A randomised, open-label, blinded endpoint post-authorisation safety study (Study A3921133; NCT02092467; database not locked and subject to change) evaluated the safety of tofacitinib 5 mg and 10 mg twice daily (BID) versus tumour necrosis factor inhibitors (TNFi) (adalimumab/etanercept) in rheumatoid arthritis (RA) patients aged ≥50 years with ≥1 cardiovascular risk factor. An ad hoc interim safety analysis of Study A3921133 reported incidence rates (IRs) per 100 patient-years (95% CIs) for fatal infections (within 28 days of treatment) and non-fatal serious infection events (SIEs), respectively: tofacitinib 5 mg BID, 0.18 (0.07 to 0.39) and 3.35 (2.78 to 4.01); tofacitinib 10 mg BID, 0.22 (0.09 to 0.46) and 3.51 (2.93 to 4.16); TNFi, 0.06 (0.01 to 0.22) and 2.79 (2.28 to 3.39).1 SIEs risk (fatal/non-fatal) was further increased with tofacitinib in patients aged >65 years versus younger patients; therefore, the European Medicines Agency recommended that older patients should receive tofacitinib when there is no suitable alternative treatment.2
Further to these recommendations, we sought to assess age-based (<65 vs ≥65 years) SIE risk in RA patients receiving tofacitinib in Phase 2, 3 and 3b/4 tofacitinib studies with a TNFi control/comparator arm,3–5 and in the US Corrona RA registry.
The clinical data set included 2180 patients (tofacitinib 5 mg BID, n=1064 (943.4 patient-years); tofacitinib 10 mg BID, n=306 (236.6 patient-years); adalimumab, n=643 (554.3 patient-years); placebo, n=167 (108.1 patient-years)). Overall, 1841 (84.4%) patients were aged <65 years and 339 (15.6%) ≥65 years. Crude IRs (patients with events/100 patient-years) and HRs were calculated for all first infections and first SIEs, overall and by age.
For all infections (online supplemental figure S1), IRs and infection risk (by HRs) were higher with active treatments versus placebo, and similar across active treatments and age groups. For SIEs (figure 1), IRs were higher in older versus younger patients for active treatments, and similar among younger patients for all treatments. For older patients, versus adalimumab, SIE IRs were similar for tofacitinib 5 mg BID and numerically higher for tofacitinib 10 mg BID, though few events occurred in this group (n=4), and the HR 95% CI was wide and included 1. Importantly, HRs revealed similar SIE risk between older and younger patients for tofacitinib 5 mg BID and adalimumab (consistent with previous studies, which report higher absolute SIE risk in older patients but similar relative risk with TNFi in older vs younger patients),6 7 while the risk was significantly greater in older versus younger patients with tofacitinib 10 mg BID.
annrheumdis-2020-218992supp001.pdf (216.9KB, pdf)
Figure 1.
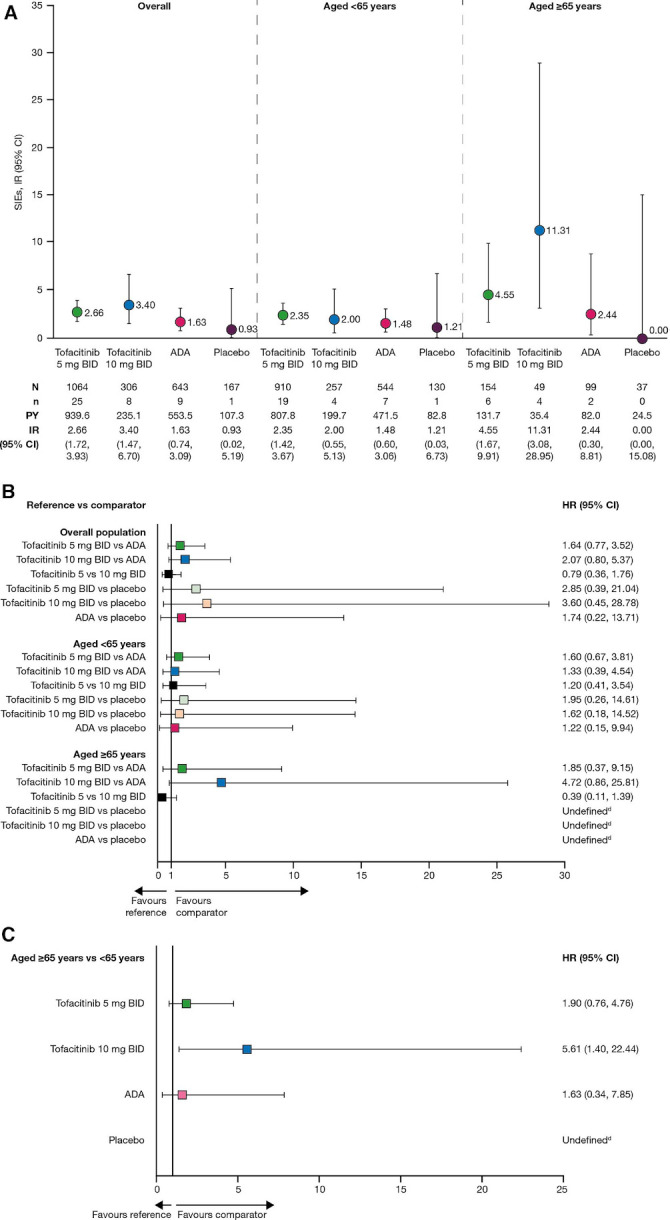
(A) IRs (95% CI) and (B) HRsa (95% CI) between treatment groups, overall and stratified by age (<65 years or ≥65 years) and (C) HRsb (95% CI) between age groups for each treatment group, for SIEs in pooled Phase 2, 3 and 3b/4 studies (months 0 to 12).c Pooled data from Phase 2 (A3921035; NCT00550446), Phase 3 (ORAL Standard; NCT00853385) and Phase 3b/4 (ORAL Strategy; NCT02187055) studies. IR=unique patients with events/100 PY. aCox proportional hazards model includes treatment as the only factor. bCox proportional hazards model includes treatment, age group (<65 years or ≥ 65 years) and treatment by age group interaction terms. cFor Study A3921035, only data within the first 3-month randomised parallel treatment period were included (before patients who were receiving ADA were switched to tofacitinib 5 mg BID after month 3). dCould not be defined as there were 0 events in the placebo group. ADA, adalimumab; BID, twice daily; IR, incidence rate; N, number of treated patients; n, number of patients with event; PY, patient-years; SIE, serious infection event.
In the registry data set (total, n=10 357; tofacitinib, n=1999; biologic disease-modifying antirheumatic drug (bDMARD), n=8358), age-/gender-standardised SIE IRs were higher in older versus younger patients, and similar between tofacitinib and bDMARD initiators for both age groups (online supplemental figure S2).
Our results are consistent with a real-world analysis of >130 000 RA patients, which reported similar adjusted SIE HRs for tofacitinib versus six of seven bDMARDs, including adalimumab.8 Limitations of the present analyses should be considered. The clinical data set included variations in sample size and patient-years of exposure between treatment and age groups, and low numbers of older patients and events in some treatment groups which led to wide 95% CIs or undefined relative risk estimates. Additionally, registry data were not matched for baseline variables beyond age/gender.
In conclusion, as would be expected, SIE incidence was higher in older versus younger patients. SIE risk was similar between age groups with tofacitinib 5 mg BID and adalimumab but higher in older versus younger patients with tofacitinib 10 mg BID, suggesting an effect modification by age for this dose. Real-world data showed similar SIE risk for patients initiating tofacitinib or bDMARDs despite limited baseline matching. These data support the globally recommended dose of 5 mg BID for RA.
Acknowledgments
Medical writing support, under the guidance of the authors, was provided by Christina Viegelmann, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, New York, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461-4).
Footnotes
Handling editor: Josef S Smolen
Contributors: KLW, DG, DH, CAC, ABS and HS contributed to the conception or design of the study. KLW, DG, DH, CAC, ABS, HS, AMO and DAP contributed to the analysis of the data. All authors were involved in the interpretation of the data and reviewed and approved the letter’s content before submission.
Funding: The clinical trial data analyses were funded by Pfizer Inc. The registry is sponsored by Corrona LLC, and the analyses based on secondary analysis of registry data were funded and sponsored by Pfizer Inc.
Disclaimer: Access to Corrona registry data was limited to Corrona, and Corrona statisticians completed all of the analysis.
Competing interests: KLW has received grant/research support from Bristol-Myers Squibb, and consulting fees from AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Galapagos, Gilead, GSK, Pfizer Inc, Roche and UCB. GC has received grant/research support and consulting fees from AbbVie, Amgen, Eli Lilly and Company, Gema Pharma, Genzyme, Novartis and Pfizer Inc. DG, DH, CAC, ABS and HS are employees and shareholders of Pfizer Inc. AMO is an employee of Corrona LLC. DAP is an employee of Corrona LLC and has equity interest. DAP also serves on the Board of Directors for the Corrona Research Foundation and has received consulting fees from AbbVie, Genentech, Novartis, Regeneron and Roche Hellas. HSK has received grant/research support from Pfizer Inc, and consulting fees from AbbVie, Amgen, Biogen, Eli Lilly and Company, Gilead, Mylan, Pfizer Inc and Sandoz-Hexal. Corrona has been supported through contracted subscriptions in the past 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Genentech, Gilead, Janssen, Merck, Novartis, Ortho Dermatologics, Pfizer Inc, Regeneron and Sun.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice and local regulations. All patients provided informed consent, and institutional review board approval was provided by all participating institutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or European Union, or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer. The Corrona data set is based on a large US multicentre study adhering to a number of institutional review boards, with complex logistics. Patients did not provide consent to raw data sharing during the data collection for this purpose, and the Corrona data sharing policies do not permit raw data sharing for this purpose. An aggregated limited data set from the current analyses is available to qualified investigators with an approved protocol. Data requests may be sent to Corrona, represented by Dr Jeffrey D Greenberg, MD, MPH, Corrona LLC, Waltham, Massachusetts, and NYU School of Medicine, New York, New York, USA. E-mail jgreenberg@corrona.org.
References
- 1. European Medicines Agency Xeljanz (tofacitinib) - summary of product characteristics, 2020. Available: https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf [Accessed 12 Jun 2020].
- 2. European Medicines Agency EMA confirms Xeljanz to be used with caution in patients at high risk of blood clots, 2020. Available: https://www.ema.europa.eu/en/documents/referral/xeljanz-article-20-procedure-ema-confirms-xeljanz-be-used-caution-patients-high-risk-blood-clots_en.pdf [Accessed 9 Mar 2020].
- 3. Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. 10.1002/art.33383 [DOI] [PubMed] [Google Scholar]
- 4. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. 10.1056/NEJMoa1112072 [DOI] [PubMed] [Google Scholar]
- 5. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. 10.1016/S0140-6736(17)31618-5 [DOI] [PubMed] [Google Scholar]
- 6. Galloway JB, Hyrich KL, Mercer LK, et al. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50:124–31. 10.1093/rheumatology/keq242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Curtis JR, Xie F, Chen L, et al. Use of a disease risk score to compare serious infections associated with anti-tumor necrosis factor therapy among high- versus lower-risk rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2012;64:1480–9. 10.1002/acr.21805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pawar A, Desai RJ, Gautam N, et al. Risk of admission to hospital for serious infection after initiating tofacitinib versus biologic DMARDs in patients with rheumatoid arthritis: a multidatabase cohort study. Lancet Rheumatol 2020;2:e84–98. 10.1016/S2665-9913(19)30137-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2020-218992supp001.pdf (216.9KB, pdf)


