Abstract
The restrictive measures required to face the recent outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may impact patterns of healthcare utilization. Our aim was to provide an insight into the change in the use of a pediatric emergency department (ED) during the SARS-CoV-2 pandemic. The medical records of the children seen in our pediatric ED during March and April 2020 were retrospectively reviewed. Consequently, these were compared to the medical records of 2018 and 2019 from the same time period and from other control periods (January–February 2019 and 2020, and July–August 2018 and 2019). The total number of ED visits declined by 73% from 2019 to 2020 (3051 vs 818). Significant variations were observed in the distribution of children between triage categories: the proportion of patients who was given a green-code showed a 0.59-fold decrease in comparison to 2019 (95% CI 0.5–0.69), while a relative increase in the proportion of yellow codes was observed (OR 1.46, 95% CI 1.2–1.78).
Conclusion: Quarantine measures significantly impacted on the total number of patients and on the reasons for visiting them in our pediatric ED. This substantial decrease in pediatric care may either be due to lower rates of acute infections because of social distancing, or to parents’ or caregivers’ reticence to risk exposure to SARS-CoV-2 in a health-care setting.
|
What is known: • A recent outbreak of a novel coronavirus responsible for a severe acute respiratory syndrome is spreading globally. • Restrictive measures may impact patterns of healthcare utilization, as observed in other previous outbreaks. | |
|
What is new: • This study shows significant variations in the distribution of children among triage categories during the COVID-19 pandemic. • Discharge diagnosis was significantly different as well, in particular a relative increase in the proportion of children presenting with traumatic injuries and a decrease of viral infections were observed. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-020-03909-9.
Keywords: COVID-19, Pediatrics, Infectious disease, Pandemic, Emergency care
Introduction
A novel type of coronavirus of unknown origin was recently identified in Wuhan (China) [1] in subjects affected by pneumonia. The virus was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and the associated disease was called 2019 coronavirus disease (COVID-19) [2]. SARS-CoV-2 rapidly spread worldwide, forcing the World Health Organization (WHO) to recognize the outbreak as a pandemic on 11 March, 2020 [3].
Italy was one of the first countries to be hit in the world, with a large number of confirmed COVID-19 cases [4]. Children, however, represented less than 3% of the total number of patients [5, 6]. As a response to the growing pandemic of COVID-19 in the country, on the 8 of March, the Italian Government imposed a national quarantine, restricting the movement of all citizens except for necessities, work, or health circumstances. Social distancing has proved to be effective in lowering the incidence of new SARS-CoV-2 infections [7, 8]. However, implementing sanitary measures at a national level may also impact several social and economic aspects of people’s routine life [9]. In particular, they can modify the patterns of healthcare utilization, as observed in other previous infectious disease outbreaks [10, 11].
As largely described, emergency department (ED) services are usually overwhelmed by an inappropriately high number of admissions of patients with non-urgent conditions, ranging from 24 to 40% of total visits [12]. Reasons for this behavior may be identified in the difficulties to access primary care providers [13], or in parents’ misperception of the severity of the illness [14]. However, some recent preliminary data estimated a reduction in the number of visits to pediatric EDs by more than 50% during the SARS-CoV-2 outbreak [15–18].
The aim of this retrospective study was to provide an insight into the changing pattern of patients’ use of a pediatric ED during the SARS-CoV-2 outbreak, comparing the number of admissions and hospitalizations to other control periods.
Material and methods
We retrospectively reviewed the medical records from the computerized database system of a tertiary level pediatric ED at the University Hospital of Central Friuli in Udine, northeastern Italy, which covers an area of 530,000 residents (Supplementary Fig. 1). It is the only pediatric ED in the city with a volume of 20,000 patients/year between the age of 0 and 16.
All visits performed during the strict lockdown phase (between 1 March and 30 April, 2020—SARS-CoV-2 period) were compared to those performed over the same time period of the two previous years (Spring 2018—Sp18 and Spring 2019—Sp19). In order to limit possible confounding effects due to yearly or seasonal changes in total attendances, we also compared the data to that of the two previous Winter (1 January–29 February 2020, W20; 1 January–28 February 2019, W19) and Summer periods (1 July–31 August 2019, Su19; 1 July–31 August 2018, Su18).
The total and daily number of visits and the hospital admission rate (defined as the number of admissions from the ED relative to the total number of visits) were identified as outcome-relevant patient statistics. We also examined demographic and clinical characteristics of the enrolled patients, including initial triage scoring (white and green codes were classified as non-urgent/delayable emergencies and yellow and red codes were classified as non-delayable urgencies/emergencies and were treated immediately—see Supplementary Table 1 for details) and discharge or admission diagnosis. Two authors extracted the data independently.
Both discharge and admission diagnoses were codified according to ICD-9-CM nomenclature [19].
Clinical features and diagnoses were grouped into different categories: (1) children presenting with acute infectious symptoms (e.g., fever, respiratory, or gastrointestinal symptoms), (2) traumatic injuries, (3) underlying or chronic diseases (e.g., febrile neutropenia in immunosuppressed children, seizures in epileptic children, newly diagnosed diabetes), (4) surgical urgencies (e.g., acute appendicitis, testicular torsion), (5) mental health disorders, and (6) other illnesses. Elective and planned hospital admissions were not included in the analysis.
The study was approved by the local Institutional Review Board (Prot. N. 017/2020_IRB, tit. III, cl. 13, fasc 32).
Statistical analysis
Continuous variables were expressed as mean (SD), while categorical variables were expressed as percentages or frequencies. The differences between groups were evaluated by t test. Fisher’s and χ-square tests were used to compare frequencies and percentages. Simple linear regression models were used to describe the trend of daily admissions of the first 4 months of 2020, 2019, and 2018 (time as independent variable). The predictive factors for assigning an urgent code (yellow or red) were identified by binomial logistic regression (standard regression analysis, probability of stepwise entry 0.05 and removal 0.1). The variables to enter in the multivariate model were chosen on the basis of the univariate analysis results (p < 0.25). The model calibration was assessed by Hosmer–Lemeshow goodness-of-fit test. The differences were presented as odds ratio (OR) with 95% confidence interval (95% CI). p value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism version 8.4.2 for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com).
Results
A total of 818 children (457 males, 55.9%; mean age 5.6 ± 4.8 years) were visited in our pediatric ED during the SARS-CoV-2 period. The main clinical and demographic characteristics of the enrolled children are shown in Table 1. No significant difference was found between the study population and the control groups (Sp18, Sp19, W19, W20, Su18, Su19) (Supplementary Table 2).
Table 1.
Demographic, triage scoring, and number of admissions of children seen in the pediatric emergency department (ED) in the months of March and April 2020
| March–April 2020 | |
|---|---|
| No. of patients | 818 |
| Males: n (%) | 457 (55.9%) |
| Mean age ± SD (years) | 5.6 ± 4.8 |
| Triage scoring: n (%) | |
| • White | 103 (12.6%) |
| • Green | 540 (66%) |
| • Yellow | 169 (20.7%) |
| • Red | 6 (0.7%) |
| No. of admission: n (%) | 64 (7.8%) |
| • General pediatric ward | 59 (92%) |
| • Neonatal intensive care unit | 3 (5%) |
| • Intensive care unit | 1 (1.5%) |
| • Surgical ward | 1 (1.5%) |
Only one child who was admitted to our pediatric ED was diagnosed with SARS-CoV-2 infection and then transferred to another hospital.
Total number and distribution of triage-scoring categories
During the SARS-CoV-2 outbreak, the total number of visits declined by 73.2% in comparison to the previous year (N = 3051). The proportion of green codes showed a 0.59-fold decrease (95% CI 0.5–0.69), while white and yellow codes showed a 1.76-fold (95% CI 1.38–2.24) decrease and a 1.46-fold (95% CI 1.2–1.78) decrease respectively (Supplementary Table 3). No significant changes over time were shown in the proportion of red codes (Supplementary Table 3). Comparisons with winter periods confirmed these results (Supplementary Table 3).
In comparison to the previous summer period, the number of children evaluated in the pediatric ED decreased by 66.6% (818 vs 2449) (Supplementary Table 3). Green codes showed a 0.66-fold decrease (95% CI 0.55–0.77), while yellow codes showed a 1.67-fold increase (95% CI 1.36–2.05). No difference was shown for white and red codes (Supplementary Table 3).
Binomial logistic regression analysis showed that the probability of assigning an urgent code (defined as yellow or red code) was 1.46 higher (95% CI 1.2–1.77) in 2020 compared to 2019 (Table 2). Children aged < 6 years (OR 1.23 95% CI 1.04–1.46) had a higher probability of receiving an urgent code, while no difference was shown for the older ones (OR 0.83; 95% CI 0.53–1.28) or between males and females (Table 2).
Table 2.
Multivariate analysis for defining predictive factors associated to urgent triage code (yellow and red) of children seen in the pediatric emergency department (ED) during the months of March–April 2020 (SARS-CoV-2), compared to the same time intervals of the two previous years (Sp19–Sp18)
| SARS-CoV-2 vs Sp19 | SARS-CoV-2 vs Sp18 | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| All patients | 1.46 | 1.2–1.77 | < 0.001 | 1.68 | 1.39–2.04 | < 0.001 |
| Age group (≤ 6 years) | 1.23 | 1.04–1.46 | 0.017 | 0.86 | 0.73–1.03 | 0.1 |
| Gender (male) | 1.53 | 0.98–1.69 | 0.366 | 1.31 | 1–1.7 | 0.457 |
All values presented in italics: p<0.05
Daily visits and time-trend overview
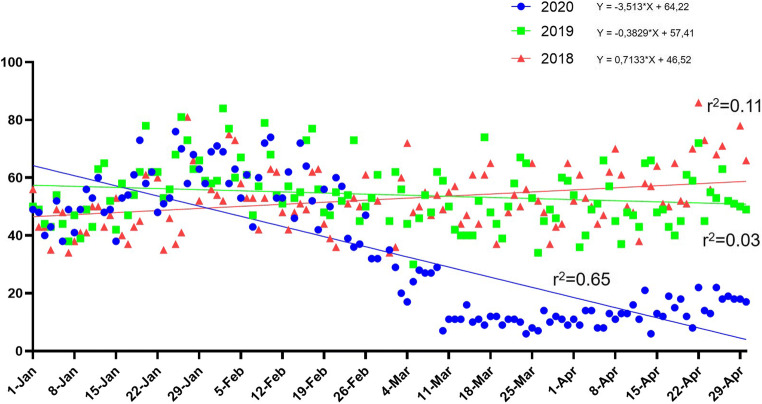
A significant decrease in the mean number of daily visits (14.6 ± 6.5/day) was observed in comparison to the same time-interval in 2019 (50.9 ± 9.6/day, p < 0.001) and 2018 (54.4 ± 10.8/day, p < 0.001). Linear regression analysis was performed on the daily admissions to our pediatric ED in the first 4 months of 2020, 2019, and 2018 which showed a statistically significant decreasing tendency in 2020 (− 3.5 visits/day, 95% CI − 3.98 to − 3.05) (Fig. 1).
Fig. 1.
Linear regression comparing the trend in the number of daily visits in the pediatric emergency department (ED) during 2020 (blue line) 2019 (green line) and 2018 (red line)
Discharge diagnosis
The distribution of ED discharge diagnosis during the SARS-CoV-2 period showed a significant reduction of acute infectious diseases in comparison to Sp19 and Sp18 (Table 3). Conversely, a relative increase in traumatic injuries and mental health disorders was observed, but no case of child abuse was registered during the lockdown phase (Table 3). The number of children with a chronic or underlying disease remained stable. Similar results were shown for other control periods (Supplementary Table 4). The distribution of specific discharge diagnosis for acute infectious disease and traumatic injuries was also analyzed to highlight the differences between 2020, 2019, and 2018 (Supplementary Table 5). In particular, during SARS-CoV-2 period, children were less frequently diagnosed with acute gastroenteritis (p = 0.007), otitis (p = 0.006), and other viral infections (p = 0.044) in comparison to Sp19 and Sp18 (Supplementary Table 5). A higher proportion of children with urinary tract infections (UTI) was also observed in comparison to previous years (p < 0.001) (Supplementary Table 5). Domestic accidents, such as minor wounds (p < 0.001), ingestion/inhalation of foreign bodies (p = 0.042), and burns (p = 0.014), were diagnosed more frequently in children who were admitted to our pediatric ED during the pandemic period compared to 2019 and 2018. However, bone fractures were less frequently observed (p = 0.007) (Supplementary Table 5).
Table 3.
Distribution of discharge diagnosis in children who were seen in the pediatric emergency department (ED) during the SARS-CoV-2 period (March–April 2020) in comparison to the same time intervals of the two previous years (Sp19–Sp18)
| SARS-CoV-2 N = 796 |
Sp19 N = 3094 |
Sp18 N = 3388 |
p | |
|---|---|---|---|---|
| Acute infectious diseases | 250 (31%) ↓ | 1580 (51%) | 1657 (49%) | < 0.001 |
| Traumatic injuries | 318 (40%) ↑ | 740 (24%) | 865 (26%) | < 0.001 |
| Underlying diseases | 27 (3%) = | 85 (3%) | 99 (3%) | 0.623 |
| Mental health disorders | 16 (2%) ↑ | 24 (0.8%) | 28 (0.8%) | 0.004 |
| Other illnesses | 185 (24%) = | 665 (21%) | 739 (22%) | 0.187 |
Differences between groups were tested by χ-square test
All values presented in italics: p<0.05
Trends in hospitalizations
Significant differences were also shown in the number of hospitalizations. The hospital admission rate increased from 3% (91/3202 ED visits) in the months of March–April 2019 to 7.8% (64/818) during the same period in 2020 (p < 0.001), with a relative 2.7-fold increase (95% CI 1.9–3.8). Similar results were shown for other control periods. A relatively lower number of patients with infectious diseases was observed compared to the same time period in 2019 and in 2018 (p = 0.019) (Table 4), but no difference in the distribution of specific diagnosis was visible (Supplementary Table 6). Comparisons with winter periods were similar, while no difference in the distribution of hospitalization diagnosis with summertime was shown (Supplementary Table 4).
Table 4.
Total number of hospitalizations according to diagnostic categories in children who were evaluated in the pediatric ED during the SARS-CoV-2 period (March–April 2020) in comparison to the same time period in the two previous years
| SARS-CoV-2 | Sp19 | Sp18 | p | |
|---|---|---|---|---|
| Admission rate | 64/818 (7.8%) ↑ | 91/3051 (3%) | 110/3266 (3.4%) | < 0.001 |
| Acute infectious diseases | 22/64 (34%) ↓ | 51/91 (56%) | 58/110 (53%) | 0.019 |
| Traumatic injuries | 14/64 (22%) | 15/91 (17%) | 15/110 (14%) | 0.371 |
| Underlying diseases | 12/64 (19%) | 8/91 (8.5%) | 15/110 (14%) | 0.194 |
| Acute surgical conditions | 7/64 (11%) | 8/91 (8.5%) | 5/110 (4%) | 0.262 |
| Mental health disorders | 3/64 (5%) | 2/91 (2%) | 3/110 (3%) | NA |
| Other illnesses | 6/64 (9%) | 7/91 (8%) | 12/110 (10%) | 0.6 |
Differences between groups were tested by χ-square test
All values presented in italics: p<0.05
Notably, during the COVID-19 pandemic, among the children discharged (n = 18) and admitted (n = 4) for UTI, 23% (5/22) were diagnosed with pyelonephritis, in comparison to 0% in 2019 and 10% (3/30) in 2018 (p = 0.015).
Discussion
This study showed a significant decline in the number of pediatric ED visits and a substantial increase in the rate of hospitalizations during the SARS-CoV-2 outbreak and national lockdown when compared to the two previous years. The reduction in ED visits was more prominent for low-acuity diseases (scored as green codes) rather than for higher triage scores (yellow and red codes).
Total number and distribution of triage-scoring categories
In recent years, overcrowding has been reported in EDs from several different countries. Moreover, one-fifth of the children under the age of 5 were reported to have been admitted to an ED at least once in their lifetime [20]. In Italy, where the national healthcare system provides free admission to the ED for children under the age of 14, non-urgent visits (namely, white and green codes) accounted for 27.6% of all ED patients and 58.2% of total pediatric attendance episodes [21]. The decrease in the number of visits during the SARS-CoV-2 period has been observed in other studies as well [15, 17, 18, 22] and it may be related to the adoption of national restrictive measures. With the closure of schools and sports activities on the 1st March, 2020, acute infections such as gastroenteritis, otitis, and other viral infections significantly decreased in comparison to previous years. A significant reduction in the mean number of episodes of acute otitis, otorrhea episodes, and prescriptions of antibiotics was also observed in a retrospective study that was recently published [23]. Similarly, French colleagues [24] documented a sharp decrease (over 70%) in the cases of acute gastroenteritis, common cold, and bronchiolitis compared to expectancy. The drop in the incidence of acute infections might seem to contrast the increased number of visits usually observed during seasonal influenza epidemics [25, 26]. However, SARS-CoV-2 does not affect children with the same frequency and severity as adults [27]. According to one of the largest study on the prevalence of COVID-19 in European children, only 8% of the patients with a confirmed infection required admission to an ICU [28].
In our study, during the SARS-CoV-2 period, a higher proportion of white codes was recorded compared to the same period over the two previous years (Sp19 and Sp18) and the winter periods. This may be explained by the fact that most of our outpatient activities were cancelled or markedly reduced; therefore, many minor procedures and non-urgent medical consultations took place in the pediatric ED and were classified as white codes. A proper reorganization of the pediatric services became necessary during the first wave of COVID-19 pandemic: national and local protocols were developed according to the WHO guidelines to provide dedicated pathways for suspected COVID-19 cases and guarantee appropriate healthcare to other patients [29, 30].
In comparison to all control periods, a relative reduction of green codes and a simultaneous increase of yellow codes were reported. The decrease of common childhood viral infections due to social distancing may partially explain this finding. In fact, this trend was observed in comparison to summer periods too, when schools are similarly closed and fewer people circulate in urban areas.
In 2011, Brousseau et al. conducted a survey on parents’ perspectives on the correct utilization of pediatric ED services [14]. Parents reported feeling anxious about their children and they did not consider non-urgent ED visits a misuse of the healthcare system, as the ED offered firsthand evaluations within the time-frame requested [14]. However, patients are likely to be more afraid than before to visit healthcare facilities due to the risk of contracting SARS-CoV-2 [31, 32], but no pediatric study has specifically addressed this point yet.
The incidence of pediatric red codes and children diagnosed with chronic/underlying diseases was not significantly affected during the COVID-19 pandemic. However, this may be in contrast with a recent case series of 12 children who received delayed hospital care [16]. Half of them were admitted to an ICU and four died. In all cases, parents reported avoiding accessing hospital services because of fear of contracting SARS-CoV-2 [16]. This was a preliminary report and the data could not be directly compared with those of previous years nor it allowed to confirm whether there was a systematic delay in accessing care for severely ill children. Therefore, even though the current findings on this issue remain controversial, it appears speculative to generalize these results. According to the Italian Diabetes Study Group, compared with the same period in 2019, during the pandemic, there were 23% fewer new diabetes cases, no differences in the incidence of acute complications, and a slightly higher frequency of severe ketoacidosis (DKA) among those who presented with DKA [33]. A recent German study [34] reported similar findings.
Discharge diagnosis
Discharge diagnosis was significantly different among studied periods. In particular, a relative increase in the proportion of children presenting traumatic injuries was observed during the SARS-CoV-2 outbreak. This finding appears in contrast with preliminary data on adult patients, showing a significant reduction in the number of injury-related admissions [35]. However, since outdoor activities were banned starting with the beginning of the quarantine, children were forced to stay at home. Therefore, in children who were admitted to our pediatric ED during the pandemic period, domestic accidents (minor wounds, ingestion/inhalation of foreign bodies, and burns) were more frequently diagnosed rather than bone fractures. Currently, domestic accidents represent one of the most frequent causes of morbidity in patients under the age of 14, and nearly half of them typically happen during play-time [36]. In particular, other reports [37] show that, in 2020, the number of accidental ingestions was higher than previous years. These data may support the hypothesis that, following stay-at-home orders, children’s environments shifted, making them face new, modifiable risks. This identifies an important area of public health education and intervention.
During the SARS-CoV-2 period, a slightly higher proportion of children with mental health disorders was diagnosed in the pediatric ED. The quarantine imposed may have worsened existing mental health problems because of the unique combination of public health crisis, social isolation, and economic recession [38]. In particular, with the closure of schools, the mental health services for adolescents with lower family incomes, who were likely to receive support especially in school settings [39], may have been disrupted. However, in many mental health centers, psychological consultations continued to be run in an urgent setting and telemedicine was set up to support patients at home. Moreover, 10/16 (62%) of the patients who were discharged with a diagnosis of mental health disorder were newly diagnosed patients, not previously evaluated by any mental health professional.
Several concerns were raised on the possibility of intrafamilial abuse during lockdown [40]. In our cohort, no case of abuse was observed, but a number of reports on this problem are emerging from different countries [41, 42]. A preliminary survey from the UK showed that there was a 1493% increase in cases of abusive head trauma [41]. Mental health issues, substance misuse, and difficult socioeconomic circumstances are often interdependent and cannot be effectively addressed in isolation.
Trends in hospitalizations
The rate of hospitalizations increased significantly too, but different patterns of admission diagnosis were observed only in comparison to spring and winter periods. This was probably due to the lower number of patients with infectious diseases, as previously discussed.
Surprisingly, in comparison to previous years, our results also showed a higher proportion of pyelonephritis among children diagnosed with UTI. In the study by Angoulvant and colleagues, UTIs were the only infection that did not decrease during the lockdown [24]. In these cases, fever, usually reported as the first and the only symptom of UTI in infants, may have been erroneously treated as a possible sign of SARS-CoV-2 infection, forcing children in quarantine and delaying further evaluation. However, more detailed data are needed to clarify this finding.
Our study has several limitations. It was a single-center retrospective analysis, and therefore the generalizability of these findings may be limited to comparable institutions. Therefore, multinational studies on this issue are needed to properly understand what really affected ED flows. Moreover, we could not evaluate the possible role of factors influencing people’s perceptions and utilization of healthcare facilities, such as family structure and socioeconomic status, scholarship, distance between the children’s house and the ED. However, despite the retrospective design, data were collected prospectively in a standardized way, with detailed clinical and epidemiological information for each patient from hospital software.
In conclusion, during the national lockdown period, our pediatric ED experienced significantly reduced volumes of children presenting low-acuity problems. This decrease in the number of visits may either be due to a reduction in the incidence of acute infectious diseases or to the fear of the potential nosocomial transmission of SARS-CoV-2 infection. However, even though parents were possibly more afraid to access healthcare facilities, red codes were not likely to be affected and urgent conditions were evaluated as usual. This study not only provides information for future SARS-CoV-2-related public health preparedness policies, but it may also provide a foundation for further research into alternative strategies to address non-urgent pediatric medical issues and alleviate emergency services.
Supplementary information
Cumulative incidence of novel Coronavirus-2 (SARS-CoV-2) infection in Italy (data from the Istituto Superiore di Sanità – ISS: COVID-19 integrated surveillance data in Italy. Update to 18 August, 2020). (JPG 101 kb)
(DOCX 15 kb)
(DOCX 15 kb)
(DOCX 16 kb)
(DOCX 16 kb)
(DOCX 16 kb)
(DOCX 14 kb)
(DOCX 33 kb)
Abbreviations
- COVID period
1 March–30 April 2020
- Spring 2019—Sp19
1 March–30 April 2019
- Spring 2018—Sp18
1 March–30 April 2018
- Winter 2020—W20
1 January–29 February 2020
- Winter 2019—W19
1 January–28 February 2019
- Summer 2019—Su19
1 July–31 August 2019
- Summer 2018—Su18
1 July–August 2018
- COVID-19
2019 coronavirus disease
- CoV
Coronavirus
- ED
Emergency department
- PCPs
Primary care physicians
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- WHO
World Health Organization
Authors’ contributions
- IL conceptualized the study, collected and analyzed data, wrote the first draft of the manuscript and contributed to the final version of the manuscript.
- CP conceptualized the study and contributed to the final version of the manuscript.
- MV collected and analyzed data and contributed to the final version of the manuscript.
- AP contributed to the final version of the manuscript
- EV contributed to the final version of the manuscript
- PC contributed to the final version of the manuscript
Data availability
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. Deidentified participant data may be available on reasonable request from IL.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was approved by the local Institutional Review Board (Prot. N. 017/2020_IRB, tit. III, cl. 13, fasc 32).
Consent to participate
N/A
Consent for publication
N/A
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ilaria Liguoro, Email: ilarialiguoro@gmail.com.
Chiara Pilotto, Email: chiara.pilotto@asufc.sanita.fvg.it.
Michela Vergine, Email: michela.vergine@asufc.sanita.fvg.it.
Anna Pusiol, Email: anna.pusiol@asufc.sanita.fvg.it.
Enrico Vidal, Email: enrico.vidal@asufc.sanita.fvg.it.
Paola Cogo, Email: paola.cogo@uniud.it.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. [cited 2020 Mar 30]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020
- 4.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases [Internet]. 2020 Feb 19 [cited 2020 Mar 30];0(0). Available from: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30120-1/abstract [DOI] [PMC free article] [PubMed]
- 5.Parri N, Lenge M, Buonsenso D (2020) Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med [DOI] [PMC free article] [PubMed]
- 6.Parri N, Magistà AM, Marchetti F, Cantoni B, Arrighini A, Romanengo M, et al. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian pediatric research networks. Eur J Pediatr. 2020;179(8):1315–1323. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen-Crowe B, McKenney M, Boneva D, Elkbuli A. A state overview of COVID19 spread, interventions and preparedness. Am J Emerg Med [Internet]. 2020 Apr 11 [cited 2020 Apr 27]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7151534/ [DOI] [PMC free article] [PubMed]
- 8.Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q et al (2020) Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA [DOI] [PMC free article] [PubMed]
- 9.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C et al (2020) The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int J Surg 16 [DOI] [PMC free article] [PubMed]
- 10.Lee SY, Khang YH, Lim HK. Impact of the 2015 Middle East respiratory syndrome outbreak on emergency care utilization and mortality in South Korea. Yonsei Med J. 2019;60(8):796–803. doi: 10.3349/ymj.2019.60.8.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paek SH, Kim DK, Lee JH, Kwak YH. The impact of Middle East respiratory syndrome outbreak on trends in emergency department utilization patterns. J Korean Med Sci. 2017;32(10):1576–1580. doi: 10.3346/jkms.2017.32.10.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carret MLV, Fassa ACG, Domingues MR. Inappropriate use of emergency services: a systematic review of prevalence and associated factors. Cad Saude Publica. 2009;25(1):7–28. doi: 10.1590/S0102-311X2009000100002. [DOI] [PubMed] [Google Scholar]
- 13.Fieldston ES, Alpern ER, Nadel FM, Shea JA, Alessandrini EA. A qualitative assessment of reasons for nonurgent visits to the emergency department: parent and health professional opinions. Pediatr Emerg Care. 2012;28(3):220–225. doi: 10.1097/PEC.0b013e318248b431. [DOI] [PubMed] [Google Scholar]
- 14.Brousseau DC, Nimmer MR, Yunk NL, Nattinger AB, Greer A. Nonurgent emergency-department care: analysis of parent and primary physician perspectives. Pediatrics. 2011;127(2):e375–e381. doi: 10.1542/peds.2010-1723. [DOI] [PubMed] [Google Scholar]
- 15.Bressan S, Buonsenso D, Farrugia R, Parri N, Oostenbrink R, Titomanlio L, et al. Preparedness and response to pediatric COVID-19 in European emergency departments: a survey of the REPEM and PERUKI networks. Ann Emerg Med [Internet]. 2020 May 15 [cited 2020 Aug 12]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7225691/ [DOI] [PMC free article] [PubMed]
- 16.Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health [Internet]. 2020 Apr 9 [cited 2020 Apr 27]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7146704/ [DOI] [PMC free article] [PubMed]
- 17.Cozzi G, Zanchi C, Giangreco M, Rabach I, Calligaris L, Giorgi R et al (2020) The impact of the COVID-19 lockdown in Italy on a pediatric emergency setting. Acta Paediatr [DOI] [PMC free article] [PubMed]
- 18.Masetti R, Corsini I, Leardini D, Lanari M, Pession A. Presentations to the emergency department in Bologna, Italy, during COVID-19 outbreak. BMJ Paediatr Open [Internet]. 2020 Jul 16 [cited 2020 Aug 17];4(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7372170/ [DOI] [PMC free article] [PubMed]
- 19.Salute M della. Il manuale ICD9CM [Internet]. [cited 2020 May 22]. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?lingua=italiano&id=1278&area=ricoveriOspedalieri&menu=classificazione
- 20.Hoot NR, Aronsky D. Systematic review of emergency department crowding: causes, effects, and solutions. Ann Emerg Med. 2008;52(2):126–136. doi: 10.1016/j.annemergmed.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vedovetto A, Soriani N, Merlo E, Gregori D. The burden of inappropriate emergency department pediatric visits: why Italy needs an urgent reform. Health Serv Res. 2014;49(4):1290–1305. doi: 10.1111/1475-6773.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M (2020) Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J [DOI] [PubMed]
- 23.Torretta S, Capaccio P, Coro I, Bosis S, Pace ME, Tosi P, et al. Incidental lowering of otitis-media complaints in otitis-prone children during COVID-19 pandemic: not all evil comes to hurt. Eur J Pediatr. 2020;20:1–4. doi: 10.1007/s00431-020-03747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angoulvant F, Ouldali N, Yang DD, Filser M, Gajdos V, Rybak A, et al. COVID-19 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non-viral infections, a time series analysis. Clin Infect Dis [Internet]. 2020 Jun 3 [cited 2020 Aug 15]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7314162/ [DOI] [PMC free article] [PubMed]
- 25.Proudlove N, Brown C. Winter planning. Seasonal Cycles Health Serv J. 2002;112(5790):24–25. [PubMed] [Google Scholar]
- 26.Glaser CA, Gilliam S, Thompson WW, Dassey DE, Waterman SH, Saruwatari M, Shapiro S, Fukuda K. Medical care capacity for influenza outbreaks, Los Angeles. Emerging Infect Dis. 2002;8(6):569–574. doi: 10.3201/eid0806.010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A et al (2020) SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr [DOI] [PMC free article] [PubMed]
- 28.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health [Internet]. 2020 Jun 25 [cited 2020 Aug 13]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7316447/ [DOI] [PMC free article] [PubMed]
- 29.Nicastro E, Mazza A, Gervasoni A, Di Giorgio A, D’Antiga L. A pediatric emergency department protocol to avoid intrahospital spread of SARS-CoV-2 during the outbreak in Bergamo. Italy J Pediatr. 2020;222:231–235. doi: 10.1016/j.jpeds.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonsenso D, Onesimo R, Valentini P, Chiaretti A, Gatto A, Attinà G, Conti G, Vento G, Cambieri A, Mercuri E, Zampino G, pedCOVID-team Children’s healthcare during corona virus disease 19 pandemic: the Italian experience. Pediatr Infect Dis J. 2020;39(7):e137–e140. doi: 10.1097/INF.0000000000002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canova V, Lederer Schläpfer H, Piso RJ, Droll A, Fenner L, Hoffmann T, et al. Transmission risk of SARS-CoV-2 to healthcare workers -observational results of a primary care hospital contact tracing. Swiss Med Wkly. 2020;150:w20257. doi: 10.4414/smw.2020.20257. [DOI] [PubMed] [Google Scholar]
- 32.Franchini S, Spessot M, Landoni G, Piani C, Cappelletti C, Mariani F, et al. Stranger months: how SARS-CoV-2, fear of contagion, and lockdown measures impacted attendance and clinical activity during February and March 2020 at an urban emergency Department in Milan. Disaster Med Public Health Prep. 2020;27:1–23. doi: 10.1017/dmp.2020.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A, Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetes* et al (2020) Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care
- 34.Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA [Internet]. 2020 Jul 20 [cited 2020 Aug 19]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2768716 [DOI] [PMC free article] [PubMed]
- 35.Forrester JD, Liou R, Knowlton LM, Jou RM, Spain DA. Impact of shelter-in-place order for COVID-19 on trauma activations: Santa Clara County, California, March 2020. Trauma Surg Acute Care Open. 2020;5(1):e000505. doi: 10.1136/tsaco-2020-000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO | World report on child injury prevention [Internet]. WHO. World Health Organization; [cited 2020 May 22]. Available from: http://www.who.int/entity/violence_injury_prevention/child/injury/world_report/en/index.html
- 37.Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F (2020) Trends in pediatric emergency department utilization after institution of COVID-19 mandatory social distancing. J Pediatr [DOI] [PMC free article] [PubMed]
- 38.Golberstein E, Wen H, Miller BF (2020) Coronavirus disease 2019 (COVID-19) and mental health for children and adolescents. JAMA Pediatr [DOI] [PubMed]
- 39.Ali MM, West K, Teich JL, Lynch S, Mutter R, Dubenitz J. Utilization of mental health services in educational setting by adolescents in the United States. J Sch Health. 2019;89(5):393–401. doi: 10.1111/josh.12753. [DOI] [PubMed] [Google Scholar]
- 40.Green P. Risks to children and young people during covid-19 pandemic. BMJ. 2020;369:m1669. doi: 10.1136/bmj.m1669. [DOI] [PubMed] [Google Scholar]
- 41.Sidpra J, Abomeli D, Hameed B, Baker J, Mankad K (2020) Rise in the incidence of abusive head trauma during the COVID-19 pandemic. Arch Dis Child 2 [DOI] [PubMed]
- 42.Campbell AM. An increasing risk of family violence during the Covid-19 pandemic: strengthening community collaborations to save lives. For Sci Int Rep. 2020;2:100089. doi: 10.1016/j.fsir.2020.100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cumulative incidence of novel Coronavirus-2 (SARS-CoV-2) infection in Italy (data from the Istituto Superiore di Sanità – ISS: COVID-19 integrated surveillance data in Italy. Update to 18 August, 2020). (JPG 101 kb)
(DOCX 15 kb)
(DOCX 15 kb)
(DOCX 16 kb)
(DOCX 16 kb)
(DOCX 16 kb)
(DOCX 14 kb)
(DOCX 33 kb)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplementary information. Deidentified participant data may be available on reasonable request from IL.