Summary
Neuroendocrine prostate cancer (NEPC) is a lethal prostate cancer subtype arising as a consequence of more potent androgen receptor (AR) targeting in castration-resistant prostate cancer (CRPC). Its molecular pathogenesis remains elusive. Here, we report that the Wnt secretion mediator Wntless (WLS) is a major driver of NEPC and aggressive tumor growth in vitro and in vivo. Mechanistic studies showed that WLS is a transcriptional target suppressed by AR that activates the ROR2/PKCδ/ERK signaling pathway to support the neuroendocrine (NE) traits and proliferative capacity of NEPC cells. Analysis of clinical samples and datasets revealed that WLS was highly expressed in CRPC and NEPC tumors. Finally, treatment with the Wnt secretion inhibitor LGK974 restricted NE prostate tumor xenograft growth in mice. These findings collectively characterize the contribution of WLS to NEPC pathogenesis and suggest that WLS is a potential therapeutic target in NEPC.
Subject areas: Biological Sciences, Cell Biology, Cancer, Transcriptomics
Graphical Abstract

Highlights
-
•
WLS is highly expressed in neuroendocrine prostate cancer clinical samples
-
•
WLS is a transcriptional target suppressed by androgen receptor
-
•
WLS drives neuroendocrine prostate cancer through the ROR2/PKCδ/ERK pathway
-
•
Wnt secretion inhibitor treatment limits neuroendocrine prostate tumor growth in mice
Biological Sciences; Cell Biology; Cancer; Transcriptomics
Introduction
Prostate cancer (PC) is a current epidemic both globally and in the United States, with roughly 1 in 9 American men developing this disease during their lifetime (Siegel et al., 2019). Androgen deprivation therapy (ADT) is the standard of care, since dependence on androgen receptor (AR) signaling is a hallmark of PC. Although tumors initially respond, the disease eventually becomes resistant to ADT and progresses to the castration-resistant prostate cancer (CRPC) stage (Wong et al., 2014). Targeting the AR axis with more potent AR pathway inhibitors (APIs), including the second-generation AR antagonist enzalutamide (ENZ), has become a cornerstone therapeutic strategy for treating patients with CRPC (Sternberg, 2019). However, the clinical benefits are limited due to rapid development of drug resistance (Ramadan et al., 2015). The highly potent API-resistant CRPC variant is a significant clinical challenge. It can rapidly develop into a phenotype similar to lethal neuroendocrine prostate cancer (NEPC), characterized by neuroendocrine (NE) differentiated cell morphology and NE marker expression with reduced or lost reliance on AR (Beltran et al., 2014). Importantly, emerging evidence indicates that the incidence of this aggressive subtype of CRPC, also called treatment-induced NEPC (t-NEPC), has significantly increased in recent years due to the widespread use of highly potent APIs such as ENZ (Beltran et al., 2016). NEPC has been reported in up to 25% of patients with advanced treatment-resistant CRPC (Aparicio et al., 2011). However, the mechanisms by which CRPC transdifferentiates into NEPC are largely unknown. Currently, NEPC remains incurable with patient survival for less than a year following diagnosis (Wang et al., 2014). These dismal facts highlight the urgent need for a greater understanding of NEPC development and progression and new targeted therapies to improve survival.
Recently, accumulating evidence suggests that the Wnt signaling pathway plays a role in PC progression to an AR-indifferent or NE phenotype (Murillo-Garzon and Kypta, 2017). The Wnt proteins are secreted lipoglycoproteins with essential roles in regulating embryonic development, neuronal patterning, cell proliferation and migration, and axon guidance. There are 19 human Wnts regulating distinctive pathways via interaction with multiple cell surface receptors, including transmembrane frizzled (FZD) receptors, low-density lipoprotein receptor (LRP)4/5/6, receptor tyrosine kinase-like orphan receptor (ROR)1/2, and receptor-like tyrosine kinase. The major branches of Wnt pathways include the canonical pathway which utilizes β-catenin signaling (Wnt/β-catenin) and noncanonical pathways including planar cell polarity (Wnt/PCP) and calcium (Wnt/Ca2+) signaling (Komiya and Habas, 2008). Multiple Wnts, including Wnt4, Wnt5A, Wnt7B, and Wnt11, and β-catenin signaling were shown to contribute to the development of API resistance in PC cells (Isaacsson Velho et al., 2020; Miyamoto et al., 2015; Zhang et al., 2018). Wnt7B and Wnt11 have been reported to induce NE differentiation and NE marker expression in PC cells (Moparthi et al., 2019; Uysal-Onganer et al., 2010). Since a large and varied number of Wnts, Wnt receptors, and Wnt-elicited signaling cascades are involved in API resistance and NE differentiation, targeting Wnt secretion instead of individual Wnts is likely to be a more promising way to block the entire Wnt signaling process in NEPC. Wnt secretion is mediated by the palmitoleation of most Wnts by O-acyltransferase porcupine (PORCN) in the ER. Palmitoleated Wnts are then transported to the cell surface for secretion by the carrier protein Wntless (WLS) (Yu et al., 2014). Recent evidence indicates that WLS promotes cellular resistance to ENZ in PC cells (Lombard et al., 2019), but WLS's function in NEPC remains to be elucidated.
Here, we investigated the role of WLS in NEPC emerging after prolonged API treatment. Using an in vitro-derived model of ENZ-resistant (ENZR) NEPC and data from PC patients, we show that WLS is transcriptionally repressed by AR, is required for NEPC growth and NE marker expression, and is highly expressed in human CRPC and NEPC. Mechanistically, silencing of WLS resulted in downregulation of the ROR2/PKCδ/ERK signaling cascade that is overexpressed in NEPC. These findings establish a functional role of WLS in the pathogenesis and progression of NEPC under the selective pressure of highly potent APIs like ENZ. Our results also identified WLS as a potential therapeutic target for this lethal form of PC.
Results
Wnt signaling and WLS expression are upregulated in ENZR NEPC cells
To model the clinical situation of CRPC transition to NEPC, we used an ENZR C4-2B (C4-2BENZR) cell line established by chronic exposure of human CRPC C4-2B cells to ENZ at gradually increasing doses to develop resistance (Liu et al., 2015). We performed expression profiling of C4-2BENZR and control C4-2B cells. Gene set enrichment analysis (GSEA) demonstrated decreased expression of androgen-responsive genes, confirming the on-target molecular effects of ENZ even in resistant cells, with strong upregulation of neuronally expressed genes (Figure 1A). To minimize interference from acute ENZ treatment that may repress active AR expressed in ENZR cells, we next characterized C4-2BENZR cells cultured in the absence of ENZ. NE markers such as CHGA and NSE showed enhanced expression in parallel to downregulated PSA expression in C4-2BENZR cells compared to controls (Figure 1B). C4-2BENZR cells also showed an NE differentiated cell morphology with an increase in per-cell number of neurites and average neurite length compared to control cells (Figures 1C and 1D). These results indicate the NE characteristics of C4-2BENZR cells, recapitulating the progression of CRPC to NEPC under the pressure of ENZ.
Figure 1.
Wnt signaling and WLS expression are upregulated in ENZR NEPC cells
(A) GSEA plots of enrichment of gene signatures related to androgen response and neuronal system in ENZR C4-2B (C4-2BENZR) cells compared to ENZ-sensitive control C4-2B cells.
(B) Western blot analysis of PSA, CHGA, and NSE protein expression in control and ENZR C4-2B cells.
(C) Representative images of control and ENZR C4-2B cell morphology. Scale bars: 50 μm.
(D) Quantification of per-cell number of neurites and average neurite length in control and ENZR C4-2B cells (n = 50 cells per cell line), which is shown as a representative of 3 independent experiments. Data represent the mean ± SEM. ∗∗p < 0.01, unpaired t test.
(E) KEGG pathway analysis of the top 57 pathways upregulated in ENZR C4-2B cells compared to controls. KEGG pathways were clustered based on functional relation, indicated by different colors (red, signal transduction; green, human diseases; light blue, cellular processes; dark blue, metabolism; purple, organismal systems). The Wnt signaling pathway is pointed out by an arrow.
(F) RT-qPCR analysis of Wnt gene expression categorized based on the ability to activate the canonical or noncanonical Wnt signaling pathways in control and ENZR C4-2B cells (n = 3). Data represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, unpaired t test.
(G) GSEA analysis of select positively enriched Wnt-related gene sets in C4-2BENZR cells compared to controls.
(H) RT-qPCR analysis of WLS mRNA expression in control and ENZR C4-2B cells (n = 3). Data represent the mean ± SEM. ∗∗p < 0.01, unpaired t test.
(I) Western blot analysis of WLS protein expression in cell lysates and secreted Wnt5A and Wnt11 protein levels in conditioned media of control and ENZR C4-2B cells.
See also Figures S1 and S2.
In searching for potential pathway(s) driving the emergent NE phenotype of C4-2BENZR cells, KEGG analysis revealed significant enrichment of the Wnt signaling pathway in C4-2BENZR cells (Figure 1E), which coincides with the prevalent activation of most Wnts in C4-2BENZR cells (Figure 1F). GSEA analysis also demonstrated several positively enriched gene signatures corresponding to Wnt-centric molecular events, including those related to both canonical and noncanonical Wnt signaling pathways, in C4-2BENZR cells (Figures 1G and S1A), further supported by the observed upregulation of multiple genes engaged in either the canonical (DKK1, LEF1 and AXIN2) or the noncanonical (VANGL2, ROR2, WNT5A and DAAM2) Wnt signaling pathways from our RNA-seq data (Figures S1B and S1C). These findings are consistent with the reported activation of both canonical and noncanonical Wnt signaling in ENZR PC cells (Chen et al., 2020; Zhang et al., 2018). Interestingly, the Wnt secretion mediator Wntless (WLS) was among the most upregulated Wnt-related genes in C4-2BENZR cells compared to controls by RNA-seq (Figure S1B), further confirmed by a 6-fold increase of WLS mRNA level in C4-2BENZR cells compared to controls by qPCR (Figure 1H). We also found higher WLS protein expression in whole cell lysates in parallel to more Wnt5A and Wnt11 proteins detected from conditioned media, reflecting their upregulation at the mRNA level, in C4-2BENZR cells compared to controls (Figures 1I and S2). Together, these results suggest activated Wnt signaling and WLS expression in ENZR NEPC cells.
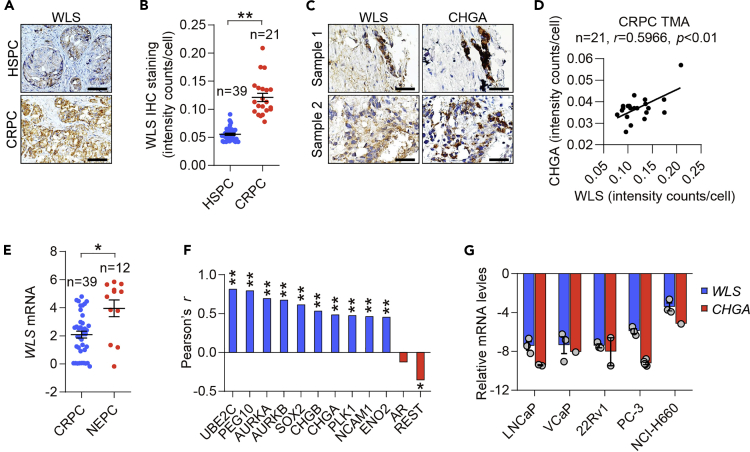
WLS is highly expressed in human CRPC and NEPC
To evaluate the clinical relevance of WLS in human CRPC and NEPC, we performed immunohistochemical (IHC) analysis of WLS expression in a tissue panel of hormone-sensitive PC (HSPC) and CRPC. We showed increased WLS protein expression in CRPC samples relative to HSPC counterparts (Figures 2A and 2B). We also demonstrated that WLS was co-expressed with the NE marker CHGA at the per-cell staining level in the CRPC cohort (Figures 2C and 2D). Next, we assessed WLS expression in RNA-seq data from CRPC and NEPC patient tumors using publicly available datasets. Analyzing the Beltran cohorts, we showed significant activation of WLS transcript in NEPC tumors, classified by NE histomorphology and an integrated NEPC score based on a set of 70 NE reference genes (Beltran et al., 2016), compared with CRPC (Figure 2E). Similarly, we revealed an upward trend of WLS mRNA levels in NEPC compared to CRPC in 2 additional independent datasets (Figure S3). Moreover, WLS demonstrated a significant positive co-expression correlation with multiple canonical NE marker genes and an inverse relationship with REST, a transcription factor lost during progression to NEPC (Lapuk et al., 2012), at the mRNA level in the Beltran dataset (Figure 2F). Further, we found a rise of normalized WLS and CHGA transcript levels toward an increasing degree of NE differentiation in a panel of human PC cell lines, with a roughly 2-fold increase for both genes in the NEPC tumor-derived NCI-H660 cell line compared to the AR-dependent, non-NEPC LNCaP cell line, by interrogating a human PC cell line RNA-seq database (Figure 2G) (Lee et al., 2018b). These data in aggregate demonstrate that WLS expression is strongly associated with PC disease severity, especially the NE phenotype, in a clinical setting.
Figure 2.
WLS is highly expressed in human CRPC and NEPC
(A) IHC images of WLS protein expression in representative CRPC versus hormone-sensitive counterpart (HSPC) patient samples from the cohorts described in (B). Scale bars: 20 μm.
(B) Quantification of WLS protein expression between HSPC (n = 39) and CRPC (n = 21) cohorts by average cell-based IHC staining intensity counts analyzed by inForm software. Data represent the mean ± SEM. ∗∗p < 0.01, unpaired t test.
(C) IHC images of WLS and CHGA protein staining in two representative CRPC patient samples. Scale bars: 20 μm.
(D) Protein co-expression correlation between WLS and CHGA in the CRPC cohort (n = 21). Average cell-based staining intensity counts for each individual protein were analyzed by inForm software to access the co-expression correlation by Pearson correlation.
(E) Quantification of WLS mRNA expression between CRPC (n = 39) and NEPC (n = 12) cohorts in the Beltran dataset. Data represent the mean ± SEM. ∗p < 0.05, unpaired t test.
(F) mRNA co-expression correlation of WLS with NE marker genes, AR and REST represented by positive (blue) or inverse (red) correlations in the Beltran dataset. ∗p < 0.05, ∗∗p < 0.01, Pearson correlation.
(G) Relative WLS and CHGA mRNA levels normalized to ACTB in human PC cell lines toward an increasing degree of NE differentiation from CellExpress.
See also Figure S3.
WLS is transcriptionally repressed by AR
Since one of the defined features of NEPC is attenuated AR signaling, we examined the possibility that inhibition of the AR pathway increases the expression of WLS in NEPC. Growing AR-positive, androgen-sensitive LNCaP and C4-2B cells in media supplemented with charcoal-stripped serum (CSS) to remove androgens resulted in a marked increase in WLS protein expression that was reversed by treatment with the synthetic androgen R1881 (Figure 3A). Treatment of LNCaP cells with ENZ also time-dependently induced WLS protein expression (Figure 3B). To determine the direct effect of AR on WLS, siRNA-mediated silencing of AR, accompanied by reduced PSA protein expression, increased WLS protein levels under both androgen-depleted and -replete conditions in LNCaP and C4-2B cells (Figure 3C). Moreover, we showed that R1881 treatment suppressed WLS mRNA level by 48% in LNCaP cells (Figure 3D), which was corroborated by an up to 10-fold increase of WLS transcript level in LNCaP cells in response to CSS media over long-term culture within a period time of 12 months (Figure 3E). This suggests that WLS expression might be repressed by AR at the transcriptional level.
Figure 3.
WLS is directly transcriptionally repressed by AR
(A) Western blot analysis of WLS and PSA protein expression in LNCaP and C4-2B cells grown in normal growth media (FBS, 5 days), CSS media (5 days), or CSS media (2 days) followed by R1881 treatment (1 nM, 72 hr).
(B) Western blot analysis of WLS protein expression in LNCaP cells grown in normal media treated with 20 μM ENZ for different times.
(C) Western blot analysis of WLS protein expression in LNCaP and C4-2B cells transfected with a scrambled siRNA or siRNA against AR (siAR) for 48 hr prior to treatment with either CSS media or CSS media supplemented with 1 nM R1881 for additional 72 hr.
(D) RT-qPCR analysis of WLS mRNA levels in LNCaP cells grown in CSS media for 48 hr followed by treatment with or without 1 nM R1881, with ethanol as a vehicle (Veh), for additional 72 hr (n = 3). Data represent the mean ± SEM. ∗p < 0.05, unpaired t test.
(E) Relative WLS mRNA expression in LNCaP cells exposed to long-term culture in CSS media measured by RNA-seq from dataset GSE8702. Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(F) Genomic browser representation of AR binding in the distal enhancer region of WLS gene from VCaP cells and PC patient samples extracted from ChIP-seq datasets GSE55062 and GSE70079, respectively. Each track depicts ChIP-seq AR binding intensity for a given sample. The sequences of androgen response element (ARE) at each locus are shown with nucleotides homologous to the canonical ARE underlined.
(G) ChIP-qPCR assays of AR occupancy of two ARE-centric enhancers of WLS gene (n = 3). Data represent the mean ± SEM. ∗p < 0.05, unpaired t test.
See also Figure S4.
To determine whether AR directly binds to the WLS gene locus to mediate its transcriptional repression, we analyzed two chromatin immunoprecipitation (ChIP)-seq datasets involving subjects from both cultured human PC cells and clinical prostate tumors. We found AR enrichment at two distinct sites upstream from the transcription start site (TSS) of WLS across the majority of samples examined. In the AR-expressed, androgen-sensitive VCaP cells, the affinity of AR association with the WLS gene locus was enhanced under androgen treatment, which was repressed in response to ENZ. In the human samples, AR was shown to be able to interact with the two regions of WLS genomic sequences in both tumor and adjacent normal prostate tissues from 6 matched pairs of samples, where the majority of samples demonstrated visibly higher AR occupancy in at least one region if not both in tumors versus in normal tissues. Of note, AR was found present at the WLS gene locus with varied levels of binding in all 13 human tumors included in the ChIP-seq dataset. Further, we identified two consensus androgen response elements (AREs), AGAAGAgagTGTTCT and GGAACAataTGTTCT (−75631∼-75645 and −95,081∼-95095, respectively, with WLS TSS set as +1), which both have high homology (10 of 12 bp and 12 of 12 bp for ARE1 and ARE2, respectively) with the canonical sequence GGT/AACAnnnTGTTCT for AR binding (Figure 3F) (Roche et al., 1992). We conducted ChIP assays coupled with quantitative PCR using primers that amplified the putative AR-binding sites and detected significant AR enrichment at the two ARE-centric regions in LNCaP cells after R1881 stimulation (Figure 3G). We also examined whether AR-V7, a constitutively active AR splice variant that was negligibly expressed in the parental C4-2B cells but highly induced in C4-2BENZR cells after prolonged ENZ treatment (Liu et al., 2018), interacts with the WLS gene locus by interrogating a ChIP-seq dataset seeking the AR-V7 association with chromatin in the CRPC LN95 cell line. We identified a putative site upstream from the TSS of WLS as likely for AR-V7 binding, which was distinct from the 2 sites validated for full-length AR binding, but we did not find any consensus AREs possessing a high sequence similarity with the canonical one in the AR-V7-occupied sequences (Figure S4). Thus, we reasoned that AR-V7 might not contribute directly to transcriptional regulation of WLS in PC cells. Collectively, these results suggest that WLS is negatively regulated by AR, possibly through direct AR binding to two AREs in the WLS enhancer region.
WLS is required for NEPC cell growth and NE marker expression
Our data suggest that WLS activation coevolves with the emergence of ENZ-induced NE differentiation in CRPC. To assess the requirement for WLS in supporting an NE phenotype in ENZR cells, we stably knocked down WLS expression using two shRNAs targeting separate, non-overlapping WLS coding regions in C4-2BENZR cells, which was confirmed by reduced protein levels of WLS and Wnt5A in cell lysates and conditioned media, respectively, as compared to controls by Western blot analysis (Figure 4A). We found that WLS knockdown (KD) yielded a marked reduction in the protein expression levels of two NE markers, NSE and CD56, in C4-2BENZR cells compared to controls (Figure 4A). Consistently, treatment of C4-2BENZR cells with LGK974, a small-molecule PORCN inhibitor blocking Wnt secretion and currently under a phase I clinical cancer therapy trial (NCT01351103) (Liu et al., 2013), also decreased the protein expression levels of NSE and CD56 in a dose-dependent manner. The successful blockade of Wnt secretion by LGK974 was confirmed by the absence of Wnt5A detection in the conditioned media of ENZR cells (Figure 4B). Moreover, we showed that addition of recombinant Wnt5A protein rescued the suppressive effect of WLS silencing on expression of the NE marker CD56 (Figure 4C), suggesting that WLS's effect on NE markers may be mediated by secretion of Wnt proteins. In addition to the expression of terminal NE markers, we also sought to determine if WLS is important in regulating the aggressive behavior of NEPC. We investigated the effects of WLS KD on the proliferation of three NE or NE-like PC cell lines, C4-2BENZR, 22Rv1 and PC-3. The 22Rv1 cell line was derived from a human prostate adenocarcinoma xenograft displaying an NE phenotype (Huss et al., 2004; Sramkoski et al., 1999), and showed upregulation of NE markers such as NSE under hypoxia or combined androgen deprivation and ENZ treatment (Lee et al., 2019; Qi et al., 2010). The 22Rv1 cell line also expresses AR and AR splice variants rendering cells resistant to ENZ (Li et al., 2013). Thus, the 22Rv1 cell line may well represent a clinically observed amphicrine phenotype of PC, classified as tumors composed of cells co-expressing AR and NE genes (Labrecque et al., 2019). The NE-like PC-3 cell line is AR negative with characteristics of prostatic small cell NE carcinoma and has been utilized in several NEPC studies (Guo et al., 2019; Tai et al., 2011). We showed that WLS KD significantly reduced proliferation and colony formation in all cell lines (Figures 4D, 4E, and S5), which expands the findings of reduced colony formation in C4-2BENZR and 22Rv1 cells after WLS silencing from a recent study (Lombard et al., 2019). Intriguingly, WLS KD also produced decreases of proliferation and colony formation seen in parental C4-2B cells to a similar extent in C4-2BENZR cells, suggesting that WLS might be indispensable for growth of PC cells regardless of their NE state (Figure S5). In addition, LGK974 treatment dose-dependently inhibited the proliferation of both C4-2BENZR and 22Rv1 cells (Figure 4F). These results indicate that WLS is essential for NE differentiation and NEPC cell growth.
Figure 4.
WLS is required for NEPC cell growth and NE marker expression
(A) Western blot analysis of WLS, NSE and CD56 protein expression and Wnt5A secretion in lysates or conditioned media of control (shCon) and WLS-KD (shWLS) C4-2BENZR cells.
(B) Western blot analysis of NSE and CD56 protein expression and Wnt5A secretion in C4-2BENZR cells treated with LGK974 at different concentrations for 7 days.
(C) Western blot analysis of CD56 protein expression in control, WLS-KD and WLS-KD/recombinant Wnt5A protein-added (100 ng/mL, 3 days) C4-2BENZR cells.
(D) Proliferation curves of control and WLS-KD C4-2BENZR and 22Rv1 cells by crystal violet staining (n = 4). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(E) Colony formation assays of control and WLS-KD C4-2BENZR and 22Rv1 cells (n = 3) with the number of colonies in respective control groups set as 100%. Representative images from each group are shown. Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(F) Proliferation curves of C4-2BENZR and 22Rv1 cells treated with LGK974 at different concentrations by crystal violet staining (n = 4). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
See also Figure S5.
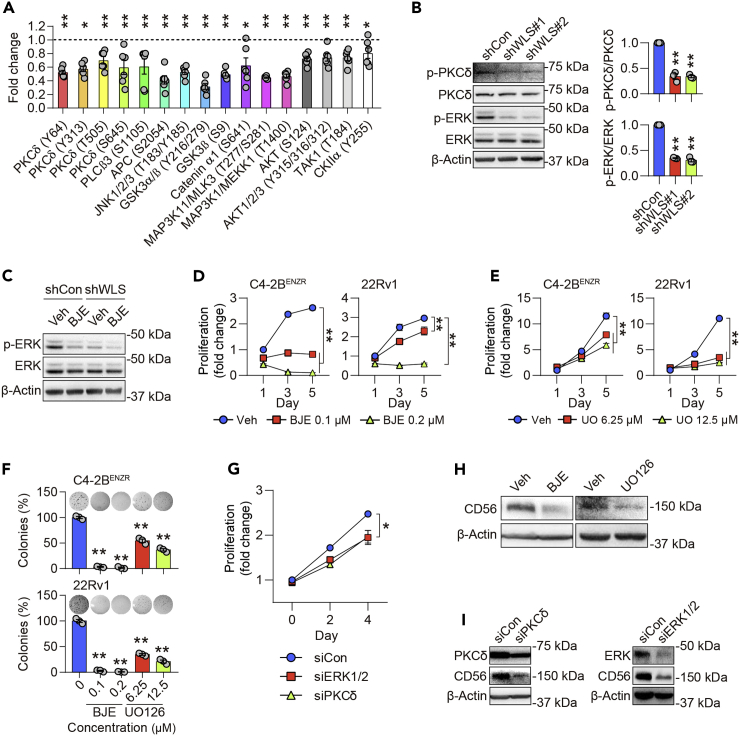
WLS induces NEPC through the ROR2/PKCδ/ERK pathway
To investigate the mechanism that mediates WLS's effect in NEPC, we first examined canonical Wnt/β-catenin pathway activity in C4-2BENZR cells. We showed increased phosphorylation levels of LRP6 in C4-2BENZR cells compared to controls (Figure S6A), an early step during activation of the canonical Wnt/β-catenin pathway (Tamai et al., 2004), which is consistent with findings from a recent report using the same ENZR cells as this study (Lombard et al., 2019). We further found marginally more accumulation of β-catenin protein in the nucleus of C4-2BENZR cells compared to controls, accompanied by higher β-catenin transcriptional activity reflected by a slight increase of TOPFlash luciferase activity after normalization with FOPFlash activity (Figures S6B and S6C). Since these results indicating very little upregulation of β-catenin did not conform to substantial activation of WLS in C4-2BENZR cells, we reasoned that the canonical Wnt/β-catenin pathway might play a minor role mediating WLS's effect in ENZ-induced NEPC.
Next, we questioned whether the noncanonical Wnt pathway involving multiple intracellular signaling cascades possibly provides a major avenue for WLS's function in NEPC. We performed an unbiased proteomic screen using a Wnt signaling phospho antibody array featuring 227 site-specific and phospho-specific antibodies important to the Wnt pathway. The levels of signaling proteins were compared in WLS-KD and control C4-2BENZR cells. Intriguingly, the screen revealed that several phosphoproteins essential in the noncanonical Wnt pathway, such as PKCδ, JNK, and AKT, were downregulated by WLS silencing compared to controls. PKCδ stands out from the list of candidate phosphoproteins because it has the most phosphorylation sites (Y64, Y313, T505, and S645) affected by WLS inhibition (Figure 5A). We validated the decline in phosphorylation levels of PKCδ protein in WLS-KD C4-2BENZR cells compared to controls (Figure 5B), with negligible changes in phosphoprotein levels of either JNK or AKT (Figure S7A). Using a candidate approach to search for effector molecule(s) downstream of PKCδ, we showed a reduction in phosphorylated ERK protein levels in WLS-KD C4-2BENZR cells compared to controls (Figure 5B). The MAPK/ERK pathway was previously demonstrated to be involved in acquiring NE properties in PC and non–small cell lung cancer cells (Chen et al., 2014; Kim et al., 2002, 2017). Consistent with observed WLS activation, the protein expression levels of total PKCδ and both active p-PKCδ and p-ERK after normalization with respective controls were higher in C4-2BENZR cells relative to control cells (Figure S7B). Following a previous report of PKCδ activation of ERK in noncancerous cells (Ueda et al., 1996), treatment with BJE-106, a third-generation PKCδ inhibitor with a 1000-fold selectivity for PKCδ over PKCɑ (Takashima et al., 2014), lessened ERK protein phosphorylation in C4-2BENZR cells, reinforcing the idea that PKCδ regulates ERK in the NEPC context (Figure S7C). Moreover, we showed that BJE-106 treatment repressed active p-ERK protein levels in C4-2BENZR cells, with such repression visibly smaller after prior WLS KD, suggesting that WLS regulates ERK through PKCδ (Figure 5C). In addition, we demonstrated that inhibition of either PKCδ with BJE-106 or ERK with UO126, a highly selective inhibitor of MEK acting upstream of ERK, suppressed proliferation and colony formation of both C4-2BENZR and 22Rv1 cells in a dose-dependent manner (Figures 5D–5F), which was paralleled by a reduction of C4-2BENZR cell proliferation under treatment with PKCδ or ERK siRNAs (Figure 5G). Further analysis of terminal NE marker expression revealed that pharmacological or siRNA-mediated inhibition of PKCδ and ERK reduced CD56 protein levels in C4-2BENZR cells compared to controls (Figures 5H and 5I).
Figure 5.
WLS mediates NEPC cell growth through the PKCδ/ERK pathway
(A) Phospho antibody array analysis of phosphoproteins implicated in Wnt signaling in WLS-KD C4-2BENZR cells. All phosphoproteins with significantly (p < 0.05) decreased levels in WLS-KD cells compared to controls are presented. All phospho signals were normalized to their total forms from a single array with 6 replicate spots (n = 6). Data represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, unpaired t test.
(B) Western blot analysis of p-PKCδ, PKCδ, p-ERK and ERK protein expression in control and WLS-KD C4-2BENZR cells, with quantification of normalized p-PKCδ and p-ERK levels presented separately (n = 3). Data represent the mean ± SEM. ∗∗p < 0.01, unpaired t test.
(C) Western blot analysis of p-ERK and ERK protein expression in control and WLS-KD C4-2BENZR cells treated with BJE-106 (BJE, 0.1 μM, 3 hr).
(D and E) Proliferation curves of C4-2BENZR and 22Rv1 cells treated with BJE-106 (D) or UO126 (UO, E) at different concentrations by crystal violet staining (n = 4). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(F) Colony formation assays of C4-2BENZR and 22Rv1 cells treated with BJE-106 or UO126 at different concentrations (n = 3). Representative images from each group are shown. Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(G) Proliferation curves of C4-2BENZR cells treated with PKCδ or ERK1/2 siRNAs by MTS assays (n = 4). Data represent the mean ± SEM. ∗p < 0.05, unpaired t test.
(H) Western blot analysis of CD56 protein expression in C4-2BENZR cells treated by BJE-106 (0.1 μM, 7 days) or UO126 (6.25 μM, 7 days).
(I) Western blot analysis of CD56 protein expression in C4-2BENZR cells treated by PKCδ or ERK1/2 siRNAs.
See also Figures S6 and S7.
WLS transports Wnts intracellularly for secretion and subsequent interaction with a variety of cell surface receptors to elicit multiple signaling pathways. Seeking the main receptor mediating WLS-Wnt signaling in NEPC, we observed increased expression of ROR2 from our RNA-seq data in C4-2BENZR cells (Figure S1B). ROR2, an orphan receptor tyrosine kinase, functions as a noncanonical Wnt receptor and binds Wnt5A, which was co-upregulated with ROR2 in the C4-2BENZR transcriptome (Figure S1B) and reported to confer antiandrogen resistance in CRPC (Ho et al., 2012; Miyamoto et al., 2015). ROR2 has been shown to activate PKCδ and ERK in different cellular contexts (Cheung et al., 2011; Xu et al., 2017). Following initial findings from RNA-seq data, we showed increased ROR2 protein expression in C4-2BENZR cells compared to controls, which was recapitulated in parental LNCaP or C4-2B cells treated with CSS media or ENZ (Figures S8A–S8C). Similar to WLS, ROR2 was upregulated in human NEPC compared with CRPC from the Beltran cohorts, and demonstrated a significant positive co-expression correlation with multiple canonical NE marker genes along with an inverse relationship with REST and AR (Figures S8D and S8E). Interestingly, we found that ROR2 protein expression was repressed when WLS was knocked down in C4-2BENZR cells (Figure 6A), supported by a positive WLS-ROR2 co-expression correlation from the Beltran CRPC/NEPC dataset (Figure 6B). Silencing of ROR2 inhibited proliferation and colony formation of C4-2BENZR cells, accompanied by reduced protein levels of CD56, p-PKCδ and p-ERK (Figures 6C–6E). We further demonstrated that transient forced expression of ROR2 reverted the suppression of proliferation and CD56 protein expression caused by WLS silencing in C4-2BENZR cells (Figures 6F and 6G), indicating ROR2's ability to overcome the effect of Wnt secretion blockade and thus rescue the NEPC phenotype. Overall, these data support the idea that WLS induces NEPC through the ROR2-dependent PKCδ/ERK pathway.
Figure 6.
ROR2 mediates WLS induction of the PKCδ/ERK pathway
(A) Western blot analysis of ROR2 protein expression in control and WLS-KD C4-2BENZR cells.
(B) Co-expression correlation between WLS and ROR2 mRNA in the Beltran dataset by Pearson correlation.
(C) Proliferation curves of C4-2BENZR cells treated with a scrambled siRNA (siCon) or ROR2 siRNAs (siROR2) by crystal violet staining (n = 4). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(D) Colony formation assays of C4-2BENZR cells treated with a scrambled siRNA or ROR2 siRNAs (n = 3). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(E) Western blot analysis of ROR2, CD56, p-PKCδ, PKC, p-ERK, and ERK protein expression in control and siRNA-mediated ROR2-KD C4-2BENZR cells.
(F) Proliferation curves of control, WLS-KD and WLS-KD/ROR2-overexpressing (OE) C4-2BENZR cells by crystal violet staining (n = 4). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(G) Western blot analysis of CD56 protein expression in control, WLS-KD and WLS-KD/ROR2-OE C4-2BENZR cells.
See also Figure S8.
WLS silencing inhibits NEPC tumor growth
To determine if the reduced proliferative capacity of WLS-silenced NEPC cells in vitro could be translated in vivo, we used 22Rv1 cells to establish subcutaneous xenograft tumors in castrated mice. We demonstrated a significant reduction in tumor growth rate and tumor weight when WLS was knocked down in tumors compared to the control group (Figures 7A and 7B). IHC analysis of tumor samples revealed lowered WLS protein expression in WLS-KD tumors, indicating effective and sustainable WLS KD effect under in vivo conditions. A 50% decrease of Ki-67+ cells, 25-fold higher cleaved caspase 3+ cells, and 63% lower CD34 staining intensity indicative of diminished tumor angiogenesis on average were found in WLS-KD tumors in comparison with controls. WLS silencing further reduced CD56, p-PKCδ, and p-ERK protein levels by an average of 30%, 26%, and 33% less staining intensity of respective proteins in WLS-KD tumors compared to controls (Figures 7C and 7D).
Figure 7.
WLS silencing inhibits NEPC tumor growth in mice
(A) Growth curves of 22Rv1 tumors (shCon, n = 14; shWLS#1, n = 14; shWLS#2, n = 12) implanted subcutaneously in castrated SCID mice. Data represent the mean ± SEM. ∗p < 0.05, one-way ANOVA.
(B) Tumor weights measured for each group in (A) at the experimental endpoint. Data represent the mean ± SEM. ∗p < 0.05, one-way ANOVA.
(C) IHC representative images of WLS, Ki-67, cleaved caspase 3 (cCas3), CD34, CD56, p-PKCδ and p-ERK in tumor samples from each group in (A). Scale bars: 20 μm.
(D) Quantitation of WLS, CD34, CD56, p-PKCδ and p-ERK IHC per-cell staining intensity and % of Ki-67+ or fold change of cCas3+ cells in tumor samples from each group in (A) (n = 15). Data represent the mean ± SEM. ∗∗p < 0.01, one-way ANOVA.
(E) Growth curves of 22Rv1 subcutaneous tumors receiving corn oil (Veh, n = 7) or LGK974 (5 mg/kg, orally, n = 8) daily in castrated nude mice. Data represent the mean ± SEM. ∗∗p < 0.01, unpaired t test.
(F) Tumor weights measured for each group in (E) at respective experimental endpoints (Veh, day 14; LGK974, day 21). Data represent the mean ± SEM. ∗∗p < 0.01, unpaired t test.
(G) Anatomic images of tumors from each group in (E) at respective experimental end points.
(H) IHC representative images of WLS, Ki-67, cCas3, CD34, CD56, p-PKCδ and p-ERK staining in tumor samples from each group in (E). Scale bars: 20 μm.
(I) Quantitation of % of Ki-67+ or fold change of cCas3+ cells and CD34, CD56, p-PKCδ and p-ERK IHC per-cell staining intensity from each group in (E) (n = 15). Data represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, unpaired t test.
To test whether a Wnt secretion inhibitor could suppress NEPC growth in vivo, we inoculated castrated mice subcutaneously with 22Rv1 cells and treated them with LGK974 through oral daily administration. After we sacrificed the mice in the control group on day 14 due to their large tumors, we continued LGK974 treatment in the treatment group for another 7 days. The average tumor volume in mice receiving LGK974 treatment was 86% smaller than that in the control group (control group vs. LGK974-treated group: 1,841 ± 341 mm3 vs. 327 ± 153 mm3 on day 14, or 1,841 ± 341 mm3 on day 14 vs. 249 ± 107 mm3 on day 21) (Figure 7E). Consistently, LGK974 treatment resulted in a 71% reduction in tumor weight (control group vs. LGK974-treated group: 1.24 ± 0.28 g on day 14 vs. 0.36 ± 0.12 g on day 21) (Figures 7F and 7G). The efficacy of LGK974 for the blockade of Wnt secretion was confirmed by significantly less Wnt5A protein stained by IHC in tumors given LGK974 compared to controls. LGK974 treatment also yielded a 21% decrease of Ki-67+ tumor cells, a 7.5-fold increase of cleaved caspase 3+ tumor cells, and 44%, 71%, 35%, and 26% lower tumor expression of CD34, CD56, p-PKCδ, and p-ERK, respectively, compared to controls by IHC analysis (Figures 7H and 7I).
In summary, our preclinical studies using ENZR NEPC models revealed the prerequisite role of WLS in the development of an NE phenotype in CRPC. WLS is activated upon relieving AR transcriptional suppression and in turn activates the ROR2/PKCδ/ERK signaling pathway to promote NE marker expression and NEPC growth. This can be effectively limited by pharmacological inhibition of the Wnt secretion pathway mediated by WLS and its associated molecules.
Discussion
In this study, we identified WLS as an important mediator and potential therapeutic target in NEPC, a poorly understood and lethal disease which has shown increasing incidence in recent years after more potent APIs, including ENZ, began to be used for CRPC treatment. Our studies present several findings with implications for patients with CRPC and NEPC. Utilizing an in vitro-derived model of ENZR CRPC that faithfully mimics t-NEPC, we demonstrated that WLS, as activated in NEPC cells, is required for the NE and proliferative phenotype of NEPC cells. We also presented the evidence that WLS is suppressed by AR likely via direct AR interaction with 2 ARE-encompassing WLS enhancers, leading to WLS upregulation in NEPC cells where AR signaling in general is absent or inhibited. Further studies are warranted to validate the functionality of these AR-binding sites for modulating the transcription of WLS. Additionally, alternative mechanisms possibly contributing to WLS upregulation in NEPC cells, especially the reported Wnt ligand-dependent ERAD (endoplasmic reticulum-associated degradation) control of WLS protein levels based on the observed concurrent induction of WLS and Wnts in C4-2BENZR cells (Glaeser et al., 2018), also merit additional exploration. We further demonstrated the feasibility and efficacy of pharmacologically targeting Wnt secretion for inhibition of NEPC tumor growth in mice. These studies expand our understanding of the molecular pathogenesis of NEPC and provide a rationale for targeting Wnt secretion for the treatment and/or prevention of NEPC emerging from high-potency API-based CRPC therapy.
WLS is a Wnt cargo receptor protein first discovered in developmental biology in Drosophila and demonstrated in mammals later, where it serves as a core component of the Wnt secretion machinery to regulate diverse processes including body axis formation, retinal angiogenesis, hair follicle formation, and bone mass regulation. WLS loss leads to intracellular accumulation of Wnts in Wnt-secreting cells without release of Wnts to the extracellular milieu, thereby impeding Wnt signaling (Banziger et al., 2006; Bartscherer et al., 2006; Fu et al., 2009; Goodman et al., 2006). Recent studies have brought WLS's role in cancer into focus owing to aberrant Wnt signaling molecularly linked to cancer development and progression. WLS has been shown to play a role in many human cancers, including glioma, bladder, gastric and colorectal cancers, and more recently PC (Augustin et al., 2012; Glaeser et al., 2018; Lombard et al., 2019; Schmid et al., 2017; Seo et al., 2018). Complementing these reports, we identified WLS as a highly expressed member of Wnt signaling from RNA-seq of an ENZR NEPC cell line, making it a top candidate for mediating NE differentiation in PC. Our observation of WLS upregulation in ENZR cells is also consistent with the findings from a recent study using the same ENZR cells as ours (Lombard et al., 2019). Mounting evidence suggests a conceptual two-stage progression to NEPC through the acquisition of an NE-like phenotype from an AR-dependent adenocarcinoma followed by the initiation of cell proliferation (Akamatsu et al., 2015). Our data indicate that WLS functions at both stages, because silencing WLS expression led to concomitant reduction in NE markers, NEPC cell proliferation, and NEPC tumor growth. In addition, our data from human specimens show the clinical relevance of WLS to aggressive PC, including CRPC and NEPC. The Beltran cohorts and 2 additional independent datasets showed that WLS tends to be increasingly expressed in clinically defined NEPC compared to CRPC tumors. In addition, WLS also has clinical relevance in PC beyond the NEPC context based on our observation of its overexpression in CRPC compared to HSPC in our cohorts. Using the same ENZR C4-2B cells as in our study, Lombard et al. recently showed that WLS silencing reduces expression of AR, AR variants, AR target genes and PSA levels in C4-2BENZR cells, and further restores ENZ sensitivity in resistant cells (Lombard et al., 2019). Coupling this report with our study, we argue that WLS may have the dual role of supporting the baseline activity and activation of AR signaling in AR-dominant PC disease stages, including CRPC, as well as heightening the potential for AR-suppressed NEPC disease progression in an androgen deprivation setting, which expands the therapeutic utility of targeting WLS for treatment of advanced PC, especially for the clinically emergent type of amphicrine tumors composed of cells exhibiting both AR and NE activity (Labrecque et al., 2019).
The transdifferentiation of CRPC into NEPC under selective pressure from APIs like ENZ requires activation of alternative survival pathways that replace AR as the driver of PC survival and growth. Our results show that inhibiting WLS suppresses a noncanonical Wnt pathway driven by the ROR2/PKCδ/ERK cascade, which is overexpressed in NEPC cells and is required for NE marker expression and NEPC cell growth. ROR2 is a favored receptor for Wnt5A to convey noncanonical Wnt5A signaling (Ho et al., 2012). Wnt5A is the most significantly activated Wnt from our RNA-seq data in ENZR NEPC cells, raising the possibility that Wnt5A is the dominant Wnt mediating WLS-Wnt signaling in NEPC. Wnt5A exhibits both tumor promoting and suppressive roles in PC, which likely depends on the combination and availability of different Wnt receptors for alterations in signaling output (Lee et al., 2018a; Ren et al., 2019; Thiele et al., 2018). We suspect that elevated expression of ROR2 in NEPC cells could release Wnt5A from association with other Wnt receptors, making it more prone to support NEPC growth. Intriguingly, we detected only a marginal increase of nuclear β-catenin level in parallel to much more WLS activation in ENZR cells, and therefore speculated that the canonical Wnt/β-catenin signaling might not play a principal role supporting WLS function in NEPC. On the other hand, using the same ENZR cells as ours, Lombard et al. recently showed that WLS silencing decreased canonical Wnt signaling evidenced by reduced levels of p-LRP6 and β-catenin target genes, including LEF1 and BAMBI, but whether the canonical Wnt signaling directly mediates WLS's observed effect on cell behaviors was inconclusive in this study (Lombard et al., 2019). Based on the findings of Lombard et al. and our findings, we argue that canonical and noncanonical Wnt signaling may both contribute to WLS's effects in ENZR NEPC, with the noncanonical Wnt signaling likely outcompeting the canonical signaling. Indeed, we revealed more drastic activation of noncanonical Wnts, such as Wnt5A, Wnt5B and Wnt11, as compared to canonical Wnts in ENZR cells. Further, these noncanonical Wnts including Wnt5A and Wnt11, which both are concomitantly implicated in NEPC, are able to inhibit the canonical Wnt/β-catenin pathway (Bisson et al., 2015; Nemeth et al., 2007). In addition, we also identified PKCδ as a hub molecule linking ROR2 to ERK for mediation of WLS's function in NEPC, as shown by a Wnt-regulated phospho antibody array. PKCδ′s controversial role in PC depends on the cellular context. PKCδ induces apoptosis in androgen-dependent PC cells via autocrine secretion of death factors, but has pro-survival effects in PC cells displaying stem cell properties, which are reported to be retained in NEPC cells as well (Gonzalez-Guerrico et al., 2005; Harris and Kerr, 2017; Kumar et al., 2014). Nevertheless, we showed that PKCδ is required for the maintenance of NE and the proliferative characteristics of NEPC cells. Our data collectively support the idea that WLS-Wnt signaling activation of the ROR2/PKCδ/ERK noncanonical Wnt pathway displaces the AR-dependent mechanism to promote the acquisition of an NE phenotype and confer growth advantages in NEPC cells.
In light of the complex transduction processes in the receiving cells, inhibition of Wnt ligand secretion in the producing cells has been considered one of the most efficacious ways to target Wnt signaling in cancer. The prevalent upregulation of Wnts and WLS in ENZR NEPC cells provides a rationale for using Wnt secretion inhibitors to treat NEPC. The orally bioavailable small-molecule inhibitor LGK974, which interferes with Wnt secretion by binding directly to and inhibiting PORCN, showed promise in an NEPC tumor xenograft mouse model in addition to in vitro settings. Notably, LGK974 treatment inhibited NEPC tumor growth as well as tumor expression of the NE marker CD56 much more drastically than the genetic silencing of WLS, which might be in large part due to global inhibition of Wnt secretion in mice including secretion by the mouse tumor microenvironment. LGK974 is currently being tested in a Phase I dose escalation clinical trial (NCT01351103) for patients with malignancies dependent on Wnt ligands. This multi-site trial is still in the recruiting phase for most of the sites participating in the trial but is currently active and set to be completed in 2022. The results of the interim analysis recently demonstrated that a combined use of LGK974 (named as WNT974 in this trial) ± spartalizumab alongside effective skin AXIN2 suppression was well tolerated in patients with several types of advanced solid tumors (Janku et al., 2020). Nevertheless, more attention to the end results of this trial would be needed for an overall assessment of the safety and potential of LGK974 for further clinical development. In a recent study, LGK974 also demonstrated growth inhibition efficacy in CRPC-derived, AR-positive VCaP xenograft prostate tumors in mice, which could mirror a precursor of NEPC if coupled to API exposure (Ma et al., 2016). Whether LGK974 treatment could prevent the transition of CRPC into NEPC merits further investigation using a recently described serial transplantation xenograft model in the presence of ENZ (Bishop et al., 2017).
In conclusion, we demonstrated the functional importance of WLS for mediating the development and progression of NEPC under the selective pressures of APIs such as ENZ. We also provided strong preclinical evidence for using Wnt secretion inhibitors for a wide-spectrum inhibition of the Wnt pathway as a potential targeted therapy for NEPC.
Limitations of the study
This study would be strengthened by including more bona fide human NEPC cell lines to consolidate the major findings and also by using a more clinically relevant animal model, such as the NEPC patient-derived xenograft model, to evaluate LGK974's effectiveness for halting NEPC growth in mice.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Boyang (Jason) Wu (boyang.wu@wsu.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The control and C4-2BENZR cell RNA-seq data generated during this study are available at GEO with the accession number GSE159548.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
This work was supported by NIH/NCI grant R37CA233658, Department of Defense Prostate Cancer Research Program grant W81XWH-19-1-0279, and WSU startup funds to B.J.W. We thank Yidi Xu (Washington State University) for providing technical assistance. We also thank Gary Mawyer for editorial assistance.
Authors contribution
T.B. and B.J.W. conceived and designed the study. T.B., J.W., L.Y., T.P., J.L., and J.G. performed the experiments. T.B., J.W., L.Y., and B.J.W. analyzed and interpreted the data. T-P.L. provide human CRPC specimens. A.C.G. provided control and ENZR C4-2B cells. T.B. and B.J.W. wrote the manuscript. B.J.W. supervised the overall research and acquired funding.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2020.101970.
Supplemental information
References
- Akamatsu S., Wyatt A.W., Lin D., Lysakowski S., Zhang F., Kim S., Tse C., Wang K., Mo F., Haegert A. The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep. 2015;12:922–936. doi: 10.1016/j.celrep.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Aparicio A., Logothetis C.J., Maity S.N. Understanding the lethal variant of prostate cancer: power of examining extremes. Cancer Discov. 2011;1:466–468. doi: 10.1158/2159-8290.CD-11-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I., Goidts V., Bongers A., Kerr G., Vollert G., Radlwimmer B., Hartmann C., Herold-Mende C., Reifenberger G., von Deimling A., Boutros M. The Wnt secretion protein Evi/Gpr177 promotes glioma tumourigenesis. EMBO Mol. Med. 2012;4:38–51. doi: 10.1002/emmm.201100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K., Pelte N., Ingelfinger D., Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Beltran H., Prandi D., Mosquera J.M., Benelli M., Puca L., Cyrta J., Marotz C., Giannopoulou E., Chakravarthi B.V., Varambally S. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H., Tomlins S., Aparicio A., Arora V., Rickman D., Ayala G., Huang J., True L., Gleave M.E., Soule H. Aggressive variants of castration-resistant prostate cancer. Clin. Cancer Res. 2014;20:2846–2850. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J.L., Thaper D., Vahid S., Davies A., Ketola K., Kuruma H., Jama R., Nip K.M., Angeles A., Johnson F. The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017;7:54–71. doi: 10.1158/2159-8290.CD-15-1263. [DOI] [PubMed] [Google Scholar]
- Bisson J.A., Mills B., Paul Helt J.C., Zwaka T.P., Cohen E.D. Wnt5a and Wnt11 inhibit the canonical Wnt pathway and promote cardiac progenitor development via the Caspase-dependent degradation of AKT. Dev. Biol. 2015;398:80–96. doi: 10.1016/j.ydbio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Chen X., Liu J., Cheng L., Li C., Zhang Z., Bai Y., Wang R., Han T., Huang C., Kong Y. Inhibition of noncanonical Wnt pathway overcomes enzalutamide resistance in castration-resistant prostate cancer. Prostate. 2020;80:256–266. doi: 10.1002/pros.23939. [DOI] [PubMed] [Google Scholar]
- Chen Y., Nowak I., Huang J., Keng P.C., Sun H., Xu H., Wei G., Lee S.O. Erk/MAP kinase signaling pathway and neuroendocrine differentiation of non-small-cell lung cancer. J. Thorac. Oncol. 2014;9:50–58. doi: 10.1097/JTO.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Cheung R., Kelly J., Macleod R.J. Regulation of villin by wnt5a/ror2 signaling in human intestinal cells. Front. Physiol. 2011;2:58. doi: 10.3389/fphys.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Jiang M., Mirando A.J., Yu H.M., Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. U S A. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser K., Urban M., Fenech E., Voloshanenko O., Kranz D., Lari F., Christianson J.C., Boutros M. ERAD-dependent control of the Wnt secretory factor Evi. EMBO J. 2018;37:e97311. doi: 10.15252/embj.201797311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guerrico A.M., Meshki J., Xiao L., Benavides F., Conti C.J., Kazanietz M.G. Molecular mechanisms of protein kinase C-induced apoptosis in prostate cancer cells. J. Biochem. Mol. Biol. 2005;38:639–645. doi: 10.5483/bmbrep.2005.38.6.639. [DOI] [PubMed] [Google Scholar]
- Goodman R.M., Thombre S., Firtina Z., Gray D., Betts D., Roebuck J., Spana E.P., Selva E.M. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Guo H., Ci X., Ahmed M., Hua J.T., Soares F., Lin D., Puca L., Vosoughi A., Xue H., Li E. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat. Commun. 2019;10:278. doi: 10.1038/s41467-018-08133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.S., Kerr B.A. Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. Stem Cells Int. 2017;2017:8629234. doi: 10.1155/2017/8629234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.Y., Susman M.W., Bikoff J.B., Ryu Y.K., Jonas A.M., Hu L., Kuruvilla R., Greenberg M.E. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc. Natl. Acad. Sci. U S A. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss W.J., Gregory C.W., Smith G.J. Neuroendocrine cell differentiation in the CWR22 human prostate cancer xenograft: association with tumor cell proliferation prior to recurrence. Prostate. 2004;60:91–97. doi: 10.1002/pros.20032. [DOI] [PubMed] [Google Scholar]
- Isaacsson Velho P., Fu W., Wang H., Mirkheshti N., Qazi F., Lima F.A.S., Shaukat F., Carducci M.A., Denmeade S.R., Paller C.J. Wnt-pathway activating mutations are associated with resistance to first-line abiraterone and enzalutamide in castration-resistant prostate cancer. Eur. Urol. 2020;77:14–21. doi: 10.1016/j.eururo.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F., de Vos F., de Miguel M., Forde P., Ribas A., Nagasaka M., Argiles G., Arance A.M., Calvo A., Giannakis M. Phase I study of WNT974 + spartalizumab in patients (pts) with advanced solid tumors. Cancer Res. 2020;80 https://cancerres.aacrjournals.org/content/80/16_Supplement/CT034 Abstract nr CT034. [Google Scholar]
- Kim J., Adam R.M., Freeman M.R. Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res. 2002;62:1549–1554. [PubMed] [Google Scholar]
- Kim J., Jin H., Zhao J.C., Yang Y.A., Li Y., Yang X., Dong X., Yu J. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene. 2017;36:4072–4080. doi: 10.1038/onc.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Shankar S., Srivastava R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014;343:179–189. doi: 10.1016/j.canlet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Labrecque M.P., Coleman I.M., Brown L.G., True L.D., Kollath L., Lakely B., Nguyen H.M., Yang Y.C., da Costa R.M.G., Kaipainen A. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Invest. 2019;129:4492–4505. doi: 10.1172/JCI128212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapuk A.V., Wu C., Wyatt A.W., McPherson A., McConeghy B.J., Brahmbhatt S., Mo F., Zoubeidi A., Anderson S., Bell R.H. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J. Pathol. 2012;227:286–297. doi: 10.1002/path.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.T., Kwon S.J., Kim J., Kwon Y.S., Lee N., Hong J.H., Jamieson C., Kim W.J., Kim I.Y. WNT5A induces castration-resistant prostate cancer via CCL2 and tumour-infiltrating macrophages. Br. J. Cancer. 2018;118:670–678. doi: 10.1038/bjc.2017.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G.T., Rosenfeld J.A., Kim W.T., Kwon Y.S., Palapattu G., Mehra R., Kim W.J., Kim I.Y. TCF4 induces enzalutamide resistance via neuroendocrine differentiation in prostate cancer. PloS one. 2019;14:e0213488. doi: 10.1371/journal.pone.0213488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.F., Lee C.Y., Lai L.C., Tsai M.H., Lu T.P., Chuang E.Y. Vol. 2018. Database; 2018. CellExpress: A Comprehensive Microarray-Based Cancer Cell Line and Clinical Sample Gene Expression Analysis Online System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chan S.C., Brand L.J., Hwang T.H., Silverstein K.A., Dehm S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Lou W., Yang J.C., Liu L., Armstrong C.M., Lombard A.P., Zhao R., Noel O.D.V., Tepper C.G., Chen H.W. Proteostasis by STUB1/HSP70 complex controls sensitivity to androgen receptor targeted therapy in advanced prostate cancer. Nat. Commun. 2018;9:4700. doi: 10.1038/s41467-018-07178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Lou W., Zhu Y., Yang J.C., Nadiminty N., Gaikwad N.W., Evans C.P., Gao A.C. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015;75:1413–1422. doi: 10.1158/0008-5472.CAN-14-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Pan S., Hsieh M.H., Ng N., Sun F., Wang T., Kasibhatla S., Schuller A.G., Li A.G., Cheng D. Targeting wnt-driven cancer through the inhibition of porcupine by LGK974. Proc. Natl. Acad. Sci. U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard A.P., Liu C., Armstrong C.M., D'Abronzo L.S., Lou W., Evans C.P., Gao A.C. Wntless promotes cellular viability and resistance to enzalutamide in castration-resistant prostate cancer cells. Am. J. Clin. Exp. Urol. 2019;7:203–214. [PMC free article] [PubMed] [Google Scholar]
- Ma F., Ye H., He H.H., Gerrin S.J., Chen S., Tanenbaum B.A., Cai C., Sowalsky A.G., He L., Wang H. SOX9 drives WNT pathway activation in prostate cancer. J. Clin. Invest. 2016;126:1745–1758. doi: 10.1172/JCI78815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto D.T., Zheng Y., Wittner B.S., Lee R.J., Zhu H., Broderick K.T., Desai R., Fox D.B., Brannigan B.W., Trautwein J. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–1356. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moparthi L., Pizzolato G., Koch S. Wnt activator FOXB2 drives the neuroendocrine differentiation of prostate cancer. Proc. Natl. Acad. Sci. U S A. 2019;116:22189–22195. doi: 10.1073/pnas.1906484116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Garzon V., Kypta R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017;14:683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- Nemeth M.J., Topol L., Anderson S.M., Yang Y., Bodine D.M. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc. Natl. Acad. Sci. U S A. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J., Nakayama K., Cardiff R.D., Borowsky A.D., Kaul K., Williams R., Krajewski S., Mercola D., Carpenter P.M., Bowtell D., Ronai Z.A. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18:23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan W.H., Kabbara W.K., Al Basiouni Al Masri H.S. Enzalutamide for patients with metastatic castration-resistant prostate cancer. Onco Targets Ther. 2015;8:871–876. doi: 10.2147/OTT.S80488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Dai Y., Yang Q., Zhang X., Guo W., Ye L., Huang S., Chen X., Lai Y., Du H. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J. Exp. Med. 2019;216:428–449. doi: 10.1084/jem.20180661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P.J., Hoare S.A., Parker M.G. A consensus DNA-binding site for the androgen receptor. Mol. Endocrinol. 1992;6:2229–2235. doi: 10.1210/mend.6.12.1491700. [DOI] [PubMed] [Google Scholar]
- Schmid S.C., Sathe A., Guerth F., Seitz A.K., Heck M.M., Maurer T., Schwarzenbock S.M., Krause B.J., Schulz W.A., Stoehr R. Wntless promotes bladder cancer growth and acts synergistically as a molecular target in combination with cisplatin. Urol. Oncol. 2017;35:544.e1–544.e10. doi: 10.1016/j.urolonc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Seo J., Kee H.J., Choi H.J., Lee J.E., Park S.Y., Lee S.H., Jeong M.H., Guk G., Lee S., Choi K.C. Inhibition of Wntless/GPR177 suppresses gastric tumorigenesis. BMB Rep. 2018;51:255–260. doi: 10.5483/BMBRep.2018.51.5.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA: Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Sramkoski R.M., Pretlow T.G., 2nd, Giaconia J.M., Pretlow T.P., Schwartz S., Sy M.S., Marengo S.R., Rhim J.S., Zhang D., Jacobberger J.W. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell. Dev. Biol. Anim. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- Sternberg C.N. Enzalutamide, an oral androgen receptor inhibitor for treatment of castration-resistant prostate cancer. Future Oncol. 2019;15:1437–1457. doi: 10.2217/fon-2018-0940. [DOI] [PubMed] [Google Scholar]
- Tai S., Sun Y., Squires J.M., Zhang H., Oh W.K., Liang C.Z., Huang J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A., English B., Chen Z., Cao J., Cui R., Williams R.M., Faller D.V. Protein kinase Cdelta is a therapeutic target in malignant melanoma with NRAS mutation. ACS Chem. Biol. 2014;9:1003–1014. doi: 10.1021/cb400837t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. A mechanism for Wnt coreceptor activation. Mol. Cel. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Thiele S., Zimmer A., Gobel A., Rachner T.D., Rother S., Fuessel S., Froehner M., Wirth M.P., Muders M.H., Baretton G.B. Role of WNT5A receptors FZD5 and RYK in prostate cancer cells. Oncotarget. 2018;9:27293–27304. doi: 10.18632/oncotarget.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y., Hirai S., Osada S., Suzuki A., Mizuno K., Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J. Biol. Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- Uysal-Onganer P., Kawano Y., Caro M., Walker M.M., Diez S., Darrington R.S., Waxman J., Kypta R.M. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol. Cancer. 2010;9:55. doi: 10.1186/1476-4598-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.T., Yao Y.H., Li B.G., Tang Y., Chang J.W., Zhang J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J. Clin. Oncol. 2014;32:3383–3390. doi: 10.1200/JCO.2013.54.3553. [DOI] [PubMed] [Google Scholar]
- Wong Y.N., Ferraldeschi R., Attard G., de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat. Rev. Clin. Oncol. 2014;11:365–376. doi: 10.1038/nrclinonc.2014.72. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ma Y.H., Pang Y.X., Zhao Z., Lu J.J., Mao H.L., Liu P.S. Ectopic repression of receptor tyrosine kinase-like orphan receptor 2 inhibits malignant transformation of ovarian cancer cells by reversing epithelial-mesenchymal transition. Tumour Biol. 2017;39 doi: 10.1177/1010428317701627. 1010428317701627. [DOI] [PubMed] [Google Scholar]
- Yu J., Chia J., Canning C.A., Jones C.M., Bard F.A., Virshup D.M. WLS retrograde transport to the endoplasmic reticulum during Wnt secretion. Dev. Cell. 2014;29:277–291. doi: 10.1016/j.devcel.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Cheng L., Li J., Farah E., Atallah N.M., Pascuzzi P.E., Gupta S., Liu X. Inhibition of the wnt/beta-catenin pathway overcomes resistance to enzalutamide in castration-resistant prostate cancer. Cancer Res. 2018;78:3147–3162. doi: 10.1158/0008-5472.CAN-17-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The control and C4-2BENZR cell RNA-seq data generated during this study are available at GEO with the accession number GSE159548.