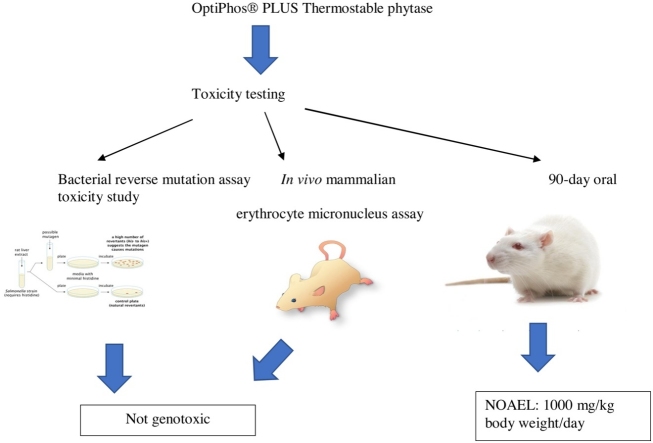
Graphical abstract
Abbreviations: 2AA, 2-aminoanthracene; 9AA, 9-aminoacridine; AAFCO, Association of American Feed Control Officials; ANOVA, analysis of variance; bw, body weight; GLP, Good Laboratory Practice; IACUC, Institutional Animal Care and Use Committee; MMS, methylmethanesulfonate; MPCE, polychromatic erythrocytes with micronuclei; NOAEL, no observed adverse effect level; NPD, 4-nitro-12-phenylenediamine; OECD, Organisation for Economic Co-Operation; PCE, polychromatic erythrocyte; SAZ, sodium azide; DMSO, Dimethyl sulfoxide
Keywords: 6-phytase, Thermostable phytase, Toxicity, Genotoxicity, Subchronic toxicity, Phytase TSP, OptiPhos PLUS
Highlights
-
•
The toxicity of Phytase TSP a new thermostable phytase was studied.
-
•
No genotoxic or toxic effects were recorded for Phytase TSP.
-
•
The NOAEL for the new Phytase TSP is 1000 mg/kg bw/d.
-
•
The studies support the safety of Phytase TSP as an animal feed additive.
Abstract
A novel 6-phytase (Phytase TSP, trade name OptiPhos® PLUS) with improved thermostability has been developed for use in animal feed. The safety of the new phytase was evaluated by testing for genotoxicity and subchronic toxicity. In in vitro and in vivo genotoxicity assays Phytase TSP concentrate was not mutagenic and did not induce biologically or statistically significant increases in the frequency of micronucleated polychromatic erythrocytes. In a subchronic toxicity study, male and female rats administered 100, 500 or 1000 mg/kg body weight/day of Phytase TSP concentrate via oral gavage for 90 days had no mortalities, and no treatment-related effects on body weight, food consumption, clinical observations or ophthalmology. Furthermore, there were no changes in haematology, clinical chemistry, urinalysis, gross pathology, organ weights or histopathology that could be attributed to the test article. Several endpoints exhibited statistically significant effects, but none was dose-related or considered to be of toxicological relevance. Based on these results, Phytase TSP concentrate (OptiPhos® PLUS) was not genotoxic and the No Observed Adverse Effect Level (NOAEL) for male and female rats was 1000 mg/kg body weight/day.
1. Introduction
Phytases (myo-inositol(1,2,3,4,5,6)hexakisphosphate phosphohydrolases) are widely used in animal nutrition to initiate the stepwise dephosphorylation of phytate (myo-inositol(1,2,3,4,5,6)hexakisphosphate), the most abundant inositol phosphate in nature [1] and the major storage form of phosphate in plant seeds [2]. This function is of pivotal importance in animal nutrition to ensure adequate availability of elementary phosphorous from the diet. Without phytases, diets of fish, pigs, and poultry would need to be supplemented with inorganic phosphorous, an expensive and nonrenewable source of the mineral, to meet nutritional phosphorous requirements [3,4]. In the recent past, a broad range of commercial phytases has become available with better catalytic performance for example by possessing a pH optimum of activity adapted to the acidic conditions of the stomach. Thermostability is also an important characteristic of phytases as animal diets are frequently pelleted at temperatures up to 90 °C possibly leading to significant loss of phytase activity.
Improvement of thermostability has been addressed by means of genetic engineering and targeted selection and a novel 6-phytase (E.C. 3.1.3.26) has been developed that preserves the favourable properties of phytases currently on the market but also exhibits drastically improved thermostability. The novel phytase named Phytase TSP, to be marketed under the brand name OptiPhos® PLUS, is encoded by the appA2P gene from the low Risk Group 1 organism Escherichia coli. The enzyme is produced in the yeast Komagataella phaffii (previously classified as Pichia pastoris) and meets the description of the feed ingredient definition of phytase from the Association of American Feed Control Officials (AAFCO).
To evaluate the general safety of Phytase TSP a battery of toxicity tests were performed including tests for potential genotoxicity as well as a 90-day subchronic repeated dose oral toxicity study in rats.
2. Materials and methods
2.1. Study locations and guidelines
The battery of toxicity tests were performed in compliance with the Organisation for Economic Co-Operation (OECD) Guidance and with the Principles of Good Laboratory Practice (GLP). The bacterial reverse mutation test and the mouse micronucleus test were conducted at TOXI−COOP ZRT. in Balatonfüred, Hungary according to OECD Guideline 471 and 474, respectively. The subchronic oral toxicity study was conducted at MediTox s.r.o. in Konárovice, Czech Republic according to OECD 408. The study design was approved by the Institutional Animal Care and Use Committee (IACUC) and the Committee for Animal Protection of the Ministry of Health of the Czech Republic (26/2018).
2.2. Test article
The novel 6-phytase used in the studies was supplied by Huvepharma Inc. (Peshtera, Bulgaria) as a concentrate representing the final product before the formulation process, i.e. Phytase TSP 161 concentrate (Batch No. 18041274,094; 300,000 phytase units per gram). One phytase unit (FTU) is defined as the amount of enzyme that releases 1 μmol of inorganic phosphate from phytate per minute under reaction conditions with a phytate concentration of 5.0 mM at pH 5.5 and 37 °C.
Phytase TSP is produced by fermentation using the non-pathogenic recombinant yeast Komagataella phaffii DSM 32854. A synthetic phytase gene appA2P was constructed based on the 6-phytase appA2 from E. coli that was identified and cloned from swine intestine bacterial isolate [[5], [6], [7]]. The active substance of AppA2P phytase produced by Komagataella phaffii DSM 32854 is secreted in the culture supernatant and modified by yeast-specific N-linked glycosylation [8]. On an SDS-PAGE the active compound migrates with apparent mobility ranging from ca. 50–75 kDa, while the calculated molecular weight based on the amino acid composition of AppA2P is 45 kDa. When treated with EndoH, AppA2P is de-glycosylated and migrates with apparent mobility close to 45 kDa.
The resulting fermentation product was filtered to remove the production strain.
2.3. Bacterial reverse mutation (Ames) test
The Ames test (Ames et al., 1975; Maron and Ames, 1983; Kier et al., 1986; Venitt and Parry, 1984; Mortelmans and Zeiger, 2000) was conducted using a plate incorporation experiment and a confirmatory pre-incubation experiment with Salmonella typhimurium TA98, TA100, TA1535 and TA1537 and Escherichia coli WP2 uvrA. Both experiments were performed in the presence and absence of metabolic activation by liver S9-mix prepared in-house from S9 fraction of phenobarbital and β-naphthoflavone-induced rat livers. S9 fraction and tester strains were provided by Trinova Biochem GmbH (Grissen, Germany; manufacturer: MOLTOX INC., Boone, NC USA). Phytase TSP concentrate was tested in both experiments at concentrations of 16, 50, 160, 500, 1600 and 5000 μg/plate. Selection of test article concentrations were based upon OECD 471 Guidance and preliminary solubility and concentration range finding tests. Treatment solutions were prepared from Phytase TSP concentrate with ultrapure water, filtered (filter paper and 0.22 μm membrane filter) and further diluted to obtain the dosing solutions. Positive controls in the absence of S9-mix were 4-nitro-1,2-phenylenediamine (NPD), sodium azide (SAZ), 9-aminoacridine (9AA) and methylmethanesulfonate (MMS); and in the presence of S9-mix, 2-aminoanthracene (2AA). Ultrapure water was the negative control and the vehicle control for the test item, SAZ and MMS. Dimethyl sulfoxide (DMSO) was the vehicle control for NPD, 9AA and 2AA. In the standard plate incorporation procedure treatments were plated in triplicate and incubated at 37 °C for 48 h. The confirmatory pre-incubation procedure included 20 min incubation at 37 °C in a shaking incubator prior to plating. Revertant colonies were counted manually, evaluated by the unaided eye. Mean, standard deviations and mutation rates were calculated.
2.4. In vivo mammalian erythrocyte micronucleus assay
Male Hsd Win: NMRI mice (Specific Pathogen Free) obtained from TOXI−COOP ZRT. (Budapest, Hungary) were acclimated for 6 days before animals in acceptable health were randomly assigned to one of the five test groups. Each treatment group consisted of five mice, except that seven mice were used in the high dose group (extra replacement mice in case of mortalities). At dosing, the average age of mice was 8 weeks old and body weight ranged from 30.3–35.1 g. Mice were housed two per cage in the high dose group and 5 per cage in the other dose groups. Polypropylene/polycarbonate type I cages with deep wood sawdust bedding were maintained at 22 ± 3 °C, 40–70 % relative humidity and a light phase from 6 a.m. to 6 p.m. Pellet diet (ssniff® SR/M-Z+H, ssniff Spezialdiäten GmbH, Experimental Animal Diets Inc., Soest, Germany) and potable water were provided ad libitum.
Phytase TSP treatments of 50, 100 and 200 mg/ml were prepared within 2 h of dosing in distilled water (aqua purificata). Doses of 500, 1000 or 2000 mg/kg body weight were administered twice orally via gavage at 24-h intervals to male mice at 10 mL/kg body weight. Doses were selected based on a preliminary range finding experiment showing no toxic effects on two male and two female mice administered 2000 mg/kg body weight. Negative control mice received distilled water via oral gavage. Positive control animals were given an intraperitoneal dose of 10 mL/kg cyclophosphamide dissolved in sterile water (aqua ad injectabilia). Five animals in the negative control, low-, mid-, and high-dose group were sampled 24 h after the second treatment. Five animals in the positive control group were sampled 24 h after the beginning of the treatment. Mice were examined for visible signs of reaction to treatment immediately after dosing and at intervals until sacrifice.
After sacrificing (cervical dislocation) bone marrow was flushed from two exposed femurs per animal with foetal bovine serum and cells were collected. After vortexing, the cell suspension was concentrated by centrifugation then smears of the cell pellet were made on standard microscope slides, which were then dried at room temperature. Smeared cells were fixed for at least 5 min in methanol, allowed to air-dry, stained with Giemsa (10 %) solution for 25 min, rinsed in distilled water, dried at room temperature for at least 12 h, and then coated with EZ-mount. One slide from each animal was coded for blind microscopic analysis.
The first 4000 polychromatic erythrocytes (PCEs) counted in the optic field per animal were assessed for micronuclei. The proportion of immature among total erythrocytes was determined for each animal by counting a total of at least 500 immature erythrocytes. Statistical analysis was performed with SPSS/PC + software using Duncan's Multiple Range test and the Mann-Whitney U test. Data were checked for a linear trend in mutant frequency with treatment dose using Microsoft Excel’s regression analysis.
2.5. Subchronic oral toxicity study
Six-week-old male and female Wistar (SPF) rats (50 per sex) from Charles River (Germany) were examined to confirm good health before random distribution into two groups with 15 males and 15 females (control and high-dose groups) and two groups with 10 males and 10 females (low- and mid-dose groups) so that body weight means for each group were comparable for each sex. Animals were identified by tail tattoo. During the study animals were housed in Macrolon Tecniplast cages (2–3 animals of the same sex/cage) with Tier Wohl Super bedding (JRS Germany) (except when in metabolic cages for urine collection). The environment was ventilated and maintained at 19−25 °C and 30–70 % relative humidity with 12-h fluorescent lighting illumination per day. Animals had free access to tap water and standard pellet diet (“Myši chov”; Sehnoutek a synové v.o.s., Czech Republic). Feed and water containers were changed and sanitized at least once weekly. Animals were acclimated for 8–11 days before starting treatment.
Test article in sterile water was prepared weekly at the testing facility and administered to animals via daily oral gavage (0.5 mL/100 g body weight) at concentrations of 0 (Control), 100 (low-dose), 500 (mid-dose) or 1000 (high-dose) mg/kg bw/d as a suspension for 90 consecutive days. Doses for the 90-d study were selected based upon earlier toxicity studies performed with “OptiPhos®”, a phytase powder currently on the market with an activity of 27,000 FTU/g, and the forerunner of OptiPhos® PLUS. Extra animals in the control and high-dose groups were retained for an additional 14-day treatment-free recovery period before sacrifice.
Individual body weights were measured and recorded at delivery, before first test article administration, and weekly throughout the main study and recovery periods. Food consumption per cage was recorded weekly. Rats were observed once daily for clinical signs, morbidity or mortality during the acclimation and recovery periods and twice daily during the administration period. Detailed clinical observations were performed before study begin and weekly thereafter. Ophthalmoscopic examination of both eyes by direct and indirect ophthalmoscopy (Keeler Professional Ophthalmoscope, Windsor, UK) was performed on all animals during acclimatisation, and at weeks 13 (administration period) and 15 (recovery period).
Blood samples were drawn from all animals during acclimatisation (week-1) and after 13 (administration period) and 15 weeks (recovery period) for haematology and plasma chemistry measurements. Samples were drawn under slight ether anaesthesia from the retro-orbital venous plexus from animals fasted for about 12−18 h. Sampling tubes contained the anticoagulants K3EDTA (haematology) or sodium citrate (coagulation) or no anticoagulant (serum biochemistry). Differential leukocyte count, lymphocyte count, monocyte count, neutrophil count, eosinophil count, basophil count, erythrocyte count, haematocrit, haemoglobin, leukocyte counts, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, mean corpuscular volume, platelet count, activated partial thromboplastin time and prothrombin time were measured. Clinical biochemistry measurements included: alanine aminotransferase, albumin, albumin/globulin ratio, alkaline phosphatase, aspartate aminotransferase, bile acids, total bilirubin, Ca, Cl, cholesterol, creatinine, gamma-glutamyl transpeptidase, glucose, lactate dehydrogenase, P, K, Na, total protein, triglycerides and urea.
Urine was collected from animals in metabolic cages during acclimatisation (week-1) and after weeks 13 (administration period) and 15 (recovery period). Macroscopic analysis and semi-quantitative biochemical analysis (Urilyzer® 100 Pro, Analyticon® Biotechnologies AG) was performed for volume, appearance, colour, specific gravity, pH, protein, bilirubin, urobilinogen, erythrocytes, ketones, nitrite, leucocytes, glucose and turbidity.
After 91 or 105 days trial, animals were euthanised by cervical dislocation under ether anaesthesia. Animals were weighed and examined externally. Cranial, thoracic and abdominal cavities were opened for macroscopic examination and any abnormalities were recorded and graded in severity using a four point system of minimal, slight, moderate, or marked, and occasional findings were recorded as present or as unilateral in paired organs. Weights were recorded for adrenal glands, brain, epididymides, heart, kidneys, liver, ovaries, prostate gland, spleen, testes, thymus, thyroid gland (including parathyroid) and uterus and cervix. Relative organ to terminal body weight ratios were calculated. Samples of the weighed organs listed above and the following organs and tissues were collected from all animals at necropsy: aorta, bone (femur including joint and cartilage), bone marrow (sternum), cecum, colon, duodenum, esophagus, eyes (incl. optic nerves), Harderian gland, ileum, jejunum, lacrimal glands, larynx, lungs (incl. main stem bronchi), mesenteric lymph nodes, pancreas, Payers patches, pituitary gland, rectum, salivary glands (mandibular, parotid), sciatic nerve, seminal vesicles, site of mammary gland, skeletal muscle, skin and subcutaneous tissue, spinal cord (3 levels examined), sternum, stomach, tongue, trachea, urinary bladder, ureters, vagina and any gross lesions. Tissues and organ samples were preserved in 4% neutral buffered formaldehyde, except that the eyes, optic nerves, testes and epididymides were first fixed in Davidson's fluid for 24 h. Organ and tissue samples were processed, wax embedded, cut to approx. 5 μm thickness, stained with haematoxylin and erythrosine. Bones were decalcified with formic acid. Full histopathology was carried out on the preserved organs and tissues of all animals in the control and high-dose groups and all gross lesions noted in any treatment groups at time of terminal sacrifice.
Statistical analyses were performed using Graph PadPrism v4. Group means and standard deviations were calculated for body and organ weights, ratios, food consumption and clinical chemistry parameters. Data of male and female rats were evaluated separately. Analysed data were tested for normality (Kolmogorov-Smirnov test) and homogeneity of variance (Bartlett’s test or F-test). Data were analysed using ANOVA followed by Dunnett’s Multiple Comparison Test. The Kruskal-Wallis test and Dunn’s Multiple Comparison test were used because of the small sample size. Recovery period data were separately analysed using t-test and the Mann-Whitney test.
3. Results
3.1. Bacterial reverse mutation assay
No relevant increases were observed in revertant colony numbers of any of the tester strains following treatment with Phytase TSP concentrate at any concentration level, either in the presence of absence of metabolic activation in the performed experiments.
The solubility test showed that at the highest test article concentration, i.e. 5000 μg/plate, the solution in water was a slightly opalescent stable suspension (not precipitating), whereas a clear solution was achieved when dissolved in phosphate buffer and top agar. The preliminary range finding experiment showed a slight inhibitory effect of unfiltered test solutions (with and without S9-mix) as indicated by decreased revertant colony counts for some concentrations between 500 and 5000 μg/plate. Several plates in this concentration range had microbial infection, which disturbed revertant colony counting and the background lawn. For filtered test solutions (filter paper and 0.22 μm membrane filter) no inhibitory effects were observed, background lawn was not affected, all samples remained within the biological variability range of the applied test system and no contamination and/or precipitation of the test article were observed. Consequently, only filtered test article was used in the mutagenicity tests. In the mutation tests the negative and positive control mutant frequencies were within acceptable ranges according to the historical controls of the laboratory and the positive control reference mutagens induced at least a 3-fold increase in the number of revertants over the negative control thereby showing the expected reversion properties of all strains and good metabolic activation of the S9-mix. Phytase TSP concentrate was considered to be non-mutagenic under the experimental conditions.
3.2. In vivo mammalian erythrocyte micronucleus assay
Phytase TSP concentrate did not induce a biologically or statistically significant increase in frequency of polychromatic erythrocytes with micronuclei (MPCE) in mice treated with 500, 1000 or 2000 mg/kg body weight. No mortalities occurred and no adverse reactions to treatment were observed in mice of any treatment group.
The number of MPCEs per 4000 PCEs for the negative control (mean 6.4, range 5–8) was within the range of historical controls of the laboratory (3–8). The positive control resulted in an increase (p < 0.01) in MPCEs and values (mean 135, range 128–144) were within the range of historical controls of the laboratory (110–149). Mean MPCEs of slides from the 500, 1000 and 2000 mg/kg body weight treatments were 5.8, 6.2 and 6.0, respectively, and it could be concluded that Phytase TSP concentrate did not induce a statistically significant increase in the frequency of MPCEs compared to the negative control. The proportion of immature cells among total erythrocytes for the 500, 1000 and 2000 mg/kg body weight treatment groups was 0.52, 0.51 and 0.49. All were below the negative control, 0.53, and the slight reduction in proportion of immature erythrocytes among total erythrocytes with increasing test-article dose demonstrated exposure to the bone marrow and gives some indication of toxicity. The test article did not induce micronucleus formation in the immature erythrocytes of mice in the study, therefore, Phytase TSP concentrate was considered to be non-genotoxic under the conditions of the study.
3.3. Subchronic oral toxicity study
Analysis of Phytase TSP concentrate formulations in sterile water confirmed the stability of formulated solutions when stored at 5 ± 2 °C for one week or at 25 ± 5 °C for 3 h.
No mortalities occurred during the study and no detailed clinical observations in any of the study animals could be attributed to exposure to Phytase TSP concentrate.
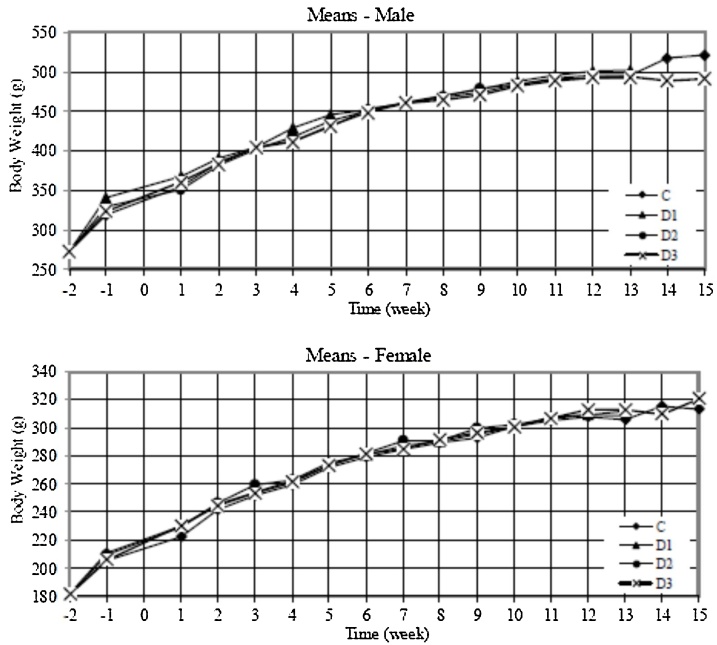
During the main study the mean body weight of male rats administered Phytase TSP concentrate was not significantly different from the negative control (Fig. 1A). During the acclimatisation period (week -1) the mean body weight of the low-dose group was significantly higher (p < 0.05) than the control and during recovery (week 14) the mean body weight of the high-dose group was significant lower (p < 0.05) than the control. These differences were transient and not seen during the main study, and therefore considered incidental and unrelated to test article treatment. The mean body weight of female rats given Phytase TSP concentrate was comparable to those in the control group during acclimatisation, the main study and recovery periods (Fig. 1B).
Fig. 1.
Mean body weights of male (A) and female (B) rats during acclimatisation (weeks -2 to 0), the 90-d main study period (weeks 0 to 13) and recovery (weeks 13 to 15). Each point during acclimatisation and the main study represents per sex a mean of 15 animals in the control (C) and high-dose groups (D3) and 10 animals in the low- and mid-dose groups (D1 and D2). Five animals per sex were retained in the control and high-dose groups during the recovery period.
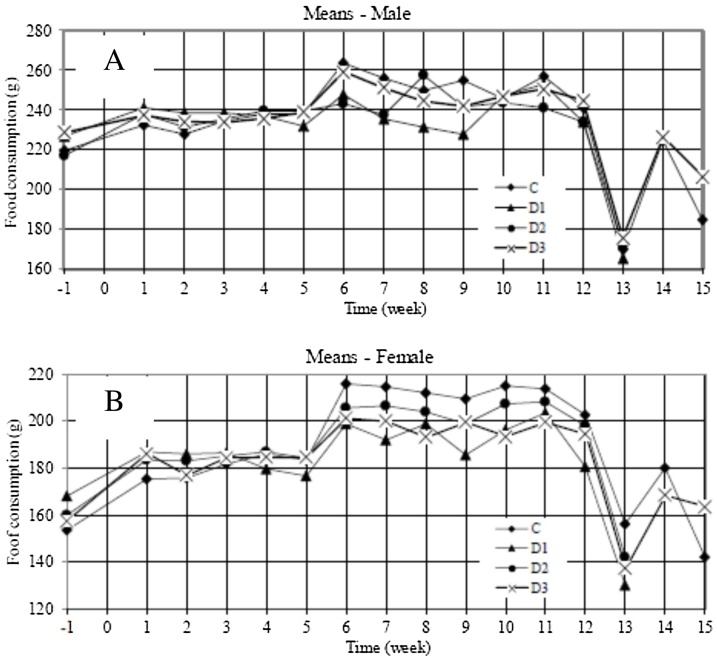
Mean weekly food consumption varied widely both within and between treatment groups over the study period, especially between weeks 6 and 12 (Fig. 2). No dose-response effect was evident and food intake fluctuations were not accompanied by differences in mean body weight. The significant differences in food intake were therefore concluded to be due to normal variability when measuring feed intake per cage and were determined to be not relevant to the safe use of Phytase TSP concentrate.
Fig. 2.
Mean weekly food consumption (g) by male (A) and female (B) rats during acclimatisation (weeks -1 to 0), the 90-d main study period (weeks 0 to 13) and recovery (weeks 13 to 15). Each point during acclimatisation and the main study represents per sex a mean of 15 animals in the control (C) and high-dose groups (D3) and 10 animals in the low- and mid-dose groups (D1 and D2). Five animals per sex were retained in the control and high-dose groups during the recovery period.
Ophthalmoscopic examination identified normal scleral, conjunctivae, lacrimation, chemosis, cornea, lens, pupil reaction to light, pupil diameter and background of the eye in all rats from all treatment groups. No opacity or ulceration was reported.
There were no test article-related effects observed in haematology and coagulation parameters in male or female rats at the end of the 90-day treatment period (Table 1). Mid-dose group males had significantly (p < 0.05) higher mean monocyte counts (MON), low-dose group males had higher neutrophil counts (NEU), and high-dose group males had lower mean corpuscular haemoglobin concentrations (MCHC). Females in the low- and high-dose groups had significantly (p < 0.05) lower mean prothrombin times (PT) and low-dose females had lower mean corpuscular haemoglobin (MCH) levels compared to the control group. The observed significant differences in MON and NEU counts, MCH and PT were sporadic, not dose-related, not accompanied by any other related clinical or histopathological changes and were therefore judged not to be related to Phytase TSP concentrate administration. The lower MCHC in high-dose males compared to the control was not accompanied by decreases in red blood cells (RBC), haemoglobin (HGB), mean corpuscular volume (MCV) or MCH, nor was it associated with histopathological or clinical signs. MCHC values in high-dose males were within the normal range defined by the animal supplier for the sex and species and there was no difference from the control following the 14-day recovery period (350.2 ± 1.8 versus 351.0 ± 3.8 for the control, data not shown). Therefore, it was concluded that the lower MCHC in high-dose males was not toxicologically relevant and not test-article related.
Table 1.
Haematology and coagulation parameters in rats following 90 days of treatment with Phytase TSP concentrate.
| Parameter (units) | Sex | Control 0 mg/kg bw/d |
Low-dose 100 mg/kg bw/d |
Mid-dose 500 mg/kg bw/d |
High-dose 1000 mg/kg bw/d |
|---|---|---|---|---|---|
| Haematology | |||||
| WBC (109/l) | M | 10.87 ± 1.99 | 10.87 ± 2.40 | 11.72 ± 4.21 | 10.96 ± 2.33 |
| F | 5.67 ± 1.10 | 6.16 ± 1.85 | 5.78 ± 1.68 | 6.15 ± 1.71 | |
| LYM (109/l) | M | 6.515 ± 1.576 | 5.670 ± 1.796 | 6.055 ± 1.719 | 6.374 ± 1.708 |
| F | 3.613 ± 0.766 | 3.837 ± 1.220 | 3.604 ± 1.149 | 4.035 ± 1.393 | |
| MON (109/l) | M | 0.399 ± 0.093 | 0.582 ± 0.235 | 0.609 ± 0.344* | 0.439 ± 0.099 |
| F | 0.157 ± 0.051 | 0.186 ± 0.076 | 0.184 ± 0.059 | 0.152 ± 0.044 | |
| NEU (109/l) | M | 3.495 ± 0.498 | 4.162 ± 0.570* | 3.743 ± 0.766 | 3.681 ± 0.667 |
| F | 1.694 ± 0.373 | 1.885 ± 0.572 | 1.725 ± 0.524 | 1.713 ± 0.360 | |
| EOS (109/l) | M | 0.275 ± 0.068 | 0.314 ± 0.076 | 0.280 ± 0.109 | 0.293 ± 0.121 |
| F | 0.138 ± 0.052 | 0.180 ± 0.068 | 0.187 ± 0.089 | 0.179 ± 0.048 | |
| BAS (109/l) | M | 0.175 ± 0.051 | 0.184 ± 0.086 | 0.230 ± 0.171 | 0.167 ± 0.068 |
| F | 0.055 ± 0.022 | 0.065 ± 0.032 | 0.062 ± 0.038 | 0.067 ± 0.033 | |
| RBC (1012/l) | M | 9.149 ± 0.306 | 9.267 ± 0.382 | 8.784 ± 0.878 | 8.899 ± 0.512 |
| F | 8.130 ± 0.272 | 8.409 ± 0.437 | 8.275 ± 0.424 | 8.157 ± 0.265 | |
| HGB (g/l) | M | 169.7 ± 4.5 | 171.3 ± 6.3 | 162.2 ± 20.3 | 165.8 ± 9.1 |
| F | 158.5 ± 5.8 | 159.8 ± 11.1 | 160.1 ± 7.5 | 158.6 ± 5.2 | |
| HCT (l/l) | M | 0.4811 ± 0.0146 | 0.4857 ± 0.0142 | 0.4612 ± 0.0565 | 0.4733 ± 0.0275 |
| F | 0.4418 ± 0.0169 | 0.4491 ± 0.0303 | 0.4475 ± 0.0222 | 0.4447 ± 0.0158 | |
| MCV (fl) | M | 52.6 ± 1.1 | 52.4 ± 2.0 | 52.2 ± 2.0 | 53.1 ± 1.3 |
| F | 54.3 ± 1.2 | 53.4 ± 1.3 | 54.2 ± 1.3 | 54.5 ± 1.4 | |
| MCH (pg) | M | 18.56 ± 0.33 | 18.50 ± 0.81 | 18.44 ± 0.78 | 18.65 ± 0.56 |
| F | 19.51 ± 0.48 | 18.96 ± 0.54* | 19.38 ± 0.47 | 19.46 ± 0.53 | |
| MCHC (g/l) | M | 353.0 ± 2.5 | 352.5 ± 3.0 | 352.0 ± 3.1 | 350.4 ± 2.9* |
| F | 358.9 ± 4.2 | 355.8 ± 2.3 | 358.2 ± 2.2 | 356.7 ± 4.0 | |
| PLT (109/l) | M | 806.9 ± 85.9 | 820.1 ± 82.2 | 862.8 ± 135.4 | 751.9 ± 70.3 |
| F | 800.1 ± 93.6 | 821.8 ± 78.3 | 789.8 ± 122.0 | 765.1 ± 108.9 | |
| Coagulation | |||||
| PT (sec) | M | 14.76 ± 1.32 | 15.51 ± 1.51 | 15.73 ± 1.59 | 14.15 ± 1.64 |
| F | 15.09 ± 0.67 | 13.26 ± 0.64* | 14.80 ± 1.20 | 14.19 ± 1.05* | |
| APTT (sec) | M | 12.56 ± 3.19 | 13.35 ± 2.30 | 14.24 ± 5.60 | 13.34 ± 2.30 |
| F | 12.21 ± 1.96 | 11.51 ± 1.82 | 11.60 ± 1.44 | 11.75 ± 2.62 | |
WBC, white blood cell count; LYM, lymphocyte count; MON, monocyte count; NEU, neutrophil count; EOS, eosinophil count; BAS, basophil count; RBC, red blood cell count; HGB, haemoglobin; HCT, Haematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; MCMC, mean corpuscular haemoglobin concentration; PLT, platelet count; PT, prothrombin time; APTT, activated partial thromboplastin time. Values are mean ± SD (n = 15 per group in the control and high-dose groups, n = 10 in the low- and mid-dose groups.
Indicates statistically significant difference from control diet p<0.05.
Compared with the control group, mean sodium levels in female rats decreased numerically with increasing test article concentration, reaching significance (p < 0.05) only in the high-dose group (Table 2). The differences in sodium levels did not persist during the recovery period, were not observed in male rats and were within the animal supplier’s range of normal values for the sex and species, therefore the effect was seen as reversible and not adverse. Measured bilirubin levels in all animals were close to or below 2 μmol/l, the method limit of detection, leading to high variability as evidenced by high standard deviation values. Significantly (p < 0.05) lower bilirubin levels were reported in mid-dose males and high-dose females, but the levels were not test article dose-related, did not persist in high-dose females following the recovery period (3.2 ± 0.4 versus 3.0 ± 0.7 μmol/l for the control, data not shown), were not supported by a corresponding decrease in bile acids, and therefore were considered not toxicologically relevant and not test article-related. Significantly (p < 0.05) higher mean lactate dehydrogenase (LDH) levels in mid-dose female compared to the control were not dose-related and not supported by any adverse signs in liver, kidneys and heart, and were therefore judged to be not toxicologically relevant and not test article-related. Low- and mid-dose females had significantly (p < 0.05) reduced alkaline phosphatase (ALP) levels compared to the control. Mean ALP values for high-dose females were not significantly different from the control and all female treatment groups were within normal range of ALP values for the sex, age and species, of 0.663–3.01 μkat/l reported by the animal supplier, therefore differences were considered to be neither dose-related nor toxicologically relevant.
Table 2.
Clinical chemistry values after treatment with Phytase TSP concentrate for 90 days.
| Parameter (units) | Sex | After the 90-day main study |
|||
|---|---|---|---|---|---|
| Control 0 mg/kg bw/d |
Low-dose 100 mg/kg bw/d |
Mid-dose 500 mg/kg bw/d |
High-dose 1000 mg/kg bw/d |
||
| Glucose (mmol/l) | M | 6.908 ± 0.436 | 7.135 ± 0.499 | 7.234 ± 0.981 | 7.028 ± 0.781 |
| F | 6.794 ± 0.712 | 6.592 ± 0.353 | 6.246 ± 0.515 | 6.642 ± 0.958 | |
| Sodium (mmol/l) | M | 144.5 ± 1.8 | 144.9 ± 1.4 | 143.7 ± 2.7 | 144.1 ± 1.4 |
| F | 144.2 ± 1.6 | 143.7 ± 1.5 | 143.0 ± 2.1 | 142.5 ± 1.3* | |
| Potassium (mmol/l) | M | 4.34 ± 0.22 | 4.38 ± 0.37 | 4.13 ± 0.33 | 4.29 ± 0.28 |
| F | 3.74 ± 0.35 | 3.86 ± 0.13 | 3.86 ± 0.33 | 3.79 ± 0.28 | |
| Chloride (mmol/l) | M | 104.1 ± 1.4 | 104.3 ± 1.9 | 102.8 ± 2.1 | 103.7 ± 1.6 |
| F | 104.0 ± 1.8 | 104.4 ± 1.8 | 103.9 ± 2.1 | 103.1 ± 1.5 | |
| Calcium (mmol/l) | M | 2.548 ± 0.068 | 2.634 ± 0.074 | 2.529 ± 0.111 | 2.531 ± 0.098 |
| F | 2.619 ± 0.082 | 2.616 ± 0.053 | 2.575 ± 0.100 | 2.545 ± 0.104 | |
| Phosphorous (mmol/l) | M | 2.261 ± 0.173 | 2.268 ± 0.117 | 2.236 ± 0.202 | 2.256 ± 0.165 |
| F | 1.859 ± 0.406 | 1.979 ± 0.196 | 1.942 ± 0.202 | 1.908 ± 0.224 | |
| Urea (mmol/l) | M | 7.53 ± 0.85 | 7.36 ± 1.35 | 7.08 ± 1.07 | 6.90 ± 1.07 |
| F | 8.11 ± 1.43 | 7.13 ± 0.91 | 7.53 ± 1.02 | 8.43 ± 1.50 | |
| Creatinine (μmol/l) | M | 29.9 ± 4.5 | 27.6 ± 2.5 | 30.4 ± 5.4 | 29.3 ± 4.1 |
| F | 42.7 ± 5.9 | 38.6 ± 6.7 | 37.9 ± 4.1 | 37.7 ± 5.8 | |
| Bilirubin (μmol/l) | M | 2.2 ± 1.0 | 2.2 ± 0.9 | 0.8 ± 1.3* | 1.5 ± 1.4 |
| F | 2.6 ± 0.9 | 1.9 ± 1.1 | 2.1 ± 1.2 | 1.4 ± 1.4* | |
| Bile acids (μmol/l) | M | 40.6 ± 20.7 | 42.2 ± 23.4 | 38.6 ± 27.2 | 32.5 ± 21.0 |
| F | 71.3 ± 37.4 | 34.6 ± 17.4 | 64.8 ± 38.4 | 83.1 ± 91.5 | |
| LDH (μkat/l) | M | 6.442 ± 3.287 | 4.957 ± 3.123 | 4.490 ± 3.539 | 6.708 ± 4.028 |
| F | 5.856 ± 1.396 | 6.438 ± 2.032 | 9.167 ± 4.909* | 5.963 ± 2.111 | |
| ALT (μkat/l) | M | 0.807 ± 0.216 | 0.862 ± 0.140 | 0.874 ± 0.167 | 0.891 ± 0.155 |
| F | 0.753 ± 0.200 | 0.674 ± 0.107 | 0.844 ± 0.620 | 0.797 ± 0.217 | |
| AST (μkat/l) | M | 1.420 ± 0.277 | 1.467 ± 0.268 | 1.593 ± 0.281 | 1.597 ± 0.175 |
| F | 1.441 ± 0.293 | 1.363 ± 0.160 | 1.846 ± 1.095 | 1.488 ± 0.289 | |
| GGT (μkat/l) | M | 0.155 ± 0.015 | 0.163 ± 0.013 | 0.149 ± 0.021 | 0.151 ± 0.013 |
| F | 0.152 ± 0.013 | 0.153 ± 0.015 | 0.174 ± 0.045 | 0.163 ± 0.018 | |
| ALP (μkat/l) | M | 1.621 ± 0.278 | 1.621 ± 0.379 | 1.970 ± 0.420 | 1.986 ± 0.556 |
| F | 1.095 ± 0.386 | 0.729 ± 0.127* | 0.811 ± 0.201* | 1.008 ± 0.143 | |
| Cholesterol (mmol/l) | M | 2.259 ± 0.425 | 1.810 ± 0.184* | 1.751 ± 0.291* | 1.856 ± 0.350* |
| F | 2.203 ± 0.737 | 2.282 ± 0.369 | 2.281 ± 0.466 | 2.088 ± 0.349 | |
| Triglycerides (mmol/l) | M | 0.877 ± 0.249 | 0.721 ± 0.22 | 0.948 ± 0.286 | 0.884 ± 0.267 |
| F | 1.115 ± 0.581 | 0.862 ± 0.218 | 0.955 ± 0.420 | 1.026 ± 0.568 | |
| Total protein (g/l) | M | 66.7 ± 3.7 | 64.2 ± 3.0 | 62.5 ± 3.5* | 63.7 ± 3.3 |
| F | 72.3 ± 2.2 | 69.1 ± 3.4* | 68.6 ± 3.7* | 68.4 ± 3.2* | |
| Albumin (g/l) | M | 13.2 ± 1.0 | 13.8 ± 0.6 | 11.4 ± 2.4* | 12.3 ± 0.9 |
| F | 15.1 ± 1.2 | 15.7 ± 1.2 | 14.9 ± 1.2 | 14.7 ± 1.0 | |
| Globulin (g/l) | M | 53.5 ± 3.4 | 50.4 ± 2.5* | 51.1 ± 2.8 | 51.5 ± 3.2 |
| F | 57.2 ± 2.1 | 53.4 ± 2.5* | 53.7 ± 3.2* | 53.7 ± 3.0* | |
| Albumin/Globulin | M | 0.247 ± 0.021 | 0.274 ± 0.010 | 0.224 ± 0.048 | 0.239 ± 0.022 |
| F | 0.265 ± 0.025 | 0.294 ± 0.014* | 0.278 ± 0.024 | 0.274 ± 0.023 | |
LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase; ALP, alkaline phosphatase. Values are mean ± SD (n = 15 per group in the control and high-dose groups, n = 10 in the low- and mid-dose groups.
Indicates statistically significant difference from control diet p<0.05.
Mean cholesterol levels of all male test-article treated groups were significantly (p < 0.05) lower than the control group, but the magnitude of the decrease was not dose related. Cholesterol levels measured in the male rats during the acclimatisation period prior to test article administration for the control, low-dose, mid-dose and high-dose groups were 2.010 ± 0.384, 1.673 ± 0.231, 1.828 ± 0.351 and 1.586 ± 0.476 mmol/l, respectively. Even prior to test-article administration the cholesterol levels in the treatment groups were numerically lower than the control group and in the high-dose group levels were significantly (p < 0.05) lower. Following the recovery period cholesterol levels in the high-dose group were numerically but not significantly lower than the control (1.850 ± 0.269 versus 2.106 ± 0.407 mmol/l in the control). The lower mean cholesterol levels in male rats in the phytase treatment groups at the end of the 90-d study were concluded to be a consequence of the lower cholesterol levels in the phytase treatment groups at the start of the study and not to be toxicologically relevant or to be due to test article-treatment. In the male rats globulin levels were significantly (p < 0.05) lower than the control in the low-dose group and total protein and albumin levels were lower in the mid-dose group. Differences were non dose-dependent and not accompanied by any other related clinical or histopathological changes and were therefore judged to be not toxicologically relevant. In all female test article-treated groups, mean levels of globulin and total protein but not albumin were significantly (p < 0.05) lower than the control and the albumin to globulin ratio was significantly (p < 0.05) higher than the control in the low-dose group. Total protein levels of samples taken during acclimatisation prior to test-article administration were not different across female groups (66.1 ± 4.7, 67.1 ± 3.5, 63.2 ± 3.0 and 66.0 ± 4.2 g/l in control, low-, mid- and high-dose groups, respectively). Following the 90-d trial the mean total protein level of all female groups increased with the highest increase in the control treatment and after the recovery period total protein levels in the control group remained significantly higher than the high-dose group (73.4 ± 1.9 versus 68.4 ± 3.4 g/l). Lower total protein levels in treatment groups were not accompanied by clinical, haematological or histopathological signs of liver damage in the female rats and therefore were considered to be not of toxicological relevance.
Urine samples collected from animals after the 90-d study period were clear and yellow, except slight turbidity was found in one female each in the low-dose and mid-dose group. Individual animal urinary pHs ranged from 5.0–9.0 in the control, mid- and high-dose groups and 6.0–9.0 in the low-dose group. Urinary volume ranged from 0.8 to 2.0 mL in the control, 0.8–3.2 ml in the low-dose, 0.6–2.0 ml in the mid-dose, and 0.6–2.3 ml in the high-dose group. Normal glucose values were observed in all urine samples. Specific gravity for male rats was 1.004 ± 0.003, 1.003 ± 0.003, 1.004 ± 0.002 and 1.006 ± 0.005 (mean ± SD) in the control, low-, mid- and high-dose groups, respectively. Specific gravity for female rats was 1.004 ± 0.003, 1.003 ± 0.005, 1.003 ± 0.004 and 1.007 ± 0.005 in the control, low-, mid- and high-dose groups, respectively. The presence of ketones ranged from 1.0–2.5 mmol/l in all treatment groups and was observed in 80 % of control animals, 90 % of low- and high-dose animals, and in 95 % of mid-dose animals. Nitrites were absent in all samples. Erythrocytes were detected in 3% of control samples (5–10 erythrocytes/μl), 40 % of low-dose (5–50 erythrocytes/μl), 75 % of mid-dose (5–300 erythrocytes/μl), and 43 % of high-dose (5–50 erythrocytes/μl). Bilirubin occurrence was 20 % in the control (17−35 μmol/l), 10 % in the low-dose (17 μmol/l), 0 in the mid-dose, and 27 % in the high-dose group (17−70 μmol/l).
Protein was present in urine from73 % of control (0.3–5.0 g/l), 65 % of low-dose (0.3–1.0 g/l), 85 % or mid-dose (0.3–5.0 g/l) and 77 % of high-dose animals (0.3–5.0 g/l). Leucocytes were present in 77 % of control (25–500 leucocytes/μl), 65 % of low-dose (25–75 leucocytes/μl), 55 % of mid-dose (25–500 leucocytes/μl), and 63 % of high-dose (25–500 leucocytes/μl) animals. Urobilinogen was detected in 33 % of control (35−70 μmol/l), 50 % of low-dose (35−70 μmol/l), 40 % of mid-dose (35−70 μmol/l), and 73 % of high-dose animals (35−140 μmol/l). The slightly increased occurrence of urobilinogen, protein, leucocytes and specific gravity in the high dose group is most likely related to increased protein intake (the test item was an enzyme). No corresponding changes proving clear toxicity were found in serum haematology and biochemistry examination.
At the end of the treatment period absolute organ weights and relative organ to body weight ratios did not support any toxicologically relevant effects related to the test article in male (Table 3) or female (Table 4) rats. Significant (p < 0.05) differences from the control were observed in low-dose males which had lower mean absolute testes weight, mid-dose males which had higher absolute and relative adrenals weight and in the high-dose males which had lower absolute and relative prostate weight, and low-dose females which had lower relative spleen weight. The differences in testes, adrenals and spleen weights were not dose-dependent and the weight of the prostate in the high-dose males was not significantly different from the control group after the recovery period, therefore it was concluded that all findings were spontaneous and incidental.
Table 3.
Organ weights of male rats following treatment with Phytase TSP concentrate for 90 days.
| Parameter (units) | Control 0 mg/kg bw/d |
Low-dose 100 mg/kg bw/d |
Mid-dose 500 mg/kg bw/d |
High-dose 1000 mg/kg bw/d |
|---|---|---|---|---|
| Absolute organ weight (g) | ||||
| Body weight | 487.10 ± 24.15 | 501.80 ± 30.44 | 492.00 ± 16.87 | 497.20 ± 39.35 |
| Liver | 15.029 ± 1.372 | 15.615 ± 1.392 | 15.461 ± 1.877 | 15.983 ± 1.567 |
| Kidneys | 3.721 ± 0.358 | 3.753 ± 0.314 | 3.544 ± 0.335 | 3.681 ± 0.217 |
| Spleen | 0.812 ± 0.098 | 0.866 ± 0.118 | 0.819 ± 0.132 | 0.874 ± 0.107 |
| Brain | 2.039 ± 0.074 | 2.029 ± 0.082 | 2.059 ± 0.153 | 2.084 ± 0.107 |
| Heart | 1.341 ± 0.148 | 1.406 ± 0.117 | 1.375 ± 0.151 | 1.395 ± 0.162 |
| Thymus | 0.326 ± 0.080 | 0.284 ± 0.048 | 0.320 ± 0.063 | 0.303 ± 0.047 |
| Adrenals | 0.0781 ± 0.0132 | 0.0885 ± 0.0124 | 0.1010 ± 0.0187* | 0.0881 ± 0.0239 |
| Thyroid gland | 0.0360 ± 0.0073 | 0.0429 ± 0.006 | 0.0390 ± 0.0071 | 0.0401 ± 0.0097 |
| Testes | 4.011 ± 0.315 | 4.376 ± 0.197* | 4.071 ± 0.273 | 4.217 ± 0.459 |
| Prostate | 1.1245 ± 0.2517 | 1.0828 ± 0.2559 | 1.1305 ± 0.3797 | 0.8142 ± 0.2029* |
| Epididymides | 1.648 ± 0.122 | 1.719 ± 0.089 | 1.680 ± 0.099 | 1.593 ± 0.121 |
| Relative organ to body weight ratio | ||||
| Liver | 30.930 ± 3.370 | 31.128 ± 2.260 | 31.379 ± 3.234 | 32.167 ± 2.185 |
| Kidneys | 7.660 ± 0.876 | 7.476 ± 0.346 | 7.196 ± 0.534 | 7.420 ± 0.358 |
| Spleen | 1.668 ± 0.205 | 1.722 ± 0.162 | 1.662 ± 0.245 | 1.755 ± 0.118 |
| Brain | 4.196 ± 0.269 | 4.053 ± 0.221 | 4.193 ± 0.390 | 4.215 ± 0.407 |
| Heart | 2.756 ± 0.303 | 2.809 ± 0.278 | 2.794 ± 0.300 | 2.819 ± 0.368 |
| Thymus | 0.671 ± 0.169 | 0.564 ± 0.075 | 0.651 ± 0.118 | 0.612 ± 0.095 |
| Adrenals | 0.1606 ± 0.0279 | 0.1767 ± 0.0255 | 0.2046 ± 0.0336* | 0.1771 ± 0.0441 |
| Thyroid gland | 0.0742 ± 0.0158 | 0.0856 ± 0.0124 | 0.0792 ± 0.0141 | 0.0812 ± 0.0215 |
| Testes | 8.252 ± 0.784 | 8.739 ± 0.477 | 8.282 ± 0.601 | 8.512 ± 0.996 |
| Prostate | 2.3048 ± 0.4891 | 2.1733 ± 0.5643 | 2.2915 ± 0.7360 | 1.6367 ± 0.4078* |
| Epididymides | 3.393 ± 0.339 | 3.435 ± 0.233 | 3.417 ± 0.233 | 3.215 ± 0.284 |
Table 4.
Organ weights of female rats following treatment with Phytase TSP concentrate for 90 days.
| Parameter (units) | Control 0 mg/kg bw/d |
Low-dose 100 mg/kg bw/d |
Mid-dose 500 mg/kg bw/d |
High-dose 1000 mg/kg bw/d |
|---|---|---|---|---|
| Absolute organ weight (g) | ||||
| Body weight | 305.7 ± 17.3 | 312.4 ± 20.1 | 309.0 ± 17.4 | 314.6 ± 11.7 |
| Liver | 11.213 ± 1.103 | 10.529 ± 0.821 | 10.693 ± 1.535 | 11.211 ± 1.245 |
| Kidneys | 2.176 ± 0.200 | 2.188 ± 0.272 | 2.195 ± 0.135 | 2.152 ± 0.166 |
| Spleen | 0.719 ± 0.082 | 0.624 ± 0.087 | 0.655 ± 0.122 | 0.681 ± 0.096 |
| Brain | 1.920 ± 0.109 | 1.910 ± 0.119 | 1.967 ± 0.093 | 1.942 ± 0.091 |
| Heart | 0.971 ± 0.063 | 0.986 ± 0.095 | 0.981 ± 0.071 | 0.97 ± 0.088 |
| Thymus | 0.340 ± 0.048 | 0.295 ± 0.045 | 0.299 ± 0.072 | 0.315 ± 0.054 |
| Adrenals | 0.1132 ± 0.0209 | 0.1274 ± 0.0187 | 0.1212 ± 0.0122 | 0.1095 ± 0.0129 |
| Thyroid gland | 0.0306 ± 0.0053 | 0.0277 ± 0.0071 | 0.0292 ± 0.0067 | 0.0310 ± 0.0039 |
| Uterus | 0.921 ± 0.247 | 0.835 ± 0.219 | 0.811 ± 0.292 | 0.789 ± 0.136 |
| Ovaries | 0.1836 ± 0.0300 | 0.1985 ± 0.0500 | 0.2016 ± 0.0322 | 0.217 ± 0.0372 |
| Relative organ to body weight ratio | ||||
| Liver | 36.676 ± 3.081 | 33.711 ± 1.605 | 34.647 ± 4.850 | 35.731 ± 4.676 |
| Kidneys | 7.107 ± 0.339 | 6.990 ± 0.541 | 7.113 ± 0.445 | 6.840 ± 0.431 |
| Spleen | 2.348 ± 0.177 | 1.993 ± 0.214* | 2.121 ± 0.391 | 2.164 ± 0.295 |
| Brain | 6.294 ± 0.424 | 6.141 ± 0.606 | 6.375 ± 0.315 | 6.180 ± 0.354 |
| Heart | 3.183 ± 0.218 | 3.163 ± 0.331 | 3.176 ± 0.176 | 3.080 ± 0.236 |
| Thymus | 1.114 ± 0.154 | 0.943 ± 0.119 | 0.962 ± 0.206 | 0.999 ± 0.163 |
| Adrenals | 0.3704 ± 0.0671 | 0.4069 ± 0.0449 | 0.3935 ± 0.0463 | 0.3476 ± 0.0352 |
| Thyroid gland | 0.1004 ± 0.0176 | 0.0885 ± 0.0216 | 0.0951 ± 0.0243 | 0.0985 ± 0.0131 |
| Uterus | 3.015 ± 0.806 | 2.714 ± 0.928 | 2.625 ± 0.939 | 2.512 ± 0.439 |
| Ovaries | 0.6003 ± 0.0908 | 0.6317 ± 0.1364 | 0.6547 ± 0.1134 | 0.6896 ± 0.1134 |
Gross pathology performed for ten animals per treatment group at the end of the main study had the following findings: kidney hydronephrosis in one male in the low-dose group (unilateral moderate), blood aspiration in the lungs of one male in the mid-dose group and one female in the high-dose group (both slight), reduced prostate gland in one male in the mid-dose group (minimal), and dilatation in the uterus of one female in the control (minimal) and mid-dose group (slight) and two females in the low- (1 minimal, 1 slight) and high-dose groups (both minimal). The findings occurred randomly in the treatment groups and were not considered to be test-article-related. Microscopic observations in tissues were considered to be of spontaneous origin and not related to the test article (Table 5).
Table 5.
Microscopic findings in rats following treatment with Phytase TSP concentrate for 90 days.
| Parameter | Control 0 mg/kg bw/d |
Low-dose 100 mg/kg bw/d |
Mid-dose 500 mg/kg bw/d |
High-dose 1000 mg/kg bw/d |
||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| KIDNEYS | ||||||||
| - cortical cyst | 1 | – | / | / | / | / | – | – |
| - cortical scar | – | – | / | / | / | / | – | 1 |
| mineralisation | – | 1 | / | / | / | / | – | – |
| - hyaline casts | 4 | 4 | / | / | / | / | 4 | 1 |
| - hydronephrosis | 2 | – | / | / | / | / | – | – |
| - venostasis | 1 | – | / | / | / | / | – | – |
| LARYNX | ||||||||
| - edema | 9 | 9 | / | / | / | / | 8 | 6 |
| - chronic inflammation | 8 | 3 | / | / | / | / | 5 | 3 |
| LIVER | ||||||||
| - focal chronic inflammation | 1 | – | / | / | / | / | – | – |
| LUNGS | ||||||||
| - blood aspiration | – | – | / | / | / | / | – | 1 |
| - focal lipidosis | 4 | 3 | / | / | / | / | 1 | – |
| LYMPH NODES (CERVICAL) | ||||||||
| - hemorrhage | – | – | / | / | / | / | 1 | – |
| - hyperplasia | – | 1 | / | / | / | / | 1 | – |
| PITUITARY GLAND | ||||||||
| - cyst | 1 | 1 | / | / | / | / | – | – |
| PROSTATE GLAND | ||||||||
| - focal chronic inflammation | – | NA | / | / | / | / | 1 | NA |
| - purulent inflammation | 2 | NA | / | / | / | / | – | NA |
| SPINAL CORD | ||||||||
| - hemorrhage | 1 | 4 | / | / | / | / | 4 | 2 |
| SPLEEN | ||||||||
| - extramedullary hemopoiesis | – | – | / | / | / | / | – | 1 |
| - siderosis | – | 3 | / | / | / | / | – | 4 |
| TESTES | ||||||||
| - tubular atrophy | – | NA | / | / | / | / | – | NA |
| THYMUS | ||||||||
| - cyst | – | – | / | / | / | / | – | 1 |
| - focal hemorrhage | – | 1 | / | / | / | / | – | – |
| UTERUS | ||||||||
| - mucosal cyst | NA | – | NA | / | NA | / | NA | 2 |
| - hydrometra | NA | 4 | NA | / | NA | / | NA | 1 |
Preserved organs and tissues from 10 control and 10 high-dose animals and all gross lesions noted in any test groups at time of sacrifice were examined. - = organ examined, no pathology findings; / = organ not examined; NA = Not Applicable.
4. Discussion
Phytases have been reported to be the most commonly used exogenous enzymes in monogastric animal feed [9] and the substantial use of phytases in poultry and swine feed is evidenced by the considerable number of phytases permitted for use as digestibility enhancers in the US and the EU. The authorised feed phytases are produced using fungal or bacterial genes originating for example from Aspergillus niger, Escherichia coli, Buttiauxella spp. and are expressed in Aspergillus niger, Aspergillus oryzae, Schizosaccharomyces pombe, Trichoderma reesei, Komagataella phaffii, and other production microogranisms [[9], [10], [11], [12]].
Some of the phytases authorised for use in feed over the last few decades are produced from genetically modified microorganisms. Recombinant DNA techniques have been used to insert phytase genes into safe non-pathogenic and nontoxigenic microorganisms for the production of large quantities of phytases with desirable characteristics such as higher bioefficacy, increased substrate affinity and improved thermal stability. Phytases are naturally ubiquitous in animal feeds, are not associated with toxicity, and once consumed are easily broken down into amino acids that are indistinguishable from those from other food sources; therefore, the primary determinant of whether a novel phytase is safe for use in feed is the production microorganism [13].
The phytase enzyme that is the subject of this paper, Phytase TSP concentrate, to be marketed under the trade name OptiPhos® PLUS, is a novel thermostable 6-phytase developed for use as an ingredient in animal feed. Studies in weaned piglets and broiler chickens have demonstrated the high in vivo bioefficacy of the phytase [14,15].
Phytase TSP concentrate is produced by a microbial source derived from a safe strain line with a history of safe use, i.e. the yeast K. phaffii, and using methods and under culture conditions that ensure controlled fermentation and prevent introduction of contamination. The production strain fulfils the criteria of Pariza and Foster [16] of a nontoxigenic and non-pathogenic microorganism and is classified as a biosafety level 1 organism and the produced phytase meets the ingredient description of the Association of American Feed Control Officers (AAFCO) (appA2 from low risk Group 1 E. coli expressed in the yeast K. phaffii a.k.a. Pichia pastoris).
Phytase TSP concentrate did not cause gene mutations in the genome of the tester strains in the bacterial reverse mutation assay when tested at levels up to 5000 μg/plate both with- and without metabolic activation. The enzyme also did not induce structural or numerical chromosomal damage in immature mouse erythrocytes in the in vivo mammalian erythrocyte micronucleus test. Under the conditions of these studies Phytase TSP concentrate was considered to be non-mutagenic with respect to gene mutations, clastogenicity and aneugenicity. These findings are in line with previous reports that phytases do not have genotoxic potential [11,17,18].
In the subchronic oral toxicity study, administration of Phytase TSP concentrate via gavage at doses up to 1000 mg/kg bw/d was well tolerated by the male and female rats and had no negative effects on clinical observations, behaviour, or ophthalmological parameters. All animals survived until scheduled necropsy. After the 90-days of test article administration mean body weights of the animals were comparable to those in the control group and there were no test article-related differences in feed consumption. There were also no treatment-related effects on haematological, coagulation, clinical chemistry, or urinalysis parameters. Organ weights and macro- and microscopic analysis revealed no adverse effects related to inclusion of Phytase TSP concentrate in the diet.
5. Conclusion
The results of the herein described studies support the safety of the novel Phytase TSP concentrate for the intended use in animal feed. The studies demonstrate an excellent safety profile for Phytase TSP concentrate and the NOAEL was recorded as 1000 mg/kg bw/d or the highest tested dose in both male and female rats.
Funding
This research was supported by Huvepharma NV, Antwerp, Belgium
Funding source declaration
The authors declare that the research presented in the manuscript referred to in the subject was supported by Huvepharma NV, Antwerp, Belgium.
CRediT authorship contribution statement
Jana Nováková: Investigation, Supervision, Formal analysis, Validation. Adél Vértesi: Investigation, Supervision, Formal analysis, Validation. Erzsébet Béres: Investigation, Supervision, Formal analysis, Validation. Spas Petkov: Funding acquisition. Katherine E. Niederberger: Data curation, Writing - original draft. Davy Van Gaver: Formal analysis. Gábor Hirka: Project administration, Resources. Zoltán Balázs: Writing - review & editing.
Declaration of Competing Interest
All authors or their employing institutions have a financial relationship with the sponsor of the studies and manuscript, Huvepharma NV, Antwerp, Belgium. Spas Petkov and Davy Van Gaver are employed by Huvepharma. Katherine E. Niederberger and Zoltán Balázs are consultants to Huverpharma. Jana Nováková, Adél Vértesi, Erzsébet Béres, and Gábor Hitka are employees of contract research organisations that conducted the studies under the sponsorship of Huverpharma.
Editor: DR. A.M Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.12.015.
Contributor Information
Jana Nováková, Email: jnovakova@meditox.eu.
Adél Vértesi, Email: adel.vertesi@toxicoop.com.
Erzsébet Béres, Email: erzsebet.beres@toxicoop.com.
Spas Petkov, Email: spas.petkov@huvepharma.com.
Katherine E. Niederberger, Email: keniederberger@leveret.ch.
Davy Van Gaver, Email: Davy.Vangaver@huvepharma.com.
Gábor Hirka, Email: gabor.hirka@toxicoop.com.
Zoltán Balázs, Email: zbalazs@leveret.ch.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.R. Greiner, U. Konietzny, Phytases: biochemistry, enzymology and characteristics relevant to animal feed use, Enzymes in Farm Animal Nutrition, pp. 96–128.
- 2.Greiner R., Konietzny U. Phytase for food application. Food Technol. Biotechnol. 2006;44:125–140. [Google Scholar]
- 3.Vats P., Banerjee U.C. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): an overview. Enzyme Microb. Technol. 2004;35:3–14. doi: 10.1016/j.enzmictec.2004.03.010. [DOI] [Google Scholar]
- 4.Casey A., Walsh G. Identification and characterization of a phytase of potential commercial interest. J. Biotechnol. 2004;110:313–322. doi: 10.1016/j.jbiotec.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez E., Han Y., Lei X.G. Cloning, sequencing, and expression of an Escherichia coli acid phosphatase/phytase gene (appA2) isolated from pig colon. Biochem. Biophys. Res. Commun. 1999;257:117–123. doi: 10.1006/bbrc.1999.0361. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez E., Wood Z.A., Karplus P.A., Lei X.G. Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/phytase expressed in Pichia pastoris. Arch. Biochem. Biophys. 2000;382:105–112. doi: 10.1006/abbi.2000.2021. [DOI] [PubMed] [Google Scholar]
- 7.Dassa J., Marck C., Boquet P.L. The complete nucleotide sequence of the Escherichia coli gene appA reveals significant homology between pH 2.5 acid phosphatase and glucose-1-phosphatase. J. Bacteriol. 1990;172:5497–5500. doi: 10.1128/jb.172.9.5497-5500.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jigami Y. Yeast glycobiology and its application. Biosci. Biotechnol. Biochem. 2008;72:637–648. doi: 10.1271/bbb.70725. [DOI] [PubMed] [Google Scholar]
- 9.Dersjant-Li Y., Awati A., Schulze H., Partridge G. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015;95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladics G.S., Han K.-H., Jang M.S., Park H., Marshall V., Dersjant-Li Y., Sewalt V.J. Safety evaluation of a novel variant of consensus bacterial phytase. Toxicol. Rep. 2020;7:844–851. doi: 10.1016/j.toxrep.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciofalo V., Barton N., Kretz K., Baird J., Cook M., Shanahan D. Safety evaluation of a phytase, expressed in Schizosaccharomyces pombe, intended for use in animal feed. Regul. Toxicol. Pharmacol. 2003;37:286–292. doi: 10.1016/s0273-2300(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 12.Zyla K. Phytase applications in poultry feeding: selected issues. J. Anim. Feed Sci. 2001;10:247–258. [Google Scholar]
- 13.Sewalt V., Shanahan D., Gregg L., La Marta J., Carrillo R. The generally recognized as Safe (GRAS) process for industrial microbial enzymes. Ind. Biotechnol. 2016;12:295–302. [Google Scholar]
- 14.Kozlowski K., Nollet L., Lanckriet A., Vanderbeke E., Mielnik P., Outchkourov N., Petkov S. Effect of different phytases derived from E. coli AppA gene on the performance, bone mineralisation and nutrient digestibility of broiler chicken. J. Appl. Anim. Nutr. 2019;7:1–9. [Google Scholar]
- 15.Wiśniewska Z., Nollet L., Lanckriet A., Vanderbeke E., Petkov S., Outchkourov N., Kasprowicz-Potocka M., Zaworska-Zakrzewska A., Kaczmarek S.A. Effect of phytase derived from the E. coli AppA gene on weaned piglet performance, apparent total tract digestibility and bone mineralization. Animals. 2020;10:121. doi: 10.3390/ani10010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pariza M.W., Foster E.M. Determining the safety of enzymes used in food processing. J. Food Prot. 1983;46:453–468. doi: 10.4315/0362-028X-46.5.453. [DOI] [PubMed] [Google Scholar]
- 17.Aureli R., Faruk M.U., Cechova I., Pedersen P.B., Elvig-Joergensen S.G., Fru F., Broz J. The efficacy of a novel microbial 6-phytase expressed in Aspergillus oryzae on the performance and phosphorus utilization in broiler chickens. Int. J. Poult. Sci. 2011;10:160–168. [Google Scholar]
- 18.Lichtenberg J., Pedersen P.B., Elvig-Joergensen S.G., Skov L.K., Olsen C.L., Glitsoe L.V. Toxicological studies on a novel phytase expressed from synthetic genes in Aspergillus oryzae. Regul. Toxicol. Pharmacol. 2011;60:401–410. doi: 10.1016/j.yrtph.2011.05.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.