). Mindfulness-based therapy in adults with an autism spectrum disorder: A randomized controlled trial. Research in Developmental Disabilities, 34(1), 246–253. 10.1016/j.ridd.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Tiger J. H., Fisher W. W., Bouxsein K. J. (2009). Therapist- and self-monitored DRO contingencies as a treatment for the self-injurious skin picking of a young man with Asperger syndrome. Journal of Applied Behavior Analysis, 42(2), 315–319. 10.1901/jaba.2009.42-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra R., Madhavan S., Sambamoorthi U. (2016). Emergency department use among adults with autism spectrum disorders (ASD). Journal of Autism and Developmental Disorders, 46(4), 1441–1454. 10.1007/s10803-015-2692-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel L. E., Griffin M., Reti I. (2010). Electroconvulsive therapy in a man with autism experiencing severe depression, catatonia, and self-injury. Journal of Electroconvulsive Therapy, 26(1), 70–73. [DOI] [PubMed] [Google Scholar]
- Weiss J. A., Lunsky Y. (2010). Group cognitive behaviour therapy for adults with Asperger syndrome and anxiety or mood disorder: A case series. Clinical Psychology & Psychotherapy, 17(5), 438–446. 10.1002/cpp.694 [DOI] [PubMed] [Google Scholar]
- Weston L., Hodgekins J., Langdon P. E. (2016). Effectiveness of cognitive behavioral therapy with people who have autistic spectrum disorders: A systematic review and meta-analysis. Clinical Psychology Review, 49, 41–54. 10.1016/j.cpr.2016.08.001 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1948, April). Preamble to the Constitution of WHO as adopted by the International Health Conference, New York; entered into force 7 April 1948 www.who.int/about/who-we-are/constitution (accessed 23 March 2020). [Google Scholar]
- Young J., Corea C., Kimani J., Mandell D.; on behalf of IMPAQ International, LLC. (2010). Autism spectrum disorders (ASDs) Services final report on environmental scan [Report to Centers for Medicare and Medicaid Services]. https://www.impaqint.com/sites/default/files/project-reports/Autism_Spectrum_Disorders.pdf
- Zerbo O., Qian Y., Ray T., Sidney S., Rich S., Massolo M., Croen L. A. (2019). Health care service utilization and cost among adults with autism spectrum disorders in a U.S. integrated health care system. Autism in Adulthood, 1(1), 27–36. 10.1089/aut.2018.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]


