Abstract
Background Prediabetes and type 2 diabetes mellitus (T2DM) are one of the major long-term health conditions affecting global healthcare delivery. One of the few effective approaches is to actively manage diabetes via a healthy and active lifestyle.
Objectives This research is focused on early detection of prediabetes and T2DM using wearable technology and Internet-of-Things-based monitoring applications.
Methods We developed an artificial intelligence model based on adaptive neuro-fuzzy inference to detect prediabetes and T2DM via individualized monitoring. The key contributing factors to the proposed model include heart rate, heart rate variability, breathing rate, breathing volume, and activity data (steps, cadence, and calories). The data was collected using an advanced wearable body vest and combined with manual recordings of blood glucose, height, weight, age, and sex. The model analyzed the data alongside a clinical knowledgebase. Fuzzy rules were used to establish baseline values via existing interventions, clinical guidelines, and protocols.
Results The proposed model was tested and validated using Kappa analysis and achieved an overall agreement of 91%.
Conclusion We also present a 2-year follow-up observation from the prediction results of the original model. Moreover, the diabetic profile of a participant using M-health applications and a wearable vest (smart shirt) improved when compared to the traditional/routine practice.
Keywords: wearable smart shirt, M-health application, type 2 diabetes mellitus, long-term conditions, prediabetes, clinical decision support, chronic conditions
Background and Significance
Type 2 diabetes mellitus (T2DM) is a chronic condition (CC) that affects the way the body processes blood sugar (glucose). T2DM is often described as a condition where the human body cannot produce enough insulin, or it resists insulin. T2DM usually develops in adults; however, it is becoming more common in children, and it is linked to obesity, inactivity, eating, and lifestyle. T2DM and its complications consume a large proportion of health budgets and require a vast amount of resources worldwide. Early detection of prediabetes might lead to reverse or a delay on the onset of T2DM. Often T2DM requires continuous monitoring of weight, diet, activity, and physiological parameters (in some cases). Wearable monitoring applications demonstrated a positive impact on the individual's overall health and well-being when used for long-term/chronic care or continuous monitoring. 1 2 3 4
Smart Patient Monitoring Applications
Wearable/remote monitoring applications are based on an integrated sensor network with Internet connectivity as a medium to transmit data such as vital signs and motion from various sensors to support remote care settings. Long-term condition (LTC) and/or (CC) monitoring applications often integrate mobile and wearable technologies. With its advantages, this approach has enabled innovative healthcare solutions to monitor patients remotely. 5 6 In ref 7 , the researchers have developed a mobile electrocardiogram (ECG) monitoring application backed by radio-frequency identification (RFID) tags to transmit data continuously to a local server. The system depended on the battery life of a mobile phone and RFID tags. Another application for identification of the health conditions, prognosis, 8 is based on fuzzy logic that monitors various signs, symptoms, and disorders. Such applications are depended on a specific use-case and limited in their scalability to holistically manage an LTC/CC due to the limitation in data collection and accuracy of the wearable body sensors. 9 The wearable sensors basically include biopotential sensors , such as ECG, electromyography and electroencephalography sensors; motion sensor units , such as accelerometers and gyroscopes; and environmental sensor units such as video cameras, vital signs monitors (such as heart rate, pulse rate, and temperature), and pressure sensors. 10
A study proposed wireless wearable T-shirt to monitor the patient's posture during rehabilitation exercise. 11 This device employed manually sewed an enameled copper wire of 1 mm diameter to a T-shirt and constituted the sensor (about 9 cm long and 2.5 cm wide with a total length of 50 cm). The copper wire was stitched with a zigzag pattern on the back and the chest, thus allowing the lengthening of the T-shirt and sensor in the sagittal plane. The study achieved an acceptable outcome in a small setting, but the impedance value of the sensor changed due to different factors such as the relaxation of the T-shirt or skin conductivity variation. The T-shirt with the sewn copper wire was washed (expecting a relaxation) after it was used, but no variation was observed. 12
Our research was conducted by combing the best-practice knowledge base with the literature analysis related to the designing of the prediabetes and T2DM theory model by emphasizing on the below key points 13 14 15 16 17 :
Detection of prediabetes has proven to delay the onset of T1DM and T2DM.
People with diabetes tend to have multiple LTCs over time.
Studies found that ethnicity and diabetes are highly correlated.
Active and healthy lifestyle tends to support good management of any LTC, particularly diabetes.
Common indicators include age, sex, weight, body mass index (BMI), and family history.
A majority of studies have found that an active and healthy lifestyle could help to manage diabetes and could delay the onset of T2DM. 18 19 20 21 Early detection of prediabetes or T2DM has led to delay in the actual onset of diabetes for up to 4 years (with active management and control). A combination of clinical, activity, and demographic data allows the proposed approach to timely detect prediabetes and T2DM earlier than traditional methods. 18 22 23 24 25
Market Available Monitoring Devices and Sensors
Wearable sensor/solution requirements were gathered after detailed market analysis for suitable solutions. The search preference was given to user-friendly, all-in-one, wireless devices, and six key areas were identified as must requirements for a successful solution ( Table 1 ). These areas include wearable (wireless body-worn sensors and devices), reliable and accurate, real-time transmission, low cost, low maintenance, and usability (user-friendly and timely processing of real-time data). 26 27 28 29
Table 1. Key capabilities identified for an efficient monitoring system/solution.
| Categories | Features |
|---|---|
| Connectivity | Bluetooth (Bluetooth class II, Bluetooth Low Energy [BLE]) Connected via local or broader area network |
| Reliability | Accurate, validated, and certified as per the national/international standards |
| Transmission | Wireless—3G, 4G, and wired |
| Size/power/cost | Small size, portable, and lightweight Low cost and low maintenance Active/hybrid power consumption (low energy) |
| Usability | User friendly Easy to understand Simple to operate |
| Processing | On-chip processing Cloud processing (with delay) Real-time processing Raw data presentation |
We used the Hexoskin smart vest 30 31 32 33 for collecting activity data and vital signs. It has integrated sensors for real-time collection of steps, activity as well as ECG, heart rate, and breaths per minute. We combined activity data and vital signs from the wearable vest (Hexoskin 30 ) and combined with the participants' demographic information after obtaining ethical approvals (AUTEC: 16/412).
This research aimed to design and develop a clinical decision support system to detect prediabetes and diabetes. 28 The early detection model was based on the local and national guidelines for prediabetes and T2DM. The developed model was uniquely trained and supported with various data, including demographics, activity, and clinical for a deep understanding of all possible contributing factors. 28 The proposed study helps to determine the prediabetes risk factor but not to make an actual diagnosis.
Methodology and Approach
Model Design and Development
A fuzzy inference system was designed and developed to early detect prediabetes and T2DM. The model 28 is based on:
Baseline values : We adopted local/ national best practice guidelines for prediabetes and T2DM to set the baseline values for activity data such as daily recommended steps, and age-based activity range. For vital signs (heart rate (HR), blood pressure (BP) measurement, we used the World Health Organization (WHO) guidelines for normal ranges of adults for each parameter, respectively.
Weighted parameters : Each parameter was weighted based on age, sex, weight, normal resting HR, and activity levels. We adopted global comparison of prediabetes guidelines and risk measures including BMI, gender, family history, BP, and ethnicity and assigned points for each “Yes” answer and for all “No” answers allocated 0 points.
Individualized parameters : Each parameter was then fed into the model to optimize the normal, mean, average, maximum, and minimum values for each individual.
Multimethodological Approach
We adopted the multimethodological approach used in information systems research. The four research phases of this project included observation, theory building, experimentation, and systems development. 34 The following text describes the details of each phase.
Observation: This phase involved reviewing case studies and field studies. 34 This research used the quantitative, experimental research approach to minimize preconceptions because technology-led diabetes self-management interventions have limited literature to support/guide the success or failure of this method. Our review spanned existing literature, programs, technology-led interventions to identify existing facts and establish the research gaps to strengthen our focus on solving technology-led self-management of T2DM. Figure 1 shows the first phase of the adopted multimethodological approach, including system review and model review to form an accurate observation.
Fig. 1.
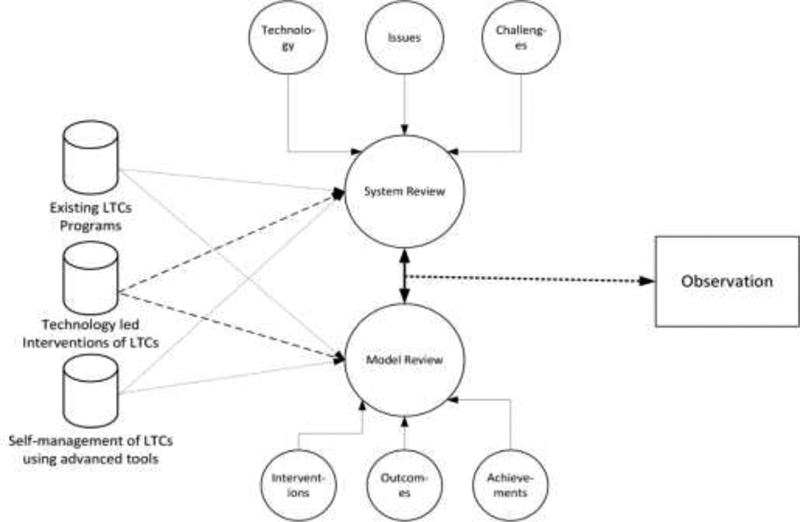
Phase 1 observation of the multimethodological approach to Information Systems Research. LTC, long-term condition.
Theory building: This phase involved the design and development of new concepts and frameworks 34 :
1) Investigation and evaluation of existing best-practice clinical guidelines, national recommendations, and care plans for diabetes
2) Designing the relationship mapping of existing information (clinical interpretation)
3) User engagement and interaction approaches for an effective data collection application
4) Analysis and mapping of best practice/standard clinical guidelines, national recommendations, and care plans for diabetes detection models
5) Review of the existing pre-build models on diabetes as a base threshold for detecting prediabetes
Experimentation: This phase dealt with the design of the experiment in this research and is motivated by the prior theory building (phase 2). 34 For the experimentation, we used the Hexoskin's 30 advanced body sensors vest as a wearable monitoring kit.
System development: An Adaptive Neuro-Fuzzy Inference System (ANFIS) was developed to interpret and detect prediabetes using vital signs and activity data. ANFIS can play an important role in the diagnosis of individualized monitoring instead of threshold-based or age-based detection. The system learns from the normal parameters for each individual using adaptive neuro-fuzzy modelling. The relationship between vital signs and activity data with set pre-diabetic conditions was established by consulting with physicians and related clinical knowledgebases.
System overview: Figure 2 shows the basic building blocks of the proposed model, including model architecture with activity data, vitals data, and demographic data fed to the knowledge base learning module for processing. The initial learning of the proposed model was based on the activity data, vital signs, and demographic information using the self-learning interpretation engine. The main learning module consists of a medical knowledge base and individualized weighted parameters combined with the national/local diabetes guidelines. We used multiple in-out combinational cross-pattern relationship to best utilize the medical knowledge base with the baseline values for each individual. The BMI was considered as a contributing factor that computed from individual height and weight to train the model.
Fig. 2.

The key building blocks of the proposed model.
Figure 3 shows a high-level framework of the interpretation engine and the proposed multilayered outcome for early detection of T2DM and prediabetes. The interpretation engine consists of four key components (individualized monitoring, evidence-based reasoning, weighted parameters, and medical knowledgebase). A multilayer concept has been introduced, as it has the potential of early detection. This mechanism is best utilized in this context by feeding a multiple input–output combinational relationship in real time. Table 2 shows the global comparison of prediabetes guidelines and risk measures, highlighting the common risk factors adopted to measure the prediabetes in adults.
Fig. 3.
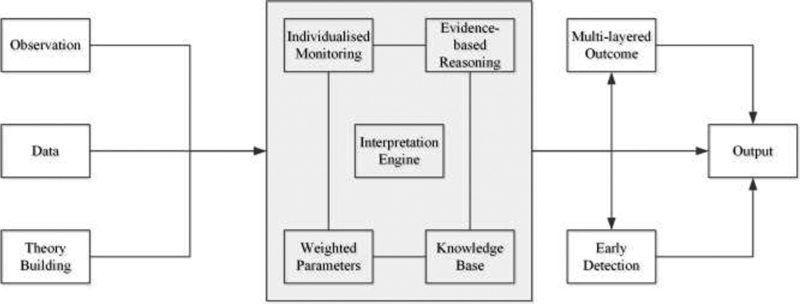
Framework of interpretation engine using multiple components: individualized monitoring, evidence-based reasoning, knowledge-base, and weighted parameters.
Table 2. Global comparison of prediabetes guidelines and risk measures 11 12 .
| Questions | NZ | UK | US |
|---|---|---|---|
| Overweight or obese (BMI) | ✓ | ✓ | Check height and weight |
| Male or female | ✓ | ✓ | ✓ |
| Inactive | Lifestyle | Activity | Activity/exercise |
| Family history | ✓ | ✓ | ✓ |
| High blood pressure | ✓ | ✓ | ✓ |
| Have given birth to a baby who weighed over 9 pounds (4kg?) | ✓ | ✓ | ✓ |
| a South Asian | ✓ (incl. Maori/ Pacific) | ✓ (incl. White European) | ✓ (incl. Asian American) |
Additional ethnicity included specific to the country's demographic population.
The interpretation of the diagnostic values and the relationship between vital signs, activity data, demographics data, and their clinical interpretation is mapped to the ANFIS rules. Moreover, clinical acceptability of assessment procedures via expert agreement analysis is evaluated. The factors that affect ongoing data collection, barriers to this intervention, accuracy, and the potential for person' self-management were also be measured. A multilayer concept has been introduced, as it has the potential of early detection. This mechanism is best utilized in this context by feeding multiple input–output combinational relationships in real-time. Table 2 shows the global comparison of prediabetes guidelines and risk measures, highlighting the common risk factors adopted to measure the prediabetes in adults.
Fuzzy rules * : The below fuzzy logic rules were produced by the original fuzzy model and published [1]. We used the same rules for the fairness of comparisons/outcomes.
If (SEX is male) and (FAMILY is No) and (BMI is Normal) and (AGE is 40-50) and (HR is Normal) and (STEPS is 5000 + ) then (Diagnosis is Normal) (1)
If (SEX is Female) and (FAMILY is No) and (BMI is Normal) and (AGE is 40-50) and (HR is Normal) and (STEPS is 5000 + ) then (Diagnosis is Normal) (1)
If (SEX is male) and (FAMILY is yes) and (BMI is Overweight) and (AGE is 51-60) and (HR is High) and (STEPS is 3001_-_5000) then (Diagnosis is Prediabetes) (1)
If (SEX is male) and (FAMILY is yes) and (BMI is Obese) and (AGE is 51-60) and (HR is Ver_High) and (STEPS is 3001_-_5000) then (Diagnosis is Prediabetes) (1)
If (SEX is male) and (FAMILY is yes) and (BMI is Obese) and (AGE is 61 + ) and (HR is High) and (STEPS is 0-3000) then (Diagnosis is Prediabetes) (1)
If (SEX is Female) and (FAMILY is yes) and (BMI is Obese) and (AGE is 61 + ) and (HR is Ver_High) and (STEPS is 0-3000) then (Diagnosis is Diabetes_Type_2) (1)
If (SEX is male) and (FAMILY is yes) and (BMI is Obese) and (AGE is 61 + ) and (HR is Ver_High) and (STEPS is 0-3000) then (Diagnosis is Diabetes_Type_2) (1)
Evaluations and Results
Data collected using market available wearable vest—{Hexoskin ( www.hexoskin.com , Montreal, Canada} 35 as Hexoskin satisfies the project requirements and allows users to download the raw data in machine-readable format. Moreover, the Hexoskin provides users with raw, processed, and/or meaningful data. The access to the application programming interfaces and raw data in machine-readable format enables the healthcare professionals, researchers, and developers to leverage the existing open platform for data mining, machine learning for various clinical, social, behavioral, and physical (activity-related) use-cases and explore the data further for enormous healthcare benefits. Hexoskin vest captures HR, HR variability (allowing to estimate stress and fatigue), HR recovery, ECG, breathing rate (RPM), minute ventilation (L/min), activity intensity, peak acceleration, steps, and cadence. The proposed model was trained and fine-tuned using 36 participant data, containing age, gender, BMI, and any significant medical condition from PhysioNet. 10 For the real-time testing, longitudinal data was collected from two participants over multiple times, activities, and durations after obtaining appropriate ethics approvals (AUTEC: 16/412).
Preprocessing of data: The data analysis tasks performed on the raw data, such as plotting data, computing descriptive statistics, performing linear correlation analysis, data fitting, removing and interpolating missing values, removing outliers, smoothing and filtering, and detrending the data
Sensors details: Analog 256Hz ECG data; Analog dual-channel 128Hz breathing sensors, and Analog 3D 64Hz acceleration. Table 3 summarizes participants' statistical information for demographic data, vital signs, and activity data and hemoglobin A1c (HbA1c) information including minimum (Min), maximum (Max), and average (AVG) for each parameter.
Table 3. Demographic information, vital signs, activity data, HbA1c, and activity data for the study participants.
| Data/Participant | Participant 1 | Participant 2 |
|---|---|---|
| Approach | Traditional | Using wearable devices/smart vest |
| Demographic information | Age—62 | Age—55 |
| Sex—male | Sex—female | |
| Weight—79 kg | Weight—62 kg | |
| Height—168 cm | Height—149 cm | |
| Vital signs | Heart rate (Min = 70; Max = 161; AVG = 115) | Heart rate (Min = 64; Max = 144; AVG = 104) |
| Breathing rate (Min = 9; Max = 33; AVG = 21) | Breathing rate (Min = 10; Max = 42; AVG = 23) | |
| Ventilation (Min = 51; Max = 65; AVG = 32) | Ventilation (Min = 44; Max = 69; AVG = 45) | |
| Activity data | Activity (Min = 0; Max = 1.9; AVG = 0.63) | Activity (Min = 0; Max = 1.1; AVG = 0.55) |
| Cadence (Min = 51; Max = 243; AVG = 132) | Cadence (Min = 69; Max = 191; AVG = 122) | |
| HbA1c (average values) | 87.5 mmol/mol | 64 mmol/mol |
| Steps and stairs | Walking, steps, and stairs | Walking, steps, and stairs |
Abbreviations: AVG, average; HbA1c, hemoglobin A1c; Max, maximum; Min, minimum.
We collected 7.25 hours (435 minutes) of data (combined, in multiple sessions over 10 months). We applied 1-minute (60 seconds) sampling window on the 435 samples for moving-window data analysis. The four possible outcome arrangements for an accurate diagnosis are true positive (TP), true negative (TN), false positive (FP), and false negative (FN).
We used Kappa analysis, which measures and allows the agreement between the human expert and the computer system using the TP, TN, FP, and FN measures. The importance of observer reliability lies in the fact that it represents the extent to which the data collected in the study are correct representations of the variables measured.
Table 4 shows the TP, TN, FP, and FN classifications derived from the expert analysis. Table 5 shows the Kappa analysis of the whole dataset. The overall Kappa value was K = 0.75, shows the K-values expressed as strength of agreement. The detection model has an accuracy of 91%, sensitivity of 94%, specificity of 90%, and predictability of 72%. The accuracy was calculated using ([TP + TN]/[sum of TP, TN, FP, and FN]), sensitivity as ([TP]/[TP + FN]), specificity as ([TN]/[TN/FP]), and predictability as ([TP]/[TP + FP]).
Table 4. Kappa analysis values for the collected data.
| Expert (+ve) | Expert (–ve) | Total | |
|---|---|---|---|
| System (+ve) | 82 (TP) | 32 (FP) | 114 |
| System (–ve) | 5 (FN) | 316 (TN) | 321 |
| Total | 87 | 348 | 435 |
Abbreviations: FN, false negative; FP, false positive; TN, true negative; TP, true positive.
Table 5. Kappa analysis of the whole dataset.
| Overall agreement | Positive agreement | Negative agreement | Agreement by chance | SE | 95% CI for K |
|---|---|---|---|---|---|
| P o | P pos | P neg | P e | SE | CI 95% |
| 0.91 | 0.82 | 0.94 | 0.64 | 0.038 | 0.82 and 0.67 |
Abbreviations: CI, confidence interval; SE, standard error.
Figure 4 shows the top five contributing factors for the T2DM for the early detection of prediabetes and T2DM using the originally proposed fuzzy model. Top five contributing factors are age, weight, HbA1c, HR, and activity (daily number of steps). The contributing factors were used in the weighted parameter module of the model.
Fig. 4.
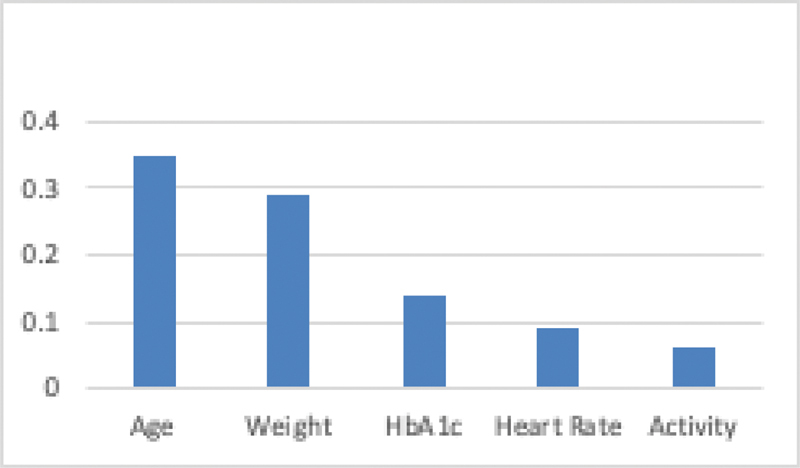
Top five contributing risk factors. HbA1c, hemoglobin A1c.
Figure 5 shows the HbA1c trend for two participants from 2016 to 2019. The original study period was from 2016 to 2017 shows participant two in good control of T2DM using the wearable vest, whereas participant one was on the traditional methods of T2DM control. In the follow-up study from 2018 to 2019, participant two ( Table 3 ) was much improved and in control (average HbA1c of 64 mmol/mol) when compared with the participant 1 (average HbA1c of 87.5 mmol/mol). The dotted (green) line shows the average T2DM predictions for the entire study period. The dash (purple) line shows the original model's predictions, predicting 2-year T2DM control based on the participant data.
Fig. 5.
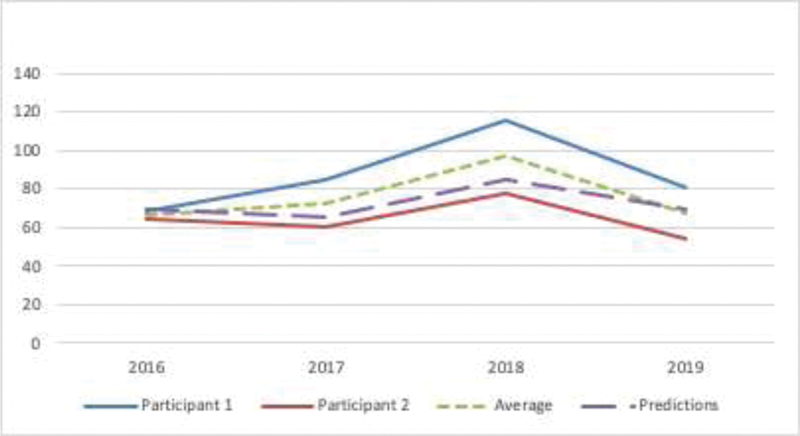
Hemoglobin A1c (HbA1c) trend for the selected participants from 2016 to 2019. Original study period 2016 to 2017 and the follow-up study period is 2018 to 2019.
From both the studies, we found that the participant two used wearable vest, smartwatch, mobile app (including other mHealth apps), motivated in self-learning, active management and found to have reasonable control and maintenance for T2DM.
Discussion and Conclusions
The collected data is mapped against the pre-build T2DM and prediabetes models and approved clinical guidelines. The proposed model is compared with a clinician's finding using “Kappa” analysis. However, the developed model is capable of detecting prediabetes and T2DM with any number of participant data. The model (and its rules engine) will self-learn and continuously tune the model for better accuracy and reliability over some time.
Data analysis of real-time data for individualized and personalized monitoring and early detection (rather than threshold-based limits) using the data clustering approach and weighted parameters mechanism is presented. Moreover, an advanced data analysis technique was applied using the identified clinical programs on the real-time data for detecting prediabetes and T2DM using fuzzy logic-based Adaptive Neuro-Fuzzy Inference model. The developed model is then deployed and compared to test the accuracy and efficiency. 4 36
In the 2-year follow-up, we found that the diabetic profile of participant 2 ( Table 3 ) was improved and in control (average HbA1c of 64 mmol/mol) when compared with the participant 1 (average HbA1c of 87.5 mmol/mol). 28 The main contributing factors were the visibility of the real-time activity data, motivation to use the smart shirt, access to real-time data via the smartphone app and cloud dashboard, timely feedback, alerts, and notifications. Data analysis was computed on-demand or as a monthly report to keep the participants motived and involved.
With the ever-growing use of smart systems, end-user acceptability is becoming an essential aspect of the design of such systems. 3 37 38 The user-awareness, as well as clinician and patient acceptance, are key factors for accepting smart systems in healthcare. Data interoperability is one of the main drawbacks of deployed solutions/systems where users are “constrained” within one application for a task and move between the applications to view/review the patient information. 19 39 40 41 Further improvements are required such as adding diet information, ethnicity, location, socioeconomic status, living status, and other social factors that could have a significant influence on the healthy and active lifestyle for managing T2DM and delaying the prediabetes. 2 3 4 27 28
Clinical Relevance Statement
The study found that the diabetic profile of a participant was much improved and in control (average HbA1c of 64 mmol/mol) when compared with the other participant (average HbA1c of 87.5 mmol/mol).
Multiple Choice Questions
-
What are the two most important factors for managing T2DM?
Weight and diet
Sleep and rest
Salt and sugar
Male and female
Correct Answer: The correct answer is option a.
-
T2DM is?
A LTC
A CC
A short-term condition
A good health condition
Correct Answer: The correct answer is option a.
-
What is the early indication of prediabetes?
Underweight
Overweight
Normal weight
Healthy weight
Correct Answer: The correct answer is option b.
Acknowledgment
We would like to thank Hexoskin for providing the advanced wearable vest (smart shirt) used in this study for the data collection. Also, we would like to thank clinicians at Auckland District Health Board and Waitemata District Health Board for their clinical advice and support.
Conflict of Interest None declared.
Protection of Human and Animals Subjects
The study was performed in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and was reviewed by the Auckland University of Technology Institutional Review Board.
FAMILY refers to the family history, STEPS are number of steps per day, and Heart Rate is based on the WHO Adult HR range.
References
- 1.Gholamhosseini H, Baig M, Maratas J, Mirza F, Lindén M. Obesity risk assessment model using wearable technology with personalized activity, calorie expenditure and health profile. Stud Health Technol Inform. 2019;261:91–96. [PubMed] [Google Scholar]
- 2.Baig M M, Gholamhosseini H, Afifi S, Mirza F.Current Challenges and Barriers to the Wider Adoption of Wearable Sensor Applications and Internet-of-Things in Health and Well-beingInternational Conference on Information Resources Management (CONF-IRM);2019Auckland, New Zealand
- 3.Baig M M, Afifi S, GholamHosseini H, Mirza F. A systematic review of wearable sensors and IoT-based monitoring applications for older adults - a focus on ageing population and independent living. J Med Syst. 2019;43(08):233. doi: 10.1007/s10916-019-1365-7. [DOI] [PubMed] [Google Scholar]
- 4.Baig M M, Afifi S, GholamHosseini H, Mirza F. Cham: Springer International Publishing; 2019. Managing long-term conditions: wearable sensors and IoT-based monitoring applications; pp. 1–5. [DOI] [PubMed] [Google Scholar]
- 5.Groat D, Kwon H J, Grando M A, Cook C B, Thompson B. Comparing real-time self-tracking and device-recorded exercise data in subjects with type 1 diabetes. Appl Clin Inform. 2018;9(04):919–926. doi: 10.1055/s-0038-1676458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groat D, Soni H, Grando M A, Thompson B, Kaufman D, Cook C B. Design and testing of a smartphone application for real-time self-tracking diabetes self-management behaviors. Appl Clin Inform. 2018;9(02):440–449. doi: 10.1055/s-0038-1660438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong-Her S, Hsiu-Sen C, Binshan L, Shih-Bin L. An embedded mobile ECG reasoning system for elderly patients. Information Technology in Biomedicine. IEEE Transactions on. 2010;14(03):854–865. doi: 10.1109/TITB.2009.2021065. [DOI] [PubMed] [Google Scholar]
- 8.Pantelopoulos A, Bourbakis N G. Prognosis-a wearable health-monitoring system for people at risk: methodology and modeling. IEEE Trans Inf Technol Biomed. 2010;14(03):613–621. doi: 10.1109/TITB.2010.2040085. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel D A, Christie A, Inglis J G, Kamen G. Experimental and modelling investigation of surface EMG spike analysis. Med Eng Phys. 2011;33(04):427–437. doi: 10.1016/j.medengphy.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu M S, Griffith L, Jolly K, Gill P, Marshall T, Gale N K. Long-term conditions, self-management and systems of support: an exploration of health beliefs and practices within the Sikh community, Birmingham, UK. Ethn Health. 2016;21(05):498–514. doi: 10.1080/13557858.2015.1126560. [DOI] [PubMed] [Google Scholar]
- 11.Morrison T, Silver J, Otis B.A single-chip encrypted wireless 12-lead ECG smart shirt for continuous health monitoring2014 Symposium on VLSI Circuits Digest of Technical Papers, Honolulu, HI, 2014, pp. 1–2. Doi: 10.1109/VLSIC.2014.6858433
- 12.Sardini E, Serpelloni M, Pasqui V. Wireless wearable T-shirt for posture monitoring during rehabilitation exercises. IEEE Trans Instrum Meas. 2015;64(02):439–448. [Google Scholar]
- 13.Powers M A, Bardsley J, Cypress M. Diabetes self-management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Educ. 2017;43(01):40–53. doi: 10.1177/0145721715588904. [DOI] [PubMed] [Google Scholar]
- 14.Lucisano J J, Routh T L, Lin J T, Gough D A. Glucose monitoring in individuals with diabetes using a long-term implanted sensor/telemetry system and model. IEEE Trans Biomed Eng. 2017;64(09):1982–1993. doi: 10.1109/TBME.2016.2619333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Association of Clinical Endocrinologists (AACE) ; American College of Endocrinology (ACE) . Garber A J, Abrahamson M J, Barzilay J I. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(01):84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 16.Chrvala C A, Sherr D, Lipman R D. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns. 2016;99(06):926–943. doi: 10.1016/j.pec.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Association A D. Standards of medical care in diabetes—2016 abridged for primary care providers. Clin Diabetes: a publication of the American Diabetes Association. 2016;34(01):3. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabesan S, Sankar R. Improving long-term management of epilepsy using a wearable multimodal seizure detection system. Epilepsy Behav. 2015;46:56–57. [Google Scholar]
- 19.Lu T-C, Fu C-M, Ma M H-M, Fang C-C, Turner A M. Healthcare applications of smart watches: a systematic review. Appl Clin Inform. 2016;7(03):850–869. doi: 10.4338/ACI-2016-03-RA-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prochaska M T, Press V G, Meltzer D O, Arora V M. Patient perceptions of wearable face-mounted computing technology and the effect on the doctor-patient relationship. Appl Clin Inform. 2016;7(04):946–953. doi: 10.4338/ACI-2016-06-LE-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels J, Haber N, Voss C. Feasibility testing of a wearable behavioral aid for social learning in children with autism. Appl Clin Inform. 2018;9(01):129–140. doi: 10.1055/s-0038-1626727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sidhu M S, Griffith L, Jolly K, Gill P, Marshall T, Gale N K. Long-term conditions, self-management and systems of support: an exploration of health beliefs and practices within the Sikh community, Birmingham, UK. Ethn Health. 2016;21(05):498–514. doi: 10.1080/13557858.2015.1126560. [DOI] [PubMed] [Google Scholar]
- 23.Kurien M, Trott N, Sanders D S. Long-term care for patients with coeliac disease in the UK: a review of the literature and future directions. J Hum Nutr Diet. 2016;29(05):617–623. doi: 10.1111/jhn.12379. [DOI] [PubMed] [Google Scholar]
- 24.Etemadi M, Inan O T, Heller J A, Hersek S, Klein L, Roy S. A wearable patch to enable long-term monitoring of environmental, activity and hemodynamics variables. IEEE Trans Biomed Circuits Syst. 2016;10(02):280–288. doi: 10.1109/TBCAS.2015.2405480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton S, Roberts S, Turner B. Delivering person centred care in long term conditions. BMJ. 2015;350:h181. doi: 10.1136/bmj.h181. [DOI] [PubMed] [Google Scholar]
- 26.Baig M M, Gholamhosseini H. Smart health monitoring systems: an overview of design and modeling. J Med Syst. 2013;37(02):9898. doi: 10.1007/s10916-012-9898-z. [DOI] [PubMed] [Google Scholar]
- 27.Baig M M, GholamHosseini H, Moqeem A A, Mirza F, Lindén M. A systematic review of wearable patient monitoring systems - current challenges and opportunities for clinical adoption. J Med Syst. 2017;41(07):115. doi: 10.1007/s10916-017-0760-1. [DOI] [PubMed] [Google Scholar]
- 28.Baig M, Mirza F, GholamHosseini H, Gutierrez J, Ullah E. Clinical decision support for early detection of prediabetes and type 2 diabetes mellitus using wearable technology. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:4456–4459. doi: 10.1109/EMBC.2018.8513343. [DOI] [PubMed] [Google Scholar]
- 29.Baig M M, GholamHosseini H, Moqeem A A, Mirza F, Lindén M. Clinical decision support systems in hospital care using ubiquitous devices: current issues and challenges. Health Informatics J. 2019;25(03):1091–1104. doi: 10.1177/1460458217740722. [DOI] [PubMed] [Google Scholar]
- 30.Hexoskin. Hexoskin Smart VestAccessed 2019 at:https://www.hexoskin.com/2019
- 31.Smith C M, Chillrud S N, Jack D W, Kinney P, Yang Q, Layton A M. Laboratory validation of Hexoskin biometric shirt at rest, submaximal exercise, and maximal exercise while riding a stationary bicycle. J Occup Environ Med. 2019;61(04):e104–e111. doi: 10.1097/JOM.0000000000001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elliot C A, Hamlin M J, Lizamore C A. Validity and reliability of the Hexoskin wearable biometric vest during maximal aerobic power testing in elite cyclists. J Strength Cond Res. 2019;33(05):1437–1444. doi: 10.1519/JSC.0000000000002005. [DOI] [PubMed] [Google Scholar]
- 33.Moriarty T A, Feito Y, Monahan J, Williamson C.Using the Hexoskin smart garment to measure cardiorespiratory variables during high intensity functional training: 2760 Board# 43 June 1 200 PM-330 PM Med Sci Sports Exerc 201850(5S):673 [Google Scholar]
- 34.Nunamaker J F, Jr, Chen M, Purdin T D. Systems development in information systems research. J Manage Inf Syst. 1990;7(03):89–106. [Google Scholar]
- 35.Villar R, Beltrame T, Hughson R L. Validation of the Hexoskin wearable vest during lying, sitting, standing, and walking activities. Appl Physiol Nutr Metab. 2015;40(10):1019–1024. doi: 10.1139/apnm-2015-0140. [DOI] [PubMed] [Google Scholar]
- 36.Baig M M, Mirza F, GholamHosseini H, Gutierrez J, Ullah E.Automated and early detection of long-term conditions using a real-time wearable patient monitoring systemPaper presented at: 35th International Conference of International Society for Quality in Health Care (ISQua);2018Kuala Lumpur, Malaysia
- 37.Nguyen H, Mirza F, Naeem M A, Baig M M. Falls management framework for supporting an independent lifestyle for older adults: a systematic review. Aging Clin Exp Res. 2018;30(11):1275–1286. doi: 10.1007/s40520-018-1026-6. [DOI] [PubMed] [Google Scholar]
- 38.Baig M M, GholamHosseini H, Linden M, Connolly M J. Review of vital signs monitoring systems – patient's acceptability, issues and challenges. Neurosci Biomed Eng. 2014;2(01):2–13. [Google Scholar]
- 39.Ancker J S, Mauer E, Kalish R B, Vest J R, Gossey J T. Early adopters of patient-generated health data upload in an electronic patient portal. Appl Clin Inform. 2019;10(02):254–260. doi: 10.1055/s-0039-1683987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Mikati H K, Yazel-Smith L, Grout R W, Downs S M, Carroll A E, Hannon T S. Clinician perceptions of a computerized decision support system for pediatric type 2 diabetes screening. Appl Clin Inform. 2020;11(02):350–355. doi: 10.1055/s-0040-1710024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karway G, Grando M A, Grimm K, Groat D, Cook C, Thompson B. Self-management behaviors of patients with type 1 diabetes: comparing two sources of patient-generated data. ACI. 2020;11(01):70–78. doi: 10.1055/s-0039-1701002. [DOI] [PMC free article] [PubMed] [Google Scholar]


