Abstract
Objectives
This study evaluated the comparative effectiveness of a tumour necrosis factor inhibitor (TNFi) versus a non-TNFi (biological disease-modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs)) as the first-line treatment following conventional synthetic DMARDs, as well as potential modifiers of response, observed in US clinical practice.
Methods
Data were from a large US healthcare registry (Consortium of Rheumatology Researchers of North America Rheumatoid Arthritis Registry). The analysis included patients (aged ≥18 years) with a documented diagnosis of rheumatoid arthritis (RA), a valid baseline Clinical Disease Activity Index (CDAI) score of >2.8 and no prior bDMARD or tsDMARD use. Outcomes were captured at 1-year postinitiation of a TNFi (adalimumab, etanercept, certolizumab pegol, golimumab or infliximab) or a non-TNFi (abatacept, tocilizumab, rituximab, anakinra or tofacitinib) and included CDAI, 28-Joint Modified Disease Activity Score, patient-reported outcomes (including the Health Assessment Questionnaire Disability Index, EuroQol-5 Dimension score, sleep, anxiety, morning stiffness and fatigue) and rates of anaemia. Groups were propensity score-matched at baseline to account for potential confounding.
Results
There were no statistically significant differences observed between the TNFi and non-TNFi treatment groups for outcomes assessed, except the incidence rate ratio for anaemia, which slightly favoured the TNFi group (19.04 per 100 person-years) versus the non-TNFi group (24.01 per 100 person-years, p=0.03). No potential effect modifiers were found to be statistically significant.
Conclusions
The findings of no significant differences in outcomes between first-line TNF versus first-line non-TNF groups support RA guidelines, which recommend individualised care based on clinical judgement and consideration of patient preferences.
Keywords: DMARDs (biologic), rheumatoid arthritis, outcomes research, TNF-alpha
Key messages.
What is already known about this subject?
American College of Rheumatology guidelines for the treatment of rheumatoid arthritis (RA) recommend a treat-to-target approach that is guided by disease stage and treatment history.
However, based on comparisons of tumour necrosis factor inhibitor (TNFi) agents versus non-TNFi agents in head-to-head randomised clinical trials, the optimal sequence of different treatment modalities following conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) is not established.
What does this study add?
Real-world, comparative evidence to aid clinical decision-making for patients who failed therapy on csDMARDs revealed only limited differences in baseline characteristics and clinical outcomes between adult patients with RA initiating treatment with a TNFi versus a non-TNFi following csDMARDs.
How might this impact on clinical practice?
The findings support RA guidelines which recommend individualised care based on clinical judgement and consideration of patient preferences.
Background
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune condition characterised by joint damage, stiffness and swelling.1 As the most common inflammatory arthritis in adults, RA affects up to 1.28–1.36 million US adults (2014 estimates).2 3 In addition to symptom relief, the aim of treatment is normalisation or improvement in physical function, health-related quality of life and social and work capacity. Inhibition of structural damage is the key marker of treatment success, for which disease-modifying antirheumatic drugs (DMARDs) remain the mainstay modality.4
American College of Rheumatology guidelines for the treatment of RA recommend a treat-to-target approach that is guided by disease stage and treatment history.2 Initial modalities for the treatment pathway comprise conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), for example, methotrexate, sulfasalazine, leflunomide and hydroxychloroquine, with the option of concomitant short-term glucocorticoids for disease flares or moderate/high disease activity.2 For patients with inadequate response or intolerance to csDMARDs, a switch to or an addition of a biologic DMARD (bDMARD) or a targeted synthetic DMARD (tsDMARD; presently only comprising the janus kinase (JAK) inhibitors) is recommended;2 bDMARD options broadly comprise tumour necrosis factor inhibitor (TNFi) and non-TNFi agents (e.g. T-cell costimulatory inhibitors, anti-B-cell agents and anti-interleukin (IL)-6 receptor monoclonal antibodies.
Presently, the optimal sequence of different treatment modalities following csDMARDs is not established; based on evidence comparing non-TNFi agents versus TNFi agents in head-to-head randomised clinical trials2; current guidelines state no definitive preference of TNFi or non-TNFi as first-line bDMARD treatment. Therefore, to aid clinical decision-making for this segment of the RA treatment pathway, the generation of comparative evidence is warranted.
Objectives
This observational study compared baseline characteristics and important clinical and patient-reported outcomes (PROs) of patients with RA initiating a TNFi versus a non-TNFi as the first-line bDMARD or tsDMARD using data from a large US healthcare registry. Any association between patient characteristics and treatment outcomes (ie, effect modification) was further assessed.
Methods
Study design
Data were prospectively collected for the period between 1 October 2001 and 31 January 2018 within a large US healthcare registry (Consortium of Rheumatology Researchers of North America (Corrona) RA Registry).5 6 Adult patients (aged ≥18 years) included in the study had a documented diagnosis of RA and a valid Clinical Disease Activity Index (CDAI) score of >2.8.7 In addition, patients were to have initiated a first-line bDMARD or tsDMARD: either a TNFi (adalimumab, etanercept, certolizumab pegol, golimumab or infliximab) or non-TNFi (abatacept, tocilizumab, rituximab, anakinra or tofacitinib) during the study period. Patients who did not have a non-remission CDAI score at baseline and a 1-year post initiation follow-up visit were excluded from analysis.
Baseline patient and disease characteristics were captured for each eligible patient, with clinical outcomes and PROs collected at 1 year postinitiation of index treatment. If a visit at 1 year was not available, then a visit within ±3 months of this timepoint was used. For patients discontinuing index treatment prior to the 1-year follow-up, values at discontinuation were used, except for binary outcomes, which were imputed.
The key clinical outcome of interest was CDAI score, which was used to assess (1) achievement of low disease activity (CDAI score of ≤10) among those with moderate or high baseline disease activity at baseline, (2) achievement of remission (CDAI score of ≤2.8) among those with low, moderate or high disease activity at baseline, (3) achievement of minimally important difference in CDAI (defined as an improvement in the CDAI score of ≥2 if the baseline CDAI score was 2.8–10.0, ≥6 if the baseline CDAI score was 10.1–22.0 and ≥11 if the baseline CDAI score was >22).8 Secondary clinical outcomes included 28-Joint Modified Disease Activity Score (mDAS28),9 which was used to assess achievement of remission (mDAS28<2.6). The rate of anaemia (defined as haemoglobin levels of <13.2 g/L for men and <11.5 g/L for women) was also of interest due to its association with inflammation associated with RA10 and exacerbated by some treatments. In the Corrona RA registry, anaemia is captured as a comorbidity or adverse event reported by physicians during the study visits.
PROs captured included the Health Assessment Questionnaire Disability Index (HAQ-DI), EuroQol-5 Dimension (EQ-5D) score,11 problem with sleep (yes or no), anxiety (yes or no), morning stiffness (presence and duration) and fatigue (visual analogue scale of 0–100).
Analysis
Propensity score matching of baseline characteristics
To account for potential confounding introduced by identified imbalanced covariates, groups were first propensity score-matched (via greedy matching without replacement) prior to statistical comparison. Standardised differences were calculated to identify baseline characteristics that were not balanced to consider for inclusion in propensity score matching. Variables that had |standardised difference|>0.1 were considered imbalanced. Imbalanced covariates with over 10% missing data were then excluded. Matching ratios were considered to maximise the sample size while balancing as many covariates as possible.
Comparison of outcomes
Following propensity score matching, outcomes at 1 year postinitiation were compared between matched TNFi and non-TNFi cohorts. Random effect logistic regression models were used for binary outcomes; random effect linear regression models were used for continuous outcomes; and rate of anaemia was analysed via a random effect Poisson regression model. Models were adjusted by baseline value for clinical outcomes and PROs, concomitant csDMARD use and prednisone use. Random effect regression models were fit with physician random effects to account for correlation of responses for patients nested within physician.
Determination of effect modifiers
To determine any association between baseline characteristics and outcomes observed (ie, effect modification), the following binary covariates that were hypothesised to influence response were selected and examined via multivariable random effect models: gender, age, race, education, smoking status, body mass index (BMI), median systolic blood pressure, history of hypertension, history of diabetes, history of anaemia, work status, private insurance, prior csDMARD use, median duration of RA, median tender joint count, median swollen joint count, median physician global assessment and median patient global assessment. Interaction terms between potential effect modifiers and treatment group were used to identify the estimated effect in each covariate group; a Bonferroni correction was applied when examining tests for statistical significance to account for assessments on multiple outcome measures, and a Bonferroni-corrected alpha level of 0.00019 was considered statistically significant. This correction was imposed due to the high number of tests performed for evaluating potential effect modifiers.
Results
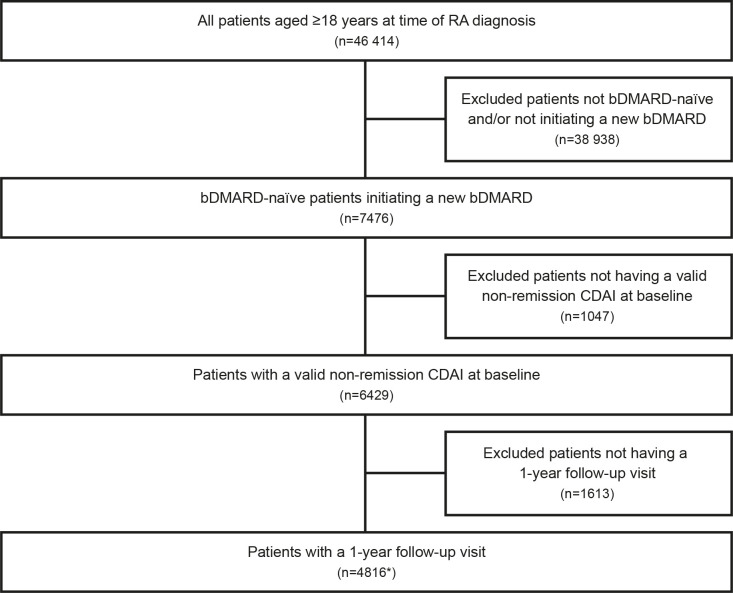
Within the Corrona RA Register, 46 414 patients aged ≥18 years were identified over the study period. Of those, 7476 patients had been initiated with an eligible medication and were bDMARD-naïve and tsDMARD-naïve. A total of 1047 patients did not have a valid non-remission CDAI score at baseline, and 1613 patients did not have a 1-year postinitiation follow-up visit. This resulted in 4816 eligible patients, comprising n=4186 that had been initiated with treatment with a TNFi and n=630 that had been initiated with treatment with a non-TNFi (figure 1).
Figure 1.
Selection of eligible patients: eligible patients were selected using the 31 January 2018 version of the RA database. *bDMARD initiations include TNFi initiations (n=4186; adalimumab: n=1464, etanercept: n=1322, certolizumab pegol: n=229, golimumab: n=139, infliximab: n=1032) and non-TNFi initiations (n=630; abatacept: n=369, tocilizumab: n=53, rituximab n=94, anakinra: n=14, tofacitinib: n=100), bDMARD, biological disease-modifying antirheumatic drug; CDAI, Clinical Disease Activity Index; DMARD, disease-modifying antirheumatic drug; RA, rheumatoid arthritis; TNFi, tumour necrosis factor inhibitor.
After consideration of standardised differences, imbalanced covariates that were selected for inclusion in the propensity score matching model included demographic variables (age, sex, insurance type, marital status, smoking status and work status) and clinical variables (BMI, baseline CDAI, duration of disease, American College of Rheumatology functional status,12 concomitant csDMARDs cardiovascular and hypertension history, and prior cancer and prednisone use).
The following imbalanced covariates had >10% missing data and were therefore excluded from the propensity score model: diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, patient global assessment, haemoglobin and number of times per week engaged in intense physical activity.
A matching ratio of 1:4 (non-TNFi:TNFi) provided an optimal balance of baseline covariates while preserving the maximal sample size for analyses of outcomes.
After propensity score matching, 2372 and 593 patients remained in the TNFi and non-TNFi groups, respectively.
Baseline characteristics
Prematching, the mean age of the TNFi group was 56.9 years versus 62.7 years for the non-TNFi group (table 1); baseline CDAI was similar between the groups (20.4). Relatively more patients in the TNFi group received concomitant csDMARDs (83.3% vs 75.6%), consistent with the clinical evidence in the guidelines which recommend TNFi in combo with csDMARDs.2 Postmatching, the groups appeared largely similar in terms of baseline characteristics (table 1); the mean ages were 61.0 and 62.3 years, with 76.8% and 79.8% female patients, and the mean duration of RA was 8.2 and 8.7 years in the TNFi and non-TNFi groups, respectively. Concomitant csDMARD use was also balanced postmatching (78.0% vs 76.7% in the TNFi and non-TNFi groups, respectively).
Table 1.
Prepropensity and postpropensity score-matched baseline characteristics
| Prematching | Postmatching | |||||
| TNFi* (n=4186) |
Non-TNFi† (n=630) |
Standardised difference | TNFi‡ (n=2372) |
Non-TNFi§ (n=593) |
Standardised difference | |
| Age (years), mean (SD) | 56.9 (12.7) | 62.7 (13.0) | –0.4476¶ | 61.0 (12.9) | 62.3 (12.8) | –0.1005¶ |
| Female, n (%) | 3202 (76.5) | 503 (79.8) | –0.0807 | 1821 (76.8) | 473 (79.8) | –0.0726 |
| White, n (%) | 3443 (82.7) | 511 (81.5) | 0.0783 | 1956 (82.8) | 483 (81.9) | 0.1025¶ |
| Duration of rheumatoid arthritis (years), mean (SD) | 7.1 (8.6) | 8.6 (9.7) | –0.1564¶ | 8.2 (9.3) | 8.7 (9.5) | –0.0602 |
| Rheumatoid factor positive, n (%) | 1862 (71.1) | 240 (69.8) | 0.0291 | 1032 (70.2) | 228 (70.4) | –0.0036 |
| Concomitant csDMARD, n (%) | 3485 (83.3) | 476 (75.6) | 0.1912¶ | 1851 (78.0) | 455 (76.7) | 0.0312 |
| Prednisone use, n (%) | 1312 (31.3) | 198 (31.4) | –0.0019 | 752 (31.7) | 185 (31.2) | 0.0109 |
| BMI, mean (SD) | 30.1 (7.2) | 29.6 (7.2) | 0.0668 | 30.0 (7.1) | 29.8 (7.3) | 0.0377 |
| CDAI score (0–76), mean (SD) | 20.4 (13.5) | 20.4 (13.2) | –0.0150 | 19.8 (13.2) | 20.1 (13.1) | –0.0256 |
| HAQ score (0–3), mean (SD) | 1.0 (0.6) | 1.1 (0.6) | –0.0744 | 1.1 (0.6) | 1.1 (0.6) | –0.0036 |
| Comorbidity history, n (%) | ||||||
| Serious infections | 631 (15.1) | 101 (16.0) | –0.0264 | 373 (15.7) | 95 (16.0) | –0.0081 |
| Cancer | 316 (7.5) | 97 (15.4) | –0.2481¶ | 289 (12.2) | 87 (14.7) | –0.0730 |
| Cardiovascular disease | 332 (7.9) | 69 (11.0) | –0.1035¶ | 238 (10.0) | 64 (10.8) | –0.0248 |
| Anaemia | 128 (3.1) | 29 (4.6) | –0.0806 | 84 (3.5) | 28 (4.7) | –0.0593 |
*Adalimumab (n=1464), etanercept (n=1322), certolizumab pegol (n=229), golimumab (n=139) and infliximab (n=1032).
†Abatacept (n=369), tocilizumab (n=53), rituximab (n=94), anakinra (n=14) and tofacitinib (n=100).
‡Adalimumab (n=759), etanercept (n=734), certolizumab pegol (n=155), golimumab (n=87) and infliximab (n=637).
§Abatacept (n=352), tocilizumab (n=49), rituximab (n=88), anakinra (n=11) and tofacitinib (n=93).
¶Standardised difference >0.1 or <–0.1.
BMI, body mass index; CDAI, Clinical Disease Activity Index; csDMARD, conventional synthetic disease-modifying antirheumatic drug; HAQ, Health Assessment Questionnaire; TNFi, tumour necrosis factor inhibitor.
Outcomes
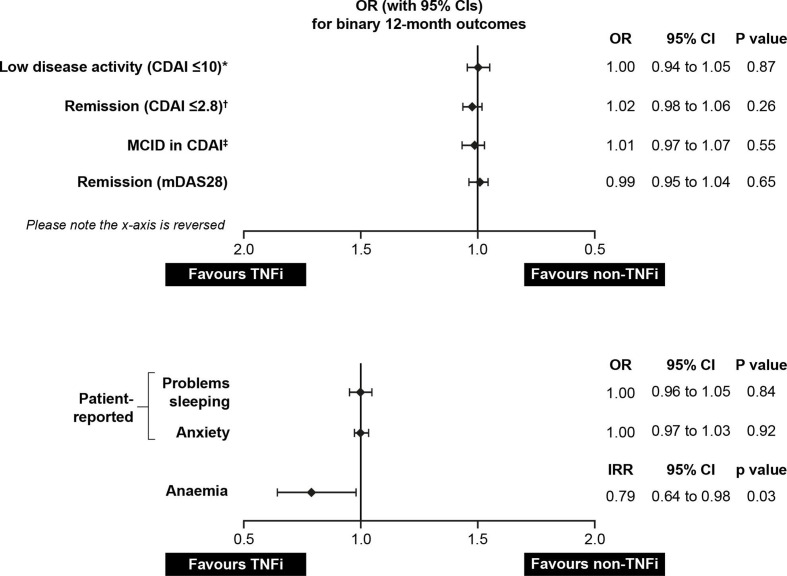
There were no statistically significant differences observed between the TNFi and non-TNFi treatment groups for binary outcomes, including achievement of low disease activity, achievement of remission (defined according to CDAI and mDAS28), achievement of minimum clinically important difference in CDAI, and problems with sleep and anxiety (figure 2). While the raw proportion of patients with anaemia was not significantly different prematching and postmatching, TNFi initiators had a lower crude incidence rate of anaemia (19.04 cases per 100 person-years) when compared with non-TNFi initiators (24.01 cases per 100 person-years, p=0.03) (figure 3 and table 2, unadjusted test not shown). This relationship persisted in adjusted analyses (adjusted incidence rate ratio=0.79, 95% CI 0.64 to 0.98).
Figure 2.
Odds ratio for binary and count outcomes for 12-month period post-TNFi/non-TNFi initiation. *Among those with moderate or high disease activity at baseline. †Among those with low, moderate or high disease activity at baseline. ‡Defined as ≥2 if baseline CDAI score of 2.8–10.0; ≥6 if baseline CDAI score of 10.1–22.0; ≥11 if baseline CDAI score of >22. CDAI, Clinical Disease Activity Index; IRR, incidence rate ratio; MCID, minimum clinically important difference; mDAS28, 28-Joint Modified Disease Activity Score; OR, odds ratio; TNFi, tumour necrosis factor inhibitor.
Figure 3.
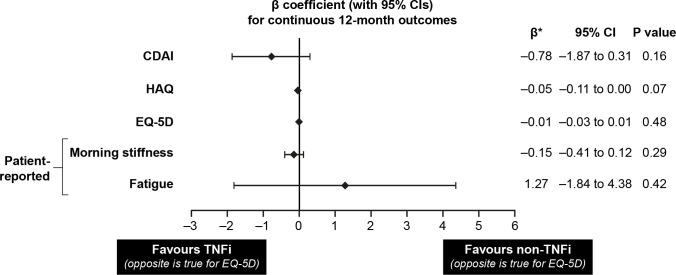
β-coefficients for continuous outcomes for 12-month period post-TNFi/non-TNFi initiation. *Change in continuous outcomes is defined as the outcome value at 1-year follow-up minus the outcome value at baseline. CDAI, Clinical Disease Activity Index; EQ-5D, EuroQol-5 Dimension; HAQ; Health Assessment Questionnaire; TNFi, tumour necrosis factor inhibitor.
Table 2.
Overall comparison of outcomes by treatment group
| TNFi initiators | Non-TNFi initiators | TNFi versus non-TNFi* | ||
| All patients | 2372 | 593 | – | – |
| Binary outcomes | Response rate n/N (%) | Response rate n/N (%) | Adjusted OR (95% CI) | P value† |
| Achievement of low disease activity (CDAI score of ≤10)‡ | 597/1498 (39.9) | 154/370 (41.6) | 1.00 (0.94 to 1.05) | 0.87 |
| Achievement of remission based on CDAI (≤2.8)§ | 363/2066 (17.6) | 82/504 (16.3) | 1.02 (0.98 to 1.06) | 0.26 |
| Achievement of MID in CDAI¶ | 940/2066 (45.5) | 227/504 (45.0) | 1.01 (0.97 to 1.07) | 0.55 |
| Achievement of remission based on mDAS28 | 449/1835 (24.5) | 118/447 (26.4) | 0.99 (0.95 to 1.04) | 0.65 |
| Patient-reported problem with sleep** | 451/1937 (23.3) | 114/496 (23.0) | 1.00 (0.96 to 1.05) | 0.84 |
| Patient-reported anxiety** | 239/1937 (12.3) | 62/496 (12.5) | 1.00 (0.97 to 1.03) | 0.92 |
| Patient-reported problem with sleep†† | 588/2363 (24.9) | 136/591 (23.0) | 1.02 (0.98 to 1.06) | 0.35 |
| Patient-reported anxiety†† | 308/2363 (13.0%) | 73/591 (12.4%) | 1.01 (0.98 to 1.04) | 0.70 |
| Count outcomes | Incidence rate (per 100 person-years) | Incidence rate (per 100 person-years) | Adjusted IRR (95% CI) | P value |
| Anaemia | 19.04 | 24.01 | 0.79 (0.64 to 0.98) | 0.03 |
| Continuous outcomes | N, mean±SD | N, mean±SD | Adjusted β coefficient (95% CI)‡‡ | P value |
| Change in CDAI | 2065, –6.8±14.0 | 504, –6.5±13.8 | –0.78 (–1.87 to 0.31) | 0.16 |
| Change in HAQ | 1732, –0.1±0.6 | 373, –0.1±0.5 | –0.05 (–0.11 to 0.00) | 0.07 |
| Change in EQ-5D | 1097, 0.0±0.2 | 357, 0.0±0.2 | –0.01 (–0.03 to 0.01) | 0.48 |
| Change in patient-reported morning stiffness (hours per day) | 1599, –0.3±2.5 | 396, –0.2±2.9 | –0.15 (–0.41 to 0.12) | 0.29 |
| Change in patient-reported fatigue | 1119, –4.7±30.1 | 363, –6.3±26.2 | 1.27 (–1.84 to 4.38) | 0.42 |
*Estimates from multivariable models.
†The reported p values are associated with the adjusted ORs.
‡Among those with moderate or high disease activity at baseline.
§Among those with low, moderate or high disease activity at baseline.
¶Defined as ≥2 if baseline CDAI score=2.8–10; ≥6 if baseline CDAI score=10.1–22; ≥11 if baseline CDAI score>22.
**Imputed as missing if patients switched to another biologic before 1-month follow-up.
††Imputed with the last observation on drug.
‡‡Change in continuous outcomes is defined as the outcome value at 1-year follow-up minus the outcome value at baseline.
CDAI, Clinical Disease Activity Index; EQ-5D, EuroQol-5 Dimension; HAQ, Health Assessment Questionnaire; IRR, incidence rate ratio; mDAS28, 28-Joint Modified Disease Activity Score; MID, minimum important difference; TNFi, tumour necrosis factor inhibitor.
For continuous variables, there were no significant differences observed between the TNFi and non-TNFi treatment groups; these outcomes included changes in CDAI score, HAQ-DI score, EQ-5D score, morning stiffness and fatigue over the 12-month postinitiation period (table 2).
Effect modification
Of the potential effect modifiers examined, none were found to be statistically significant at a Bonferroni-corrected alpha level of 0.00019.
Discussion
The present study, which was based on observations in US clinical practice, revealed only limited differences in baseline characteristics and clinical outcomes (but not PROs) between adult patients with RA initiating treatment with a TNFi versus a non-TNFi. At baseline, the relatively larger proportion of patients in the TNFi group receiving concomitant csDMARDs supports clinical evidence in guidelines which recommend TNFi in combination with csDMARDs.2 Insofar as a lower crude incidence rate of anaemia was found for TNFi initiators versus the non-TNFi initiators, these results can be viewed as being largely inconclusive for two reasons. First, the disproportionately low usage of IL-6 and JAK inhibitor treatments in this study was insufficient to uphold the observed results. Second, a large body of evidence supports the association between IL-6 inhibitors and increased haemoglobin levels13–15 in patients with RA, with JAK inhibitors having a neutral association.16–18 Supplementary to these findings was the observation that no patient characteristics impacted treatment effect on RA outcomes; however, longer follow-up may indicate if the post-treatment observation is clinically meaningful.
The findings from this real-world study are consistent with findings from systematic reviews of data from randomised controlled trials, which have also shown no significant differences between TNFi and non-TNFi treatments in patients initiated with a bDMARD.19 20 While in monotherapy separate comparative effectiveness studies21 revealed clinical superiority of IL-6 inhibitors to TNFs, this was not observable in the present study, given the disproportionately low IL-6 use in the non-TNFi cohort. Similarly, other real-world22 and trial-based comparative effectiveness19 23 24 studies in RA patients with previous anti-TNF exposure real-world evidence point to similar outcomes between TNFi cycling and switching to a non-TNFi.
This study is the first to attempt to address the dearth of comparative evidence, in terms of baseline patient characteristics and treatment outcomes, of TNFi and non-TNFi treatment approaches to address the inadequacy of initial csDMARD therapy for RA in a real-world setting. Robust statistical comparison methodologies were applied to cohorts, with differences corrected for at baseline.
The Corrona RA Registry is the largest disease-based registry in the USA, with broad geographical presence in rural and urban areas. It comprises data from academic and private practices and includes patients from all socioeconomic and racial strata. In addition, external validation of the registry data to different data sources lend further support to the generalisability and credibility of the data.25 Considering this and the long time frame over which the study was conducted, findings can be considered largely representative of the RA patient population in the USA as has been demonstrated in a study comparing the characteristics of Corrona patients with a Medicare database.25 However, as with any retrospective observational study, the level of generalisability is difficult to quantify.
To minimise the potential for channelling bias of different kinds of patients into the treatment regimen arms, a propensity-matching approach was used; as such, it is possible that some residual channelling existed that was not detected. Results may have been influenced by the effect of pooling index treatments into two categories: TNFi and non-TNFi. While mechanisms of action are similar for all TNFi included, distinct mechanisms of action exist within the non-TNFi group. It is possible that individual comparisons would wield different outcomes and effect modifiers, though the extent to which this would alter conclusions cannot be quantified without further investigation.
The study provides an indication of the comparative effectiveness of TNFi versus non-TNFi as the first-line treatment after csDMARD for adult patients with RA, addressing the limited evidence and resulting lack of directive provided in current treatment guidelines. Although new entrants to the bDMARD treatment market are not reflected in the current data, it can be reasonably expected that findings would have been similar to the current and historical real world, in addition to the trial-based evidence in the literature. Further investigation into the comparative effects of individual TNFi and non-TNFi treatments is warranted, as well as investigation of the comparative effects of individual TNFi after failure of one or more prior TNFi.
Conclusions
In this large US registry study, patients showed similar improvements in clinical outcomes and PROs after 1 year of treatment regardless of whether initiated with a TNFi or a non-TNFi. The findings of no significant differences in outcomes between first-line TNF and first-line non-TNF groups support RA guidelines which recommend individualised care based on clinical judgement and consideration of patient preferences.2
Footnotes
Handling editor: Josef S Smolen
Correction notice: This article has been corrected since it published Online First. Figure 2 has been corrected.
Contributors: All authors were involved with study conceptualisation, design and reporting. DAP, CJE, TB and KE conducted the data analysis.
Funding: Medical writing assistance, under the direction of the authors, was provided by Gauri Saal, MA Economics, and Samantha Webster, and editorial support was provided by Sinead Stewart, all of Prime (Knutsford, UK); the study was funded by Sanofi and Regeneron Pharmaceuticals, Inc, according to Good Publication Practice guidelines (http://annals.org/aim/article/2424869).
Disclaimer: The sponsors were involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript. The authors had unrestricted access to study data, were responsible for all content and editorial decisions, and received no honoraria related to the development of this publication.
Competing interests: This study was sponsored by Corrona, LLC, and the analysis was funded by Regeneron Pharmaceuticals, Inc., and Sanofi. Access to study data was limited to Corrona employees, and Corrona statisticians completed all of the analysis; all authors contributed to the interpretation of the results. Corrona has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Crescendo, Eli Lilly and Company, Genentech, Gilead, GSK, Janssen, Merck, Momenta Pharmaceuticals, Novartis, Pfizer Inc, Regeneron Pharmaceuticals, Inc., Roche, Sun, UCB and Valeant. TK is an employee of and stockholder in Regeneron Pharmaceuticals, Inc. GSJ and SB are former employees of Regeneron Pharmaceuticals, Inc. SF is an employee of and stockholder in Sanofi. JC and RP are former employees of Sanofi.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Corrona is reviewed by the New England Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data generated by the present research can be made available as soon as possible, wherever legally and ethically possible.
References
- 1. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2016;68:1–25. 10.1002/acr.22783 [DOI] [PubMed] [Google Scholar]
- 3. Hunter TM, Boytsov NN, Zhang X, et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int 2017;37:1551–7. 10.1007/s00296-017-3726-1 [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 5. Corrona LLC Corrona rheumatoid arthritis registry, 2019. Available: https://www.corrona.org/registries/rheumatoid-arthritis [Accessed Jan 2019].
- 6. Kremer J. The CORRONA database. Ann Rheum Dis 2005;64(Suppl 4):iv37–41. 10.1136/ard.2005.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aletaha D, Nell VPK, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. 10.1186/ar1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curtis JR, Yang S, Chen L, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res 2015;67:1345–53. 10.1002/acr.22606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bentley MJ, Greenberg JD, Reed GW. A modified rheumatoid arthritis disease activity score without acute-phase reactants (mDAS28) for epidemiological research. J Rheumatol 2010;37:1607–14. 10.3899/jrheum.090831 [DOI] [PubMed] [Google Scholar]
- 10. Nikolaisen C, Figenschau Y, Nossent JC. Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol 2008;35:380–6. [PubMed] [Google Scholar]
- 11. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med 2001;33:337–43. 10.3109/07853890109002087 [DOI] [PubMed] [Google Scholar]
- 12. Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992;35:498–502. 10.1002/art.1780350502 [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto M, Fujii T, Hamaguchi M, et al. Increase of hemoglobin levels by anti-IL-6 receptor antibody (tocilizumab) in rheumatoid arthritis. PLoS One 2014;9:e98202. 10.1371/journal.pone.0098202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isaacs JD, Harari O, Kobold U, et al. Effect of tocilizumab on haematological markers implicates interleukin-6 signalling in the anaemia of rheumatoid arthritis. Arthritis Res Ther 2013;15:R204. 10.1186/ar4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burmester GR HO, Dong Q, Stanislav M, et al. Unique changes in hemoglobin with sarilumab versus adalimumab are independent of better disease control in patients with rheumatoid arthritis (RA) [abstract 1528]. Arthritis Rheumatol 2018;70. [Google Scholar]
- 16. Schulze-Koops H, Strand V, Nduaka C, et al. Analysis of haematological changes in tofacitinib-treated patients with rheumatoid arthritis across phase 3 and long-term extension studies. Rheumatology (Oxford) 2017;56:46–57. 10.1093/rheumatology/kew329 [DOI] [PubMed] [Google Scholar]
- 17. Huizinga TW, Kay J, Harigai M, et al. e48 Effects of baricitinib on haematological laboratory parameters in patients with rheumatoid arthritis. Rheumatology (Oxford) 2018;57 10.1093/rheumatology/key075.589 [DOI] [Google Scholar]
- 18. Burmester GR, Kremer J, van den Bosch F, et al. 242 A phase III randomised placebo-controlled double-blind study of upadacitinib (ABT-494), a selective JAK-1 Inhibitor, in patients with active rheumatoid arthritis with inadequate response to conventional synthetic DMARDs. Rheumatology (Oxford) 2018;57 10.1093/rheumatology/key075.466 [DOI] [Google Scholar]
- 19. Choy E, Freemantle N, Proudfoot C, et al. Evaluation of the efficacy and safety of sarilumab combination therapy in patients with rheumatoid arthritis with inadequate response to conventional disease-modifying antirheumatic drugs or tumour necrosis factor α inhibitors: systematic literature review and network meta-analyses. RMD Open 2019;5:e000798. 10.1136/rmdopen-2018-000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jansen JP, Buckley F, Dejonckheere F, et al. Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with an inadequate response to conventional DMARDs--a systematic review and network meta-analysis. Health Qual Life Outcomes 2014;12:102. 10.1186/1477-7525-12-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choy E, Freemantle N, Proudfoot C, et al. Indirect treatment comparison of the efficacy and safety of sarilumab monotherapy in rheumatoid arthritis patients with inadequate response to conventional disease-modifying antirheumatic drugs. Adv Ther 2019;36:817–27. 10.1007/s12325-019-00912-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrold LR, Reed GW, Kremer JM, et al. The comparative effectiveness of abatacept versus anti-tumour necrosis factor switching for rheumatoid arthritis patients previously treated with an anti-tumour necrosis factor. Ann Rheum Dis 2015;74:430–6. 10.1136/annrheumdis-2013-203936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guyot P, Taylor P, Christensen R, et al. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther 2011;13:R204. 10.1186/ar3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyot P, Taylor PC, Christensen R, et al. Indirect treatment comparison of abatacept with methotrexate versus other biologic agents for active rheumatoid arthritis despite methotrexate therapy in the United Kingdom. J Rheumatol 2012;39:1198–206. 10.3899/jrheum.111345 [DOI] [PubMed] [Google Scholar]
- 25. Curtis JR, Chen L, Bharat A, et al. Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken) 2014;66:1790–8. 10.1002/acr.22377 [DOI] [PMC free article] [PubMed] [Google Scholar]