The septum pellucidum (“translucent wall”) is a thin, triangular membranous structure that runs sagittally from the corpus callosum down to the fornix and separates the left and right lateral ventricles of the brain. The septum pellucidum consists of two laminae of both white and gray matter. During fetal development there is a space between the two laminae called the cavum septum pellucidum, which disappears during infancy in most individuals. The septum pellucidum is the anatomic site of origin of a spectrum of uncommon neuroepithelial tumors that include central neurocytoma, subependymoma, and low-grade glioneuronal tumors morphologically resembling either dysembyroplastic neuroepithelial tumor (DNT) or rosette-forming glioneuronal tumor (RGNT). Multiple case series or individual case reports have described DNT-like or RGNT-like low-grade glioneuronal tumors of the septum pellucidum and lateral ventricle, which are distinct from the typical cortical and fourth ventricular locations of DNT and RGNT, respectively [1, 5, 16]. Molecular analysis of these tumors has revealed absence of IDH1/2 mutation and co-deletion of chromosomes 1p/19q that genetically define oligodendroglioma [5, 16]. Additionally, they have been found to lack alterations in FGFR1, BRAF, MYB, and MYBL1 that characterize the majority of DNT, RGNT, and other low-grade neuroepithelial tumors [5].
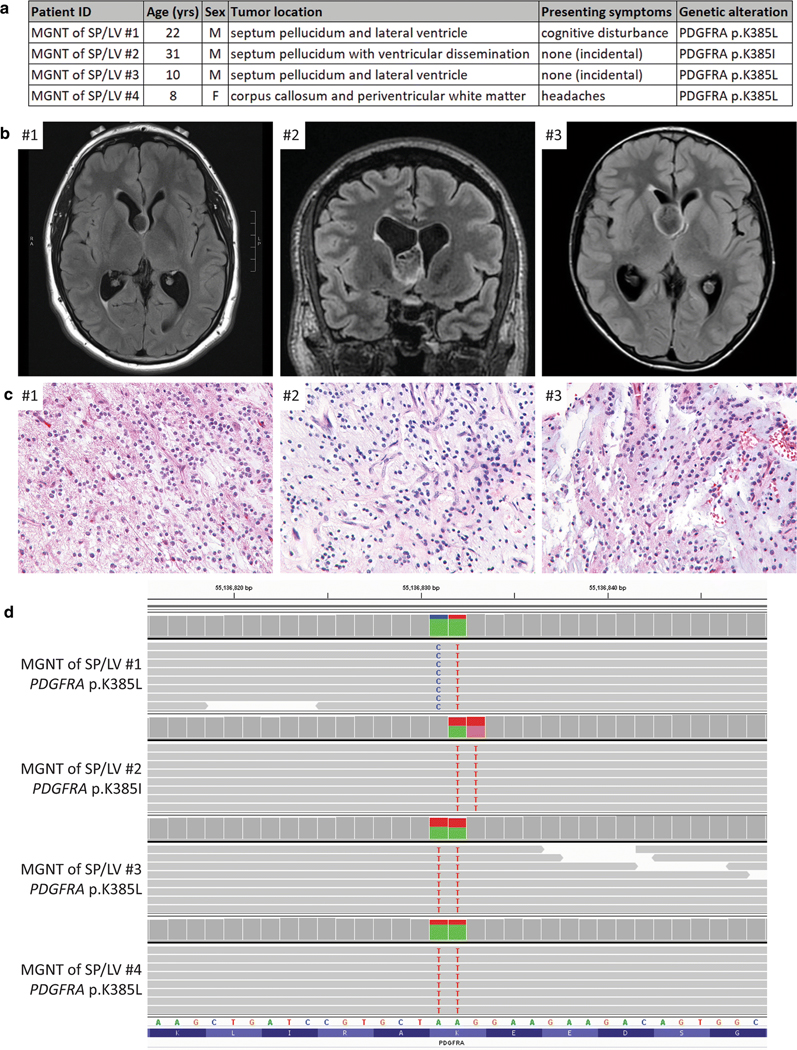
To investigate the molecular pathogenesis of these enigmatic low-grade glioneuronal neoplasms of the septum pellucidum and lateral ventricle with DNT-like or RGNT-like histologic features, we assembled a cohort of tissue specimens from four patients (Fig. 1a and Supplementary Table 1 [Online Resource 1]). The three male and one female patients ranged in age at time of initial surgery from 8–31 years. One patient had presented with cognitive disturbance, one with headaches, and two were asymptomatic with brain imaging that had been performed for unrelated indication. Pre-operative imaging demonstrated non-enhancing, T2-hyperintense, lobulated masses centered in the septum pellucidum (n=3) or genu of the corpus callosum (n=1) (Fig. 1b, Supplementary Table 2, and Supplementary Figure 1 [Online Resources 1 and 2]). Multi-nodularity typical of cortically-based DNT was absent in all cases. Two patients had localized disease, one patient had an additional small focus of dissemination in the posterior horn of the lateral ventricle (#3), and one patient had extensive dissemination throughout the ventricular system (#2).
Figure 1.
Myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle is defined by a recurrent PDGFRA p.K385 hotspot mutation. a, Table of the clinicopathologic features of the four patients with MGNT of SP/LV and the identified PDGFRA mutations. b, Pre-operative magnetic resonance imaging for patients #1–3. c, Tumor histology of cases #1–3. Hematoxylin and eosin staining, 40x magnification. d, Snapshot from the Integrated Genome Viewer showing sequencing reads containing a dinucleotide substitution at codon p.K385 of the PDGFRA gene in all four cases. Reference transcript NM_006206.
All four cases were histologically characterized by a low-grade proliferation of neoplastic glial cells with oligodendroglial cytology (i.e. uniform round nuclei with delicate chromatin) in a mucin-rich stroma (Fig. 1c, Supplementary Table 3, and Supplementary Figure 2 [Online Resources 1 and 2]). A prominent or at least focal feature in all cases was columnar arrangement of the oligodendroglioma-like cells along with ganglion cells “floating” in mucin-filled lacunae. All cases lacked the multi-nodularity that is typical of cortically-based DNT. Two of the cases (#2 and #3) additionally demonstrated well-formed neurocytic rosettes, composed of tumor cells surrounding central areas of neuropil. No Rosenthal fibers, eosinophilic granular bodies, or calcifications were observed in any of the cases. Mitotic activity, significant nuclear pleomorphism, necrosis, and microvascular proliferation were uniformly absent. The neoplastic glial cells demonstrated GFAP and OLIG2 positivity by immunostaining, while the floating neurons and neurocytic rosettes showed synaptophysin positivity. CD34-positive ramified cells were not seen in the two evaluated cases. The Ki-67 labeling index was uniformly low (less than 5%).
Genomic DNA was extracted from formalin-fixed, paraffin-embedded tumor tissue, and targeted capture-based next-generation DNA sequencing was performed as previously described using the UCSF500 Cancer Panel [7–9, 12, 13], which assesses approximately 500 cancer-associated genes for mutations, copy number alterations, and structural variants including gene fusions (Supplementary Table 4 [Online Resource 1]). All four cases demonstrated dinucleotide substitutions at codon p.K385 in the PDGFRA gene, three resulting in a lysine to leucine change (p.K385L) and one resulting in a lysine to isoleucine change (p.K385I) (Fig. 1d and Supplementary Table 5 [Online Resource 1]). The 20–44% allele frequencies of these PDGFRA mutations were consistent with being heterozygous somatic variants in each case. PDGFRA encodes the platelet-derived growth factor receptor alpha, a transmembrane receptor tyrosine kinase known to signal through the Ras-Raf-MAP kinase and PI3-kinase-Akt-mTOR pathways. While PDGFRA alterations including gene amplification, intragenic deletions/rearrangements, and missense mutations are known to be common in high-grade diffuse gliomas in both children and adults, these PDGFRA alterations invariably co-occur with other pathogenic alterations such as p.K27M mutation of H3F3A or HIST1H3B genes in diffuse midline gliomas or TERT promoter mutation and CDKN2A deletion in IDH-wildtype glioblastomas [10, 11]. PDGFRA mutation has not been identified as the solitary genetic driver in any CNS tumor entity to date. Of note is a recent study of 91 low-grade neuroepithelial tumors in children and young adults that identified two cases with solitary PDGFRA alterations, one of which was morphologically classified as “DNT” and the other as “oligoastrocytoma” [14]. Both tumors were located in the temporal lobe with precise location not further specified, and both tumors harbored similar dinucleotide substitutions at codon p.K385 as seen in the four tumors in this series, one resulting in p.K385L and the other p.K385I. These mutations all result in substitution from a basic amino acid (lysine) to a hydrophobic amino acid (leucine or isoleucine) at a codon that is located in the extracellular ligand binding domain. While the precise functional consequence of this recurrent p.K385 mutation in PDGFRA is uncertain at present, it is very likely to be an oncogenic, gain-of-function mutation that causes constitutive activation of the kinase domain in the absence of ligand. Interestingly, mutations at codon p.K385 of PDGFRA appear to be specific to glial and glioneuronal tumors, as other human tumor types with frequent PDGFRA mutations, such as gastrointestinal stromal tumors, have not been found to harbor variants at this codon (Catalog of Somatic Mutations In Cancer database, version 85 release).
Besides PDGFRA mutation, no additional pathogenic mutations, amplifications, deletions, or structural variants were identified involving any of other assayed genes that included IDH1, IDH2, H3F3A, HIST1H3B, HIST1H3C, MYB, MYBL1, ATRX, TERT (including promoter region), CIC, FUBP1, TP53, MDM2, MDM4, PPM1D, ACVR1, BRAF, RAF1, PTPN11, KRAS, NF1, MAP2K1, PRKCA, FGFR1–3, NTRK1–3, EGFR, MET, ALK, PTEN, PIK3CA, PIK3R1, AKT1, TSC1, TSC2, MTOR, CDKN2A, CDKN2C, RB1, CDK4, CDK6, CCND1–3, BCOR, BCORL1, NF2, RELA, and YAP1. No chromosomal copy number gains, losses, or focal amplifications or deletions were identified in any of the tumors (Supplementary Table 6 and Supplementary Figure 3 [Online Resources 1 and 2]).
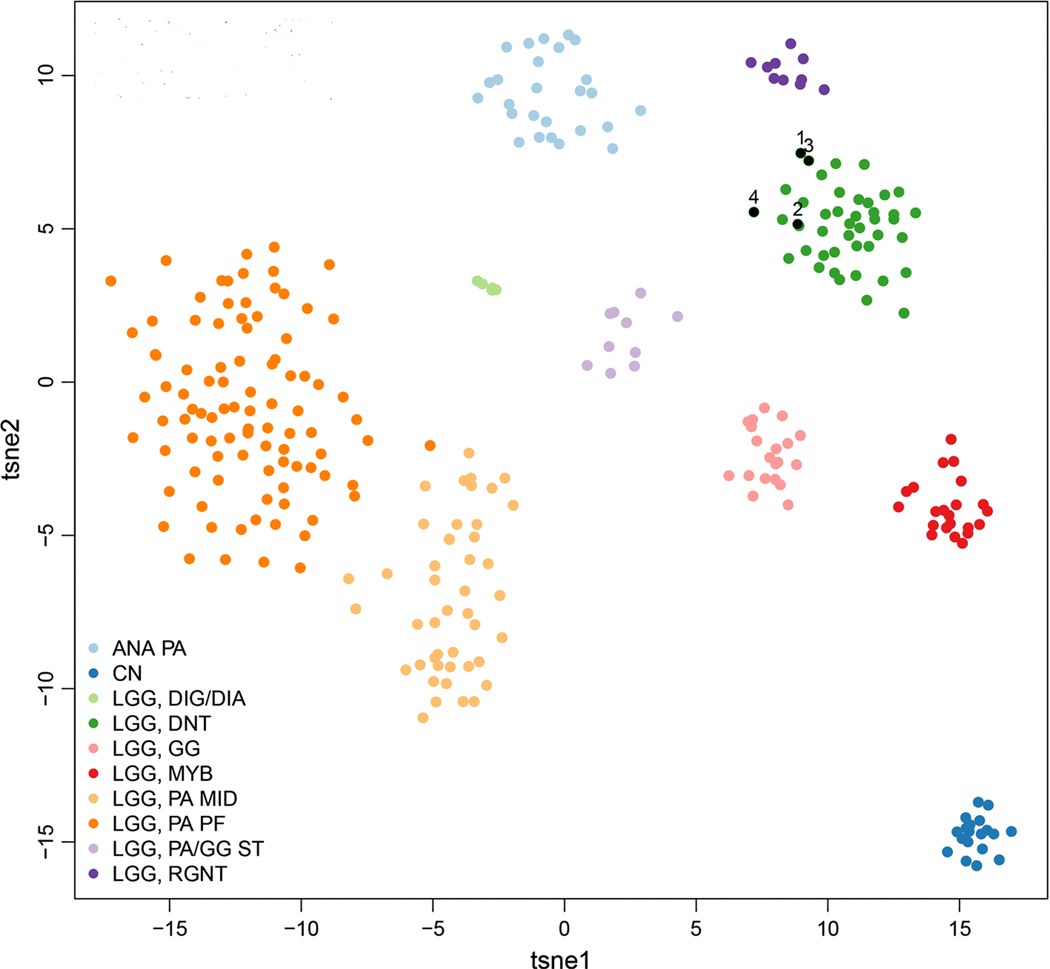
Genome-wide DNA methylation profiling was performed as previously described [3] using the Illumina MethylationEPIC BeadChip (850k array) to further characterize these four myxoid glioneuronal tumors of the septum pellucidum and lateral ventricle with PDGFRA p.K385 mutation. Unsupervised clustering of DNA methylation patterns alongside 300 previously characterized CNS neoplasms encompassing a spectrum of low grade glial and glioneuronal tumor entities revealed that each of the four tumors closely clustered with cortically-based dysembyroplastic neuroepithelial tumors and did not cluster with rosette-forming glioneuronal tumors, central neurocytomas, or other neuroepithelial tumor entities (Fig. 2).
Figure 2.
Myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle is defined by a DNT-like methylation profile. Unsupervised clustering of DNA methylation patterns for the four MGNT of SP/LV (black circles numbered 1 through 4), alongside 300 previously characterized glial and neuronal neoplasms. Shown is a two-dimensional representation of pairwise sample correlations using the 10,000 most variably methylated probes by t-distributed stochastic neighbor embedding (tSNE) dimensionality reduction. Reference methylation classes are: ANA PA, anaplastic pilocytic astrocytoma. CN, central neurocytoma. LGG DIG/DIA, desmoplastic infantile ganglioglioma/astrocytoma. LGG DNT, dysembyroplastic neuroepithelial tumor. LGG GG, ganglioglioma. LGG MYB, low grade glioma with MYB or MYBL1 rearrangement. LGG PA MID, midline pilocytic astrocytoma. LGG PA PF, posterior fossa pilocytic astrocytoma. LGG PA/GG ST, supratentorial/hemispheric pilocytic astrocytoma and ganglioglioma. LGG RGNT, rosette-forming glioneuronal tumor.
Together, these findings suggest that such DNT-like or RGNT-like low-grade glioneuronal neoplasms of the septum pellucidum and lateral ventricles may be a distinct entity, for which we propose the terminology “myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle, PDGFRA-mutant” (MGNT of SP/LV). While these four tumors all shared morphologic overlap and clustered by genome-wide methylation analysis with DNT, they lacked the cortical location, the multinodular architecture, and FGFR1 or BRAF mutation, gene fusion, or kinase domain tandem duplication that characterize the vast majority of DNT [14, 15, 17]. While two of these four tumors shared morphologic overlap with RGNT, they did not cluster by genome-wide methylation analysis with RGNT and also lacked the location in the fourth ventricle and combination of FGFR1 plus PIK3CA or PIK3R1 alterations that characterize the majority of RGNT [4, 6]. In addition, they lacked the KIAA1549-BRAF fusion that is typical of pilocytic astrocytoma and diffuse leptomeningeal glioneuronal tumor, the BRAF mutation or fusion that is typical of ganglioglioma, and the MAP2K1 mutation that is typical of multinodular and vacuolating neuronal tumor of the cerebrum (MVNT) [12–14, 17]. No PRKCA fusions or kinase domain mutations were identified in any of the cases, providing genetic distinction from papillary glioneuronal tumor and chordoid glioma of the third ventricle [2, 7].
In summary, we have identified a recurrent PDGFRA p.K385 hotspot mutation as the solitary pathogenic alteration in DNT-like and RGNT-like low-grade glioneuronal tumors of the septum pellucidum, which together with their stereotypic anatomic location, should help facilitate accurate diagnosis of this distinct neuroepithelial tumor entity for which we propose the name “myxoid glioneuronal tumor of the septum pellucidum and lateral ventricle, PDGFRA-mutant”.
Supplementary Material
Acknowledgements
D.A.S. is supported by NIH Director’s Early Independence Award (DP5 OD021403) and the UCSF Physician-Scientist Scholar Program. B.C.B. is supported by an NCI Outstanding Investigator Award (R35 CA220481).
Footnotes
Data availability
Scanned image files of the H&E stained slides from which representative images are presented are available for downloading and viewing at the following link: https://figshare.com/projects/Myxoid_glioneuronal_tumor_of_the_septum_pellucidum_and_lateral_ventricle/35651. Sequencing and methylation data files are available from the authors upon request.
Compliance with ethical standards
This study was approved by the Committee on Human Research of the University of California, San Francisco, with a waiver of patient consent.
Conflict of interest
The authors declare that they have no competing interests related to this study.
References
- 1.Baisden BL, Brat DJ, Melhem ER, Rosenblum MK, King AP, Burger PC (2001) Dysembryoplastic neuroepithelial tumor-like neoplasm of the septum pellucidum: a lesion often misdiagnosed as glioma: report of 10 cases. Am J Surg Pathol 25:494–499 [DOI] [PubMed] [Google Scholar]
- 2.Bridge JA, Liu XQ, Sumegi J et al. (2013) Identification of a novel, recurrent SLC44A1-PRKCA fusion in papillary glioneuronal tumor. Brain Pathol 23:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capper D, Jones DTW, Sill M et al. (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellezam B, Theeler BJ, Luthra R, Adesina AM, Aldape KD, Gilbert MR (2012) Recurrent PIK3CA mutations in rosette-forming glioneuronal tumor. Acta Neuropathol 123:285–287 [DOI] [PubMed] [Google Scholar]
- 5.Gessi M, Hattingen E, Dorner E et al. (2016) Dysembryoplastic neuroepithelial tumor of the septum pellucidum and the supratentorial midline: histopathologic, neuroradiologic, and molecular features of 7 cases. Am J Surg Pathol 40:806–811 [DOI] [PubMed] [Google Scholar]
- 6.Gessi M, Moneim YA, Hammes J et al. (2014) FGFR1 mutations in Rosette-forming glioneuronal tumors of the fourth ventricle. J Neuropathol Exp Neurol 73:580–584 [DOI] [PubMed] [Google Scholar]
- 7.Goode B, Mondal G, Hyun M et al. (2018) A recurrent kinase domain mutation in PRKCA defines chordoid glioma of the third ventricle. Nat Commun 9:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iorgulescu JB, Van Ziffle J, Stevers M et al. (2018) Deep sequencing of WNT-activated medulloblastomas reveals secondary SHH pathway activation. Acta Neuropathol 135:635–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kline CN, Joseph NM, Grenert JP et al. (2017) Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro-Oncol 19:699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay A, Burford A, Carvalho D et al. (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32:520–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paugh BS, Zhu X, Qu C et al. (2013) Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res 73:6219–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pekmezci M, Stevers M, Phillips JJ et al. (2018) Multinodular and vacuolating neuronal tumor of the cerebrum is a clonal neoplasm defined by genetic alterations that activate the MAP kinase signaling pathway. Acta Neuropathol 135:485–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pekmezci M, Villanueva-Meyer JE, Goode B et al. (2018) The genetic landscape of ganglioglioma. Acta Neuropathol Commun 6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qaddoumi I, Orisme W, Wen J et al. (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera B, Gayden T, Carrot-Zhang J et al. (2016) Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol 131:847–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong J, Liu Y, Chu SG et al. (2012) Dysembryoplastic neuroepithelial tumor-like neoplasm of the septum pellucidum: review of 2 cases with chromosome 1p/19q and IDH1 analysis. Clin Neuropathol 31:31–38 [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Wu G, Miller CP et al. (2013) Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.