Abstract
Diabetes mellitus is a metabolic syndrome characterized by elevated blood glucose. The α-glucosidase enzyme is responsible for the hydrolysis of carbohydrates. This in silico study aimed to evaluate the inhibitory effects of the isolated compounds from Allium sativum L. on α-glucosidase. At first, sulfur and phenolic compounds of A. sativum L. were obtained from PubChem database, and α-glucosidase enzyme structure was obtained from Protein Data Bank. Toxicity class of compounds and the Lipinski parameter were predicted by Toxtree and Protox II and the Swiss ADME tools, respectively. Finally, the molecular interaction analysis between α-glucosidase and compounds from A. sativum L. was performed by AutoDock 4.2.6. Molecular interactions were investigated using Discovery Studio Visulizer and Ligplot 2.1 program. All of the selected sulfur and phenolic compounds from A. sativum L. followed the Lipinski’s rules, had an acceptable binding energy, and lacked toxicity; therefore, they were appropriate candidates for α-glucosidase inhibition. Among these compounds, methionol and caffeic acid showed the lowest binding energy, and the highest inhibitory effect on α-glucosidase enzyme with − 3.9 and − 4.8 kcal/mol, respectively. These compounds also indicated the lower binding energy than the standard inhibitor (miglitol). Among the sulfur and phenolic compounds in A. sativum L., methionol and caffeic acid were predicted to be the powerful inhibitors, due to having more hydrogen binds and hydrophobic interactions with the active site of α-glucosidase.
Keywords: In silico, α-glucosidase inhibition, Molecular docking, Allim sativum L.
Introduction
Diabetes mellitus is a metabolic syndrome characterized by causing high blood sugar (Hudson et al. 2002; Kato et al. 2012; Matsui et al. 2001; Trapero and Llebaria 2012), which is one of the most common diseases in the world, that is still among the leading causes of mortality and morbidity (Kitabchi et al. 2009; Worrall et al. 2005). By 2030, it is estimated that the prevalence of diabetes will have 69% and 20% increase in developing and developed countries, respectively (Hati et al. 2015). Elevated blood glucose levels induce vascular damage, which increases the risk of coronary heart disorders and myocardial infarction (Gerich 2003). Therefore, controlling the blood sugar could ultimately reduce the risk of cardiovascular disease (Hanefeld et al. 2004; Holman 2006; Stratton et al. 2000; Wright et al. 2002).
Antidiabetic drugs fall into several categories including sulfonylureas, bioguanidines, insulin mimetics (glucagon-like peptide analogues), and α-glucosidase inhibitors. However, due to the unacceptable side effects of some of these drugs, their application is reduced. Accordingly, the discovery of new antidiabetic drugs is still an important field of study in medicine (Kato et al. 2012).
α-glucosidase enzymes (extinction coefficient 3.2.1.20) are active in the small intestinal villi. These enzymes are responsible for hydrolyzing carbohydrates (Kimura et al. 2004). Inhibition of these enzymes in humans could present an effective measure to control the blood sugar in type II of diabetes (Ross et al. 2004). Drugs including acarbose, voglibose, and miglitol, which are useful for inhibiting the α-glucosidase, cause problems; such as diarrhea, bloating, and abdominal pain. Therefore, the discovery of novel compounds with the potential inhibitory effect on α-glucosidase is a growing field of study, and has been considered by many researchers in recent years (Dabhi et al. 2013; Kato et al. 2012; Rengasamy et al. 2013; van de Laar 2008). In this regard, previously many attempts have been given for in silico and in vitro study of α-glucosidase inhibition; such as the use of dihydropyridines (Yousuf et al. 2020), flavonoids (Proença et al. 2017), alkaloids (Zafar et al. 2016), benzimidazole derivatives (Zawawi et al. 2016), and morning glory resin glycosides (Rosas-Ramírez et al. 2018).
Natural compounds isolated from plants, animals and microorganisms are very important for lowering the blood sugar. Garlic (Allium sativum L.) belongs to the order Asparagales and has an important role in the diet and medicine (Benkeblia 2004). Garlic has various therapeutic applications including hyperlipidemia, anti-sclerotic, hypoglycemic, antihypertensive, antimicrobial and anticancer. The role of garlic in lowering blood sugar is due to its sulfur (Kimbaris et al. 2006) and phenolic compounds (Kallel et al. 2014). Allicin (dialylthiosulfate) is one of the most important thiosulfate compounds in garlic, which together with garlic sulfur compounds have the antibacterial and antifungal properties. They also provide defense mechanisms against the pest attacks (Lanzotti et al. 2012; Rastogi and Arunachalam 2011). Important polyphenolic compounds of garlic; such as caffeic acid, ferulic acid and p-coumaric acid have antioxidant effects, prevent oxidative stress and could inhibit the damages imposed to the DNA content of cells by suppressing the free radicals (Bozin et al. 2008).
Previously, the in silico approach has been used for investigating the potential inhibitors of α-glucosidase, and has provided valuable insights in this area of medical study (Zafar et al. 2016). The field of bioinformatics has shown to represent a potential ground to investigate the molecular interaction between a receptor molecule and its ligands by using the in silico tools (Haghighi et al. 2019; Haghighi and Moradi 2020; Nabati et al. 2020). Molecular docking is one of the most widely used methods for rapid screening and finding the effective composition (Jhong et al. 2015). Molecular docking is a simulation approach that predicts the molecules that bind properly to an enzyme. These in silico studies could reduce the costs of research by diminishing the random trial and error, and save the time before testing at in vivo and in vitro environments. Also, by having a detailed prediction of the study outcome, the requirement for animal sacrifice would be decreased (Gilson and Zhou 2007; Kitchen et al. 2004). Therefore, in this study, the interaction of the most important sulfur-containing and phenolic compounds from garlic with α-glucosidase were investigated through in silico approach by application of bioinformatics tools.
Methodology
At first, the important sulfur-containing and phenolic compounds in garlic were fetched from the literature. The 3D structures of the compounds were harvested from the PubChem chemical compounds database (http://pubchem.ncbi.nlm.nih.gov). An appropriate crystallographic structure of α-glucosidase enzyme (PDB ID 3A4A) with the resolution of 1.6oA was obtained from protein data bank (http://www.rcsb.org).
Determination of pharmacological properties of compounds by Lipinski parameter
The potential effective sulfur and phenolic compounds of garlic were evaluated using the Lipinski parameter to inhibit the activity of α-glucosidase enzyme. All of the compounds showed to have the properties required by Lipinski’s parameter; therefore, they were predicted to have an optimal adsorption. The SwissADME database (http://www.swissadme.ch/index.php) was used to obtain the Lipinski properties of the compounds.
Evaluating the toxicity of the selected compounds
Garlic contains many sulfur and phenolic compounds. Among the most highlighted sulfur compounds; allicin (with two sulfur atoms) and methionol (with one sulfur atom) are prominent for their potential therapeutic application. Phenolic compounds; such as ferulic acid, caffeic acid and p-coumaric acid are also found in this plant. Lack of toxicity is one of the critical factors for choosing a compound as a therapeutic candidate. Therefore, in this study, the toxicity of each of the sulfur and phenolic compounds; such as liver toxicity, carcinogenicity, immunotoxicity, mutagenicity and the toxicity class of the compounds were examined using Protox tool (https://bio.tools/protox) (Drwal et al. 2014) and Toxtree 2.5.4 program (Enoch et al. 2008).
Molecular interaction analysis
Autodock tool (Harris et al. 2008) was used to investigate the molecular interaction between α-glucosidase enzyme and the selected ligands. Before the docking analysis, the structure of enzyme was optimized by removing the excess ligands, then all of the compounds as well as the enzyme molecule were optimized for energy using the Chimera 1.7 program (Pettersen et al. 2004). Polar hydrogens were added to the protein and their Kollman charges were determined.
The partial load of the compounds was calculated using Compute Gasteiger in AutoDock 4.2.6 tool. Finally, the molecular interactions and the type of bindings between the selected compounds and α-glucosidase enzyme were investigated using Discovery Studio visualizer and Ligplot 2.1 programs (Laskowski and Swindells 2011).
Results and discussion
Pharmacological properties of the selected compounds
In this study, the structure of Allitridin (C6H10S3, pubchem ID: 16315), Methyl propyl disulfide (C4H10S2, pubchem ID: 16592), Allicin (C6H10OS2, pubchem ID: 65036), Diisoamyl trisulfide (C10H22S3, pubchem ID: 15667721), as the sulfur compound and Methionol (C4H10OS, pubchem ID: 10448), Ferulic acid (C10H10O4, pubchem ID: 445858), Caffeic acid (C9H8O4, pubchem ID: 689043), and p-coumaric acid (C9H8O3, pubchem ID: 637542) as the phenolic compounds were collected.
The structure of pharmacological properties of the selected compounds in garlic were studied following the Lipinski’s rule (Tice 2001). According to this rule, for an oral drug to be well absorbed and permeable in the body, it must have a number of characteristics including a molecular weight of less than 500 Daltons, a hydrogen bond donor less than or equal to 10, and a logp (coefficient of octanol to water) of less than five. As presented by Table 1, it appears that all of these compounds were following the Lipinski’s parameter; therefore, they are not expected to have a problem in terms of absorption and permeability.
Table 1.
Investigation of pharmacologic parameters (Drug-like) for sulfur and phenolic compounds in garlic according to Lipinski rule
| Compound | Hydrogen bond donors (≤ 5) | Hydrogen bond acceptors (≤ 10) | Molecular mass (< 500) | Logp (< 5) |
|---|---|---|---|---|
| Allitridin | 0 | 0 | 178.34 | 2.64 |
| Methyl propyl disulfide | 0 | 0 | 122.25 | 1. 8 |
| Allicin | 0 | 1 | 162.27 | 1.31 |
| Diisoamyl trisulfide | 0 | 0 | 238.48 | 4.72 |
| Methionol | 1 | 1 | 106.19 | 0.55 |
| Ferulic acid | 2 | 4 | 194.18 | 1.51 |
| Caffeic acid | 3 | 4 | 180.16 | 1.15 |
| p-coumaric acid | 2 | 3 | 164.16 | 1.46 |
Analysis for toxicity of the selected compounds
The compounds were also evaluated for their toxicity and toxicity class. The criterion for selecting a compound as a drug candidate is the safety of the compound and the compound should not show any toxicity (Tshikalange et al. 2005). As shown in Table 2, none of the compounds exhibited any toxicity, other than ferulic acid (immunotoxicity). The toxicity class of the compounds also indicates a measure of the total toxicity. Class I is the most toxic and the most dangerous class is toxicity, and class VI is the least toxic one. Table 2 presents that none of the compounds are in class I, so this indicates that most of the sulfur and phenolic compounds in garlic do not have toxicity for human body.
Table 2.
Evaluation of toxicity and toxicity class of the selected garlic compounds
| Compounds | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Mutagenicity | Toxicity Class |
|---|---|---|---|---|---|
| Allitridin | N.H | N.C | N.I | N.M | 3 |
| Methyl propyl disulfide | N.H | N.C | N.I | N.M | 3 |
| Allicin | N.H | N.C | N.I | N.M | 4 |
| Diisoamyl trisulfide | N.H | N.C | N.I | N.M | 4 |
| Methionol | N.H | N.C | N.I | N.M | 3 |
| Ferulic acid | N.H | N.C | I | N.M | 4 |
| Caffeic acid | N.H | N.C | N.I | N.M | 5 |
| p-coumaric acid | N.H | N.C | N.I | N.M | 5 |
N.H;no hepatotoxicity, N.C;no carcinogenicity, N.I;no immunotoxicity, N.M; no mutagen, I, immunotoxicity
Molecular interaction analysis
The results of molecular interaction between the selected compound with the α-glucosidase enzyme, obtained by the molecular docking assay, are provided in Table 3. Due to the appropriate binding energy of these compounds, all of them showed to have a good binding with the enzyme. Binding energy, hydrogen and hydrophobic interactions between compounds and the enzyme are also presented in Table 3.
Table 3.
Interaction and binding energy of sulfur and phenolic compounds with amino acids of α-glucosidase
| Compounds | Binding energy (Kcal/mole) |
Hydrogen interaction | Hydrophobic interaction |
|---|---|---|---|
| Allitridin | − 3.1 | – | Arg442 |
| Methyl propyl disulfide | − 2.9 | – | Phe159 |
| Allicin | − 3.1 | – | Tyr72, val 216 |
| Diisoamyl trisulfide | − 3.2 | Arg446 | Asp442,Val216,Phe178 |
| Methionol | − 3.9 | Gln182, Asp69 | His112, Phe178, Val109, Asp215, Glu277, Val216, Phe159, Tyr72, Arg213,Asp352, His351, Arg446, Arg442 |
| Ferulic acid | − 3.7 | Asp69, Gln279, Glu277 | His112, Val216, Asp215, Glu277, Val216, Phe159, Tyr72,Arg213,Asp352, His351, Arg442, |
| Caffeic acid | − 4.8 | Arg213, Tyr24, Arg446, Asp69, Asp352, Glu277, Asp215 | His112, Phe178, Phe159, Met70, His351, Val216, Tyr72, Arg442 |
| p-coumaric acid | − 3.7 | Asp69, Arg446, Tyr24 | Arg442, Asp215, Val216, Tyr72 |
| Miglitol (standard compound) | − 3.8 | Asp69, Gln182, Glu277, Gln279 | Arg446, Tyr72, His112, Val216, Asp215, Phe178, Phe303, Tyr158, Phe159 |
The results of molecular docking study indicated that all of the sulfur and phenolic compounds in garlic could bind to the active site of the α-glucosidase and inhibit the activity of this enzyme. According to the results of docking analysis, the binding energy of the studied compounds are different; therefore, the amount of binding energy varies from − 2.9 to − 4.8 kcal /mole. The lower the binding energy level (negative), the binding between the receptor (enzyme) and the ligands (compound or inhibitor) will be stronger. Among the selected sulfur compounds, methionol with a value of -3.9 kcal/mol, and among phenolic compounds, caffeic acid with a value of − 4.8 kcal/mol showed the lowest binding energy, therefore its predicted that they may provide the highest inhibitory effect.
Previous studies have shown that several amino acids, including Asp69, Glu277, Asp352, Arg446 and Gln182, play a key role in the interaction of the enzyme and inhibitor at the active site of α-glucosidase (Jhong et al. 2015). This study also found that compounds with lower binding energies interact more with these amino acids. Miglitol was considered as a standard α-glucosidase inhibitor. Docking results showed that miglitol with binding energy of − 3.8 kcal/mol has a significant binding energy and shows hydrogen binds with the of Gln279, Gln182, GLU277 and Asp69 amino acids.
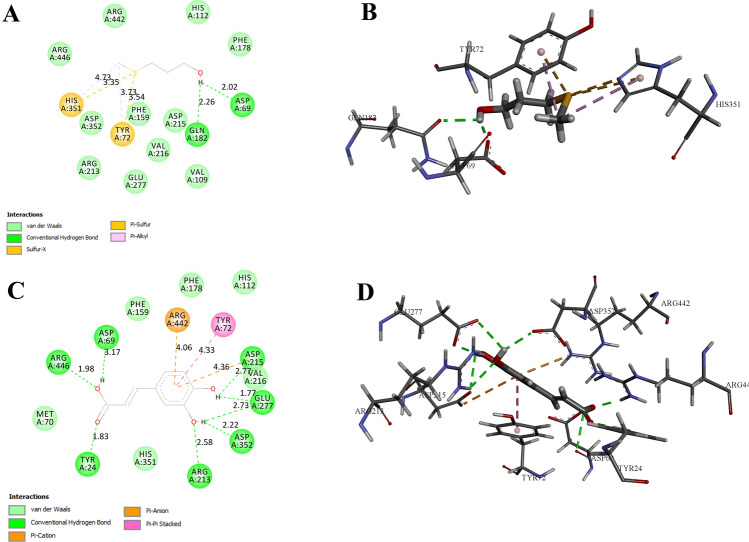
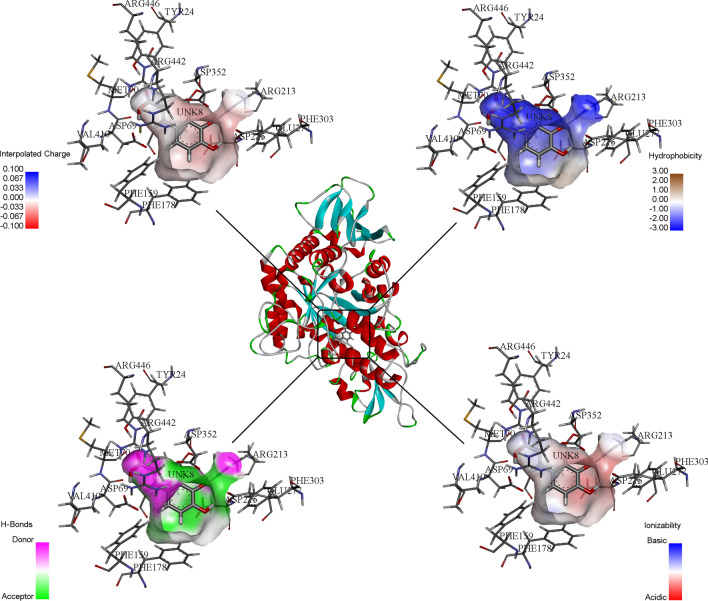
The complex of methionol with the two amino acids Asp69 and Gln182, and the complex of caffeic acid with the amino acids Asp352, Glu277, Asp215, Asp69, Arg446, Tyr24 and Arg213, also have hydrogen binding interaction (Fig. 1a, b). Also, due to the formation of more hydrogen and hydrophobic bindings, these two compounds had the lower binding energy and stronger binding to the enzyme than other compounds. Figure 2 shows a three-dimensional image of the ionization, hydrophobic, polar, and hydrogen binding reactions between the enzyme and caffeic acid molecules.
Fig. 1.
The two-dimentional (2D) and three-dimentional (3D) plot of α-glucosidase interactions with the selected potential inhibitors. a 2D presentation of the molecular interactions between the enzyme and methionol. b 3D presentation of the molecular interactions between the enzyme and methionol. c 2D presentation of the molecular interactions between the enzyme and caffeic acid. d 3D presentation of the molecular interactions between the enzyme and caffeic acid
Fig. 2.
Three-dimensional display of the receptor and caffeic acid ligand surface interactions in the active site of α-glucosidase, regarding polarity, hydrogen binding, hydrophobic and ionize surface of interactions
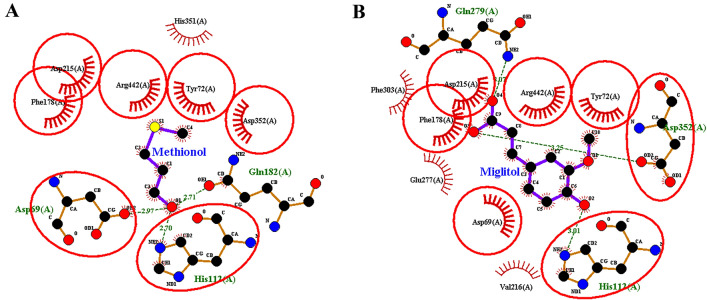
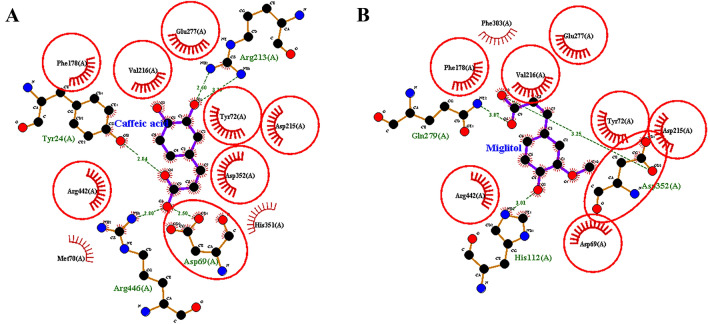
The overlap degree of methionol and caffeic acid with miglitol indicated that common amino acids were involved among these compounds and miglitol. The overlap between methionol and miglitol indicates that the two compounds are common in seven amino acids, including His112, Asp352, Tyr72, Arg442, Phe178, Asp215, and Asp69 (Fig. 3a, b). The overlap between caffeic acid and miglitol also indicated interacting with eight common amino acids; including Glu277, Asp352, Tyr72, Arg442, Phe178, Asp215, Val216, and Asp69 (Fig. 4a, b).
Fig. 3.
Degree of overlap between (a) methionol (b) miglitol (standard inhibitor) in the active site of α-glucosidase enzyme
Fig. 4.
Degree of overlap between (a) caffeic acid (b) miglitol (standard inhibitor) in the active site of α-glucosidase enzyme
Extensive studies have shown that phenolic compounds have a major effect for inhibiting the α-glucosidase enzyme. By isolating phenolic compounds in 2016, Camargo et al. reported that these compounds, including caffeic acid, catechin, and quercetin, have a potential inhibitory against α-glucosidase (de Camargo et al. 2016). Mohammad et al. reported that the ethanolic extract of Orthosiphon stamineus contains flavonoids, terpenoids and saponins, of which phenolics ones; such as caffeic acid and its derivatives had a significant effect on the α-glucosidase (Mohamed et al. 2012).
A previous in silico study of the phenolic compounds has indicated that the combination of caffeic acid with a binding energy of − 4.9 kcal/mol and binding to the amino acids Asp69, Glu277, and Arg442 could provide a good inhibitory effect on α-glucosidase (Rasouli et al. 2017). In the current study, caffeic acid also showed to have the lowest binding energy among phenolic compounds of garlic. In another study on the synthesis of sulfur compounds and their inhibitory potential, compounds such as methazolamid, acetazolamide and timolol (which have sulfur atoms in their structure with binding energies of − 4.3, − 4.8 and 4.5, kcal/mol, respectively) showed the significant inhibition of α-glucosidase enzyme (Gollapalli et al. 2019). In the current study, the complex of methionol of sulfur compounds showed the lower binding energy, too.
Conclusions
The results of this study showed that all of the important compounds in Allium sativum L. have the high potential pharmaceutical properties; such as good absorption (following Lipinski parameter), lack of toxicity and good binding energy. Based on the results of molecular docking presented by this study, it could be concluded that among the eight compounds of Allium sativum L., methionol and caffeic acid, are more effective for inhibiting the α-glucosidase enzyme. These compounds also presented lower binding energies compared to miglitol; therefore, they are likely to be stronger inhibitors than miglitol. However, in order to further investigate these compounds, it is necessary to perform in vitro and in vivo studies to complete the in silico prediction.
Acknowledgements
We wish to thank University of Isfahan, and Zanjan University of Medical Sciences for their supports.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Moradi, Email: Moradimuhamad@gmail.com, Email: moradi.mo.biotech@ast.ui.ac.ir.
Behrooz Johari, Email: Dr.johari@zums.ac.ir.
References
- Benkeblia N. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) LWT-food. Sci Technol. 2004;37:263–268. [Google Scholar]
- Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) Food Chem. 2008;111:925–929. doi: 10.1016/j.foodchem.2008.04.071. [DOI] [Google Scholar]
- Dabhi AS, Bhatt NR, Shah MJ. Voglibose: an alpha glucosidase inhibitor. JCDR. 2013;7:3023. doi: 10.7860/JCDR/2013/6373.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Camargo AC, Regitano-d’Arce MAB, Biasoto ACT, Shahidi F. Enzyme-assisted extraction of phenolics from winemaking by-products: antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016;212:395–402. doi: 10.1016/j.foodchem.2016.05.047. [DOI] [PubMed] [Google Scholar]
- Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R. ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014;42:W53–W58. doi: 10.1093/nar/gku401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch S, Hewitt M, Cronin M, Azam S, Madden J. Classification of chemicals according to mechanism of aquatic toxicity: an evaluation of the implementation of the Verhaar scheme in. Toxtree Chemos. 2008;73:243–248. doi: 10.1016/j.chemosphere.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163:1306–1316. doi: 10.1001/archinte.163.11.1306. [DOI] [PubMed] [Google Scholar]
- Gilson MK, Zhou H-X. Calculation of protein-ligand binding affinities. Annu Rev Biophys Biomol Struct. 2007;36:21–42. doi: 10.1146/annurev.biophys.36.040306.132550. [DOI] [PubMed] [Google Scholar]
- Gollapalli M, et al. Synthesis of benzothiazole derivatives as a potent α-glucosidase inhibitor. Bioorgan Chem. 2019;85:33–48. doi: 10.1016/j.bioorg.2018.12.021. [DOI] [PubMed] [Google Scholar]
- Haghighi O, Moradi M (2020) In silico study of the structure and ligand interactions of alcohol dehydrogenase from cyanobacterium Synechocystis Sp. PCC 6803 as a key enzyme for biofuel production. Appl Biochem Biotechnol:1–22 [DOI] [PubMed]
- Haghighi O, Davaeifar S, Zahiri HS, Maleki H, Noghabi KA (2019) Homology Modeling and molecular docking studies of glutamate dehydrogenase (GDH) from Cyanobacterium Synechocystis sp. PCC 6803. Int J Pep Res Ther:1–11
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–16. doi: 10.1016/S0195-668X(03)00468-8. [DOI] [PubMed] [Google Scholar]
- Harris R, Olson AJ, Goodsell DS. Automated prediction of ligand-binding sites in proteins. Proteins Struct Funct Bioinform. 2008;70:1506–1517. doi: 10.1002/prot.21645. [DOI] [PubMed] [Google Scholar]
- Hati S, et al. Design, synthesis and biological evaluation of small molecules as potent glucosidase inhibitors. Eur J Med Chem. 2015;100:188–196. doi: 10.1016/j.ejmech.2015.04.059. [DOI] [PubMed] [Google Scholar]
- Holman RR. Long-term efficacy of sulfonylureas: a United Kingdom Prospective Diabetes Study perspective. Metabolism. 2006;55:S2–S5. doi: 10.1016/j.metabol.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hudson BI et al (2002) Glycation and diabetes: the RAGE connection. Curr Sci:1515–1521
- Jhong CH, Riyaphan J, Lin SH, Chia YC, Weng CF. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors. 2015;41:242–251. doi: 10.1002/biof.1219. [DOI] [PubMed] [Google Scholar]
- Kallel F, Driss D, Chaari F, Belghith L, Bouaziz F, Ghorbel R, Chaabouni SE. Garlic (Allium sativum L.) husk waste as a potential source of phenolic compounds: influence of extracting solvents on its antimicrobial and antioxidant properties. Ind Crops Prod. 2014;62:34–41. doi: 10.1016/j.indcrop.2014.07.047. [DOI] [Google Scholar]
- Kato A, et al. α-1-C-butyl-1, 4-dideoxy-1, 4-imino-l-arabinitol as a second-generation iminosugar-based oral α-glucosidase inhibitor for improving postprandial hyperglycemia. J Med Chem. 2012;55:10347–10362. doi: 10.1021/jm301304e. [DOI] [PubMed] [Google Scholar]
- Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum) Ultrason Sonochem. 2006;13:54–60. doi: 10.1016/j.ultsonch.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kimura A, Lee J-H, Lee I-S, Lee H-S, Park K-H, Chiba S, Kim D. Two potent competitive inhibitors discriminating α-glucosidase family I from family II. Carbohyd Res. 2004;339:1035–1040. doi: 10.1016/j.carres.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- Lanzotti V, Barile E, Antignani V, Bonanomi G, Scala F. Antifungal saponins from bulbs of garlic, Allium sativum L. var. Voghiera Phytochem. 2012;78:126–134. doi: 10.1016/j.phytochem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. Washington, DC: ACS Publications; 2011. [DOI] [PubMed] [Google Scholar]
- Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J Agric Food Chem. 2001;49:1948–1951. doi: 10.1021/jf001251u. [DOI] [PubMed] [Google Scholar]
- Mohamed EAH, et al. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complem Alternat Med. 2012;12:176. doi: 10.1186/1472-6882-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabati F, Moradi M, Mohabatkar H. In silico analyzing the molecular interactions of plant-derived inhibitors against E6AP, p53, and c-Myc binding sites of HPV type 16 E6 oncoprotein. Mol Biol Res Commun. 2020;9:71–82. doi: 10.22099/mbrc.2020.36522.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Proença C, et al. α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure–activity relationship study. J Enzyme Inhib Med Chem. 2017;32:1216–1228. doi: 10.1080/14756366.2017.1368503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli H, Hosseini-Ghazvini SM-B, Adibi H, Khodarahmi R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017;8:1942–1954. doi: 10.1039/C7FO00220C. [DOI] [PubMed] [Google Scholar]
- Rastogi L, Arunachalam J. Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. Mater Chem Phys. 2011;129:558–563. doi: 10.1016/j.matchemphys.2011.04.068. [DOI] [Google Scholar]
- Rengasamy KR, Aderogba MA, Amoo SO, Stirk WA, Van Staden J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss Food Chem. 2013;141:1412–1415. doi: 10.1016/j.foodchem.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Rosas-Ramírez D, Escandón-Rivera S, Pereda-Miranda R. Morning glory resin glycosides as α-glucosidase inhibitors: in vitro and in silico analysis. Phytochemistry. 2018;148:39–47. doi: 10.1016/j.phytochem.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Ross SA, Gulve EA, Wang M. Chemistry and biochemistry of type 2 diabetes. Chem Rev. 2004;104:1255–1282. doi: 10.1021/cr0204653. [DOI] [PubMed] [Google Scholar]
- Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice CM. Selecting the right compounds for screening: does Lipinski’s Rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manag Sci Formerly Pest Sci. 2001;57:3–16. doi: 10.1002/1526-4998(200101)57:1<3::AID-PS269>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Trapero A, Llebaria A. A prospect for pyrrolidine iminosugars as antidiabetic α-glucosidase inhibitors. Washington, DC: ACS Publications; 2012. [DOI] [PubMed] [Google Scholar]
- Tshikalange T, Meyer J, Hussein A. Antimicrobial activity, toxicity and the isolation of a bioactive compound from plants used to treat sexually transmitted diseases. J Ethnopharmacol. 2005;96:515–519. doi: 10.1016/j.jep.2004.09.057. [DOI] [PubMed] [Google Scholar]
- van de Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc Health Risk Manag. 2008;4:1189. doi: 10.2147/VHRM.S3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall E, Basu S, Hanson K. Is malaria a disease of poverty? A review of the literature. Trop Med Int Health. 2005;10:1047–1059. doi: 10.1111/j.1365-3156.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Wright A, Burden AF, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–336. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- Yousuf H, et al. Dihydropyridines as potential α-amylase and α-glucosidase inhibitors: synthesis, in vitro and in silico studies. Bioorg Chem. 2020;96:103581. doi: 10.1016/j.bioorg.2020.103581. [DOI] [PubMed] [Google Scholar]
- Zafar M, Khan H, Rauf A, Khan A, Lodhi MA. In Silico study of alkaloids as α-glucosidase inhibitors: hope for the discovery of effective lead compounds. Front Endocrinol. 2016;7:153. doi: 10.3389/fendo.2016.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawawi NKNA, et al. Benzimidazole derivatives as new α-glucosidase inhibitors and in silico studies. Bioorgan Chem. 2016;64:29–36. doi: 10.1016/j.bioorg.2015.11.006. [DOI] [PubMed] [Google Scholar]