Abstract
Perfluorooctane sulfonate (PFOS), an environmentally persistent pollutant, has been revealed to elicit hepatic toxicity. In the current study, we investigated the protective role of grape seed proanthocyanidin extract (GSPE) against PFOS-caused steatohepatitis in mice. Animals were exposed intragastrically to PFOS (10 mg/kg/day), GSPE (150 mg/kg/day), or their combination. After 21 days of treatment, mice exposed to PFOS exhibited steatosis, oxidative stress, and inflammation in the liver. Nevertheless, simultaneous administration of GSPE resumed the declined serum hepatic enzyme activities and histological abnormalities in PFOS-exposed mice. Furthermore, GSPE supplementation reduced the contents of triglyceride (TG) and total cholesterol (TC) and expression of lipid metabolism-associated genes CD36 and fatty acid-binding protein 4 (FABP4) in the liver of mice treated with PFOS. Moreover, GSPE suppressed the generation of lipid peroxidative product malondialdehyde and restored the activity of superoxide dismutase in the liver of PFOS-exposed mice. In addition, GSPE repressed the PFOS-induced hepatic overproduction of proinflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Our results demonstrate that GSPE attenuates PFOS-caused steatohepatitis in mice by regulating lipid metabolism, oxidative stress, and inflammatory response.
1. Introduction
Steatohepatitis is a common histological finding that involves a variety of etiologies. Toxicant-associated steatohepatitis is one of the most frequent causes of steatohepatitis [1]. Perfluorooctane sulfonate (PFOS), a member of a family of perfluoroalkyl substances (PFAS), has widely aroused public attention due to its ubiquitous distribution, environmental persistence, high bioaccumulation, and potential toxicity [2]. PFOS has been detected in populations worldwide [3], and the consumption of contaminated foods and drinking water and inhalation of indoor dust are the predominant exposure pathways of PFOS to nonoccupationally exposed people [4]. Owing to the long half-life of elimination in people (approximately 5 years) [5], PFOS accumulates in the body and poses a threat to human health, particularly for occupationally exposed persons. Tests in rodents have revealed that PFOS exposure caused hepatic steatosis [6–9]. In humans, monitoring studies have also reported that serum PFOS is significantly associated with dyslipidemia [10, 11]. Furthermore, PFAS exposure is correlated with increased risk of steatohepatitis and fibrosis in children diagnosed with nonalcoholic fatty liver disease (NAFLD), suggesting that PFOS may be a toxicant contributing to nonalcoholic steatohepatitis (NASH) [12].
Nutrition is a powerful protector against environmental chemical insults [13]. Proanthocyanidins are a class of polyphenols that possess a wide range of health benefits [14]. Grape seed proanthocyanidin extract (GSPE) is a major source of proanthocyanidins. The physiological activities of proanthocyanidins include reduction in oxidative stress, inflammation, and metabolic syndrome which provide multiorgan protection from various drug- and chemical-induced toxicities [15, 16]. Although several studies have shown the protective effect of GSPE on hepatotoxicity induced by diverse environmental pollutants [17–21], it remains unknown whether GSPE exerts hepatoprotection against PFOS-caused liver damage. This study hypothesizes that GSPE can attenuate PFOS-evoked steatohepatitis via improving hepatic lipid metabolism, oxidative stress, and inflammation.
2. Materials and Methods
2.1. Experimental Animals
Specific pathogen-free male Kunming mice weighing 20 to 22 g were procured from the Laboratory Animal Science Department of Jiangxi University of Traditional Chinese Medicine. The mice were housed at 25°C on a light/dark cycle of 12 h : 12 h with unrestricted diet and water intake. All procedures for animal experimentation were conducted in compliance with the guidelines of Nanchang University Laboratory Animal Research Ethics Committee (no. 20130916).
2.2. Treatments
After 1-week adaptation to the laboratory environment, the randomly selected mice were intragastrically treated once daily with PFOS (10 mg/kg/day, ≥98%, Sigma-Aldrich, USA), GSPE (150 mg/kg/day, ≥95%, Sciphar Biotechnology, China), or their combination, with 6 mice in each group. PFOS and GSPE were dissolved in distilled water. Control animals received only the equivalent volume of water. The doses of PFOS and GSPE were chosen based on the reported studies [6, 9, 20, 21]. After 21 consecutive days of administration, mice were weighed and fasting blood samples were drawn under anesthesia from the retroorbital venous plexus for liver function analysis. The mice were sacrificed by cervical dislocation, and the livers were excised, weighed, and frozen in liquid nitrogen for biochemical measurement or fixed with 10% buffered formalin for histological evaluation.
2.3. Biochemical Analysis
Activities of lactate dehydrogenase (LDH), alanine transaminase (ALT), and aspartic acid transaminase (AST) in serum and contents of triglyceride (TG) and total cholesterol (TC) in homogenized livers were detected utilizing the colorimetric assay kits acquired from Jiancheng Bioengineering Institute (China).
2.4. Histopathology
The formalin-fixed liver specimens were dehydrated in alcohol, infiltrated with paraffin, sliced at 5 μm thickness, and stained with hematoxylin-eosin to observe hepatic morphological changes.
2.5. Real-Time Quantitative PCR
Liver RNA obtained by TRIzol was converted to cDNA with a cDNA synthesis kit purchased from TaKaRa Biotechnology (China). Quantitative SYBR Green PCR reactions were conducted using an ABI Prism 7500 apparatus. The primers used for amplification were designed as follows: CD36: 5′-GCCAAGCTATTGCGACATGA-3′ (forward), 5′-GGCATTGGCTGGAAGAACAA-3′ (reverse); FABP4: 5′-CTTTGTGGGAACCTGGAAGC-3′ (forward), 5′-ATGATCATGTTGGGCTTGGC-3′ (reverse); GAPDH: 5′-GGCAAATTCAACGGCACAGT-3′ (forward), 5′-GTCTCGCTCCTGGAAGATGG-3′ (reverse). The mRNA expression of CD36 and FABP4 in each sample was normalized to the corresponding GAPDH. Relative quantification was calculated using the 2−ΔΔCT method.
2.6. Oxidative Stress Assessment
Malondialdehyde (MDA) and hydrogen peroxide (H2O2) generation and superoxide dismutase (SOD) activity in the liver homogenates were determined to assess hepatic lipid peroxidation, oxidant damage, and antioxidative defense using the colorimetric detection kits supplied by Jiancheng Bioengineering Institute (China).
2.7. Proinflammatory Cytokine Determination
The hepatic contents of proinflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) were determined using commercial ELISA test kits (Boster Biological Technology, China) following the guidance of the manufacturer.
2.8. Statistical Analysis
Experimental data were analyzed statistically by the one-way analysis of variance and Tukey's post hoc comparisons using GraphPad Prism 8.2.1 software. The results were given as means ± SD of 4 mice. P value <0.05 was defined as statistically significant.
3. Results
3.1. GSPE Lowered Liver Index and Serum Liver Enzyme Activities in Mice Exposed to PFOS
To observe the ameliorative role of GSPE in PFOS-caused hepatic injury, liver function parameters AST, ALT, and LDH were detected in mice. As illustrated in Figure 1, intragastric exposure to PFOS for 21 consecutive days resulted in a conspicuous elevation in serum AST, ALT, and LDH activities, with an increase in relative liver weight (P < 0.05). Nevertheless, the increased liver index and enzyme activities were resumed by combined treatment with GSPE in mice exposed to PFOS (P < 0.05). Treatment with GSPE alone did not influence the liver function of mice (P > 0.05).
Figure 1.
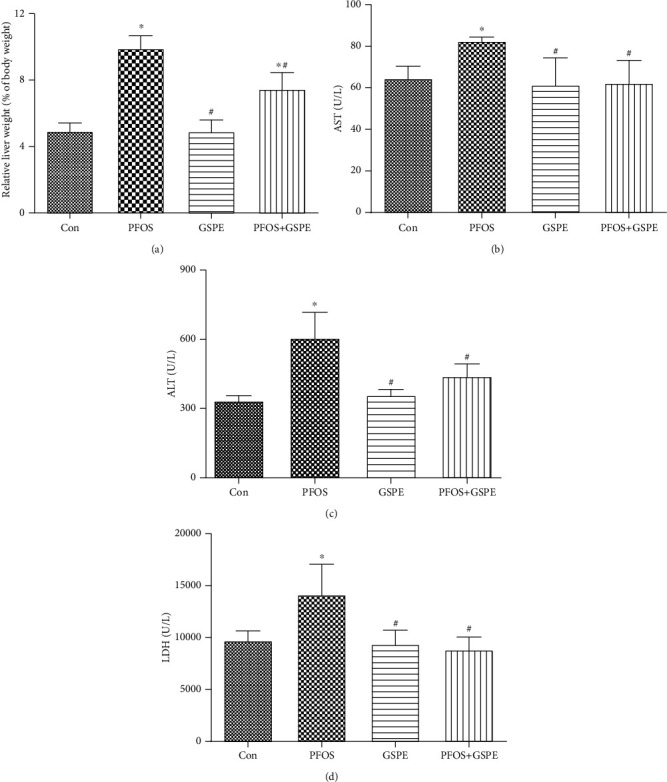
Effects of GSPE on relative liver weight and serum AST, ALT, and LDH activities in PFOS-exposed mice. Values are mean ± SD. ∗P < 0.05 compared to the control group; #P < 0.05 compared to the PFOS group.
3.2. GSPE Improved the Histopathologic Abnormalities in the Liver of PFOS-Exposed Mice
As displayed in Figure 2, morphological examination exhibited evident histologic features of steatohepatitis in the liver of mice administrated with PFOS, including architectural disorder, hepatocyte swelling, lipid droplet formation, and inflammatory cell infiltration. However, the pathologic abnormalities were ameliorated by supplementation of GSPE. Mice given only GSPE had the morphological structure of a normal liver.
Figure 2.
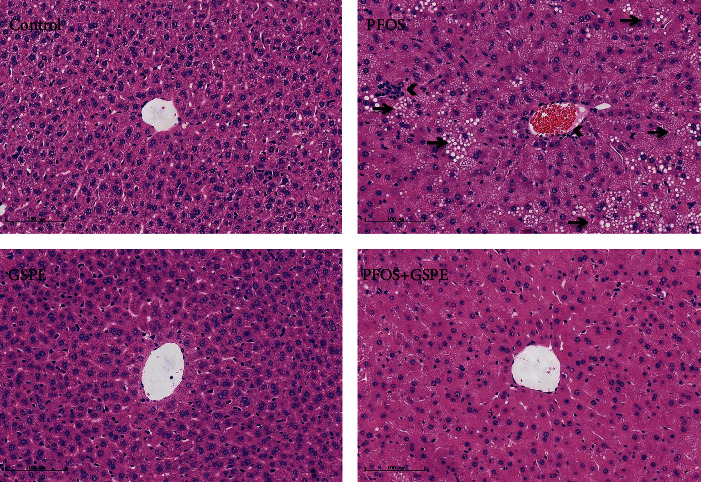
GSPE ameliorated PFOS-induced histological changes in the liver. Arrows indicate steatosis, and arrowheads indicate inflammatory cell infiltration. Scale bar: 100 μm. Magnification: 200x.
3.3. GSPE Corrected Hepatic Lipid Metabolic Disturbance in Mice Exposed to PFOS
To further explore the therapeutic effect of GSPE on PFOS-induced steatohepatitis, levels of TC and TG and expression of lipid metabolism-associated genes CD36 and FABP4 were analyzed in the liver of mice. As shown in Figure 3, the PFOS challenge induced a marked elevation in hepatic TC and TG contents (P < 0.05). Correspondingly, the expression of CD36 and FABP4 mRNA in the liver was notably upregulated after 21 days of PFOS exposure (P < 0.05). However, the increased lipid content and CD36 and FABP4 expression in PFOS-exposed mice were significantly reduced by simultaneous treatment with GSPE (P < 0.05).
Figure 3.
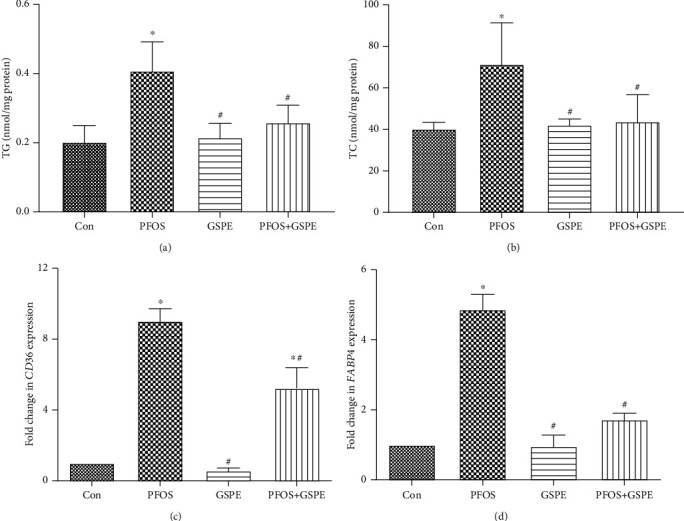
Effects of GSPE on the contents of TG and TC and mRNA expression of CD36 and FABP4 in the liver of PFOS-exposed mice. Values are mean ± SD. ∗P < 0.05 compared to the control group; #P < 0.05 compared to the PFOS group.
3.4. GSPE Antagonized PFOS-Initiated Oxidative Stress in the Liver
As can be seen in Figure 4, compared with the control group, the generation of oxidative stress markers MDA and H2O2 was enhanced and the hepatic activity of the antioxidant enzyme, SOD, was inhibited in PFOS-administrated mice (P < 0.05). Nonetheless, simultaneous GSPE administration reversed the increased levels of MDA and H2O2 and compensated for reduced SOD activity (P < 0.05). GSPE alone did not affect any oxidative stress indicators measured.
Figure 4.
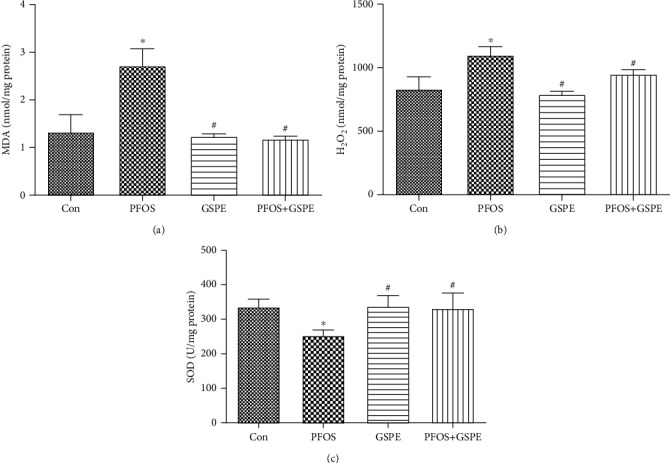
Effects of GSPE on MDA, H2O2, and SOD levels in the liver of PFOS-treated mice. Values are mean ± SD. ∗P < 0.05 compared to the control group; #P < 0.05 compared to the PFOS group.
3.5. GSPE Attenuated PFOS-Caused Inflammation in the Liver
As presented in Figure 5, significantly increased IL-6 and TNF-α expressions were observed in the liver of mice exposed to PFOS compared to the control group (P < 0.05). Compared with the PFOS group, the increased IL-6 and TNF-α were restored by combined administration with GSPE (P < 0.05). GSPE alone did not influence the production of these two inflammatory mediators (P > 0.05).
Figure 5.
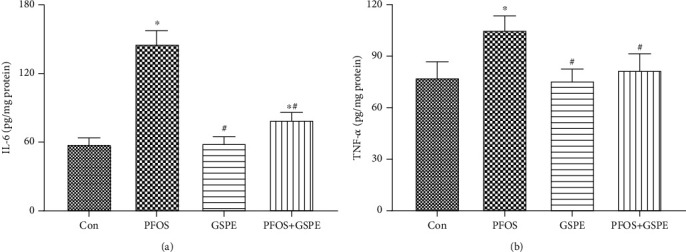
Effects of GSPE on the hepatic expression of IL-6 and TNF-α in mice exposed to PFOS. Values are mean ± SD. ∗P < 0.05 compared to the control group; #P < 0.05 compared to the PFOS group.
4. Discussion
There is increasing evidence that PFOS exerts detrimental effects on health. Considering that the liver is a main target organ for toxic insult arising from PFOS exposure, it is necessary to explore the hepatoprotection against PFOS-induced hepatic damage. Healthful nutrition intervention may be the most advisable strategy against the vulnerability to toxic chemicals. The antioxidant and anti-inflammatory properties of natural bioactive components in functional food can effectively prevent or moderate environmentally associated diseases [13, 22]. In the present study, mice exposed to PFOS exhibited a phenotype of nonalcoholic steatohepatitis, characterized by hepatic steatosis, hepatocyte swelling, and inflammatory infiltrate. Nevertheless, the biochemical and histological abnormalities caused by PFOS were significantly rescued by supplementation with GSPE, implying a hepatoprotective role of GSPE from PFOS-evoked toxicity.
Multiple mechanisms are linked to the pathogenesis and progression of NASH [23]. It has been suggested that PFOS-induced hepatic steatosis involves the disturbance of lipid metabolism [8, 9, 24, 25]. NAFLD is due to excessive fat accumulation in hepatocytes. The lipotoxicity is manifested by hepatocellular ballooning, and triacylglycerols are the major lipid component in ballooned hepatocytes. In NAFLD, increased uptake and accumulation of fatty acids propel the synthesis and storage of triglycerides in hepatocytes [26]. Undue lipid deposition in the liver can exert prooxidative and proinflammatory effects that account for the features of NASH, and the formation of toxic metabolites during lipid metabolism is mainly responsible for the generation of ROS and induction of inflammatory mediators [27, 28].
Grape polyphenols can be proposed as a potential remedy for metabolic syndrome and NAFLD [29, 30]. It has been reported that GSPE ameliorates hepatic lipid metabolism dysfunction in diabetic mice, as well as in rats treated with a high-fat diet, CCl4, and lead [17, 19, 31, 32]. CD36 and FABP4 are key transporters for fatty acid uptake. High hepatic levels of FABP4 have been proposed to be a predictive factor for NASH progression [33]. In NASH mice, FABP4 expression increased in the liver and overexpression of FABP4 in hepatocytes induced an elevation of proinflammatory cytokines. However, the inhibition of FABP4 significantly abrogated NASH-related inflammation [34]. CD36 directly contributes to the development and progression of fatty liver through regulating the uptake of fatty acids by hepatocytes, and the perturbation of hepatic CD36 attenuates fatty liver and associated inflammation in mice fed a high-fat diet [35]. In the present study, PFOS exposure gave rise to lipid dysmetabolism revealed by elevated contents of TG and TC and upregulated expression of lipid metabolic genes CD36 and FABP4 in the liver. However, GSPE treatment remarkably reduced lipid accumulation and CD36 and FABP4 expression in the liver of PFOS-exposed mice, suggesting that GSPE can reverse hepatic steatosis through modulating lipid metabolism.
Oxidative stress seems to be a critical factor in the pathogenesis of NASH [23]. In the process of hepatic lipid overload, free fatty acids accumulate in the liver and initiate lipid peroxidation. As a consequence of oxidative stress, lipid peroxidation leads to severe injury in the steatotic liver [23]. In NASH patients, lipid peroxidative product MDA in erythrocytes significantly increased, and the oxidative stress in plasma was positively correlated with the severity of steatosis [36]. In this study, PFOS exposure triggered hepatic oxidative stress, which was evidenced by increased MDA generation and declined SOD activity. These results were consistent with previous findings in rodents [37–39]. Likewise, in vitro toxicity assessment demonstrated that PFOS increased ROS formation in rat hepatocytes and human HepG2 cells [39, 40]. At present, lifestyle intervention is the main therapeutic option for NASH, and antioxidative treatment may be an attractive candidate [23]. It has been suggested that antioxidant supplementation can ameliorate diet-induced NASH [41]. As a potent free radical scavenger and oxidative inhibitor, GSPE offers stronger protection than vitamin C and E against hepatic lipid peroxidation [42]. Studies have shown that GSPE attenuates fluoride-caused hepatotoxicity by inhibiting oxidative damage in the mouse liver and human embryo hepatocytes [18, 43]. In our experiment, PFOS-induced hepatic oxidative stress and lipid peroxidation were dramatically alleviated by GSPE administration in mice, demonstrating a hepatoprotective potential of GSPE as a natural antioxidant in oxidative damage caused by PFOS exposure.
Inflammation is a hallmark of NASH. It is critical for the development and progression of NASH [27]. Oxidant stress is recognized to exert a pathogenic effect in steatosis-related inflammatory response [44]. Oxidative stress and consequent lipid peroxidation can activate various transcription factors, which lead to an elevation in inflammatory mediators such as TNF-α, IL-6, and IL-1. These proinflammatory cytokines are of importance to recruit leukocytes into inflamed tissues [23, 45]. Consistent with previous studies [37, 38], exposure of mice to PFOS in this study resulted in hepatic inflammation manifested by inflammatory cell infiltration, which may be linked to the augmented generation of IL-6 and TNF-α. Polyphenols have been proposed as adjuvant therapy for inflammation [45]. It has been shown that GSPE alleviates hepatic inflammation triggered by thioacetamide and lipopolysaccharide via upregulating the expression of proinflammatory factors [46, 47]. In the current experiment, supplementation with GSPE observably repressed the PFOS-induced overproduction of IL-6 and TNF-α in hepatic tissue, indicating that GSPE exerted anti-inflammatory activity in mice exposed to PFOS.
5. Conclusion
GSPE supplementation can reduce TG and TC accumulation, CD36 and FABP4 expression, MDA and H2O2 generation, and IL-6 and TNF-α production in the liver of mice exposed to PFOS, suggesting that GSPE can serve as a candidate for the prevention and therapy of PFAS-induced steatohepatitis via regulating lipid metabolism, oxidative stress, and inflammatory response. Our results provide a promising pharmacological strategy that may contribute to the protection against toxicant-associated steatohepatitis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81860112).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Tao Huang and Yurong Zhang contributed equally to this work.
References
- 1.Joshibarve S., Kirpich I. A., Cave M. C., Marsano L., Mcclain C. J. Alcoholic, nonalcoholic, and toxicant-associated steatohepatitis: mechanistic similarities and differences. Cellular and molecular gastroenterology and hepatology. 2015;1(4):356–367. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochoaherrera V., Field J. A., Lunavelasco A., Sierraalvarez R. Microbial toxicity and biodegradability of perfluorooctane sulfonate (PFOS) and shorter chain perfluoroalkyl and polyfluoroalkyl substances (PFASs) Environmental Science: Processes & Impacts. 2016;18(9):1236–1246. doi: 10.1039/c6em00366d. [DOI] [PubMed] [Google Scholar]
- 3.Domingo J. L. Health risks of dietary exposure to perfluorinated compounds. Environment International. 2012;40:187–195. doi: 10.1016/j.envint.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y. G., Wong C. K. C., Wong M. H. Environmental contamination, human exposure and body loadings of perfluorooctane sulfonate (PFOS), focusing on Asian countries. Chemosphere. 2012;89(4):355–368. doi: 10.1016/j.chemosphere.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Olsen G. W., Burris J. M., Ehresman D. J., et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental Health Perspectives. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagley B. D., Chang S. C., Ehresman D. J., et al. Perfluorooctane sulfonate-induced hepatic steatosis in male Sprague-Dawley rats is not attenuated by dietary choline supplementation. Toxicological Sciences. 2017;160(2):284–298. doi: 10.1093/toxsci/kfx185. [DOI] [PubMed] [Google Scholar]
- 7.Das K. P., Wood C. R., Lin M. T., et al. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology. 2017;378:37–52. doi: 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang X., Xie G., Wu X., Su M., Yang B. Effect of prenatal PFOS exposure on liver cell function in neonatal mice. Environmental Science and Pollution Research. 2019;26(18):18240–18246. doi: 10.1007/s11356-019-05245-4. [DOI] [PubMed] [Google Scholar]
- 9.Wan H. T., Zhao Y., Wei X., Hui K. Y., Giesy J. P., Wong C. K. C. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochimica et Biophysica Acta. 2012;1820(7):1092–1101. doi: 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Geiger S. D., Xiao J., Ducatman A. M., Frisbee S. J., Innes K. E., Shankar A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere. 2014;98:78–83. doi: 10.1016/j.chemosphere.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Barregard L., Xu Y., et al. Associations between perfluoroalkyl substances and serum lipids in a Swedish adult population with contaminated drinking water. Environmental Health. 2020;19(1):p. 33. doi: 10.1186/s12940-020-00588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin R., McConnell R., Catherine C., et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: an untargeted metabolomics approach. Environment International. 2020;134:p. 105220. doi: 10.1016/j.envint.2019.105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petriello M. C., Newsome B. J., Dziubla T. D., Hilt J. Z., Bhattacharyya D., Hennig B. Modulation of persistent organic pollutant toxicity through nutritional intervention: emerging opportunities in biomedicine and environmental remediation. Science of The Total Environment. 2014;491-492:11–16. doi: 10.1016/j.scitotenv.2014.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapiero H., Tew K. D., Ba G. N., Mathe G. Polyphenols: do they play a role in the prevention of human pathologies? Biomedicine & Pharmacotherapy. 2002;56(4):200–207. doi: 10.1016/S0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguezperez C., Garciavillanova B., Guerrahernandez E., Verardo V. Grape seeds proanthocyanidins: an overview of in vivo bioactivity in animal models. Nutrients. 2019;11(10):p. 2435. doi: 10.3390/nu11102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagchi D., Ray S. D., Patel D., Bagchi M. Protection against drug- and chemical-induced multiorgan toxicity by a novel IH636 grape seed proanthocyanidin extract. Drugs Under Experimental and Clinical Research. 2001;27(1):3–15. [PubMed] [Google Scholar]
- 17.Yang D., Jiang H., Lu J., et al. Dietary grape seed proanthocyanidin extract regulates metabolic disturbance in rat liver exposed to lead associated with PPARα signaling pathway. Environmental Pollution. 2018;237:377–387. doi: 10.1016/j.envpol.2018.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Niu Q., He P., Xu S., et al. Fluoride-induced iron overload contributes to hepatic oxidative damage in mouse and the protective role of grape seed proanthocyanidin extract. Journal of Toxicological Sciences. 2018;43(5):311–319. doi: 10.2131/jts.43.311. [DOI] [PubMed] [Google Scholar]
- 19.Dai N., Zou Y., Zhu L., Wang H., Dai M. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. Journal of Medicinal Food. 2014;17(6):663–669. doi: 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W., Xu C., Sun X., et al. Grape seed proanthocyanidin extract protects against perfluorooctanoic acid-induced hepatotoxicity by attenuating inflammatory response, oxidative stress and apoptosis in mice. Toxicology Research. 2016;5(1):224–234. doi: 10.1039/C5TX00260E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long M., Liu Y., Cao Y., Wang N., Dang M., He J. Proanthocyanidins attenuation of chronic lead-induced liver oxidative damage in Kunming mice via the Nrf2/ARE pathway. Nutrients. 2016;8(10):p. 656. doi: 10.3390/nu8100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennig B., Petriello M. C., Gamble M. V., et al. The role of nutrition in influencing mechanisms involved in environmentally mediated diseases. Reviews on environmental health. 2018;33(1):87–97. doi: 10.1515/reveh-2017-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koek G. H., Liedorp P. R., Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clinica Chimica Acta. 2011;412(15-16):1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Wang Y., Liang Y., et al. PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Scientific Reports. 2015;4(1):p. 4582. doi: 10.1038/srep04582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv Z., Li G., Li Y., et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environmental Toxicology. 2013;28(9):532–542. doi: 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- 26.Fiorucci S., Biagioli M., Distrutti E. Future trends in the treatment of non-alcoholic steatohepatitis. Pharmacological Research. 2018;134:289–298. doi: 10.1016/j.phrs.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Bessone F., Razori M. V., Roma M. G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cellular and Molecular Life Sciences. 2019;76(1):99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filozof C., Goldstein B. J., Williams R. N., Sanyal A. J. Non-alcoholic steatohepatitis: limited available treatment options but promising drugs in development and recent progress towards a regulatory approval pathway. Drugs. 2015;75(12):1373–1392. doi: 10.1007/s40265-015-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Ramiro I., Vauzour D., Minihane A. M. Polyphenols and non-alcoholic fatty liver disease: impact and mechanisms. The Proceedings of the Nutrition Society. 2016;75(1):47–60. doi: 10.1017/S0029665115004218. [DOI] [PubMed] [Google Scholar]
- 30.Akaberi M., Hosseinzadeh H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytotherapy Research. 2016;30(4):540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 31.Yin M., Zhang P., Yu F., et al. Grape seed procyanidin B2 ameliorates hepatic lipid metabolism disorders in db/db mice. Molecular Medicine Reports. 2017;16(3):2844–2850. doi: 10.3892/mmr.2017.6900. [DOI] [PubMed] [Google Scholar]
- 32.Baiges I., Palmfeldt J., Blade C., Gregersen N., Arola L. Lipogenesis is decreased by grape seed proanthocyanidins according to liver proteomics of rats fed a high fat diet. Molecular & Cellular Proteomics. 2010;9(7):1499–1513. doi: 10.1074/mcp.M000055-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coilly A., Desterke C., Guettier C., Samuel D., Chiappini F. FABP4 and MMP9 levels identified as predictive factors for poor prognosis in patients with nonalcoholic fatty liver using data mining approaches and gene expression analysis. Scientific Reports. 2019;9(1):p. 19785. doi: 10.1038/s41598-019-56235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong H., Kim J.-W., Yang M.-S., et al. Beneficial effects of Korean red ginseng in the progression of non-alcoholic steatohepatitis via FABP4 modulation. The American Journal of Chinese Medicine. 2018;46(7):1581–1607. doi: 10.1142/S0192415X18500817. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C. G., Tran J. L., Erion D. M., Vera N. B., Febbraio M., Weiss E. J. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice. Endocrinology. 2016;157(2):570–585. doi: 10.1210/en.2015-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loguercio C., De Girolamo V., de Sio I., et al. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. Journal of Hepatology. 2001;35(5):568–574. doi: 10.1016/S0168-8278(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 37.Lv Z., Wu W., Ge S., et al. Naringin protects against perfluorooctane sulfonate-induced liver injury by modulating NRF2 and NF-κB in mice. International Immunopharmacology. 2018;65:140–147. doi: 10.1016/j.intimp.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Han R., Hu M., Zhong Q., et al. Perfluorooctane sulphonate induces oxidative hepatic damage via mitochondria-dependent and NF-κB/TNF-α-mediated pathway. Chemosphere. 2018;191:1056–1064. doi: 10.1016/j.chemosphere.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 39.Wan C., Han R., Liu L., et al. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicology and Applied Pharmacology. 2016;295:85–93. doi: 10.1016/j.taap.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Khansari M. R., Yousefsani B. S., Kobarfard F., Faizi M., Pourahmad J. In vitro toxicity of perfluorooctane sulfonate on rat liver hepatocytes: probability of distructive binding to CYP 2E1 and involvement of cellular proteolysis. Environmental Science and Pollution Research. 2017;24(29):23382–23388. doi: 10.1007/s11356-017-9908-2. [DOI] [PubMed] [Google Scholar]
- 41.Rezazadeh A., Yazdanparast R., Molaei M. Amelioration of diet-induced nonalcoholic steatohepatitis in rats by Mn-salen complexes via reduction of oxidative stress. Journal of Biomedical Science. 2012;19(1):p. 26. doi: 10.1186/1423-0127-19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagchi D., Garg A., Krohn R. L., Bagchi M., Balmoori J., Stohs S. J. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. General Pharmacology-the Vascular System. 1998;30(5):771–776. doi: 10.1016/S0306-3623(97)00332-7. [DOI] [PubMed] [Google Scholar]
- 43.Niu Q., Mu L., Li S., Xu S., Ma R., Guo S. Proanthocyanidin protects human embryo hepatocytes from fluoride-induced oxidative stress by regulating iron metabolism. Biological Trace Element Research. 2016;169(2):174–179. doi: 10.1007/s12011-015-0409-1. [DOI] [PubMed] [Google Scholar]
- 44.Ikura Y., Ohsawa M., Suekane T., et al. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43(3):506–514. doi: 10.1002/hep.21070. [DOI] [PubMed] [Google Scholar]
- 45.Hussain T., Tan B., Yin Y., Blachier F., Tossou M. C. B., Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxidative Medicine and Cellular Longevity. 2017;2016:9. doi: 10.18508/endo5463.7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Li J., Li S., et al. Ameliorative effect of grape seed proanthocyanidin extract on thioacetamide-induced mouse hepatic fibrosis. Toxicology Letters. 2012;213(3):353–360. doi: 10.1016/j.toxlet.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Pallarès V., Fernández-Iglesias A., Cedó L., et al. Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radical Biology and Medicine. 2013;60:107–114. doi: 10.1016/j.freeradbiomed.2013.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


