Abstract
Objective
To describe the clinical, neurological, neuroimaging, and cerebrospinal fluid (CSF) findings associated with encephalopathy in patients admitted to a COVID-19 tertiary reference center.
Methods
We retrospectively reviewed records of consecutive patients with COVID-19 evaluated by a consulting neurology team from March 30, 2020 through May 15, 2020.
Results
Fifty-five patients with confirmed SARS-CoV-2 were included, 43 of whom showed encephalopathy, and were further divided into mild, moderate, and severe encephalopathy groups. Nineteen patients (44%) had undergone mechanical ventilation and received intravenous sedatives. Eleven (26%) patients were on dialysis. Laboratory markers of COVID-19 severity were very common in encephalopathy patients, but did not correlate with the severity of encephalopathy. Thirty-nine patients underwent neuroimaging studies, which showed mostly non-specific changes. One patient showed lesions possibly related to CNS demyelination. Four had suffered an acute stroke. SARS-CoV-2 was detected by RT-PCR in only one of 21 CSF samples. Two CSF samples showed elevated white blood cell count and all were negative for oligoclonal bands. In our case series, the severity of encephalopathy correlated with higher probability of death during hospitalization (OR = 5.5 for each increment in the degree of encephalopathy, from absent (0) to mild (1), moderate (2), or severe (3), p < 0.001).
Conclusion
In our consecutive series with 43 encephalopathy cases, neuroimaging and CSF analysis did not support the role of direct viral CNS invasion or CNS inflammation as the cause of encephalopathy.
Keywords: COVID-19, Encephalopathy, Cerebrospinal fluid, Critical care
Introduction
COVID-19 is a multisystem disease that usually targets the respiratory and cardiovascular systems. Neurological manifestations are common, occurring in up to 36.4% of cases [1]. The precise mechanism of neurologic involvement in COVID-19 remains incompletely understood, and may include neuroinvasion by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), cytokine storm, hypoxic, and vascular injury, as well as endothelial dysfunction [2]. Central nervous system invasion has not been consistently demonstrated.
Encephalopathy is a major complication of SARS-CoV-2 infection [3]. In previous studies, decreased consciousness was observed in 7.5–19.6% of COVID-19 patients [1, 4, 5], and in 14.8–38.9% of severe cases [1, 5]. Agitation and confusion occur commonly in patients with acute respiratory distress syndrome in COVID-19 [6]. In a previous series of consecutive COVID-19 patients evaluated by neurologists in a dedicated COVID-19 tertiary referral hospital, encephalopathy was the leading reason for a neurology consultation [7].
Objective
To describe the underlying conditions associated with encephalopathy in patients admitted to a COVID-19 reference center, we include systemic illness, intravenous sedative use, cerebrospinal fluid (CSF), and neuroimaging findings.
Methods
In March 2020, part of the Hospital das Clínicas da FMUSP was designated as a 900 bed (250 ICU) referral center dedicated to clinically suspected or confirmed COVID-19 patients. A neurology consult team consisting of seven neurologists and eight neurology residents (four PGY-2 and four PGY-3) provided on demand evaluations in the ICUs and wards. We retrospectively reviewed records of consecutive patients with COVID-19 evaluated by the neurology team from March 30, 2020 through May 15, 2020.
We selected cases with confirmed COVID-19, by positive SARS-CoV2 RT-PCR in nasopharyngeal swab or tracheal secretions or positive IgM or IgG serology by immunochromatography.
We classified patients in four groups according to the severity of the encephalopathy:
No encephalopathy—fully awake, preserved sustained and basic attention, without neuropsychiatric disturbances or psychomotor slowing.
Mild encephalopathy—awake or easily arousable patient, with preserved basic attention but impaired sustained attention. Patients with preserved sustained attention and level of consciousness, but presenting psychiatric, behavioral symptoms, or psychomotor slowing were also included in this group.
Moderate encephalopathy—awake or easily arousable patient, with impaired basic and sustained attention.
Severe encephalopathy—comatose patients or patients requiring vigorous stimuli to be aroused. Patients with severe psychomotor agitation were also included in this group.
Basic attention was defined as the ability to concentrate on a simple task, such as counting from 1 to 20, reciting the months in direct order or forward-order digit span. Sustained or complex attention described the patient’s ability to focus on a complex task that requires information processing, such as counting from 20 to 1, reciting the months of the year backwards, or reverse-order digit span.
We did not include patients with pre-existing encephalopathy without significant worsening during the acute illness, encephalopathy occurring immediately after cardiopulmonary resuscitation, incomplete clinical or neurological evaluation, and fully awake patients with severe aphasia precluding attention evaluation. Patients still receiving intravenous sedatives were not excluded, since clinicians noted signs that warranted a neurology consultation despite sedation.
Data collection
We performed a systematic chart review for all enrolled patients collecting clinical data and neurological exam findings.
Outcome and additional neurological diagnoses established during follow-up were checked for all patients on June 1, 17 days after the evaluation period ended. Only laboratory tests collected within 3 days of the evaluation were included. CSF analysis included interleukin 6 (IL-6) quantification (electrochemiluminescence—Cobas® e 411, Roche), presence of oligoclonal bands (isoelectric focusing followed by immunoblotting), and CSF-SARS-CoV-2-RT-PCR (Abbot m2000 system Invitrogen Superscript IV and IDT primers and probes with 40 copies/mL as the detection limit). Chest CT findings were classified as suggestive of viral pneumonia or not, based on radiology reports.
Brain computed tomography (CT), CT angiography, magnetic resonance imaging (MRI), and MRI angiography in encephalopathy patients were systematically reviewed by a trained neuroradiologist for acute changes. MRI was acquired in a 1.5 T GE scanner, including T1, T2, FLAIR, DWI, and susceptibility-weighted images and, when possible, FLAIR and T1-weighted images post gadolinium injection.
The study was approved by the hospital’s ethics committee and is registered at http://plataformabrasil.saude.gov.br, certification number 33563420.0.0000.0068.
Statistical analysis
In the primary analysis, we applied multivariate ordinal logistic regression with the degree of encephalopathy as the endpoint. Predictors were selected based on previously described mortality predictors in COVID19 [8, 9]: age, gender, hypertension, diabetes, lymphocyte count, and C-reactive protein. We included two additional predictors to account for a potential contribution of neuroinflammation: CSF white blood cell count and CSF total protein. Intravenous sedation was also included as a predictor. In a secondary predetermined analysis, we applied logistic regression using encephalopathy degree as a predictor and mortality as an endpoint.
Additionally, we performed an exploratory analysis. We applied multivariate ordinal logistic regression with the degree of encephalopathy as the endpoint and other laboratory parameters (platelets, BUN, d-dimer, presence of renal dysfunction, hypernatremia, and hyponatremia) as independent variables. Prior medical history was compared between groups using Mann-Whitney test.
Results
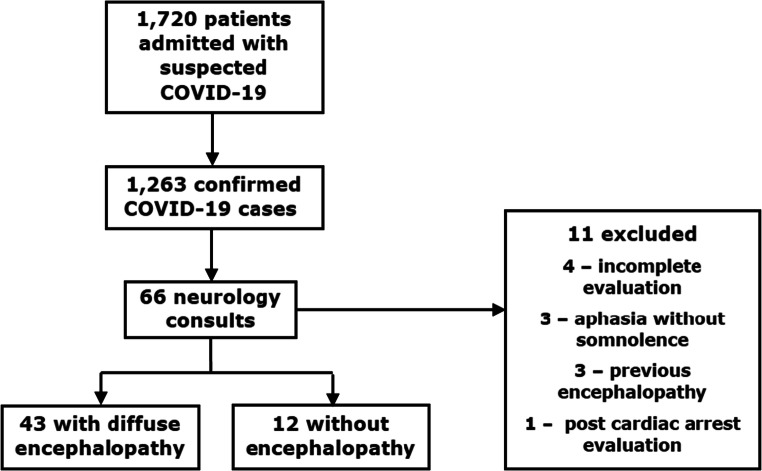
In the period between March 30 and May 15, 1720 patients were admitted to the COVID-19 dedicated facility, with 1263 confirmed SARS-CoV-2 infections. As of June 30, 812 of 1263 (64.3%) COVID-19 patients had been discharged, 412 (32.6%) had died, and 39 (3.1%) were still in the hospital. The neurology team evaluated a total of 66 COVID-19 patients. Eleven patients were excluded. Fifty-five patients were included in the final analysis (Flowchart 1). All patients were also included in a previous study that evaluated reasons for neurology consultation in Hospital das Clínicas [7]. Detailed clinical and ancillary data of patients with encephalopathy were not provided in that report.
Flowchart 1.
From March 30 to May 15: 1720 patients admitted to the COVID dedicated facility; 1263 confirmed SARS-CoV-2 cases throughout the hospital; 66 COVID-19 patients received a neurology consultation, 43 with acute encephalopathy; 11 patients were excluded
Patients were classified according to encephalopathy severity as follows: no encephalopathy, 12 cases; mild encephalopathy, 12 cases; moderate encephalopathy, 18 cases; severe encephalopathy, 13 cases.
Of the 43 encephalopathy cases, 33 consultations were requested due to acute altered mental state. In six cases, consultations were requested following abnormal brain CT results. Three consultations were requested to evaluate if a previously diagnosed neurological disease could contribute to the patient’s current status and one for suspected acute focal deficit.
Of the 12 cases without encephalopathy, five consultations were requested to evaluate patients with neurological conditions preceding COVID-19 infection, which remained stable (two with previously operated tumors, one with myasthenia gravis, one with epilepsy, and one with a history of central venous thrombosis). Three were requested for headache, three for peripheral neuropathies, and one for somatoform disorder.
Demographic data is shown in Table 1, including the median number of days from initial respiratory symptoms to admission and days from admission to neurology consultation. Five patients (12%) developed COVID-19 while already hospitalized for other conditions.
Table 1.
Demographic data and prior medical history of COVID positive patients with mild, moderate, and severe encephalopathy, all encephalopathy cases, and cases without encephalopathy
| Mild | Moderate | Severe | Encephalopathy cases | Cases without encephalopathy | P value | |
|---|---|---|---|---|---|---|
| Number of cases | 12 | 18 | 13 | 43 | 12 | - |
| Men | 8 (67%) | 12 (67%) | 9 (69%) | 29 (68%) | 6 (50%) | 0.17* |
| Age (years) | 58.5 (+/− 15.9) | 65.1 (+/− 12.9) | 58.2 (+/− 15.8) | 61.2 (+/− 14.7) | 55.0 (+/− 9.8) | 0.15* |
| Over 60 years of age | 5 (33%) | 11 (61%) | 7 (54%) | 22 (51%) | 3 (25%) | - |
| Days from initial respiratory symptoms to admission | 7.0 (5.0–12.0) | 2.5 (1.3–4.8) | 5.0 (1.0–7.0) | 3.5 (2.0–7.8) | 6.5 (4.5–8.0) | - |
| Days from admission to initial neurology consultation | 4.0 (1.5–6.0) | 10.0 (3.3–19.8) | 6.0 (2.0–10.0) | 5.0 (3.0–16.0) | 11.5 (3.3–21.8) | - |
| Prior medical history | ||||||
| Hypertension | 9 (75%) | 14 (78%) | 8 (62%) | 31 (72%) | 8 (67%) | 0.61* |
| Diabetes | 6 (50%) | 8 (44%) | 5 (39%) | 19 (44%) | 4 (33%) | 0.92* |
| Heart disease | 4 (33%) | 2 (11%) | 2 (15%) | 8 (19%) | 0 (0%) | 0.68** |
| Pulmonary disease | 0 (0%) | 2 (11%) | 2 (15%) | 5 (11%) | 1 (8%) | 0.52** |
| Impaired renal function | 3 (25%) | 4 (22%) | 4 (31%) | 11 (26%) | 3 (25%) | 0.76** |
| Alcohol abuse | 2 (17%) | 1 (6%) | 1 (8%) | 4 (9%) | 0 (0%) | 0.96** |
| Smoking | 1 (8%) | 4 (22%) | 5 (39%) | 10 (23%) | 2 (17%) | 0.19** |
| Cancer (in the previous 5 years) | 0 (0%) | 2 (11%) | 3 (23%) | 5 (12%) | 2 (17%) | 0.40** |
| Immuno-suppressive drug use | 1 (8%) | 0 (0%) | 3 (23%) | 4 (9%) | 3 (25%) | 0.78** |
| Prior neurologic disease | 6 (50%) | 5 (28%) | 3 (23%) | 14 (33%) | 4 (33%) | 0.33** |
| Ischemic stroke | 4 (33%) | 3 (17%) | 1 (8%) | 8 (19%) | 0 (0%) | 0.97** |
| Epilepsy | 3 (25%) | 0 (0%) | 1 (8%) | 4 (9%) | 3 (25%) | 0.06** |
| Dementia | 3 (25%) | 1 (6%) | 1 (8%) | 5 (12%) | 0 (0%) | 0.93** |
Data are median (interquartile range), mean (standard deviation) or n/N (%)
*Primary analysis, ordinal logistic regression
**Exploratory analysis, Mann-Whitney test
Clinical data
Arterial hypertension was the most common comorbidity in the encephalopathy group: 31 of 43 (72%), followed by diabetes in 19 (44%), renal disease in 11 (26%), and smoking in 10 (23%). No patient had HIV or AIDS. Fourteen (33%) had a pre-existing neurologic condition (eight had a history of stroke, four of epilepsy, five had cognitive decline, and one had been diagnosed with primary CNS vasculitis).
Three of 32 (9%) encephalopathy patients reported hyposmia and 16 of 36 (44%) reported mental status changes, such as somnolence or confusion, prior to admission to the referral center. Three encephalopathy (7%) patients reported seizures before admission, one of whom had a prior history of epilepsy. Three patients in the non-encephalopathy group had a history of epilepsy and presented breakthrough seizures before admission. Of the patients with confirmed COVID-19 and encephalopathy, chest CT was typical of viral infection in 37/39 cases (95%), compared to 8/12 (67%) in the non-encephalopathy group.
Mechanical ventilation, dialysis, vasopressor, and sedative use is shown in Table 2. During hospitalization, 19 (44%) patients with encephalopathy received intravenous sedatives for mechanical ventilation, six (14%) of whom were still under sedation on neurological evaluation. All these patients received midazolam and fentanyl. Three of the 19 (16%) also received propofol, and six (32%) also received dexmedetomidine. For the 13 patients no longer receiving intravenous sedatives on neurological evaluation, the median number of days from initiation of IV sedatives to IV sedative cessation was 9.0 (IQR 6.0–14.0), ranging from three to 42 days. Neurology evaluation occurred a median of 8.0 (IQR 5.0–11.0) days after sedation cessation (Table 2).
Table 2.
Clinical data leading to neurology consultation and patient outcome.
| Mild | Moderate | Severe | Encephalopathy cases | Cases without encephalopathy | |
|---|---|---|---|---|---|
| Number of cases | 12 | 18 | 13 | 43 | 12 |
| Intravenous sedative use | |||||
| Any time since admission | 2 (17%) | 10 (56%) | 7 (54%) | 19 (44%) | 4 (33)% |
| Still in use at time of evaluation | 0 (0%) | 0 (0%) | 6 (46%) | 6 (14%) | 0 (0%) |
| Days from sedatives cessation to evaluation | 4.5 (3.8–5.3) | 8.0 (5.5–10.5) | 11.0 (11.0–11.0) | 8.0 (5.0–11.0) | 16.0 (11.0–18.0) |
| Days from sedative (initiation/withdrawal) | 11.5 (8.8–14.3) | 9.5 (6.3–13.5) | 6.0 (6.0–6.0) | 9.0 (6.0–14.0) | 10.0 (8.0–14.0) |
| Sedatives in use at evaluation | 0 (0%) | 0 (0%) | 6 (46%) | 6 (14%) | 0 (0%) |
| Midazolam | 2 (100%) | 10 (100%) | 7 (100%) | 19 (100%) | 4 (33%) |
| Propofol | 0 (0%) | 1 (10%) | 2 (29%) | 3 (16%) | 0 (0%) |
| Fentanyl | 2 (100%) | 10 (100%) | 7 (100%) | 19 (100%) | 4 (33%) |
| Dexmedetomidine | 2 (100%) | 1 (10%) | 3 (43%) | 6 (32%) | 1.0 (8%) |
| Mechanical ventilation (MV) | |||||
| Any time since admission | 2 (17%) | 10 (56%) | 7 (54%) | 19 (43%) | 5 (42%) |
| MV at neurological evaluation | 0 (0%) | 4 (22%) | 5 (39%) | 9 (21%) | 0 (0%) |
| Days on MV | 10.5 (7.75–13.25) | 16.0 (13.5–19.3) | 13.0 (13.0–13.0) | 15.0 (13.0–17.0) | 13.0 (11.0–19.0) |
| Days from MV cessation to initial evaluation | 5.5 (4.75–6.25) | 6.5 (4.5–10.0) | 4.0 (4.0–4.0) | 6.0 (4.0–7.0) | 10.0 (5.0–17.0) |
| Other clinical data | |||||
| Required IV vasopressors | 2 (17%) | 8 (44 %) | 8 (62%) | 18 (42%) | 5.0 (42%) |
| On dialysis | 2 (17%) | 4 (22%) | 5 (39%) | 11 (26%) | 2.0 (17%) |
| On antipsychotics | 4 (33%) | 3 (17%) | 5 (39%) | 12 (28%) | 3 (25%) |
| Outcome | |||||
| Discharged | 10 (84%) | 10 (56%) | 1 (8%) | 21 (49%) | 9 (75%) |
| Death | 0 (0%) | 5 (28%) | 6 (46%) | 11 (26%) | 1 (8%) |
Data are median (IQR), mean (SD) or n/N (%). N is the total number of patients with available data and n the number of presenting the finding
Table 3 presents neurological exam findings and clinical data obtained from chart review closest to the neurology consultation. Four (9%) patients had preserved level of consciousness and attention, but were included in the mild encephalopathy group due to marked psychomotor slowing or acute psychiatric symptoms (psychosis and hypervigilance) without previous psychiatric disease.
Table 3.
Clinical data from the neurological evaluation
| Mild | Moderate | Severe | Encephalopathy cases | Cases without encephalopathy | |
|---|---|---|---|---|---|
| Total | 12 | 18 | 13 | 43 | 12 |
| Clinical data at neurology consultation | |||||
| Heart rate > 100 bpm | 3 (25%) | 9 (50%) | 4 (31%) | 16 (37%) | 2 (17%) |
| Mean blood pressure < 70 mmHg | 0 (0%) | 1 (6%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Oxygen saturation < 90% | 0 (0%) | 1 (6%) | 1 (8%) | 2 (5%) | 0 (0%) |
| Receiving supplemental O2 | 6 (50%) | 14 (78%) | 10 (77%) | 30 (70%) | 6 (50%) |
| Glasgow coma scale (mean) | 14.0 (14.0–14.5) | 13.0 (8.5–14.0) | 11.0 (6.0–13.0) | 14.0 (9.5–14.0) | 15.0 (15.0–15.0) |
| Richmond agitation–sedation scale (mean) | 0 (0–0) | − 0.5 (− 3.3 to 0) | − 5.0 (− 5.0 to − 4.3) | − 1.0 (− 5.0 to 0) | 0 (0–0) |
| Neurological exam | |||||
| Behavioral changes | 4 (33%) | 0 (0%) | 0 (0%) | 4 (9%) | 0 (0%) |
| Psychomotor agitation | 0 (0%) | 0 (0%) | 1 (8%) | 1 (2%) | 0 (0%) |
| Oriented to time and space | 6 (46%) | 0 (0%) | 0 (0%) | 6 (14%) | 12 (100%) |
| Nuchal rigidity | 0/9 (0%) | 0/13 (0%) | 0/3 (0%) | 0/25 (0%) | 0/8 (0%) |
| Quadriparesis | 2 (17%) | 2 (11%) | 1 (8%) | 5 (12%) | 1 (8%) |
| Increased deep tendon reflexes | 1 (8%) | 1 (6%) | 0 (0%) | 2 (5%) | 0 (0%) |
| Myoclonus | 2 (17%) | 2 (11%) | 2 (15%) | 6 (14%) | 0 (0%) |
| Other neurological diagnoses | |||||
| Acute neurovascular disease | 0 (0%) | 3 (17%) | 1 (8%) | 4 (9%) | 1 (8%) |
| Seizures in patients without prior epilepsy | 0 (0%) | 0 (0%) | 3 (23%) | 3 (7%) | 0 (0%) |
| Seizures in patients with prior epilepsy | 2 (17%) | 0 (0%) | 0 (0%) | 2 (5%) | 3 (25%) |
Data are median (IQR), mean (SD) or n/N (%). N is the total number of patients with available data and n the number of patients presenting the finding
Five patients had overt seizures; two of these had breakthrough seizures attributed to pre-existing epilepsy. Electroencephalogram could not be performed due to restrictions imposed by COVID-19.
Four patients, aged 45 to 59 years, had suffered acute ischemic strokes that could at least partially account for drowsiness. Three of these patients had focal deficits noted in the setting of slow neurologic recovery following IV sedative discontinuation. Of these four patients, two suffered malignant middle cerebral artery infarctions, one had an occipital and midbrain infarct, and the other one had a temporal-parietal infarct with hemorrhagic transformation (Table 3).
Laboratory test findings are presented in Table 4, including lymphocytes, platelets, creatinine, blood urea nitrogen (BUN), sodium, and C-reactive protein (CRP) for all patients. All encephalopathy patients had CRP above 5 mg/L (median 90.2; IQR 49.3–174.4 mg/L).
Table 4.
Laboratory and CSF studies
| Mild | Moderate | Severe | Encephalopathy cases | Cases without encephalopathy | OR | P value | |
|---|---|---|---|---|---|---|---|
| Total | 12 | 18 | 13 | 43 | 12 | - | - |
| Blood tests values (median) | |||||||
| Lymphocytes/mm3 | 1020 (705–1257) | 1476 (65–2010) | 980 (680 – 1460) | 1150 (645–1680) | 1400 (1100–2000) | 1.000 (CI 0.999–1.001 | 0.33* |
| C-reactive protein (mg/L) | 71.3 (46.7–168.3) | 92.7 (45.7–156.7) | 115.0 (69.4–176.9) | 90.2 (49.3–174.4) | 36.4 (11.4–176.4) | 1.002 (CI 0.996–1.008) | 0.46* |
| BUN (mg/dL) | 22.7 (15.5–36.2) | 52.6 (22.4–68.1) | 64.5 (28–81.8) | 35.5 (21.7–81.8) | 13.1 (10.0–19.9) | 1.015 (CI 1.002–1.028) | 0.03** |
| D-dimer (ng/ml) | 1974.0 (801.5–5231) | 4444.0 (1594–14,950) | 4716.5 (2411.3–14,827.8) | 4222.0 (1538.5–8891.3) | 1469.0 (488.0–3775.3) | 1.000 (CI 0.999–1.001) | 0.37** |
| Platelets 103/mm3 | 255 (166–326) | 270 (213–364) | 235 (189–254) | 242 (173–320) | 292 (218–369) | 0.999 (CI 0.995–1.003) | 0.66** |
| Abnormal blood tests | |||||||
| Creatinine >1.2 (males); > 0.9 (females) mg/dL | 5 (42%) | 12 (67%) | 10 (77%) | 27 (63%) | 5 (42%) | 0.796 (CI 0.186–3.397) | 0.76** |
| Sodium > 145 mEq/L | 0 (0%) | 7 (39%) | 3 (23%) | 10 (23%) | 0 (0%) | 1.903 (CI 0.425–8.518) | 0.40** |
| Sodium < 135 mEq/L | 2 (17%) | 2 (11%) | 2 (15%) | 6 (14%) | 1 (8%) | 2.988 (CI 0.474–18.858) | 0.24** |
| NT-proBNP > 125 pg/mL | 3/3 (100%) | 7/8 (88%) | 4/4 (100%) | 14/15 (93%) | 3/6 (50%) | - | - |
| Creatinine phosphokinase > 190 (men); > 167 (women) U/L | 0/8 (0%) | 6/13 (46%) | 3/11 (27%) | 9/31 (29%) | 3/10 (30%) | - | - |
| ALT > 120 U/L | 0/12 (0%) | 3/17 (18%) | 0/12 (0%) | 3/41 (7%) | 0/11 (0%) | - | - |
| Indirect bilirubin > 0.6 mg/dL | 0/10 (0%) | 0/16 (0%) | 0/11 (0%) | 0/37 (0%) | 0/10 (0%) | - | - |
| Albumin < 3.4 g/dL | 7/9 (78%) | 13/14 (93%) | 5/7 (71%) | 25/30 (83%) | 6/6 (100%) | - | - |
| Prothrombin time ratio > 1.4 | 0/11 (0%) | 1/16 (6%) | 1/12 (8%) | 2/39 (5%) | 0/10 (0%) | - | - |
| Cerebrospinal fluid findings | |||||||
| White blood cells > 4/mm3 | 0/8 (0%) | 1/8 (13%) | 1/7 (14%) | 2/23 (9%) | 0/3 (0%) | - | - |
| Protein > 40 mg/dL | 2/8 (25%) | 3/8 (38%) | 4/7 (57%) | 9/23 (39%) | 2/3 (67%) | - | |
| Protein > 40 mg/dL without diabetes | 0/8 (0%) | 2/8 (25%) | 3/7 (43%) | 5/23 (22%) | 1/3 (33%) | - | - |
| Glucose < 50 mg/dL | 1/8 (13%) | 1/8 (13%) | 1/7 (14%) | 3/23 (13%) | 0/3 (0%) | - | - |
| Lactate > 22 mg/dL | 0/8 (0%) | 3/8 (38%) | 2/7 (29%) | 5/23 (22%) | 0/3 (0%) | - | - |
| Elevated CSF gamma-globulin | 0/6 | 1/6 (17%) | 0/6 (0%) | 1/18 (5%) | 1/2 (50%) | - | - |
| Oligoclonal bands present | 0/8 (0%) | 0/8 (0%) | 0/8 (0%) | 0/23 (0%) | 0/3 (0%) | - | - |
| Interleukin 6 > 7.8 pg/mL | 0/1 (0%) | 1/2 (50%) | 2/2 (100%) | 3/5 (60%) | 0/1 (0%) | - | - |
| PCR for SARS-CoV-2 positive | 0/6 (0%) | 0/8 (0%) | 1/7 (14%) | 1/21 (4%) | 0/3 (0%) | - | - |
Data are median (IQR), mean (SD) or n/N (%). N is the total number of patients with available data and n the number of positive patients. OR, odds ratio
*Primary analysis, ordinal logistic regression
**Exploratory analysis, ordinal logistic regression
Neuroimaging
Thirty-nine of 43 encephalopathy patients underwent neuroimaging studies: brain CT 38, CT angiography 14, brain MRI nine, MRI angiography three. Neuroimaging was normal or showed only chronic changes in 29/38 (76%) of CTs and 4/9 (44%) of MRIs. Thirty of the 39 patients (77%) did not present acute changes in neuroimaging studies.
Neuroimaging findings potentially associated with COVID-19 were seen in five patients: acute ischemic stroke in the four described patients and one previously reported case [33] displayed multiple rounded T2/FLAIR hyperintensities possibly related to CNS demyelination. Four patients had acute brain imaging abnormalities probably not associated with COVID-19: a pregnant woman with convexity subarachnoid hemorrhage (SAH), Wernicke’s encephalopathy findings and pontine myelinolysis, a man with newly diagnosed brain metastasis from lung cancer, a transplant recipient with tacrolimus-associated posterior reversible encephalopathy syndrome, and a patient with a convexity subarachnoid hemorrhage alone. In the first SAH case, the finding could be attributed to Wernicke’s encephalopathy or concurrent extrapontine myelinolysis. The second patient with convexity SAH did not have a history of trauma, coagulopathy, or vascular abnormalities on CT angiography. He underwent a brain CT after slow neurological recovery following a week of sedation and mechanical ventilation due to COVID-19 related acute respiratory syndrome, and did not display any other atypical findings that could relate to the SAH. At the time of the evaluation, he displayed a c-reactive protein of 30.3 mg/L and d-dimer of 14,898 ng/mL, with normal coagulation profile.
Cerebrospinal fluid
Lumbar puncture was performed in 23 encephalopathy patients and in three patients without encephalopathy who presented with headache. All three patients without encephalopathy showed normal CSF cell count and mildly elevated protein in two patients (42 and 48 mg/dL).
Among encephalopathy patients, two (9%) had an elevated CSF white blood cell (WBC) count. All 26 samples were negative for oligoclonal bands (Table 4).
Interleukin 6 was quantified in seven CSF samples from six patients (five encephalopathy patients and one non-encephalopathy patient). Interleukin 6 level values were in normal reference ranges (< 7.8 pg/mL) in three patients, including one patient without encephalopathy (2.13 pg/mL), one patient with mild encephalopathy (3.44 pg/mL), and one with moderate encephalopathy (7.32 pg/mL). Three patients in the encephalopathy group had elevated IL-6 levels. One of these was case report #2 (below), with moderate encephalopathy, and two IL-6 measures (52.99 pg/mL and 25.32 pg/mL). The two other patients had severe encephalopathy (19.57—case report #1, below—and 12.15 pg/mL).
Case report #1
A woman with peripheral eosinophilia, positive CSF SARS-CoV-2-RT-PCR, and elevated CSF IL-6—SARS-CoV-2 was detected in the CSF of one patient (out of 21): a woman in her 50s who developed fever during hospitalization for acute aortic dissection complicated by renal failure requiring dialysis, blood eosinophilia, disseminated candidiasis, and central venous catheter-related bloodstream infection. A nasal swab test was positive for SARS-CoV-2. Two days after onset of fever, she developed encephalopathy. A lumbar puncture, obtained 10 days after onset of fever, revealed 15 cells/mm3 (38% lymphocytes, 8% monocytes, 22% neutrophils, 31% eosinophils, and 1% macrophages), protein of 54 mg/dL, interleukin 6 of 19.57 pg/mL (cut-off 7.8 pg/mL), and a positive CSF SARS-COV-RT-PCR. A peripheral blood cell count also revealed elevated leukocytes count with 48% eosinophils. Brain CT was normal, and MRI was not obtained.
Case report #2
A man presents with EBV meningoencephalitis, CSF pleocytosis, and elevated CSF IL-6. A man over the age of 70 with positive nasal swab SARS-CoV-2-RT-PCR was transferred to our center due to encephalopathy 28 days after respiratory symptom onset.
Brain CT was normal. CSF analysis revealed 256/mm3 cells (90% mononuclear cells), a total protein 115 mg/dL, glucose 46 mg/dL, lactate 25.2 mg/dL, and IL-6 52.99 pg/mL. A CSF multiplex PCR assay detected EBV and CSF-SARS-CoV-2-PCR was negative. Additional lumbar punctures were performed 3 and 7 days after the initial exam, and showed persistent CSF pleocytosis, elevated IL-6 (25.32 pg/mL) and elevated total protein, but negative EBV-PCR. The patient fully recovered after 1 month.
Statistical analysis
None of the variables showed a correlation with the severity of encephalopathy in the primary ordinal logistic regression analysis. On the secondary predetermined analysis, the degree of encephalopathy correlated with higher probability of death during hospitalization; OR of 5.5 for each increment in the degree of encephalopathy (p < 0.001).
On exploratory analysis of prior medical history and other laboratory tests, only BUN correlated with the severity of encephalopathy (OR = 1.015, CI: 1.002–1.028, p = 0.027). P values were not corrected for multiple comparisons.
Discussion
We report the findings of encephalopathy in 43 patients from 66 neurology consults in 1263 COVID-19 patients. The presentation of encephalopathy ranged from behavioral changes and mild attention deficit to severely impaired consciousness. Its degree correlated with mortality
Our series represents cases in the COVID-19 dedicated center presenting with more severe neurological manifestations, or those in which clinicians could not attribute symptoms to systemic dysfunctions alone. These cases would represent the sample of patients most likely to present neurological disease caused by direct central nervous system viral invasion. However, evidence of neuroinvasion and neuroinflammation were largely absent. Although clinical factors, including renal impairment and prolonged sedative use were highly prevalent in moderate and severe encephalopathy cases, many patients displayed encephalopathy without clear metabolic dysfunction or sedative use, especially in mild encephalopathy cases. The only consistent findings in these patients were laboratory signs of systemic inflammation.
Neurological examination showed few additional findings other than encephalopathy. Some patients presented myoclonus, attributed to toxic and metabolic disorders. In two patients, abnormal movements noted on neurological exam suggested focal seizures. Only one patient in this series presented severe psychomotor agitation on examination. In other patients, agitation had been controlled with antipsychotics and intravenous sedatives by the time of the neurology evaluation.
The moderate encephalopathy group was the largest group in our series, comprising patients seen later in the disease course, many of whom had spent prolonged periods under sedation and mechanical ventilation. These patients were easily arousable, but had severely compromised level of attention, in some cases unable to engage in basic attention testing.
Although encephalopathy is a common finding in COVID-19 inpatients, the precise underlying mechanisms remain incompletely understood. Case reports or small-case series that evaluated SARS-CoV-2 RNA detection in CSF samples showed conflicting results: while some studies detected viral RNA with RT-PCR or next-generation sequencing [10–14], other studies reported negative findings [15–20]. Generalizability of findings is possibly limited by publication bias. Our study included 24 CSF SARS-CoV-2-RT-PCR tests. We found only one SARS-CoV-2-positive sample, which may have resulted from shedding of viral genetic material from plasma to CSF in a patient with damaged blood–brain barrier. Our findings suggest that SARS-CoV-2 RNA detection rates in encephalopathy cases may be lower than expected from case reports, but consistent with other larger case series of neurologic disease [6, 21–23]. Other studies demonstrated the presence of anti-SARS-CoV-2 antibodies in the CSF of patients with neurologic disease and negative CSF-RT-PCR [19, 20], suggesting that immunological assays may be superior to molecular testing to demonstrate SARS-CoV-2 CNS involvement. In other infectious diseases, such as Lyme neuroborreliosis, neurosyphilis, West Nile virus encephalitis and Japanese encephalitis, immunological assays appear to be superior to molecular testing in blood or CSF, which may be related to low pathogen loads or transient CNS infections [24].
Alternatively, encephalopathy may be caused by CNS immune-mediated processes without viral invasion. Our series is in agreement with larger studies showing that CSF cell and biochemistry counts are largely normal in COVID-19 patients with neurological symptoms [5, 6, 19, 21]. However, elevated CSF WBC, protein levels, and positive oligoclonal bands were described in case reports [3]. Oligoclonal bands were absent in all 24 CSF samples in our series. Only two patients (8.3% of cases) showed increased CSF WBC. In both cases, alternative explanations not related to SARS-CoV-2 CNS infection (case reports #1 and #2) were postulated.
Cytokine storm is a potential major cause of acute respiratory distress syndrome and multiple organ dysfunction in COVID-19 patients [25]. Cytokine serum levels, including IL-1, IL-6, and TNF-α, correlate with systemic COVID-19 severity [26, 27]. Recent studies demonstrated elevated CSF IL-6 levels in patients with encephalopathy, negative CSF SARS-CoV-2-RT-PCR, and normal CSF cell count, suggesting a potential role of IL-6 as a biomarker in these patients [23, 28]. We found markedly elevated IL-6 levels in two patients with known inflammatory CNS disease, both of which were possibly not COVID-related–in case report #1, elevated IL-6 was probably the result of viral meningoencephalitis; the patient in case report 2#, had bloodstream infection, disseminated candidiasis and elevated levels of eosinophils in both blood and CSF, which implied systemic inflammation from other causes compromised the blood-brain barrier.
Although most COVID-19 neuroimaging studies evaluated patients with acute cerebrovascular disease, suspected myelopathy and miscellaneous neurologic syndromes, neuroimaging was requested to evaluate mental status changes in 37–73% of patients [29–32]. In our series, the majority of patients had brain CTs or MRIs, most of which did not display acute changes. Infarcts (10.3% of neuroimaging studies) were the most common acute findings, in keeping with other retrospective studies [31, 32]. Only one case, reported elsewhere [33], had acute demyelinating-like lesions potentially related to COVID-19. In other COVID-19 patients, neuroimaging findings could be explained by other neurological diseases; however, their association with COVID-19 cannot be completely ruled out. In our series, we did not observe other previously described neuroimaging findings, such as punctate hemorrhagic foci, cortical swelling, and leptomeningeal enhancement [34]. Since only a few patients in this series underwent MRI, these findings could be missed on brain CT.
Conclusion
In our series, encephalopathy in COVID-19 manifested in various degrees of severity which were associated with increased mortality and may be influenced by factors such as sedative drug use, organ system dysfunction, and systemic inflammation. Head CT showed mainly non-specific changes, and MRI may be preferable, though not easily obtainable in critically ill patients, to document more subtle findings related to encephalopathy in COVID-19 patients. CSF analysis in our series did not detect viral genetic material or inflammatory changes, suggestive of CNS viral invasion or immune-mediated processes, in the majority of encephalopathy cases. Future studies should clarify the role of cytokines, CSF-SARS-CoV-2 immunology, and other inflammatory biomarkers, particularly in mild and moderate cases of COVID-19 associated encephalopathy.
Authors’ contributions
Raphael de Luca e Tuma: Conceptualized the paper, collected data, participated in consensus meetings, analyzed data and wrote the initial draft; Bruno Fukelmann Guedes: Participated in consensus meetings, analyzed data and wrote the initial draft; Rafael Bernhart Carra: Participated in consensus meetings, collected data data and wrote the initial draft; Bruno Diógenes Iepsen: Participated in consensus meetings, collected data data and wrote the initial draft; Júlia Chartouni Rodrigues: Participated in consensus meetings, collected data data and wrote the initial draft; Antonio Edvan Camelo Filho: Data collection and analysis; Gabriel Taricani Kubota: Data collection and analysis; Maíra Medeiros Honorato Ferrari: Data collection and analysis; Adalberto Studart-Neto: Data collection and analysis; Mariana Hiromi Manoel Oku: Data collection and analysis; Sara Terrim: Data collection and analysis; Cesar Castello Branco Lopes: Data collection and analysis; Carlos Eduardo Borges Passos Neto: Data collection and analysis; Matheus Dalben Fiorentino: Data collection and analysis; Julia Carvalhinho Carlos De Souza: Data collection and analysis; José Pedro Soares Baima: Data collection and analysis; Tomás Fraga Ferreira Da Silva: Data collection and analysis; Iago Navas Perissinotti: Completed the statistical analysis; Maria da Graça Martin: Reviewed the neuroimaging studies; Marcia Rubia Rodrigues Gonçalves: Participated in consensus meetings and reviewed the manuscript for intellectual content; Ida Fortini: Participated in consensus meetings and reviewed the manuscript for intellectual content; Jerusa Smid: Participated in consensus meetings and reviewed the manuscript for intellectual content; Tarso Adoni: Participated in consensus meetings and reviewed the manuscript for intellectual content; Leandro Tavares Lucatto: Reviewed the neuroimaging studies; Ricardo Nitrini: Participated in consensus meetings, wrote and reviewed the manuscript for intellectual content and supervised the study; Helio Rodrigues Gomes: Participated in consensus meetings, wrote and reviewed the manuscript for intellectual content and supervised the study; Luiz Henrique Martins Castro: Participated in consensus meetings, wrote and reviewed the manuscript for intellectual content and supervised the study. The corresponding author takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. He has full access to all of the data and has the right to publish any and all data separate and apart from any sponsor.
Compliance with ethical standards
As stated in the Methods section of the manuscript, this study was approved by the hospital’s ethics committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Statistical analysis completed by Iago Perissinotti
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed]
- 2.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg RK, Paliwal VK, Gupta A (2020) Encephalopathy in patients with COVID-19: A review. J Med Virol. 10.1002/jmv.26207 [DOI] [PubMed]
- 4.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet Lond Engl. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E et al (2020) Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed]
- 6.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studart-Neto A, Guedes BF, Tuma R d L e et al (2020) Neurological consultations and diagnoses in a large, dedicated COVID-19 university hospital. Arq Neuropsiquiatr. 10.1590/0004-282x20200089 [DOI] [PubMed]
- 8.Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- 9.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingues RB, Mendes-Correa MC, Vilela de Moura Leite FB et al (2020) First case Of SAR-Coronavirus-2 sequencing in the cerebrospinal fluid of a patient with suspected CNS demyelinating disease. 10.21203/rs.3.rs-31801/v1 [DOI] [PMC free article] [PubMed]
- 13.Sun T, Guan J Novel coronavirus and the central nervous system. Eur J Neurol. 10.1111/ene.14227 [DOI] [PubMed]
- 14.Zhou L, Zhang M, Wang J, Gao J (2020) Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis:101642. 10.1016/j.tmaid.2020.101642 [DOI] [PMC free article] [PubMed]
- 15.Al Saiegh F, Ghosh R, Leibold A, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. 2020;91:846–848. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 16.Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci Off J Neurosurg Soc Australas. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilinc D, van de Pasch S, Doets AY et al (2020) Guillain-Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. 10.1111/ene.14398 [DOI] [PMC free article] [PubMed]
- 18.Sancho-Saldaña A, Lambea-Gil Á, Liesa JLC, et al. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med Lond Engl. 2020;20:e93–e94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andriuta D, Roger P-A, Thibault W et al (2020) COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J Neurol. 10.1007/s00415-020-09975-1 [DOI] [PMC free article] [PubMed]
- 20.Benameur K, Agarwal A, Auld SC et al (2020) Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis 26. 10.3201/eid2609.202122 [DOI] [PMC free article] [PubMed]
- 21.Neumann B, Schmidbauer ML, Dimitriadis K, et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci. 2020;418:117090. doi: 10.1016/j.jns.2020.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Destras G, Bal A, Escuret V, et al. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1:e149. doi: 10.1016/S2666-5247(20)30066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexopoulos H, Magira E, Bitzogli K et al (2020) Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm 7 [DOI] [PMC free article] [PubMed]
- 24.He T, Kaplan S, Kamboj M, Tang Y-W. Laboratory diagnosis of central nervous system infection. Curr Infect Dis Rep. 2016;18:35. doi: 10.1007/s11908-016-0545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coperchini F, Chiovato L, Croce L, et al. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farhadian S, Glick LR, Vogels CBF, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:248. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain R, Young M, Dogra S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremer S, Lersy F, de Sèze J, et al (2020) Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology 202222. 10.1148/radiol.2020202222
- 31.Mahammedi A, Saba L, Vagal A et al (2020) Imaging in neurological disease of hospitalized COVID-19 patients: an Italian multicenter retrospective observational study. Radiology:201933. 10.1148/radiol.2020201933 [DOI] [PMC free article] [PubMed]
- 32.Radmanesh A, Raz E, Zan E, et al. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. AJNR Am J Neuroradiol. 2020;41:1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopes CCB, Brucki SMD, Passos Neto CEB et al (2020) Acute disseminated encephalomyelitis in COVID-19: presentation of two cases and review of the literature. Arq Neuropsiquiatr. 10.1590/0004-282X20200186 [DOI] [PubMed]
- 34.Kandemirli SG, Dogan L, Sarikaya ZT et al (2020) Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology:201697. 10.1148/radiol.2020201697 [DOI] [PMC free article] [PubMed]