Abstract
Fabry disease (FD) is an X-linked lysosomal storage disorder caused by a deficiency in the enzyme α-galactosidase A due to mutations in the GLA gene. This leads to an accumulation of globotriaosylceramide (GL-3) in many tissues, which results in progressive damage to the kidneys, heart, and nervous system. We present the molecular and clinical characteristics and long-term outcomes of FD patients from a multidisciplinary clinic at the University of California, Irvine treated with agalsidase beta enzyme replacement therapy (ERT) for 2–20 years. This cohort comprised 24 adults (11 males, 13 females) and two male children (median age 45; range 10–68 years). Of the 26 patients in this cohort, 20 were on ERT (12 males, 8 females). We describe one novel variant not previously reported in the literature in a patient with features of ‘classic’ FD. The vast majority of patients in this cohort presented with symptoms of ‘classic’ FD including peripheral neuropathic pain, some form of cardiac involvement, angiokeratomas, corneal verticillata, hypohidrosis, tinnitus, and gastrointestinal symptoms, primarily abdominal pain. The majority of males had clinically evident renal involvement. An annual eGFR reduction of −1.88 mL/min/1.73 m2/yr during the course of ERT was seen in this cohort. The most common renal presentation was proteinuria, and one individual required a renal transplant. Other common findings were pulmonary involvement, lymphedema, hearing loss, and significantly, three patients had strokes. Notably, there was a high prevalence of endocrine dysfunction and low bone mineral density, including several with osteoporosis.
While enzyme replacement therapy (ERT) cleared plasma GL-3 in this cohort, there was limited improvement in renal function or health-related quality of life based on the patient-reported SF-36 Health Survey. Physical functioning significantly declined over the course of ERT treatment, which may be, in part, due to the late initiation of ERT in several patients. Further delineation of the phenotypic and genotypic spectrum in patients with FD and the long-term outcome of ERT will help improve management and treatment options for this disease.
Keywords: Fabry disease, Enzyme replacement therapy, Agalsidase beta, Globotriaosylceramide, GL-3, GLA gene
1. Introduction
Fabry Disease (FD, OMIM#301500) is an inherited, X-linked lysosomal storage disorder caused by mutations in the GLA gene that result in deficient or absent α-galactosidase A enzyme [1]. This consequently leads to the progressive deposition of globotriaosylceramide (GL-3) in several tissues throughout the body, notably in endothelial cells, smooth muscle cells, podocytes, and cardiomyocytes [2]. The absence of this enzyme may lead to multi-organ involvement including progressive renal disease, cardiac disease, cerebrovascular disease, neuropathic pain, corneal verticillata, angiokeratomas, tinnitus, hearing loss, pulmonary involvement, and gastrointestinal complaints, including abdominal pain, constipation, and diarrhea. FD is pan-ethnic and the second most prevalent lysosomal storage disorder with an estimated incidence of 1 in 40,000 to 117,000 males worldwide, though newborn screening initiatives have revealed that the incidence is much higher [[3], [4], [5], [6]]. This is likely due to the fact that early studies referred to the incidence of ‘classic’ FD, while newborn screening may identify individuals on the full phenotypic spectrum. Symptoms typically present early during childhood or adolescence. Affected males with little or absent α-galactosidase A activity (<1% of mean normal) may present with clinical features of ‘classic’ FD. Mutations that result in residual enzyme activity typically cause a ‘later-onset’ form of the disease with variable manifestations. Heterozygous females typically present with milder symptoms and later-onset and less commonly have ‘classic’ FD due to residual enzyme activity and X-chromosome inactivation pattern.
Management and guidelines of a newly diagnosed individual with FD includes documentation of symptoms, consideration of enzyme replacement therapy (ERT), and monitoring of disease progression [7,8]. ERT is provided to supplement or replace α-galactosidase A, and to slow the rate of disease progression. Thus, early identification of affected individuals is important in order to initiate ERT as early as possible [[8], [9], [10]]. Clinical evaluation during childhood is recommended if a familial mutation is known [11]. In the absence of a family history, a timely diagnosis of FD may be difficult due to the variation and unpredictability of clinical manifestations that can often be misdiagnosed as childhood growing pains. The addition of FD to newborn screening programs worldwide has allowed for the early detection of the disorder [12].
There are two types of enzyme replacement therapy: agalsidase alfa (Replagal) and agalsidase beta (Fabrazyme); however only agalsidase beta is an approved ERT by the US Food and Drug Administration (FDA) [13,14]. There is some discussion on the most appropriate infusion as well as the efficacy and safety of the infusions, but studies have shown no adverse effects in switching therapies or an increased benefit of using one over the other [15,16]. There is one oral pharmacological chaperone therapy, migalastat (Galafold), that has been approved by the FDA [17]. This treatment is only available for adults with FD who have amenable gene variants that lead to misfolded α-galactosidase A enzyme that can be stabilized by migalastat. Once stabilized, protein folding is improved and trafficking of the α-galactosidase A enzyme to the lysosome is restored. Other treatments currently being investigated in clinical trials include plant-based ERT, gene therapy, and substrate reducing therapies.
We describe a cohort of 24 adults and two children with FD, 13 males and 13 females, who range in age from 10 to 68 years (mean age 46.1 ± 16.3). The majority of participants were on agalsidase beta ERT (20/26; 12 males, 8 females). Each participant demonstrated different response rates of symptoms and disease progression with ERT, including two individuals who developed adverse infusion reactions.
The aim of this study is to present the variable molecular and clinical features in a cohort of FD patients on ERT, treatment outcomes across organ systems, and highlight patients with previously unreported variants, to help improve treatment options and patient care.
2. Methods
2.1. Participants
This cohort comprised 13 male and 13 female patients, including two male children. All participants had a confirmed molecular diagnosis of FD and most also had recorded enzyme activity levels measured. GLA variants are listed using transcript NM_000169.2. These patients were followed over the course of a one to 12 year period in an outpatient multidisciplinary clinic at the University of California, Irvine. Earlier data were obtained from review of past medical records and the Fabry Registry, if available. Written IRB informed consent approved by UC Irvine (#2008–6631) was obtained from each participant, and all procedures were performed according to The Code of Ethics of the World Medical Association.
2.2. Clinical evaluations
Clinical surveillance visits were conducted every six months and included evaluations by a clinical geneticist, a cardiologist, and a nephrologist. The standard of care for patients with renal and cardiac disease from FD did not vary when compared to the management of other etiologies. At each visit, the following components were documented:
-
•
Quality of life surveys, including a Brief Pain Inventory (BPI) and the SF-36 Health Survey. These provided a scale for pain and represented an attempt to document how pain has influenced the quality of life of each individual. The SF-36 Health survey measures physical function, social function, physical role, emotional role, mental health, energy, pain, and general health perception. Scores ranged from 0 (worst) to 100 (best).
-
•
The development or change in the status of clinical features specific to FD including cardiac, renal, pulmonary, and nervous system involvement, in addition to angiokeratomas, acroparesthesia, GI health, hyper or hypohidrosis, hearing loss and tinnitus, lymphedema, and corneal whorling.
-
•
Plasma and urine GL-3 levels and plasma Lyso-GL-3 to assess overall glycolipid burden.
-
•
Renal status and disease progression were monitored by labs: serum creatinine levels to measure the mean estimated glomerular filtration rate (eGFR), in addition to urine creatinine, urine protein, and urine albumin/micro-albumin to measure proteinuria and albuminuria. Renal biopsy was only performed if clinically indicated.
Evaluations performed at less frequent intervals:
-
•
Cardiac status was determined by echocardiography (with strain), electrocardiograms, and holter monitor screened at annual intervals in males and less frequently in females and children. Cardiac MRI was conducted every 2–3 years or as needed.
-
•
Brain imaging was performed by MRI, MRA, and CT scan.
-
•
Ophthalmologic exams were performed for evaluation of corneal whorls, vessel tortuosity, and lenticular opacities.
-
•
Audiometry was performed for evaluation of sensorineural hearing loss and tinnitus, as needed.
-
•
Dual-energy X-ray absorptiometry (DXA) & the World Health Organization (WHO) classification of bone mineral density were used to classify severity. T-scores between −1.0 and −2.5 SD were categorized as osteopenia and T-scores below −2.5 SD were categorized as osteoporosis.
2.3. ERT infusions
Agalsidase beta (Fabrazyme) recommended dosage is 1 mg/kg body weight given every two weeks as an intravenous infusion [14]. The majority of the participants in this cohort received the infusions without incident, and some have transitioned to home infusions. To determine the effectiveness of ERT, we measured the presence of agalsidase beta IgG antibodies, plasma and urine GL-3 levels, and plasma Lyso-GL-3 levels at each six-month visit. Two individuals experienced adverse reactions to ERT infusions (patient 15 and patient 22); they both tested negative for IgE antibodies and showed no change in IgG antibodies.
2.4. Statistical analysis
We used linear mixed-effects regression analysis with varying intercepts and slopes to evaluate linear time trends of serum creatinine, eGFR, and health-related quality of life scores (SF-36 Health Survey), starting with the initiation of ERT (time = 0). The significance level for all statistical tests was set to α = 0.05. All analyses were carried out using the R language and environment for statistical computing, version 3.6.3.
3. Results
Of the 26 patients included in our study, there were 13 males and 13 females between ages 10–68 years (mean age 46.1 ± 16.3 years). This cohort consisted of five families in addition to eleven unrelated individuals. The majority of males (12/13, 92%) and the majority of females (8/13, 62%) were treated with ERT. Ages at initiation of ERT were between 6 and 65 years (mean age 39.0 ± 18.3) (Supplemental Table 1). The majority of patients tolerated ERT well, except for two individuals who had adverse reactions (patient 15 and patient 22). Both patients were IgE-negative and showed no changes in IgG antibodies; patient 22 was consistently IgG-positive throughout the course of ERT. Patient 15 had the most significant adverse event during the last infusion attempt, which resulted in intubation in the cardiac intensive care unit due to difficulty breathing, tightness in chest, rigors, and convulsions, and has not been on ERT since. Only three males were routinely IgG-positive (patients 12, 13, 24) and two males had one single IgG-positive test in the past, but had not had another one since (patient 1 and patient 7).
Clinical features of each patient are described in Supplemental Table 2. Notably, there was clinically evident renal involvement in 50% of participants; the most common feature was varying levels of proteinuria. One individual required a renal transplant. Seventy-nine percent of patients showed evidence of some form of cardiac involvement, including 46% with left ventricular hypertrophy. Two males and one female had a history of a stroke, and one male had a history of one TIA and one stroke. The most commonly observed clinical feature was peripheral neuropathic pain (20/26, 77%), followed by angiokeratomas (18/26, 69%), tinnitus (17/26, 65%), gastrointestinal symptoms, including abdominal pain, constipation, and diarrhea (16/26, 62%), and hypohidrosis/anhidrosis (16/26, 62%). Additional common findings of FD in this cohort include corneal verticillata (15/26, 58%), pulmonary involvement (6/12, 50%), lymphedema (11/26, 42%), and hearing loss (9/26, 35%). The adult males in this cohort had higher frequencies of clinical manifestations than adult females in all categories with the exception of neuropathic pain, white matter lesions, and corneal verticillata (Table 1).
Table 1.
Clinical manifestations of FD in this cohort.
| Adult Females N = 13 (%) |
Adult Males N = 11 (%) |
Pediatric Males N = 2 (%) |
Total N = 26 (%) |
|
|---|---|---|---|---|
| Mean Age at Diagnosis (Years) | 36.2 | 34.3 | 3.5 | 32.8 ± 16.3 |
| Currently On ERT | 8 (62) | 10 (91) | 2 (100) | 20 (77) |
| Mean Age ERT Initiated (Years) | 51 | 37.5 | 6.5 | 39.0 ± 18.3 |
| Renal Involvement | 5 (38) | 7 (64) | 1 (50) | 13 (50) |
| Cardiac Involvement | 9/12 (75) | 9/10 (90) | 1 (50) | 19/24 (79) |
| White matter Lesions | 3/10 (30) | 1/6 (17) | N/A | 4/16 (25) |
| Neuropathic Pain | 10 (77) | 8 (73) | 2 (100) | 20 (77) |
| Corneal Verticillata | 10 (77) | 4 (36) | 1 (50) | 15 (58) |
| Angiokeratomas | 7 (54) | 11 (100) | 0 (0) | 18 (69) |
| Hypohidrosis | 5 (38) | 10 (91) | 1 (50) | 16 (62) |
| GI Involvement | 8 (62) | 8 (73) | 0 (0) | 16 (62) |
| Low BMD | 2/6 (33) | 5/8 (63) | N/A | 7/14 (50) |
| Hearing Loss | 3 (23) | 6 (55) | 0 (0) | 9 (35) |
| Tinnitus | 7 (54) | 9 (82) | 1 (50) | 17 (65) |
| Vertigo | 4/10 (40) | 6/10 (60) | 0/1 (0) | 10/21 (48) |
| Pulmonary Involvement | 0/5 (0) | 5/6 (83) | 1/1 (100) | 6/12 (50) |
| Depression | 3 (23) | 3 (27) | 0 (0) | 6/26 (23) |
Numbers provided in fractions indicate that not all patients had the corresponding assessment.
3.1. Clearance of GL-3 in plasma
Plasma-GL-3, plasma Lyso-GL-3, and urine GL-3 are biomarkers used as surrogate endpoints to monitor Fabry disease severity and progression in both treated and untreated patients. Longitudinal analysis of plasma GL-3 concentration in 17 of our patients on ERT is depicted in Fig. 1. The data showed significant reduction in GL-3 of −0.25 μg/mL per year (95% CI: −0.478 to −0.030, p = 0.026) with ERT treatment.
Fig. 1.
Plasma GL-3 clearance in patients on ERT. A linear mixed-effects regression analysis of plasma GL-3 with time as a predictor and varying intercepts and slopes. The figure shows observed GL-3 measurements (dots) together with model-estimated time trajectories of GL-3 for each participant (colored lines) and for the population (black line). Note that observations at time < 0 were excluded from the analysis. N = 17.
While there was less data available for the deacetylated form, plasma Lyso-GL-3, the majority showed a reduction or stabilization of plasma Lyso-GL-3 concentration at last visit compared to that of the earlier time points (N = 11, data not shown).
3.2. Renal involvement
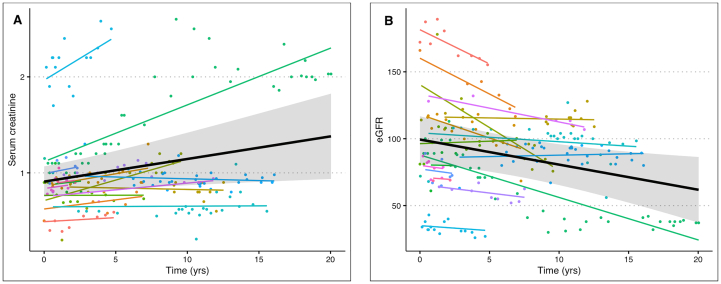
There was clinically evident renal disease in 50% (13/26) of participants as defined by albuminuria, hypertension or reduced eGFR. Renal biopsies were not done routinely. The majority demonstrated proteinuria and/or albuminuria as the sole renal manifestation. Ten patients had elevated urine albumin-to-creatine ratios, nine with microalbuminuria (5 females, 4 males) and one male with macroalbuminuria. Hypertension was seen in 32% (8/25) of patients and managed with angiotensin-converting enzyme inhibitors (ACEI) (8/26), angiotensin receptor blockers (ARB) (4/26), beta-blockers (4/26), and diuretics (6/26). Two had stage I chronic kidney disease (CKD) (patient 1 and patient 12), two had stage III CKD (patient 14 and patient 17), and one had stage IV CKD and is on hemodialysis (patient 22). Patient 23 underwent a deceased donor renal transplant due to ESRD at age 52 years and has had normal kidney function since. For those on ERT, only some patients maintained serum creatinine and estimated glomerular filtration rate (eGFR) within normal limits for age and sex. However, the data showed significant increase in serum creatinine levels of +0.02 mg/dL per year (95% CI: 0.01–0.04, p = 0.015) for both males and females on ERT in this cohort (Fig. 2A) and +0.038 mg/dL per year (95% CI: 0.01–0.06, p = 0.003, data not shown) for males alone. Further, the data showed an annual decrease in eGFR of −1.88 mL/min/1.73m2 (95% CI: −3.36 to −0.40, p = 0.013) on ERT in both males and females (Fig. 2B), and −3.16 mL/min/1.73m2 (95% CI: −4.97 to −1.35, p = 0.001, data not shown) for males alone.
Fig. 2.
Serum creatinine and estimated glomerular filtration rate (eGFR) of patients on ERT. The figure shows observed serum creatinine measurements (A) and eGFR measurements (B) together with model-estimated time trajectories for each participant (colored lines) and for the population (black lines). Note that observations at time < 0 were excluded from both analyses in figs. A-B. N = 18.
3.3. Cardiac involvement
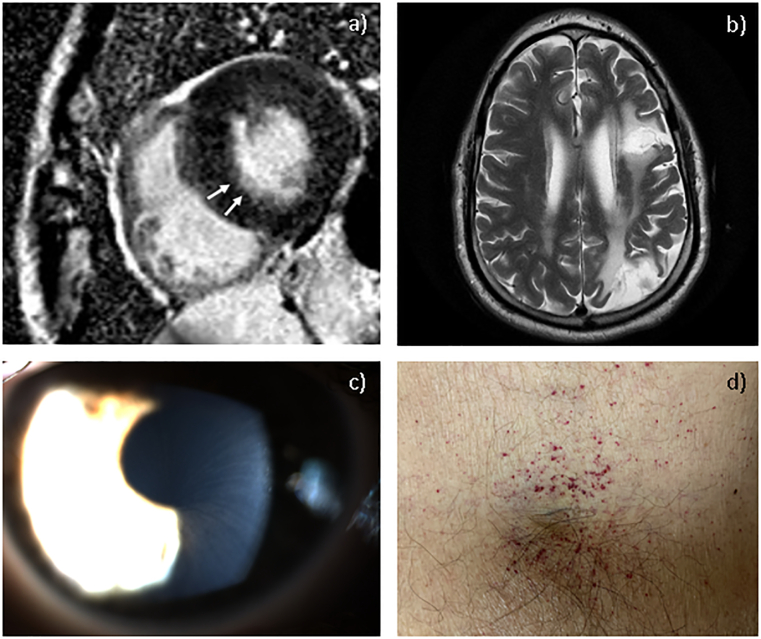
In our cohort, 79% (19/24) of participants had some form of cardiac involvement. Left ventricular hypertrophy (LVH) was seen in 46% (11/24) of patients, with severity ranging from borderline to severe and one confined to the septum. Severe LVH of patient 24 is shown in Fig. 3A. Of the adult males, only one was not found to have clinically evident cardiac involvement, and while his past ECGs met voltage criteria for LVH, his echocardiograms were normal showing no evidence of LVH. Left ventricular ejection fraction (LVEF) was one of the measures used to monitor cardiac disease progression of patients on ERT. LVEF was within normal range at baseline prior to ERT. Some patients fell below normal range over the course of treatment with the lowest recorded being 43% in a male patient. Of the individuals in our cohort who had cardiac imaging, 25% (6/24) had valvular abnormalities, including one patient with aortic valve sclerosis, two patients had thickened mitral valve leaflets, one patient with mild Systolic Anterior Motion (SAM) abnormality of the mitral valve, one with mitral valve prolapse, and one patient required mitral valve annuloplasty after experiencing lower extremity edema, shortness of breath and dyspnea on exertion (patient 22). Other cardiac manifestations are described in Table 2. Of those who underwent cardiac MRI, only one showed possible fibrosis of the myocardium (Fig. 3A). Those with cardiac abnormalities on echocardiogram, cardiac MRI, and/or ECG did not consistently have abnormal cardiac biomarkers, such as brain natriuretic peptide (BNP), troponin, and soluble suppression of tumourigenicity 2 (ST2), though limited data was available. Adult FD patients can have elevated lipid levels, particularly high HDL cholesterol, which may be non-responsive to long-term ERT [18]. Dyslipidemia was present in 48% (10/21) of patients in our cohort, and managed with diet, fish-oil and statins.
Fig. 3.
Clinical features in patients with FD. (A) Cardiac MRI showing severe concentric hypertrophy of the left ventricle with reduced chamber size. There is subendocardial linear hyperenhancement in the basal inferolateral wall suggesting myocardial scar (white arrow), consistent with FD (patient 24); (B) Axial brain MRI section following TIA event (patient 22); (C) Corneal verticillata seen by slit lamp examination. Courtesy: Dr. Pinakin Davey, Western University of Health Sciences; (D) Cluster of angiokeratomas in the groin region (patient 24).
Table 2.
Characterization of cardiac involvement in FD patients in this cohort.
| Adult Females |
Adult Males |
Pediatric Males | Total (%) | |
|---|---|---|---|---|
| Cardiac Involvement | 9/12 (75) | 9/10 (90) | 1/2 (50) | 19/24 (79) |
| Echocardiogram Findings | ||||
| Left Ventricular Hypertrophy | 5/12 | 7/10 | 0/2 | 11/24 (46) |
| Borderline | 1 | 2 | 0 | |
| Mild | 2 | 3 | 0 | |
| Moderate | 0 | 1 | 0 | |
| Severe | 0 | 1 | 0 | |
| Septal | 1 | 0 | 0 | |
| Valvular Abnormality | 2/12 | 4/10 | 0/2 | 6/24 (25) |
| Aortic Valve | 1 | 0 | 0 | |
| Mitral Valve | 1 | 4 | 0 | |
| Left Atrium Dilation | 2/12 | 2/10 | 0/2 | 4/24 (17) |
| Right Atrium Dilation | 1/12 | 0/10 | 0/2 | 1/24 (4) |
| Electrocardiogram Findings | ||||
| Conduction Abnormalities | 3/12 | 6/10 | 0/2 | 9/24 (38) |
| Right Bundle Branch Block | 2 | 5 | 0 | |
| Anterior Fascicular Block | 1 | 1 | 0 | |
| First Degree Atrioventricular Block | 1 | 0 | 0 | |
| Intraventricular Conduction Delay | 0 | 2 | 0 | |
| Short PR Interval | 1 | 2 | 0 | |
| Rhythm Abnormalities | 5/12 | 8/10 | 1/2 | 14/24 (58) |
| Sinus Bradycardia | 5 | 8 | 1 | |
| Sinus Arrhythmia | 1 | 0 | 0 | |
| Premature Ventricular Complexes | 0 | 2 | 0 | |
| Premature Atrial Contractions | 0 | 1 | 0 | |
| Repolarization Abnormalities | 5/12 | 3/10 | 0/2 | 8/24 (33) |
| T Wave Changes | 2 | 1 | 0 | |
| ST/STT Changes | 3 | 2 | 0 | |
| Left Axis Deviation | 0/12 | 1/10 | 0/2 | 1/24 (4) |
One male and one female in this cohort were excluded due to no cardiac imaging records available.
3.4. Cerebrovascular complications, neurologic, and neuropsychiatric symptoms
White matter lesions were present in 25% (4/16) of participants who had brain imaging. One male in this cohort (patient 22) has a history of two cerebrovascular accidents, one transient ischemic attack (TIA) at age 52 years and one stroke at age 60 years. Brain MRI of patient 22 showed multifocal and adjacent gliosis within the left cerebral hemisphere, high left frontal and parietal lobes, as well as subcortical and periventricular deep white matter T2 hyperintensity without mass effect that is most pronounced within the parietal occipital region (Fig. 3B). The stroke impacted his eyesight and word-finding ability. Patient 22 only began ERT at the age of 57 years, and passed away at the age of 63 years due to post-operative complications of abdominal surgery. Another male (patient 15) and one female (patient 14) in this cohort have a history of TIAs while on ERT. They were not taking low dose aspirin at the time.
With regard to neuropathic manifestations, 77% (20/26) of patients reported peripheral neuropathic pain, manifesting as burning, tingling, or numbness, often triggered by temperature change or strenuous activity. Of the 17 participants for whom data on the brief pain inventory (BPI) survey was available, the majority reported worsening or inconsistent pain symptoms over the duration of treatment with ERT. Decreased quality of life has been associated with FD, in part due to the debilitating episodic pain crises and burden associated with having a chronic illness. Health-related quality of life was collected using the SF-36 Health survey. Bodily pain scores obtained from the SF-36 survey also showed no improvement over the course of ERT, though this was not significant (p = 0.19; Fig. 4C). For the majority of SF-36 respondents, pain worsened over time, which was similar to what was reported in the BPI survey. Notably, there was a significant decline in physical functioning (Est. = −1.40, 95% CI: −2.73 to −0.06, p = 0.040) for patients in this cohort. The remaining health dimensions did not show significant change over time (Fig. 4A-H).
Fig. 4.
SF-36 health-related quality of life component scores of patients on ERT. The SF-36 Health survey measures (A) physical function, (B) physical role, (C) pain, (D) general health perception, (E) vitality, (F) social function, (G) emotional role, (H) mental health. Scores ranged from 0 (worst) to 100 (best). Observed values (dots) are shown together with model-estimated trajectories for individual patients (colored lines) and the population (black lines).
Of the adult patients in this cohort, seven reported depression (7/24; 29%). One patient reported a history of depression that correlated with pain symptoms. Another patient had a history of depression with no current pain symptoms based on responses in the BPI and SF-36 Health Survey. Of note, one patient in this cohort had a history of two suicide attempts. On the other hand, one patient's testimonial described noticeable improvement in anxiety, fatigue, and executive function (termed “Fabry fog”) since initiation of ERT.
3.5. Ophthalmologic findings
Corneal verticillata, or corneal whorling, is a unique clinical feature in patients of both sexes with FD and is often used as a diagnostic tool. It is a result of GL-3 deposits and has not been linked to vision loss. Corneal verticillata was noted in 58% (15/26) of individuals, at a higher prevalence in females than males in our cohort (Fig. 3C). Vessel tortuosity is also associated with FD and is exacerbated by renal complications [19,20]. Only one patient was found to have vessel tortuosity (patient 22); this individual had stage IV CKD.
3.6. Skin manifestations
Angiokeratomas are common in Fabry patients as they occur when GL-3 accumulates in dermal endothelial cells and lead to secondary ectasia. In our cohort, 69% (18/26) had angiokeratomas (Fig. 3D).
Hypohidrosis is also a common feature of FD and may be a predisposition to acroparesthesia. In this cohort, 62% (16/26) experienced hypohidrosis or anhidrosis. Of those with hypohidrosis, 89% experienced acroparesthesia. Lymphedema of the extremities, particularly in the feet, was present in 42% (11/26) of patients in this study.
3.7. Gastrointestinal involvement
Gastrointestinal symptoms are common among FD patients and can manifest as diarrhea, constipation, nausea, vomiting, incontinence, and abdominal pain. In our cohort, gastrointestinal symptoms were present in 62% (16/26) and the most common complaint was abdominal pain. Previous studies have shown significantly improved gastrointestinal pain with ERT [21]. This was also reported by the patients in our study in the patient health survey. The majority of those on ERT reported either improved or stable abdominal pain and frequency of diarrhea based on their survey responses at last visit compared to earlier survey responses. Specifically, six patients reported improvement in abdominal pain and four reported that symptoms were stable (N = 20). Eleven reported improvement in diarrhea and one reported that symptoms were stable (N = 20). One patient's testimonial described a frequency of diarrhea multiple times daily prior to ERT that has now significantly improved since ERT initiation. He also reported noticeable return of symptoms during the time of the ERT shortage. On the other hand, worsening or recent onset of abdominal pain (3/20) and diarrhea (2/20) over the course of ERT treatment were also reported by participants in this study.
3.8. Auditory and vestibular involvement
Sensorineural hearing loss, predominantly in the high frequencies, is a common manifestation of FD and correlates with neuropathic and vascular damage [22]. In our cohort, 35% (9/26) of patients reported a history of hearing loss. The majority of those who underwent audiology assessments were found to have bilateral mild to moderate sensorineural hearing loss. Additionally, 65% (17/26) of patients reported tinnitus. Some patients do not use hearing aids due to amplification by the presence of tinnitus. Vertigo was reported in 48% of our cohort in those who recorded their response in the health survey (10/21).
3.9. Pulmonary involvement
Respiratory involvement in FD typically includes obstructive lung disease, however patients may develop interstitial restrictive lung disease. In our cohort, 50% (6/12) of those who have had spirometry testing were found to have reduced lung capacity. Of those, four had obstructive lung disease, one had possible obstructive lung disease, and one had restrictive lung disease.
3.10. Endocrine and other clinical manifestations
There is limited data in the literature that describes endocrine system involvement in FD. In our cohort, patients reported type II diabetes, hypothyroidism, hyperthyroidism, hyperparathyroidism, and vitamin D deficiency (Supplemental Table 2). Patient 8 has a history of hyperthyroidism secondary to Graves' disease, and a past history of a 1.9 mm benign thyroid nodule. Patient 21 has type II diabetes and hypothyroidism. Patient 22 has acquired hypothyroidism and hyperparathyroidism secondary to renal disease. One of the pediatric participants (patient 9) was diagnosed with growth hormone deficiency and a small pituitary gland. Not all participants in this study have had an endocrine evaluation, so this study may have missed those with subclinical endocrine dysfunction.
Low Bone Mineral Density (BMD) has been noted more recently to be a common feature of FD [23]. Impaired renal function leads to vitamin D deficiency and may be one of the contributing factors to reduced BMD in FD patients. Fifty percent of patients in this cohort who had DXA scans (7/14) were found to have low BMD. Of those, 57% (4/7) had osteopenia (T score < 1.5) and 43% (3/7) had osteoporosis (T score < 2.5). Notably, two of the seven patients (29%) had early-onset low BMD at age 32 years (patient 6) and age 36 years (patient 13). There were no prior DXA scans to measure the rate of bone loss in these two young adult males. Additionally, patient 13 spends up to 40 h per week in a sensory deprivation floatation tank, which may have contributed to bone loss (described below in section 3.13. Supplemental Treatments). Only one patient endured an ankle fracture at the age of 65 years due to a mechanical fall; none of the remaining participants reported bone fractures. Of those with low BMD, four patients had concurrent vitamin D deficiency; of those four, one had secondary hyperparathyroidism due to renal disease. All patients in this cohort were prescribed calcium and vitamin D supplementation. Those with osteoporosis were managed by an endocrinologist and prescribed bisphosphonates.
Hypertension is also a common adverse event in adult FD patients. Cardiac, renal and cerebrovascular complications increase the risk of uncontrolled hypertension in individuals with FD [24]. Hypertension was present in 32% (8/25) of patients in our cohort, the majority but not all of these individuals had some form of clinically evident renal involvement. Patients were managed on ACEIs, ARBs, beta-blockers, and diuretics.
3.11. Novel GLA variant and genotype-phenotype correlations
Our study describes 16 unique variants in the 26 total participants, including one novel variant and one atypical ‘later-onset’ cardiac variant (Table 3; Fig. 5). The novel variant c.1226_1231delCCACAG (p.P409_G411delinsR), an in-frame deletion located within a hot-spot, was identified in a 63-year-old male (patient 22) who was diagnosed later in adulthood at 57 years old following a renal biopsy that revealed stage IV chronic kidney disease. He is discussed earlier with renal, pulmonary, and cardiac involvement and a history of two cerebrovascular accidents. Additional FD-related symptoms in patient 22 include angiokeratomas, corneal verticillata, gastrointestinal involvement, lymphedema, hypohidrosis, acroparesthesia, Raynaud's syndrome, hearing loss, tinnitus, and low bone mineral density (Supplemental Table 2). Cardiac work-up for chest pain revealed a 95% blockage of the left anterior descending coronary artery requiring stent placement, which resolved except for occasional reports of arrhythmia. Endocrine dysfunction in this patient includes acquired hypothyroidism and hyperparathyroidism secondary to renal disease. Patient 22 was particularly significant among our participants because of the history of one TIA and one stroke; brain MRI revealed significant gliosis and nonspecific white matter signal changes (Fig. 3B). Patient 22 is now deceased due to post-operative complications of abdominal surgery for abdominal abscess and intra-abdominal infection.
Table 3.
GLA variant details.
| Fam. No. | Patient No. | Ethnicity | Nucleotide Change | Protein Change | Position | Type | Phenotype |
|---|---|---|---|---|---|---|---|
| 1 | 1,2,3,4,5,6 | Hispanic | c.983G > T | G328V | Exon 6 | Missense | Classic |
| 2 | 7,8 | Caucasian | c.1250 T > G | L417R | Exon 7 | Missense | Classic |
| 3 | 9,10,11 | Caucasian | c.132G > T | W44C | Exon 1 | Missense | Classic |
| 4 | 12 | Caucasian | c.568delG | A190PfsX2 | Exon 4 | Small Deletion | Classic |
| 5 | 13,14 | Caucasian | c.1041_1042insG | A348GfsX27 | Exon 7 | Small Insertion | Classic |
| 6 | 15 | Hispanic | c.706 T > C | W236R | Exon 5 | Missense | Classic |
| 7 | 16 | Caucasian | c.680G > A | R227Q | Exon 5 | Missense | Classic |
| 8 | 17 | Caucasian | c.816C > A | N272K | Exon 6 | Missense | Classic |
| 9 | 18 | Caucasian | c.730G > A | D244N | Exon 5 | Missense | Classic/ Later Onset |
| 10 | 19 | Asian | c.639 + 919G > A | IVS4 + 919G > A | Intron 4 | Splicing | Later Onset |
| 11 | 20 | Asian | c.427G > C | A143P | Exon 3 | Missense | Classic |
| 12 | 21 | Hispanic | c.639 + 4A > T | IVS4 + 4A > T | Intron 4 | Splicing | Classic |
| 13 | 22 | Caucasian | c.1226_1231delCCACAG | P409_G411delinsR | Exon 7 | Small Deletion | Classic |
| 14 | 23 | Hispanic | c.1088G > A | R363H | Exon 7 | Missense | Later Onset |
| 15 | 24,25 | Hispanic | c.1072_1074delGAG | E358del | Exon 7 | Small Deletion | Classic |
| 16 | 26 | Caucasian | c.1246C > T | Q416X | Exon 7 | Nonsense | Classic |
Novel variant is bolded.
Fig. 5.
Unique variants identified in the GLA gene in this cohort. A schematic representation of GLA showing the position of all variants identified, with novel variant in red. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Though the rest of our cohort harbors variants that have been previously reported in the literature, we present novel clinical data for certain recurrent variants. Family 3 (patients 9, 10, 11) harbor the W44C variant, a previously reported variant in a Chinese family of 12 affected members, all of whom experienced paroxysmal pain of limb extremities [25]. Interestingly, our family is of European/mixed descent, with no known Asian ancestry, and present with multi-organ involvement in addition to peripheral pain. We report one patient with the c.639 + 919G > A ‘later-onset’ cardiac variant, who, in addition to mild left ventricular hypertrophy, experiences other FD-related symptoms including angiokeratomas, gastrointestinal issues, pulmonary involvement, and hypohidrosis with heat intolerance. His mother, who also harbors the familial variant, has a history of strokes and presented at age 76 years with hypertrophic cardiomyopathy. Another example of potential genotype-phenotype correlation in our cohort is Family 1; all three females who harbor the G328V variant have audiologic involvement.
3.12. Pediatric patients
There were two male pediatric participants in this study (patient 7 and patient 9). Both patients were diagnosed before the age of five and treated with ERT at or before the onset of symptoms. Patient 9 is a seven year-old male with the W44C variant. This patient was diagnosed with FD at age 3 and began ERT at age 7 years soon after an evaluation for reactive hyperemia index of 0.94 (Reference RHI value >1.68) using an EndoPAT (Itamar Medical) device performed on a research basis, which was consistent with low peripheral vascular endothelial function. He lacked ‘classic’ FD symptoms at the time of the procedure, but ERT was recommended due to concern for deteriorating endothelial function. Initial urine GL-3 level of 569 μg/mmol creatinine resolved during the course of this study while on ERT. He is developmentally normal, however he has a past medical history remarkable for growth hormone deficiency and a small pituitary gland and has been managed on somatropin since 3 years of age.
Patient 7 from Family 2 first presented with acroparesthesia and hypohidrosis at 4 years of age and began ERT by 6 years of age. Prior to initiation of ERT, urine GL-3 level was at 370 μg/mmol and was cleared to 0 μg/mmol in less than two years on ERT. Notably, spirometry testing revealed mild obstructive airway disease, though it has not yet affected routine activity. Patient 7 and his affected mother (patient 8) present with variable symptoms of FD as shown in Supplemental Table 2.
3.13. Supplemental treatments
Several individuals in this cohort sought alternative sources for pain management. Effective pain management could impact the psychological consequences of FD, as there is a high rate of depression that is often linked to the effects of long-term pain. Patient 13 had a five-year history of frequent, severe pain crises, including in response to ERT infusion, which has led the patient to seek alternative pain management strategies in conjunction with ERT. This includes floatation in a sensory deprivation tank used for the treatment of pain and anxiety by limiting stimulation from gravity, sound and light while immersed in water baths saturated with Epsom salts to allow for buoyancy in a dark and soundless room. Patient 15 and Patient 16 also report benefit from this float tank. Important to note, extended time in the float tank may have had an adverse effect on patient 13's bone density, as he reports spending up to 40 h per week in the float tank. There was a variety of medications used in this cohort for the management of neuropathic pain. Patient 13 and 15 are prescribed cannabidiol (CBD). Patient 15 and patient 26 use non-prescription topical and oral CBD as needed during pain crises. Use of tetrahydrocannabinol (THC) was also reported. Six patients are prescribed opioid narcotic analgesics, eight patients are managed with over-the-counter analgesics, two are on antidepressants, and one on an anticonvulsant (carbamazepine).
4. Discussion
The benefit, safety, and risk of ERT have been previously investigated, though the long-term impact continues to be studied [[26], [27], [28], [29], [30]]. ERT is recommended in order to curtail disease progression or when there is already evidence of disease progression and vital organ damage. In this study, 20 of the 26 participants and all but one male (patient 15) were consistently on ERT.
All participants receiving ERT however, demonstrated a different response rate of disease progression. While ERT was shown to be effective in clearing plasma GL-3 and, in many patients maintaining serum creatinine and eGFR within normal limits in this cohort, we identified that many patients continued to have impairment of their renal function and health-related quality of life despite standard of care management. For patients with chronic kidney disease from FD, standard of care did not vary when compared to that of other etiologies. The standard management includes the evaluation for possible reversible causes of renal disease (i.e. dehydration, reduced renal perfusion, obstruction, nephrotoxicity), management of blood pressure to a target ≤130/80, maximally tolerated dose of renin-angiotensin-aldosterone system inhibitors (i.e. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aldosterone receptor antagonist), smoking cessation, dietary interventions (particularly reduced sodium and protein restricted diet), treatment of metabolic acidosis, management of anemia of CKD with erythropoiesis-stimulating agents to target Hg 10–11.5 g/dL, management of mineral and bone disorders as per 2012 KDIGO guidelines, initiation of renal replacement therapy in individuals with uremic symptoms and eGFR 5–15 mL/min/1.73 m2 or in those with eGFR <5 mL/min/1.73 m2, and referral for kidney transplant evaluation in those with eGFR 20–25 mL/min/1.73 m2.
The late initiation of ERT may have contributed to those with renal disease progression, as shown previously [31]. Furthermore, nearly all of the adult patients in this cohort had major organ involvement at baseline prior to initiation of ERT. Typically, renal function in FD gradually deteriorates to end-stage renal disease in the third to fifth decade in males [32,33]. Only one patient progressed to severe CKD requiring hemodialysis, therefore, compared to the natural history data, it is possible that ERT contributed to the prevention of renal failure by slowing disease progression. Though vital organ damage is not typically seen in pediatric patients (some cases have been documented), studies have shown evidence for the benefit of the early initiation of ERT in potentially reversing renal damage [10,29]. Patient 12 initiated ERT at age 11 years, however, developed renal involvement by age 24 years [34]. Therefore, we believe earlier initiation of ERT is required to limit deterioration of the major organs. Our two pediatric participants initiated ERT at ages 6 and 7 years, respectively, and one reported significant improvement of pain symptoms.
Genotype-phenotype association is difficult to establish in FD for many reasons. Most GLA variants are unique (private mutations) and there are few family studies. Even within families, the phenotype may vary depending on the sex, age of onset, and other genetic and epigenetic factors. There are several reports in the literature of ‘later-onset’ isolated hypertrophic cardiomyopathy in individuals with the recurrent c.639 + 919G > A variant [6,[35], [36], [37]]. While this is often considered an atypical later-onset cardiac disease variant, patient 19 presented with several other ‘classic’ FD symptoms. Studies have explored the use of Amiloride as a potential treatment for FD by modulating alternative splicing in the context of the c.639 + 919G > A variant [35]. In our cohort we identified one novel variant c.1226_1231delCCACAG (p.P409_G411delinsR) in patient 22 with a severe, ‘classic’ FD phenotype [38]. This variant is absent in The Genome Aggregation Database (gnomAD), is located in a mutational hot-spot, and thus predicted to be pathogenic [39]. Two of the 26 individuals in this cohort harbor variants amenable to migalastat, an oral chaperone therapy, but chose not to switch from ERT. Long-term outcome studies for FD patients who have switched to migalastat will be of interest for future studies.
In this cohort, nearly one third of adult patients reported depression and anxiety. Based on the patient-reported SF-36 Health Survey and Brief Pain Inventory survey, there is no significant improvement in health-related quality of life over the course of ERT. Depression, sleep apnea, and anxiety disorders are linked to both pathology and the effects of long-term pain [40]. It has been suggested that psychological counseling can have an impact on reversing or reducing effects, an important consideration to optimize patient care as needed [41]. Importantly, studies have proposed pain as an indicator for the need to initiate ERT [42]. The majority of our cohort experienced Fabry crises or acroparasthesia. There are few data on nervous system involvement of FD in women [43]. Some studies have shown hippocampal volume loss and white matter lesions in female patients with FD [44]. While we showed white matter lesions in three females and one male in this cohort, we did not include brain imaging, electrical conduction, or quantitative sensory testing for every participant in this study. Further studies assessing the outcomes of neuropathy and CNS findings with ERT are warranted.
Cardiac manifestation can be the primary and only symptom of FD in some patients and can present as early as childhood. Our study showed a higher prevalence of cardiac manifestations in both the males and females compared to previous studies [34,[45], [46], [47]]. Cardiac involvement was observed in 83% of male patients, including one pediatric male patient with sinus bradycardia, and 75% of female patients in this cohort. Left ventricular hypertrophy (LVH) was seen in 70% of males and 42% of females. This data provides supporting evidence that female patients, even those with no obvious signs or symptoms of FD, should be monitored closely for evidence of cardiac involvement and be considered for ERT. The standard of care for patients with hypertrophic cardiomyopathy due to FD did not vary when compared to the management of other etiologies and followed AHA/ACC guidelines.
We highlight the variable endocrine dysfunction and low bone mineral density in this cohort, including several with osteoporosis, which provides further evidence for the inclusion of adequate endocrine work-up in the ongoing management of all patients with FD [48].
There is a need for comprehensive, multidisciplinary evaluation and management of the multi-organ system involvement. Furthermore, there is a need for more and improved biomarkers to monitor disease progression. Early identification of endothelial function or other pathologies can provide insight into when ERT should be initiated in order to slow the advancement of adverse effects [49]. Additional tools and biomarkers for measuring endothelial function and other indicators of future pathologies, such as proteomics, are needed to help prevent the deterioration of vital organs [[50], [51], [52]]. Newborn screening for FD has been implemented in several states and will lead to earlier detection. Early detection is important to proactively monitor for complications of the disease, initiate ERT, and prevent co-morbidity progression [[53], [54], [55], [56]].
Since most of our participants were on ERT, we could not make comparisons between long-term outcomes of those on ERT compared to those not on ERT. Also, with the small sample size, we could not compare multiple age-matched individuals or genotype-specific individuals other than our reported families. Another limitation to this study is the long-term analysis of plasma-GL-3 over time rather than plasma Lyso-GL-3, due to the fact that we had more data points for plasma-GL-3. Plasma Lyso-GL-3 has been recently determined to be a more accurate biomarker that correlates more closely with disease phenotype in male and in female patients, with higher levels associated with increased clinical manifestation [57].
5. Conclusion
The variability of symptoms and disease progression in patients with FD, even within the same family, complicates the discernment of a genotype-phenotype correlation. ERT has been available for the treatment of FD since 2001 and has shown improved outcomes, especially when started prior to organ damage, and may reverse fatal disease progression. In this cohort, agalsidase beta infusions have been effective in clearing GL-3 levels. However, renal involvement and health-related quality of life has continued to progress in adult patients diagnosed late despite ERT infusions.
Eight females were on ERT in this study and demonstrated significant symptoms of FD, and in some cases the disease progressed to a similar degree as in the males. This study further demonstrates that females may develop significant FD symptoms and should be managed appropriately and treated as more than carriers.
Documentation of novel variants can contribute to future genotype-phenotype associations between the severity and progression of FD. Genotype-phenotype correlations and long-term outcome data on ERT and/or other FD treatments are needed, especially now that the newborn screening has led to an increase in diagnoses and earlier detection in individuals with FD.
Authors' contributions
V.K. conceived the study and supervised the overall direction and implementation of the study. All authors contributed to acquisition of data. V.K., M.D.C., D.R., D.T., Z.A.D., and G.L. analyzed the data. D.R. performed statistical analyses. M.D.C. and E.C. drafted the manuscript with input from all authors. All authors provided critical feedback and approved the final manuscript for publication.
Funding
The Fabry registry was funded by Sanofi Genzyme. This funding source had no role in data collection or analysis.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the patients and their health care providers for their contribution to the study. The Fabry registry was funded by Sanofi Genzyme. This work was supported by CTSI grant UL1TR000124UCLA (Clinical and Translational Science Institute), Los Angeles, CA, USA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2020.100700.
Appendix A. Supplementary data
Supplementary material
References
- 1.Brady R.O., Gal A.E., Bradley R.M., Martensson E., Warshaw A.L., Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276(21):1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- 2.Duve C. Exploring cells with a centrifuge. Science. 1975;189(4198):186–194. doi: 10.1126/science.1138375. [DOI] [PubMed] [Google Scholar]
- 3.Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 4.Desnick R.J., Ioannou Y.A., Eng C.M. Alpha-galactosidase A deficiency: Fabry disease. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Kinzler K.E., Vogelstein B., editors. The Metabolic and Molecular Bases of Inherited Diseases. 8 ed. McGraw-Hill; New York, NY: 2001. pp. 3733–3774. [Google Scholar]
- 5.Matern D., Gavrilov D., Oglesbee D., Raymond K., Rinaldo P., Tortorelli S. Newborn screening for lysosomal storage disorders. Semin. Perinatol. 2015;39(3):206–216. doi: 10.1053/j.semperi.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Spada M., Pagliardini S., Yasuda M. High incidence of later-onset fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006;79(1):31–40. doi: 10.1086/504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eng C.M., Germain D.P., Banikazemi M., Warnock D.G., Wanner C., Hopkin R.J., Bultas J., Lee P., Sims K., Brodie S.E., Pastores G.M., Strotmann J.M., Wilcox W.R. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006 Sep;8(9):539–548. doi: 10.1097/01.gim.0000237866.70357.c6. 16980809 [DOI] [PubMed] [Google Scholar]
- 8.Ortiz A., Germain D.P., Desnick R.J. Fabry disease revisited: management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018;123(4):416–427. doi: 10.1016/j.ymgme.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Arends M., Wijburg F.A., Wanner C. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol. Genet. Metab. 2017;121(2):157–161. doi: 10.1016/j.ymgme.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Warnock D.G., Thomas C.P., Vujkovac B. Antiproteinuric therapy and Fabry nephropathy: factors associated with preserved kidney function during agalsidase-beta therapy. J. Med. Genet. 2015;52(12):860–866. doi: 10.1136/jmedgenet-2015-103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisi E.C., McCandless S.E. Newborn screening for lysosomal storage disorders: views of genetic healthcare providers. J. Genet. Couns. 2016;25(2):373–384. doi: 10.1007/s10897-015-9879-8. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins P.V., Klug T., Vermette L., Raburn-Miller J., Kiesling J., Rogers S. Incidence of 4 lysosomal storage disorders from 4 years of newborn screening. JAMA Pediatr. 2018;172(7):696–697. doi: 10.1001/jamapediatrics.2018.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Replagal® Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000369/WC500053612.pdf (2014), Accessed 19th Oct 2017.
- 14.Fabrazyme® Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103979s5135lbl.pdf (2010), Accessed 19th Oct 2017.
- 15.El Dib R., Gomaa H., Carvalho R.P. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst Rev. 2016;7(7):CD006663. doi: 10.1002/14651858.CD006663.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripeau D., Amartino H., Cedrolla M. Switch from agalsidase beta to agalsidase alfa in the enzyme replacement therapy of patients with Fabry disease in Latin America. Cambio de agalsidasa beta por agalsidasa alfa en la terapia de reemplazo enzimática de pacientes con enfermedad de Fabry en Latinoamérica. Medicina (B Aires) 2017;77(3):173–179. [PubMed] [Google Scholar]
- 17.Amicus Therapeutics. Galafold™ (Migalastat) Capsules, for Oral Use: US Prescribing Information. 2018. https://www.fda.gov/. Accessed 18 Oct 2018.
- 18.Stepien K.M., Hendriksz C.J. Lipid profile in adult patients with Fabry disease - Ten-year follow up. Mol Genet Metab Rep. 2017;13:3–6. doi: 10.1016/j.ymgmr.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivley M.D., Wallace E.L., Warnock D.G., Benjamin W.J. Conjunctival lymphangiectasia associated with classic Fabry disease. Br. J. Ophthalmol. 2018;102(1):54–58. doi: 10.1136/bjophthalmol-2016-310088. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T.T., Gin T., Nicholls K., Low M., Galanos J., Crawford A. Ophthalmological manifestations of Fabry disease: a survey of patients at the Royal Melbourne Fabry Disease Treatment Centre. Clin. Exp. Ophthalmol. 2005;33(2):164–168. doi: 10.1111/j.1442-9071.2005.00990.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox W.R., Feldt-Rasmussen U., Martins A.M. Improvement of Fabry disease-related gastrointestinal symptoms in a significant proportion of female patients treated with Agalsidase Beta: data from the Fabry registry. JIMD Rep. 2018;38:45–51. doi: 10.1007/8904_2017_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ries M., Kim H.J., Zalewski C.K. Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain. 2007;130(Pt 1):143–150. doi: 10.1093/brain/awl310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mersebach H., Johansson J.O., Rasmussen A.K. Osteopenia: a common aspect of Fabry disease. Predictors of bone mineral density. Genet Med. 2007;9(12):812–818. doi: 10.1097/gim.0b013e31815cb197. [DOI] [PubMed] [Google Scholar]
- 24.Kleinert J., Dehout F., Schwarting A. Prevalence of uncontrolled hypertension in patients with Fabry disease. Am. J. Hypertens. 2006;19(8):782–787. doi: 10.1016/j.amjhyper.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z.X., Zhang Y., Bu D.F., Zhang W., Yuan Y. Novel GLA gene mutations in two Chinese families with classic Fabry disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi (Chinese journal of medical genetics). 2005;22(5):489–492. [PubMed] [Google Scholar]
- 26.Skrunes R., Tøndel C., Leh S. Long-term dose-dependent Agalsidase effects on kidney histology in Fabry disease. Clin. J. Am. Soc. Nephrol. 2017;12(9):1470–1479. doi: 10.2215/CJN.01820217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck M., Hughes D., Kampmann C. Long-term outcomes with agalsidase alfa enzyme replacement therapy: Analysis using deconstructed composite events. Mol Genet Metab Rep. 2017;14:31–35. doi: 10.1016/j.ymgmr.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germain D.P., Elliott P.M., Falissard B. The effect of enzyme replacement therapy on clinical outcomes in male patients with Fabry disease: A systematic literature review by a European panel of experts. Mol Genet Metab Rep. 2019;19:100454. doi: 10.1016/j.ymgmr.2019.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spada M., Baron R., Elliott P.M. The effect of enzyme replacement therapy on clinical outcomes in paediatric patients with Fabry disease - a systematic literature review by a European panel of experts. Mol. Genet. Metab. 2019;126(3):212–223. doi: 10.1016/j.ymgme.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Germain D.P., Arad M., Burlina A. The effect of enzyme replacement therapy on clinical outcomes in female patients with Fabry disease - a systematic literature review by a European panel of experts. Mol. Genet. Metab. 2019;126(3):224–235. doi: 10.1016/j.ymgme.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Germain D.P., Waldek S., Banikazemi M. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J. Am. Soc. Nephrol. 2007;18(5):1547–1557. doi: 10.1681/ASN.2006080816. [DOI] [PubMed] [Google Scholar]
- 32.Branton M., Schiffmann R., Kopp J.B. Natural history and treatment of renal involvement in Fabry disease. J. Am. Soc. Nephrol. 2002;13(Suppl. 2):S139–S143. [PubMed] [Google Scholar]
- 33.Jaurretche S., Antogiovanni N., Perretta F. Prevalence of chronic kidney disease in fabry disease patients: Multicenter cross sectional study in Argentina. Mol Genet Metab Rep. 2017;12:41–43. doi: 10.1016/j.ymgmr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson H.C., Hopkin R.J., Madueme P.C. Arrhythmia and clinical cardiac findings in children with Anderson-Fabry disease. Am. J. Cardiol. 2017;120(2):251–255. doi: 10.1016/j.amjcard.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Chang W.H., Niu D.M., Lu C.Y., Lin S.Y., Liu T.C., Chang J.G. Modulation the alternative splicing of GLA (IVS4+919G>A) in Fabry disease. PLoS One. 2017;12(4):e0175929. doi: 10.1371/journal.pone.0175929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu T.R., Hung S.C., Chang F.P. Later onset Fabry disease, cardiac damage Progress in silence: experience with a highly prevalent mutation. J. Am. Coll. Cardiol. 2016;68(23):2554–2563. doi: 10.1016/j.jacc.2016.09.943. [DOI] [PubMed] [Google Scholar]
- 37.Lin H.Y., Huang C.H., Yu H.C. Enzyme assay and clinical assessment in subjects with a Chinese hotspot late-onset Fabry mutation (IVS4 + 919G→A) J. Inherit. Metab. Dis. 2010;33(5):619–624. doi: 10.1007/s10545-010-9166-7. [DOI] [PubMed] [Google Scholar]
- 38.Arends M., Wanner C., Hughes D. Characterization of classical and nonclassical Fabry disease: a multicenter study. J. Am. Soc. Nephrol. 2017;28(5):1631–1641. doi: 10.1681/ASN.2016090964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talbot A., Hammerschlag G., Goldin J., Nicholls K. Sleep disturbance, obstructive sleep Apnoea and abnormal periodic leg movements: very common problems in Fabry disease. JIMD Rep. 2017;31:37–44. doi: 10.1007/8904_2016_549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ali N., Gillespie S., Laney D. Treatment of depression in adults with Fabry disease. JIMD Rep. 2018;38:13–21. doi: 10.1007/8904_2017_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Politei J.M., Bouhassira D., Germain D.P. Pain in Fabry disease: practical recommendations for diagnosis and treatment. CNS Neurosci Ther. 2016;22(7):568–576. doi: 10.1111/cns.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Üçeyler N., Kahn A.K., Kramer D. Impaired small fiber conduction in patients with Fabry disease: a neurophysiological case-control study. BMC Neurol. 2013;13:47. doi: 10.1186/1471-2377-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lelieveld I.M., Böttcher A., Hennermann J.B., Beck M., Fellgiebel A. Eight-Year Follow-Up of Neuropsychiatric Symptoms and Brain Structural Changes in Fabry Disease. PLoS One. 2015;10(9):e0137603. doi: 10.1371/journal.pone.0137603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta A., Ricci R., Widmer U. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry outcome survey. Eur. J. Clin. Investig. 2004;34(3):236–242. doi: 10.1111/j.1365-2362.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- 46.Linhart A., Lubanda J.C., Palecek T. Cardiac manifestations in Fabry disease. J. Inherit. Metab. Dis. 2001;24(Suppl. 2):75–95. doi: 10.1023/a:1012428009627. [DOI] [PubMed] [Google Scholar]
- 47.Kampmann C., Baehner F., Whybra C. Cardiac manifestations of Anderson-Fabry disease in heterozygous females. J. Am. Coll. Cardiol. 2002;40(9):1668–1674. doi: 10.1016/s0735-1097(02)02380-x. [DOI] [PubMed] [Google Scholar]
- 48.Faggiano A., Pisani A., Milone F. Endocrine dysfunction in patients with Fabry disease. J. Clin. Endocrinol. Metab. 2006;91(11):4319–4325. doi: 10.1210/jc.2006-0858. [DOI] [PubMed] [Google Scholar]
- 49.Demuth K., Germain D.P. Endothelial markers and homocysteine in patients with classic Fabry disease. Acta Paediatr. Suppl. 2002;91(439):57–61. doi: 10.1111/j.1651-2227.2002.tb03112.x. [DOI] [PubMed] [Google Scholar]
- 50.Moerland M., Kales A.J., Schrier L., van Dongen M.G., Bradnock D., Burggraaf J. Evaluation of the EndoPAT as a tool to assess endothelial function. Int J Vasc Med. 2012;2012:904141. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riccio E., Sabbatini M., Capuano I., Pisani A. Early biomarkers of Fabry nephropathy: a review of the literature. Nephron. 2019;143(4):274–281. doi: 10.1159/000502907. [DOI] [PubMed] [Google Scholar]
- 52.Matafora V., Cuccurullo M., Beneduci A. Early markers of Fabry disease revealed by proteomics. Mol. BioSyst. 2015;11(6):1543–1551. doi: 10.1039/c4mb00707g. [DOI] [PubMed] [Google Scholar]
- 53.Hopkin R.J., Jefferies J.L., Laney D.A. The management and treatment of children with Fabry disease: a United States-based perspective. Mol. Genet. Metab. 2016;117(2):104–113. doi: 10.1016/j.ymgme.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Ramaswami U., Whybra C., Parini R. Clinical manifestations of Fabry disease in children: data from the Fabry outcome survey. Acta Paediatr. 2006;95(1):86–92. doi: 10.1080/08035250500275022. [DOI] [PubMed] [Google Scholar]
- 55.Desnick R.J., Banikazemi M. Fabry disease: clinical spectrum and evidence-based enzyme replacement therapy. Nephrol Ther. 2006;2(Suppl. 2):S172–S185. [PubMed] [Google Scholar]
- 56.Pintos-Morell G., Beck M. Fabry disease in children and the effects of enzyme replacement treatment. Eur. J. Pediatr. 2009;168(11):1355–1363. doi: 10.1007/s00431-009-0937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rombach S.M., Dekker N., Bouwman M.G. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim. Biophys. Acta. 2010;1802(9):741–748. doi: 10.1016/j.bbadis.2010.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material