Abstract
Background
The liver is a key regulator of systemic energy homeostasis and can sense and respond to nutrient excess and deficiency through crosstalk with multiple tissues. Regulation of systemic energy homeostasis by the liver is mediated in part through regulation of glucose and lipid metabolism. Dysregulation of either process may result in metabolic dysfunction and contribute to the development of insulin resistance or fatty liver disease.
Scope of review
The liver has recently been recognized as an endocrine organ that secretes hepatokines, which are liver-derived factors that can signal to and communicate with distant tissues. Dysregulation of liver-centered inter-organ pathways may contribute to improper regulation of energy homeostasis and ultimately metabolic dysfunction. Deciphering the mechanisms that regulate hepatokine expression and communication with distant tissues is essential for understanding inter-organ communication and for the development of therapeutic strategies to treat metabolic dysfunction.
Major conclusions
In this review, we discuss liver-centric regulation of energy homeostasis through hepatokine secretion. We highlight key hepatokines and their roles in metabolic control, examine the molecular mechanisms of each hepatokine, and discuss their potential as therapeutic targets for metabolic disease. We also discuss important areas of future studies that may contribute to understanding hepatokine signaling under healthy and pathophysiological conditions.
Keywords: Hepatokine, Liver, Obesity, Nutrient, Insulin sensitivity, Glucose homeostasis
1. Introduction
The prevalence of obesity continues to increase worldwide due to complex behavioral, genetic, and environmental factors. Obesity is a major contributor to metabolic diseases including type 2 diabetes, hypertension, and cardiovascular disease [[1], [2], [3]]. Tissue crosstalk through autocrine, paracrine, and endocrine signals are critical regulators of energy and nutrient homeostasis [[4], [5], [6]]. While adipose tissues and pancreas are well known for their endocrine function, the liver has more recently been identified to contribute to the endocrine control of metabolism by producing liver-derived factors or hepatokines [[7], [8], [9], [10], [11], [12], [13]]. The liver can sense and respond to nutrient overabundance and deficit by secreting hepatokines, which signal energy status and help regulate nutrient availability to multiple peripheral tissues and the central nervous system (CNS). While not meant to be an exhaustive list, in this review we summarize recent findings about key hepatokines and highlight their roles in physiology and metabolic disease.
1.1. The liver and hepatokines
In humans and other higher organisms, systemic metabolism is controlled by complex pathways that regulate energy expenditure and nutrient intake. The liver is a major regulator of energy homeostasis by sensing nutrient availability and altering energy and metabolite production needed for other tissues. The liver regulates systemic glucose homeostasis through hepatic glucose production and glycogen storage. During the postprandial state, the liver increases glucose uptake in response to elevated plasma glucose and insulin levels and then converts glucose into glycogen or utilizes it for de novo lipogenesis. During this time, hepatic glucose production is also reduced due to sufficient circulating glucose availability [12,14]. In response to fasting, however, the liver increases hepatic glucose production via glycogenolysis and gluconeogenesis to supply glucose as a fuel source for non-hepatic tissues including the brain and skeletal muscle [15,16]. In addition to glucose production, the liver also supplies ketones from oxidation of lipids, and supplies lipids to peripheral tissues through very low-density lipoprotein (VLDL) production. This regulation of energy production, utilization, and storage by the liver is essential for maintaining physiological energy homeostasis. Dysregulation of any of these various pathways may result in metabolic dysfunction that can contribute to the development of insulin resistance or fatty liver disease.
Insulin resistance occurs when insulin is unable to properly (1) stimulate glucose uptake into metabolic tissues (skeletal muscle and adipose tissues), (2) suppress hepatic glucose production, and/or (3) reduce adipose tissue lipolysis [17]. During hepatic insulin resistance, excess storage of ectopic fat in the liver can contribute to the development of non-alcoholic fatty liver disease (NAFLD) [[17], [18], [19], [20]]. NAFLD, more recently proposed to be termed metabolic-associated fatty liver disease (MAFLD) [19], covers a spectrum of liver disorders that can progress to steatohepatitis (NASH) and cirrhosis [21]. NAFLD is the most common chronic liver disease worldwide and a risk factor for type 2 diabetes, obesity, and cardiovascular disease (CVD) [8,12,22]. NAFLD and type 2 diabetes are both multi-system diseases that involve perturbations in crosstalk between peripheral tissues (the liver, adipose tissue, and skeletal muscle) and the CNS. Disruption in the ability of these tissues to communicate manifests as dysregulation of lipid handling and mitochondrial function in the liver, excessive cytokine and lipid release by adipose tissue, ectopic fat deposition in skeletal muscle, and disruption of endocrine signaling in homeostatic neurons in the hypothalamus [23].
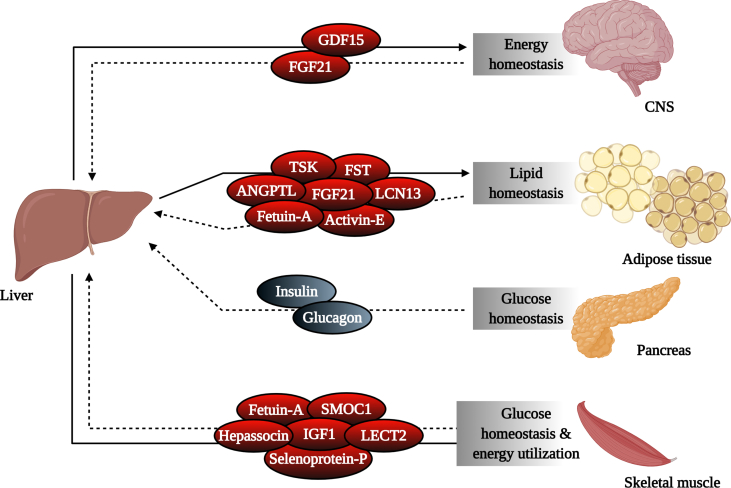
The liver communicates with other organs including the CNS, adipose tissues, and skeletal muscle in part by producing hepatokines, which are essential for transmitting information regarding the metabolic status of the liver (Figure 1). In recent years, several hepatokines have been identified and examined for their roles in the development of obesity, insulin resistance, and NAFLD [24]. While altered expression levels of certain hepatokines are considered biomarkers of metabolic dysfunction, expression of other hepatokines fluctuates dynamically with physiological states (fed, fasted, etc.) reflecting their important roles in maintaining metabolic homeostasis. The function of these protein-encoded hepatokines in physiology and metabolic disease are explored below.
Figure 1.
Hepatic production of secreted factors controls nutrient and energy homeostasis. The liver plays a central role in regulating systemic energy balance by sensing nutrient availability and altering metabolite and energy production used by various organ systems. The liver communicates with these tissues in part through the production of hepatokines, which are responsible for transmitting information regarding the metabolic status of the liver to target organs, including the CNS, adipose tissues, and muscle. This information is communicated back to the liver in a feedforward loop through hepatokines and other secreted factors, including those produced by the pancreas, to maintain energy balance in response to constant changes in nutrient status. CNS, central nervous system; GDF15, growth differentiation factor 15; FGF21, fibroblast growth factor 21; TSK, Tsukushi; FST, follistatin; ANGPTL, angiopoietin-like; LCN13, lipocalin 13; SMOC1, SPARC-related modular calcium-binding protein-1; IGF1, insulin-like growth factor 1; LECT2, leukocyte cell-derived chemotaxin 2.
1.1.1. Activin-E
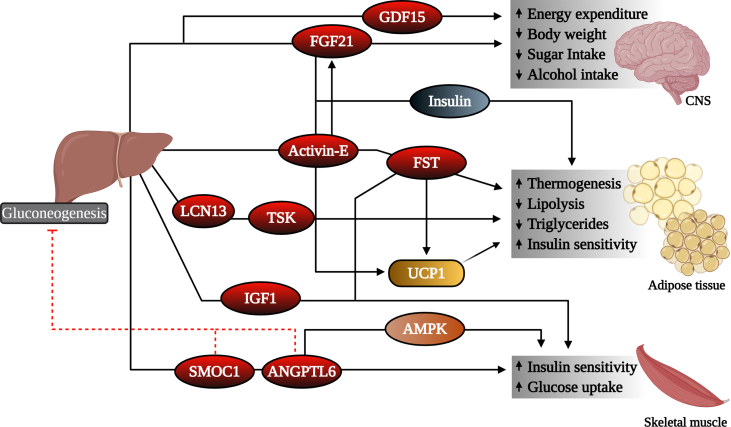
Activins are members of the transforming growth factor-β (TGFβ) superfamily and disulfide-linked homo- or heterodimers of the β-subunits of inhibin/activin A and B [25,26]. Activins were initially identified as stimulators of follicle-stimulating hormone secretion, but several studies revealed that activins play multiple roles in various cells and tissues. Activin subunits and receptors are expressed ubiquitously and believed to exert autocrine and paracrine effects. Activin-E (also called inhibin subunit beta E) was recently identified in humans and rodents and, unlike other activins that are expressed throughout the body, activin-E is expressed and secreted primarily by the liver [27,28]. Activin-E is elevated in the livers and serum of humans with obesity and NAFLD (Table 1) [29] and was recently identified as an important regulator of energy expenditure and insulin sensitivity in rodents [30] (Figure 2). Expression levels of activin-E are closely related to those of other thermogenic genes in subcutaneous white adipose tissue, and activin-E directly enhances the expression of uncoupling protein 1 (UCP1) and fibroblast growth factor (FGF21), two important regulators of adipose thermogenesis, in cultured brown pre-adipocytes [30]. Activin-E expression increases during fasting and is markedly suppressed by loss of the insulin receptor in the liver [10], suggesting dynamic regulation. Furthermore, circulating levels of activin-E are elevated in high-fat diet-fed mice and are thought to contribute to resistance to body weight gain [28,30]. Supporting a potential role for activin-E in human diseases, activin-E levels in humans are increased in individuals with obesity and insulin resistance [31]. Thus, obesity may reflect an activin-E resistant state (Figure 3). Further studies are required to assess the metabolic effects of activin-E and explore its potential as a pharmacological agent for treating insulin resistance and NAFLD.
Table 1.
Known hepatokines and their role in metabolism and disease.
| Hepatokine | Target organs | Metabolic roles | Human serum concentration in insulin resistance | Reference |
|---|---|---|---|---|
| Activin-E | Liver and adipose tissue | Increases energy expenditure and insulin sensitivity through brown and beige adipocyte activation | Increased | [[28], [29], [30]] |
| ANGPTL3 | Liver, adipose tissue, skeletal muscle, and brain cardiac tissue | Regulates plasma triglycerides by inhibiting LPL and increasing insulin resistance Dissociates active dimeric LPL to monomers |
Increased | [37,39,40,42,45,46] |
| ANGPTL4 | Liver, adipose tissue, skeletal muscle, and brain | Enhances hepatic glucose production and promotes disruption of glucose metabolism | Debated | [59,60,[67], [68], [69], [70], [71],74,75,78,83] |
| ANGPTL6 | Various peripheral tissues | Regulates glucose and lipid metabolism and insulin sensitivity | Increased | [89,91,92,94] |
| ANGPTL8 | Liver, adipose tissue, skeletal muscle, and brain cardiac tissue | Regulates plasma triglycerides by binding ANGPTL3, inhibiting LPL, and increasing insulin resistance Downregulates ANGPTL4 |
Debated | [38,59,81,100] |
| Fetuin-A | Liver and skeletal muscle | Promotes adipose tissue inflammation and insulin resistance Inhibits liver and skeletal muscle insulin receptor phosphorylation |
Increased | [12,[107], [108], [109], [110], [111], [112],117,119] |
| FGF21 | Adipose tissue and brain | Regulates energy homeostasis by increasing energy expenditure, improving insulin sensitivity, decreasing plasma triglycerides, and decreasing sugar intake | Increased | [129,[133], [134], [135], [136], [137], [138], [139], [140], [141], [142],169] |
| Follistatin | Various peripheral tissues | Serves as a novel energy deprivation signal | Increased | [177,182,[188], [189], [190]] |
| GDF15 | Adipose tissue, skeletal muscle, liver, and brain | Regulates energy homeostasis by mediating increased energy expenditure and reduced body weight gain | Increased | [195,199,200,[202], [203], [204], [205]] |
| Hepassocin | Liver, adipose tissue, and skeletal muscle | Promotes insulin resistance and hepatic lipid accumulation | Increased | [29,[208], [209], [210]] |
| IGF1 | Skeletal muscle | Improves insulin sensitivity in skeletal muscle | Decreased | [214,219,[225], [226], [227], [228], [229]] |
| LECT2 | Skeletal muscle, liver, and adipose tissue | Impairs insulin signaling and promotes hepatic lipid accumulation | Increased | [230,239,241] |
| Lipocalin 13 | Adipose tissue and liver | Regulates glucose metabolism by enhancing insulin sensitivity and regulating expression of gluconeogenic genes | Decreased | [245, 248, 249] |
| Selenoprotein-P | Various peripheral tissues | Impairs insulin signaling and augments glucose metabolism | Increased | [251,252] |
| SMOC1 | Liver and skeletal muscle | Improves glycemic control by suppressing hepatic glucose output and skeletal muscle glucose uptake and inhibiting hepatic glucagon-stimulated insulin signaling | Decreased | [257,259] |
| Tsukushi | Various peripheral tissues | Likely improves energy homeostasis by regulating thermogenesis Regulates cholesterol homeostasis |
Increased | [[260], [261], [262], [263]] |
Figure 2.
Mechanism for physiological effects of hepatokines in target tissues. Hepatokines elicit diverse effects on target tissues. Some hepatokines signal the CNS to regulate body weight homeostasis and nutrient intake, while others act on adipose tissues and muscle to regulate lipid and glucose homeostasis. GDF15, growth differentiation factor 15; FGF21, fibroblast growth factor 21; FST, follistatin; LCN13, lipocalin 13; TSK, Tsukushi; IGF1, insulin-like growth factor 1; UCP1, uncoupling protein 1; SMOC1, SPARC-related modular calcium-binding protein-1; ANGPTL6, angiopoietin-like 6; AMPK, 5′ adenosine monophosphate-activated protein kinase.
Figure 3.
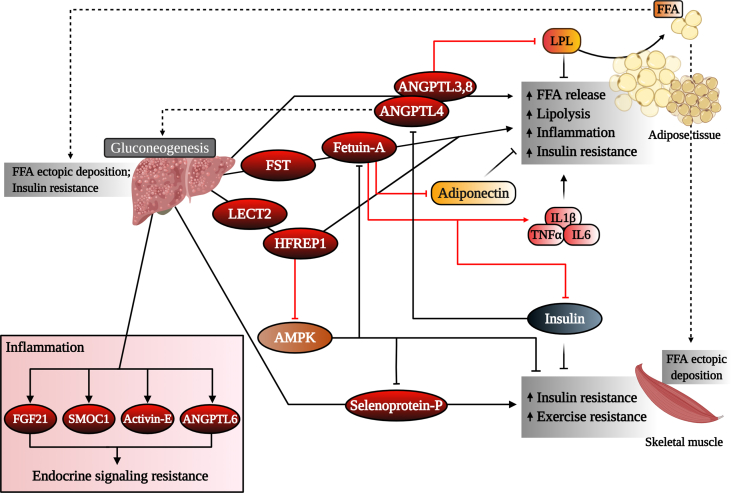
Regulation of hepatokine function during obesity and diabetes. Signaling of certain hepatokines to target tissues including skeletal muscle and adipose tissues may result in impaired lipid and glucose homeostasis, ultimately leading to metabolic dysregulation. Some hepatokines increase in the circulation under inflammation conditions, which may result in endocrine-signaling resistance. ANGPTL3, 8, 4, angiopoietin-like 3, 8, 4; LPL, lipoprotein lipase; FFA, free fatty acid; FST, follistatin; LECT2, leukocyte cell-derived chemotaxin 2; HFREP1, hepassocin; IL1β, interleukin-1 beta; TNF-α, tumor necrosis factor alpha; IL6, interleukin-6; AMPK, 5′ adenosine monophosphate-activated protein kinase; FGF21, fibroblast growth factor 21; SMOC1, SPARC-related modular calcium-binding protein-1; ANGPTL6, angiopoietin-like 6.
1.1.2. Angiopoietin-like proteins
White adipose tissue (WAT) is a major site of lipid deposition and production. Triglyceride-rich lipoproteins travel in the circulation until they reach metabolic tissues that express lipoprotein lipase (LPL) on the luminal surface of capillary endothelial cells. LPL is a critical enzyme that hydrolyzes lipoprotein triglycerides (TGs) into fatty acids that are then taken up by tissues (adipose tissue and muscle) [32]. Fatty acids taken up by target cells are either re-esterified for storage (white adipose tissue) or utilized for energy (muscle). Angiopoietin-like proteins (ANGPTL1-8) are a group of secreted glycoproteins that have emerged as important regulators of lipid metabolism in part through post-translational regulation of LPL activity. ANGPTLs are structurally similar to angiopoietin family proteins as they contain an N-terminal coiled-coil structure and a C-terminal fibrinogen-like domain [33]. Members of the ANGPTL family function as regulators of LPL activity in WAT (Figure 3).
ANGPTL3 is exclusively expressed and secreted by the liver and has emerged as an important regulator of plasma triglyceride levels due to its inhibition of LPL in oxidative tissues [34] (Figure 3). ANGPTL3 positively correlates with plasma glucose, insulin, and HOMA-IR levels in patients with insulin resistance [35,36] (Table 1). ANGPTL3 directly binds LPL with its N-terminal coiled-coil domain and promotes dissociation of active LPL dimers into inactive monomers [37]. This inhibitory action results in increased storage of lipoprotein-derived fatty acids in WAT [38]. ANGPTL3 is proteolytically cleaved by proprotein convertases, which allows complex formation with ANGPTL8 to orchestrate the inhibition of LPL and the dysregulation of carbohydrate and lipid homeostasis [37,39,40]. Furthermore, inhibition of ANGPTL3 reduces VLDL lipid content and size in an endothelial lipase-dependent manner [41]. ANGPTL3 likely augments carbohydrate homeostasis by inducing lipolysis in adipose tissue [42], attenuating de novo lipogenesis [43], and upregulating ANGPTL4 expression [44], factors that result in decreased glucose uptake and insulin sensitivity. ANGPTL3 is upregulated by liver X receptor (LXR) [45] and downregulated by several factors, including insulin [42,46], leptin [42], peroxisome proliferator-activated receptor-β (PPARβ) [47], statins [48], and thyroid hormone [49,50]. Individuals with loss of function alleles in ANGPTL3 exhibit reduced levels of plasma triglycerides, LDL-, and HDL-cholesterol [51,52]. Mice lacking ANGPTL3 have increased LPL activity and reduced circulating levels of triglycerides and free fatty acids (FFA) [[53], [54], [55]], a phenotype that has been reproduced pharmacologically in monkeys and humans [56]. Recent findings from a phase 3 clinical trial and findings in rodents suggest that combinatorial treatment of ANGPTL3 inhibitor evinacumab with other lipid-lowering therapies, including statins and PCSK9 inhibitors, result in significant reductions in hyperlipidemia compared to lipid-lowering therapies alone [57,58]. These data support the possibility that inhibiting ANGPTL3 represents a therapeutic strategy for the treatment of obesity-related metabolic dysfunction.
ANGPTL4, also referred to as fasting-induced adipose factor (FIAF), is the most studied ANGPTL family member. In humans and mice, ANGPTL4 is predominantly expressed in adipose tissue and the liver and meagerly in the skeletal muscle and heart [[59], [60], [61]]. ANGPTL4 plays important roles in regulating energy homeostasis and triglyceride metabolism [61,62], angiogenesis [63], and cancer cell invasion [64,65]. Angptl4 mRNA expression in the liver increases during fasting via PPARα and is suppressed during refeeding [66]. In addition to nutritional status, ANGPTL4 expression can also be modulated by various metabolic challenges including hypoxia and exercise [67,68]. ANGPTL4 regulates many metabolic processes through its N-terminal (nANGPTL4) and C-terminal (cANGPTL4) domains. Full-length ANGPTL4 and nANGPTL4 increase circulating triglyceride concentrations in a nutrient-dependent manner by inhibiting LPL activity and suppressing clearance of triglyceride-rich lipoproteins by WAT [[69], [70], [71], [72], [73]] (Figure 3). Furthermore, ANGPTL4 induces adipose tissue lipolysis, resulting in increased circulating FFA likely for redistribution to oxidative tissues [71,74,75]. This increase in plasma FFA and triglycerides is often associated with ectopic lipid deposition in the liver and skeletal muscle [29]. To this end, the binding and inhibition of LPL activity is limited to nANGPLT4 and full-length ANGPTL4, while cANGPTL4 (and to a lesser extent nANGPTL4) is involved in regulating several non-lipid metabolic-related processes [72].
ANGPTL4 is also upregulated in adipose tissue under fasted conditions [75], which leads to decreased LPL levels in capillaries in adipose tissue allowing for preferential triglyceride uptake into oxidative tissues such as skeletal muscle [75,76]. Specifically, in the context of exercise, ANGPTL4 selectively inhibits LPL in WAT to redirect FFAs for catabolism in skeletal muscle, increasing its oxidative capacity [77]. Overexpression of ANGPTL4 in rodents maintains glucose tolerance but results in hyperlipidemia and hepatic steatosis [78]. Mice that lack ANGPTL4 fed a high-fat diet have increased body weight and visceral fat mass compared with wild-type mice on the same diet [79]. Thus, loss of ANGPTL4 results in increased LPL activity and triglyceride uptake, leading to an increase in body weight, emphasizing its role in the redistribution of lipoprotein-derived FFAs [77,80]. A recent study demonstrated that ANGPTL3 and ANGPTL4 have opposite association patterns with body weight, diabetes status, and glucose control parameters across a wide range of body mass indexes (BMI) [69]. While it is widely accepted that ANGPTL4 correlates strongly with fasting glucose levels, the influence of ANGPTL4 on glucose metabolism and insulin resistance remains unresolved (Table 1). Overexpression of ANGPTL4 in mice causes hepatic steatosis and the mice show improved hepatic and systemic insulin sensitivity [29]. Several studies reported that plasma levels of ANGPTL4 were elevated in patients with type 2 diabetes and obese non-diabetic humans compared to non-obese non-diabetic humans [69,[81], [82], [83], [84]]. This increase in ANGPTL4 may result in a suboptimal lipid profile (increased triglycerides and decreased high-density lipoprotein (HDL)) [78,84]. One study, however, reported that circulating levels of ANGPTL4 were lower in patients with type 2 diabetes than non-diabetic subjects [78]. Human genetic studies of ANGPTL4 variants support some of the mice findings. Carriers of a low-frequency missense variant ANGPTL4, E40K, exhibited lower circulating triglycerides, increased HDL cholesterol, lower fasting glucose levels, and reduced risk of developing type 2 diabetes compared with non-carriers [[85], [86], [87], [88], [89]]. These human and rodent genetic studies paved the way for developing novel ANGPTL4 antagonists to treat metabolic dysfunction. Preclinical studies in rodents and non-human primates demonstrated that short-term treatment with a monoclonal antibody against ANGPTL4 resulted in a marked reduction in plasma triglyceride-rich lipoproteins without major side effects [80,90]. Additional late stage clinical trials are needed to define a conclusive role for targeting ANGPTL4 to treat dyslipidemia in humans.
ANGPTL6, also called angiopoietin-related growth factor, is involved in glucose, lipid, and energy metabolism [91,92]. ANGPTL6 is secreted into the circulation predominantly by the liver and is expressed at relatively low levels in other tissues. Mice lacking ANGPTL6 have higher incidences of obesity, increased lipid accumulation in the liver and skeletal muscle, increased insulin resistance, and reduced energy expenditure [91]. Conversely, overexpression of ANGPTL6 leads to elevated energy expenditure, insulin sensitivity, and protection from high-fat diet-induced obesity and hepatic steatosis [91]. A recent study showed that ANGPTL6 increased adenosine monophosphate-activated protein kinase (AMPK) activity to improve insulin signaling in skeletal muscle [33] (Figure 2). ANGPTL6 can lower gluconeogenesis in the liver by decreasing the expression of glucose-6-phosphatase by reducing FoxO1 activity in the PI3K/AKT signaling pathway [93,94]. Together, these data suggest that ANGPTL6 acts as a protective factor that may regulate energy and glucose homeostasis.
While there is limited information on whether these beneficial effects of ANGPTL6 occur in humans, recent studies demonstrated that levels of circulating ANGPTL6 increase in individuals with obesity and type 2 diabetes and positively correlate with fasting plasma glucose levels [95,96] (Table 1). Additionally, high-fat diet fed mice showed higher expression of ANGPTL6 in the liver and serum [97]. Consistent with the disruption of other endocrine signaling pathways during obesity, these data may also indicate impairments in ANGPTL6 signaling (resistance) under obese and/or diabetic conditions (Figure 3).
ANGPTL8 (also called lipasin, Td26, and betatrophin) is a more recently explored ANGPTL that negatively regulates glucose and lipid metabolism. Sources of ANGPTL8 differ among species but it is mainly produced by the liver and adipose tissue in humans and mice [98]. Expression levels can be regulated by various stimuli including hypoxia, nutritional availability [99], non-esterified free fatty acids [100], and thyroid hormone expression [101]. Circulating levels of ANGPTL8 in metabolic disease are controversial, with several studies reporting both positive and negative correlations with obesity, type 2 diabetes, NAFLD, and dyslipidemia [83,[102], [103], [104], [105], [106], [107]] (Table 1). ANGPTL8 is homologous to ANGPTL3 and by itself has no measurable effect on LPL activity. However, as previously stated, recent studies demonstrated that ANGPTL8 requires ANGPTL3 or ANGPTL4 to elicit effects on LPL [37,39,61]. ANGPTL8 and ANGPTL3 co-expression and complex formation allow the maximal inhibition of LPL [38,61] (Figure 3). Conversely, complex formation of ANGPTL8 and ANGPTL4 impairs ANGPTL4's ability to inactivate LPL [61]. ANGPTL8 expression is upregulated when ANGPTL4 expression is downregulated [98], suggesting that ANGPTL8 acts as a physiological inhibitor of ANGPTL4. ANGPTL8 also affects appetite by altering the activity of neuropeptide-Y (NPY) in the hypothalamus [108]. Similar to ANGPTL3, ANGPTL8 serves as a potential biomarker for treating metabolic dysfunction.
Despite their common architecture, each ANGPTL family member possesses distinct physiological functions and are involved in several processes influencing glucose and lipid metabolism. While the biological mechanisms of ANGPTL members discussed above have not been completely defined, it is clear that ANGPTL3, ANGPTL4, ANGPTL6, and ANGPTL8 play vital roles in governing the activity of LPL and energy metabolism.
1.1.3. Fetuin-A
Fetuins are glycoproteins that mediate the transport of a wide variety of cargo substances present in the bloodstream. Fetuin-A (alpha2-HS-glycoprotein) was the first hepatokine identified suggested to regulate metabolic homeostasis through multi-organ crosstalk and among the most important hepatokines regulating human metabolism. Predominantly synthesized and secreted from the liver, fetuin-A is a multi-faceted protein that is an endogenous inhibitor of the insulin receptor tyrosine kinase in the liver, adipose tissue, and skeletal muscle [12,[109], [110], [111], [112], [113], [114]] (Figure 3). Patients with insulin resistance, obesity, and NAFLD have high circulating fetuin-A levels, making it a potential marker of disease prediction and diagnosis [12,[115], [116], [117], [118]] (Table 1). Purified rat fetuin-A dose-dependently inhibits insulin receptor tyrosine kinase activity, altering insulin signaling and ultimately resulting in insulin resistance [109] (Figure 3). Its involvement in insulin resistance is further supported by fetuin-A-deficient mice that have decreased body fat, improved insulin sensitivity, and resistance to high-fat diet-induced body weight gain [119]. Fetuin-A can be upregulated by NFκB and ERK1/2 following increased circulating levels of free fatty acids and glucose, respectively [120]. Interestingly, fetuin-A enhances the secretion of proinflammatory cytokines in monocytes and adipose tissue and can act as an endogenous ligand and scaffold protein for toll-like receptor 4 to promote lipid-induced proinflammatory responses and insulin resistance [[121], [122], [123]] (Figure 3). Furthermore, fetuin-A is a negative regulator of the adipokine adiponectin and organokines work in concert to regulate insulin resistance (Figure 3) [121,124,125]. Conversely, adiponectin has been shown to inhibit fetuin-A expression through the AMPK pathway, and it was recently suggested that hypoadiponectinemia in patients with metabolic syndrome may result in increased circulating fetuin-A [118]. In patients with type 2 diabetes, 12 weeks of calorie restriction significantly decreased circulating fetuin-A concentrations, resulting in improved visceral fat, plasma glucose, blood pressure, and lipid profiles [126]. The anti-diabetic drug pioglitazone decreases both mRNA and circulating levels of fetuin-A in individuals with type 2 diabetes [127]. Interestingly, fetuin-B, which also increases in humans with liver steatosis and patients with type 2 diabetes, has also been shown to impair insulin action in myotubes and hepatocytes and cause glucose intolerance in mice [128].
While regulation of fetuin-A remains largely unknown, this multi-faceted hepatokine is a desirable target for obesity and insulin resistance that acts as a vital link between metabolic syndrome and inflammatory responses.
1.1.4. FGF21
Fibroblast growth factor 21 (FGF21) is a promising pharmacological therapeutic for metabolic dysfunction due to its pleotropic effects on maintaining energy homeostasis in rodents and humans. FGF21, unlike traditional FGFs, is an endocrine hormone produced and secreted predominantly by the liver [129,130]. Pharmacological administration of FGF21 decreases plasma glucose and lipid levels, improves insulin and leptin sensitivity, decreases hepatic steatosis, increases energy expenditure [[131], [132], [133], [134], [135], [136]], and decreases sugar and alcohol intake in mice [[137], [138], [139], [140], [141], [142]]. FGF21 signals to a receptor complex comprised of FGF receptor 1c (FGFR1c) and its co-receptor β-klotho (KLB) [[143], [144], [145], [146]]. FGF21 directly binds to KLB, which then facilitates binding to FGFR1c and then initiates downstream signaling events [[146], [147], [148], [149]]. While the specific signaling cascade of FGF21 has not been fully determined, activation of the FGF21/FGFR1c/KLB receptor complex results in the phosphorylation of ERK1/2 and FRS2α [135]. FGF21 expression is regulated by nutrient signals and subsequently functions to regulate nutrient homeostasis. Classically recognized as a fasting hormone regulated by the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) [150,151], several recent studies confirmed that hepatic FGF21 increases in response to other nutritional stimuli, including conditions of macronutrient imbalance [152], and in response to alcohol intake [138,140,153].
Under fasted conditions, FGF21 increases hepatic gluconeogenesis, β-oxidation, and ketogenesis in mice [142,154]. FGF21 facilitates the transition from the fasted to refed state by enhancing insulin-stimulated glucose uptake [154]. Hepatic and circulating FGF21 levels also increase in response to low-protein content or amino acid deprivation [152,[155], [156], [157], [158], [159], [160]] in an ATF4-dependent mechanism [155,161,162]. This robust increase in FGF21 results in increased energy expenditure and lower body weight gain [155,156,160,163] (Figure 2), which may function physiologically to achieve the protein leverage effect without increasing body weight [155,164]. FGF21 also regulates macronutrient-specific intake and sweet-taste preference in response to the activation of the hepatic glucose-sensitive transcription factor, carbohydrate responsive element-binding protein (ChREBP) [137]. Excess simple sugar intake increases plasma FGF21 levels in humans and rodents [137,138]. As such, maximal levels of circulating FGF21 increase in response to a combination of low-protein and high-carbohydrate conditions [152,158,165]. Various signals induce the expression of hepatic FGF21 in wild-type mice, and while a lack of FGF21 exacerbates liver and metabolic dysfunction in response to high-fat diet [166], overexpression of FGF21 is highly protective [135]. Altogether, FGF21 seems to serve as a protective factor that limits hepatic toxicity [141,167] and dysfunction [168].
Administration of FGF21 analogs to obese and type 2 diabetic subjects improves dyslipidemia, decreases body weight, improves fasting insulin levels, and was recently thoroughly reviewed [136] (Figure 2). FGF21's metabolic effects are achieved through actions on different tissues [141]. FGF21 directly signals adipose tissue to improve insulin sensitivity [169,170], while it signals the CNS to increase energy expenditure and decrease body weight [169,171,172] (Figure 2). Recent research demonstrated that FGF21 signaling to glutamatergic neurons lowers carbohydrate intake and non-nutritive sweet-taste preference in mice [173]. Interestingly, FGF21 signaling to the ventromedial hypothalamus (VMH) is required for its effects on lowering carbohydrate intake but not non-nutritive sweet-taste preference or body weight. FGF21 enhances the activity of VMH glucose-sensing neurons in response to glucose presumably to regulate carbohydrate intake [173].
In humans, circulating FGF21 levels also increase with obesity [[174], [175], [176]], type 2 diabetes [[176], [177], [178]], and NAFLD [179,180], making it a marker for metabolic disorders [141] (Table 1). Given the demonstrated beneficial effects of FGF21, this increase in circulating FGF21 levels may reflect impaired endocrine signaling that results in FGF21 resistance, similar to insulin or leptin resistance [141] (Figure 3). Overall, FGF21 has a promising therapeutic potential for treating type 2 diabetes and NAFLD.
1.1.5. Follistatin
Follistatin (FST) was first identified in the 1980s as a secreted glycoprotein that is found in most tissues throughout the body. FST acts as a multi-faceted hepatokine that is involved in reproduction [181], muscle growth [182], cancer development [183], and metabolism [184]. FST acts as an inhibitor of members of the TGF-β superfamily, including myostatin and activins [185,186], and can function as a promoter of skeletal muscle growth [187]. Most of FST's effects on reproductive health and cancer development are related to its interactions with activins and follicle-stimulating hormone, while its effects on muscle growth result from interactions with myostatin [186]. FST is secreted by a broad range of cell types, and plasma levels are thought to result from paracrine/autocrine signaling spillover [188]. However, recent work indicated that the liver is a key contributor to circulating levels of FST in humans [[188], [189], [190]]. Patients with type 2 diabetes have slightly elevated levels of circulating FST and plasma levels associated with glycemic parameters in these patients [188,191,192] (Table 1). Similarly, serum levels of FST are also significantly increased in individuals with NAFLD [193]. FST is present in three main isoforms, FST288, FST303, and FST315, with FST315 being the predominant isoform found in the circulation [185,194,195]. Although the physiological role of circulating FST is not fully understood, it is thought that FST is acutely regulated by the glucagon-to-insulin ratio [190]. Under conditions of prolonged fasting or increased energy demands (such as exercise), FST is significantly upregulated in response to increased and decreased amounts of glucagon and insulin, respectively [190]. Unlike other “energy deprivation” hepatokines that function to improve the metabolic profile, the function of physiological increases in FST during energy deprivation is not yet fully understood. Overexpression of FST in mice resulted in insulin resistance in WAT, increased hepatic glucose production, and impaired whole-body glucose tolerance, consistent with higher expression in patients with type 2 diabetes [77,196]. Furthermore, knockdown of FST in mice improved insulin sensitivity [29,196].
Although increased chronic levels of FST may be metabolically detrimental, acute expression of FST during exercise appears to be beneficial as it results in FFA and glucose uptake in skeletal muscle. FST has also been shown to promote brown adipocyte differentiation in mice [197]. A recent study demonstrated that mice overexpressing FST have increased expression of thermogenic markers, including UCP1, in WAT and BAT [198]. FST functions to increase the phosphorylation of MAPK/ERK1/2 proteins in WAT and increase the expression of Myf5 protein in BAT, which increases UCP1 expression in both tissues [198]. While there is a potential therapeutic role for acute FST treatment, more research is needed to determine the acute and chronic effects of FST in metabolic dysfunction.
1.1.6. GDF15
Growth differentiation factor 15 (GDF15), also called macrophage inhibitory cytokine-1, is associated with numerous biological processes and diseases including cancer, cardiovascular disease, and obesity [[199], [200], [201], [202]]. Physiological levels of circulating GDF15 are low in healthy humans but increase in disease states and during pregnancy [203] (Table 1). While GDF15 is not highly expressed in the human liver under basal conditions, it is highly expressed in the liver, adipose, and intestine in mice [[204], [205], [206]]. GDF15 belongs to the TGF-β superfamily and is upregulated by several inflammatory or stress-related proteins, including interleukin (IL)-1β, IL-2, and tumor necrosis factor (TNF) alpha. Its role in regulating energy homeostasis and body weight was first observed in 2006. The expression of human GDF15 (hGDF15) improved glucose tolerance and insulin sensitivity, lowered body weight and leptin levels, and increased oxidative metabolism and energy expenditure in mice [207,208] (Figure 2). GDF15 transgenic mice also exhibited a longer lifespan when fed both normal chow and high-fat diets, suggesting its role as a survival factor [209]. Experimental and clinical data have supported its role in regulating body weight and energy homeostasis [[210], [211], [212], [213]] (Figure 2). Interestingly, GDF15 production is induced by the anti-diabetic drug metformin and is partially responsible for improvements in the metabolic health of patients taking this drug [214].
GDF15's receptor, glial-derived neurotropic factor receptor-α family (GFRAL), was recently identified, and multiple studies highlighted its importance in GDF15-mediated improvements in energy homeostasis [205,212,213]. GFRAL is exclusively expressed in the human brainstem and is responsible for the metabolic effects of GDF15 on regulating appetite [201,215]. GDF15 binds with high affinity to GFRAL and its co-receptor RET, which induces phosphorylation of RET, leading to downstream signaling through the AKT, ERK1/2, and phospholipase C (PLCγ) pathways [201,213,215]. Administration of hGDF15 to GFRAL-deficient mice had no effect on body weight, energy expenditure, serum leptin and insulin levels, and food intake compared to wild-type mice given hGDF15 [213]. There have been conflicting results on the relative importance of food intake on weight reduction in GDF15 transgenic mice vs mice administered GDF15. Two studies found that GDF15 transgenic mice were protected against obesity and had increased insulin sensitivity and energy expenditure without any changes in food consumption compared to control mice [207,208].Conversely, administration of GDF15 to wild-type mice produced responses similar to those in transgenic mice; however, these mice exhibited consistent reduction in food intake [201,213]. This discrepancy may have been due to differences in the mode and duration of elevated GDF15 levels. A recent study found that GDF15 induced anorexia through nausea and emesis in mice and musk shrews, respectively [216]. While more research is needed to determine the mechanism by which GDF15 fully regulates energy homeostasis, targeting the GDF15/GFRAL axis to alter food intake and body weight represents a promising therapeutic strategy in patients with obesity or those with anorexia and/or cachexia caused by other chronic diseases.
1.1.7. Hepassocin
Hepassocin (also called fibrinogen-like protein 1 and hepatocyte-derived fibrinogen-related protein 1, HFREP1) is a hepatokine involved in the development of insulin resistance and obesity [217]. Hepassocin has mitogenic activity on hepatocytes, serving as a regulator of hepatic growth and proliferation [218]. Hepassocin is secreted predominantly from the liver, but is also expressed in adipose tissue where it is suggested to regulate lipid metabolism [219]. Plasma levels of hepassocin are associated with fasted plasma glucose, HOMA-IR, and prediabetes and increase in patients with type 2 diabetes (Table 1) [217,220]. Several studies demonstrated that hepatic expression of hepassocin increased in mice with high-fat diet-induced NAFLD [217,220,221]. Hepassocin increases the phosphorylation of ERK1/2, leading to hepatic lipogenesis and the development of hepatic steatosis [217,218]. Hepassocin has also been implicated in skeletal muscle insulin resistance through an AMPK-dependent mechanism [29] (Figure 3). Administration of recombinant hepassocin or hepatic overexpression of hepassocin in mice induces insulin resistance in the liver and skeletal muscle, while loss of hepassocin improves high-fat diet-induced insulin resistance in wild-type and ob/ob mice [217] (Figure 3). Liver injury in mice enhances the expression of hepassocin in adipose tissues, suggesting crosstalk between the liver and adipose tissue, although its role in obesity remains obscure [219]. Together, these reports suggest that hepassocin may be a useful biomarker for obesity and metabolic dysregulation.
1.1.8. Insulin-like growth factor 1
Insulin-like growth factors (IGFs) are structurally and functionally related to insulin and play an important role in enhancing insulin sensitivity [222]. While insulin regulates metabolism primarily in an endocrine manner, IGFs promotes cell growth, proliferation, differentiation, and survival in an endocrine, paracrine, and autocrine fashion. The two main IGFs, IGF1 and IGF2, bind the IGF1 receptor (IGF1R), which elicits phosphorylation of intracellular adaptor proteins and activation of MAPK and PI3K/AKT signaling pathways [223]. IGFs are regulated by a family of IGF-binding proteins (IGFBP) that can bind IGFs with equal or greater affinity than IGF1R [224,225]. IGFBP1 is elevated in patients with type 2 diabetes and has been shown to counteract the hypoglycemic effect of insulin through inhibition of IGF1 [224]. While IGF2 functions predominantly in early embryogenesis [226], IGF1 is secreted into the circulation by the liver in response to growth hormone stimulation [227]. IGF1 acts primarily on skeletal muscle, where it elicits its insulin-sensitizing effects [228] (Figure 2). While insulin and IGF1 regulate metabolism in response to nutrient availability [229,230], the role of IGF1 in obesity and insulin resistance is not fully known. There is evidence that low plasma levels of IGF1 predict the development of type 2 diabetes and correlate with insulin resistance and increased risk of metabolic syndrome and cardiovascular disease [[231], [232], [233]] (Table 1). Administration of IGF1 in humans results in decreased blood glucose levels and improved insulin sensitivity in those with and without type 2 diabetes [[234], [235], [236]]. Studies have also shown a reduction in IGF levels in patients with obesity and NAFLD [237,238]. Altogether, it is clear that IGF1 plays an important role in maintaining insulin sensitivity; however, the full mechanism by which it does so has not been completely resolved.
1.1.9. LECT2
Leukocyte cell-derived chemotaxin 2 (LECT2) is an energy-sensing hepatokine that is positively associated with hepatic inflammatory signaling, obesity, NAFLD, and insulin resistance [239,240]. LECT2 functions in liver regeneration, immune modulation, bone growth, neural development, glucose metabolism, metabolic syndrome, and cancer [[241], [242], [243], [244], [245], [246]]. LECT2 is expressed in the liver, adipose tissue, neurons, and white blood cells and is often found in the circulation. Decreased expression of LECT2 is observed during tissue inflammation, fibrosis, or pathology, suggesting that LECT2 functions positively under normal conditions. Interestingly, tissues normally lacking LECT2 exhibit increased expression of LECT2 in disease settings, indicating that improper upregulation of LECT2 may contribute to pathological conditions [246,247]. For example, mice that were fed high-fat diets or exercised had increased or decreased serum and hepatic levels of LECT2, respectively [239]. LECT2-deficient mice showed improved insulin sensitivity in skeletal muscle, while administration of recombinant LECT2 in mice led to impaired insulin signaling and induced insulin resistance in skeletal muscle [239] (Figure 3).
LECT2 alters insulin signaling through the JNK pathway in myocytes, and the expression of LECT2 in the liver is negatively regulated by the energy-depletion sensor AMPK [239,248,249]. LECT2-deficient mice exhibited increased glucose tolerance and insulin sensitivity [239]. These mice also exhibited increased insulin-stimulated AKT phosphorylation in skeletal muscle but not in the liver or adipose tissue, suggesting that loss of LECT2 selectively improves insulin sensitivity in skeletal muscle [239]. Moreover, LECT2 treatment impaired insulin signaling in differentiated 3T3-L1 cells by decreasing levels of the insulin receptor and AKT phosphorylation, reducing insulin-stimulated glucose uptake [250]. Interestingly, there is a positive relationship between circulating LECT2 levels, BMI, and insulin resistance in humans [239] (Table 1). Recent research demonstrated that acute high-fat diet overfeeding increased circulating concentrations of LECT2 in healthy men [251]. While few studies have shown the effects of LECT2 contribution to insulin resistance and obesity, more work is needed to determine whether LECT2 can be used as a potential target for the treatment of obesity-related insulin resistance.
1.1.10. Lipocalin 13
Lipocalin (LCN) proteins are secreted proteins that bind small hydrophobic ligands through their conical β barrels [252,253]. Some LCN family members, including major urinary protein 1, retinol-binding protein 4, LCN2, and LCN13, have been shown to regulate energy metabolism [[254], [255], [256]]. LCN13 in particular is secreted from multiple tissues including the liver to regulate glucose homeostasis and improve insulin sensitivity [254]. Plasma levels of LCN13 decrease in patients with obesity and type 2 diabetes [257], and both mRNA and plasma levels of LCN13 are low in mice with genetic or high-fat diet-induced obesity [254,257]. Expression and secretion of LCN13 in mice vary in response to changes in metabolic states, suggesting a role for this hepatokine in nutrient sensing and regulation [254]. While the factors regulating LCN13 expression are unknown, it is well established that LCN13 regulates glucose metabolism in adipocytes and hepatocytes by enhancing insulin signaling, insulin-stimulated glucose uptake, and insulin suppression of glucose production [254,258]. LCN13 has also been shown to regulate glucose metabolism independent of insulin by regulating the expression of key gluconeogenic genes including glucose-6-phosphate [258].
In contrast to glucose metabolism, LCN13 also decreases hepatic steatosis in obese mice. Mice overexpressing LCN13 are resistant to high-fat diet-induced hepatic steatosis and hyperlipidemia [259]. LCN13 likely regulates lipid metabolism by downregulating the expression of key liver lipogenic genes including ChREBP and PPARγ and upregulating hepatic expression of carnitine palmitoyltransferase-1α (CPT1α) to decrease lipogenesis and increase fatty acid β-oxidation [259]. Interestingly, knockdown of LCN13 or administration of recombinant LCN13 protein in mice have no significant effects on body weight or adipose tissue mass compared with control animals [257]. The molecular mechanisms of LCN13's action on glucose and lipid metabolism are largely unknown. It has been hypothesized that LCN13 binds to small hydrophobic bioactive molecules that regulate insulin sensitivity and glucose metabolism to control their transportation, activation, release, and/or clearance [254]. It is possible that LCN13 binds and activates LCN13 receptors on the plasma membranes of target cells to activate downstream signaling pathways involved in metabolic responses [254]. While it remains unclear whether LCN13's metabolic effects translate to humans, LCN13 may have therapeutic potential for the treatment of type 2 diabetes, NAFLD, and other obesity-related disorders.
1.1.11. Selenoprotein P
Selenium is an essential nutrient that is strongly related to carbohydrate and lipid metabolism. The natural forms of selenium that are present physiologically are selenocysteine and selenoproteins. Selenoprotein P (SeP) is a glycoprotein that is secreted predominantly from the liver and is upregulated by selenium and glucose supplies but downregulated by insulin and adiponectin [260,261]. SeP is responsible for the distribution of selenium to other organs and tissues [262]. SeP is associated with insulin resistance and increased serum triglyceride, and patients with NAFLD, obesity, and type 2 diabetes have high circulating levels of SeP [260,263,264] (Table 1). These findings coincide with studies in mice that demonstrate increased hepatic expression of SeP in response to high-fat diet feeding, NAFLD, and type 2 diabetes [29,260,265]. Wild-type mice treated with SeP exhibited whole-body glucose intolerance and insulin resistance [260], whereas SeP knockout mice showed improved glucose tolerance and insulin resistance [260]. Thus, it is possible that elevated circulating SeP contributes to insulin resistance. SeP expression increased in mice in response to ER stress and JNK activation, while activation of AMPK protected against SeP production (Figure 3). Although studies demonstrating a marked role of SeP in the development of insulin resistance and type 2 diabetes are limited, studies in animals have suggested that SeP could be a promising future drug target.
1.1.12. SMOC1
SPARC-related modular calcium-binding protein-1 (SMOC1) is a hepatokine recently identified to improve glucose homeostasis in rodents [266]. SMOC1 belongs to a family of matricellular proteins including secreted protein acidic and rich in cysteine (SPARC), SMOC2, and testican-1 [267]. SMOC1 is known to regulate cell matrix interactions by binding to cell surface receptors, including laminins, C-reactive protein, and tenascin-C [266,268]. SMOC1 is widely expressed in multiple tissues and generally localized at the basement membrane of cells. However, SMOC1 is highly expressed and secreted by the liver and regulates glucose homeostasis [266]. Plasma levels of SMOC1 decrease in patients with insulin-resistance compared with insulin-sensitive individuals and correlate with hepatic and peripheral insulin sensitivity (Table 1). Hepatic and circulating SMOC1 levels are induced in response to glucose in hyperglycemic high-fat diet-fed mice through a ChREBP-dependent mechanism. Loss of hepatic SMOC1 impairs glycemic control in lean mice, whereas acute SMOC1 administration and chronic hepatic SMOC1 overexpression improves glycemic control in lean and obese mice. Interestingly, SMOC1 has no effect on food intake, body weight, or energy expenditure. SMOC1 improves glucose homeostasis by (1) acutely suppressing glucose output from the liver, (2) increasing glucose uptake in skeletal muscle, and (3) inhibiting cAMP response element-binding protein (CREB)-dependent gluconeogenesis and glucagon-stimulated insulin signaling in hepatocytes (Figure 2). Administration of a long-lasting SMOC1-Fc fusion protein improved glycemic control, cholesterol, and NAFLD activity scores in lean and obese mice up to 4 weeks following the final administration of SMOC1-Fc [266]. While the effects of this fusion protein have not yet been described in humans, SMOC1 represents a promising therapeutic for treating obesity and its related complications, including insulin resistance and NASH.
1.1.13. Tsukushi
Tsukushi (TSK) is an inducible hepatokine that regulates energy expenditure and is strongly associated with NAFLD, NASH, and obesity [269,270]. TSK was recently identified as an atypical member of the small leucine-rich proteoglycan family that regulates developmental processes in various organisms [[269], [270], [271]]. Plasma levels of TSK have been linked to NAFLD and NASH pathologies [269,270]. TSK regulates energy expenditures at least in part through brown fat sympathetic innervation. Hepatic and plasma levels of TSK were strongly induced by different stimuli that increased thermogenesis and energy expenditure, including adrenergic agonists and cold exposure [271]. Mice lacking TSK exhibit elevated core body temperatures and are unable to adequately suppress energy expenditure during starvation, leading to greater body weight loss. TSK-deficient mice were resistant to diet-induced obesity, insulin resistance, and hepatic steatosis as a result of enhanced sympathetic activation and brown fat thermogenesis [271]. These results suggest that TSK deficiency protects from high-fat diet-induced obesity and metabolic disorders. However, these results with TSK knockout mice and TSK administration were not reproduced in a subsequent study [272]. Instead, the latter study found that TSK regulates systemic cholesterol homeostasis by reducing circulating high-density lipoprotein cholesterol, lowering cholesterol efflux capacity, and decreasing the conversion of cholesterol to bile in the liver [272]. The discrepancies in findings between groups may have been due to the differences in TSK knockout mouse models that were generated and/or variations in diet composition that were used. More studies are required to understand the role of TSK in regulating energy expenditure.
2. Conclusion
The regulation of whole-body energy homeostasis by the liver is a complex process that involves tissue crosstalk through the production of hepatokines and changes in hepatic metabolism in response to metabolic cues and substrates. The liver responds to energy influx and demand by secreting hepatokines that act on various tissues to facilitate energy utilization or storage. These hepatokines can act in isolation or in conjunction with other factors to coordinate systemic metabolic processes. Their concentrations and effects are altered in response to metabolic stressors, and dysregulation in their signaling can result in pathologies including obesity, type 2 diabetes, and NAFLD.
Beneficial hepatokines that improve metabolic dysfunction include activin-E, ANGPTL4, ANGPTL6, FGF21, GDF15, IGF-1, LCN13, SMOC1, and TSK. These hepatokines improve whole organism energy homeostasis by accomplishing one or more of the following (1) increasing energy expenditure through brown and beige adipocyte activation, (2) increasing insulin sensitivity, (3) increasing glucose uptake, (4) decreasing plasma triglyceride concentrations and regulating cholesterol homeostasis, (5) decreasing body weight, and (6) lowering food intake. Chronic decreased levels of detrimental hepatokines, including ANGPTL3, ANGPTL8, fetuin-A, hepassocin, LECT-2, and SeP aid in maintaining insulin sensitivity, lipid homeostasis, and healthy body weight by decreasing systemic inflammation and insulin resistance.
Important areas of future studies include (1) determining the mechanisms by which hepatokines and other organokines may integrate to regulate metabolism through inter-organ crosstalk, (2) understanding how preclinical evidence for hepatokines may translate to human studies, (3) determining how hepatokines contribute to disease progression, and 4) understanding the regulation and mechanisms of action of each hepatokine under healthy and pathophysiological conditions.
Author contributions
S.O.J. and M.J.P. wrote the paper. M.J.P. is responsible for the integrity of its content.
Acknowledgments
This study was funded by the National Institutes of Health R01DK106104 (M.J.P.), R01AA027654 (M.J.P.), and F31 DK117515 (S.O.J.), the Veterans Affairs Merit Review Program I01BX004634 (M.J.P.), and the Carver College of Medicine at the University of Iowa (M.J.P.).
Conflict of interest
The authors have no conflicts to declare.
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Bornfeldt K.E., Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metabolism. 2011;14(5):575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. The Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo-Armengol J., Fajas L., Lopez-Mejia I.C. Inter-organ communication: a gatekeeper for metabolic health. EMBO Reports. 2019;20(9) doi: 10.15252/embr.201947903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Priest C., Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nature Metabolism. 2019;1(12):1177–1188. doi: 10.1038/s42255-019-0145-5. [DOI] [PubMed] [Google Scholar]
- 6.Oishi Y., Manabe I. Organ system crosstalk in cardiometabolic disease in the age of multimorbidity. Frontiers in Cardiovascular Medicine. 2020;7(64) doi: 10.3389/fcvm.2020.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esfahani M., Baranchi M., Goodarzi M.T. The implication of hepatokines in metabolic syndrome. Diabetes Metab Syndr. 2019;13(4):2477–2480. doi: 10.1016/j.dsx.2019.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Iroz A., Couty J.P., Postic C. Hepatokines: unlocking the multi-organ network in metabolic diseases. Diabetologia. 2015;58(8):1699–1703. doi: 10.1007/s00125-015-3634-4. [DOI] [PubMed] [Google Scholar]
- 9.Ennequin G., Sirvent P., Whitham M. Role of exercise-induced hepatokines in metabolic disorders. American Journal of Physiology. Endocrinology and Metabolism. 2019;317(1):E11–E24. doi: 10.1152/ajpendo.00433.2018. [DOI] [PubMed] [Google Scholar]
- 10.Smati S., Regnier M., Fougeray T., Polizzi A., Fougerat A., Lasserre F. Regulation of hepatokine gene expression in response to fasting and feeding: influence of PPAR-alpha and insulin-dependent signalling in hepatocytes. Diabetes & Metabolism. 2020;46(2):129–136. doi: 10.1016/j.diabet.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature Reviews Endocrinology. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 12.Stefan N., Haring H.U. The role of hepatokines in metabolism. Nature Reviews Endocrinology. 2013;9(3):144–152. doi: 10.1038/nrendo.2012.258. [DOI] [PubMed] [Google Scholar]
- 13.Pocai A., Obici S., Schwartz G.J., Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metabolism. 2005;1(1):53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Krssak M., Brehm A., Bernroider E., Anderwald C., Nowotny P., Dalla Man C. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004;53(12):3048–3056. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- 15.Roden M., Bernroider E. Hepatic glucose metabolism in humans—its role in health and disease. Best Practice & Research Clinical Endocrinology & Metabolism. 2003;17(3):365–383. doi: 10.1016/s1521-690x(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 16.Leone T.C., Weinheimer C.J., Kelly D.P. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proceedings of the National Academy of Sciences of the U S A. 1999;96(13):7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 18.Hodson L., Karpe F. Hyperinsulinaemia: does it tip the balance toward intrahepatic fat accumulation? Endocr Connect. 2019;8(10):R157–R168. doi: 10.1530/EC-19-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eslam M., Sanyal A.J., George J., Sanyal A., Neuschwander-Tetri B., Tiribelli C. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 20.Calori G., Lattuada G., Ragogna F., Garancini M.P., Crosignani P., Villa M. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145–152. doi: 10.1002/hep.24356. [DOI] [PubMed] [Google Scholar]
- 21.Katsarou A., Moustakas I.I., Pyrina I., Lembessis P., Koutsilieris M., Chatzigeorgiou A. Metabolic inflammation as an instigator of fibrosis during non-alcoholic fatty liver disease. World Journal of Gastroenterology. 2020;26(17):1993–2011. doi: 10.3748/wjg.v26.i17.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison A.E., Zaccardi F., Khunti K., Davies M.J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: a meta-analysis with bias analysis. Liver International. 2019;39(3):557–567. doi: 10.1111/liv.13994. [DOI] [PubMed] [Google Scholar]
- 23.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. 2019;1(1):30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han H.S., Kang G., Kim J.S., Choi B.H., Koo S.H. Regulation of glucose metabolism from a liver-centric perspective. Experimental & Molecular Medicine. 2016;48(3):e218. doi: 10.1038/emm.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T., Asashima M., Eto Y., Takio K., Uchiyama H., Moriya N. Isolation and characterization of native activin B. Journal of Biological Chemistry. 1992;267(23):16385–16389. [PubMed] [Google Scholar]
- 27.Hashimoto O., Tsuchida K., Ushiro Y., Hosoi Y., Hoshi N., Sugino H. cDNA cloning and expression of human activin betaE subunit. Molecular and Cellular Endocrinology. 2002;194(1–2):117–122. doi: 10.1016/s0303-7207(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 28.Chabicovsky M., Herkner K., Rossmanith W. Overexpression of activin beta(C) or activin beta(E) in the mouse liver inhibits regenerative deoxyribonucleic acid synthesis of hepatic cells. Endocrinology. 2003;144(8):3497–3504. doi: 10.1210/en.2003-0388. [DOI] [PubMed] [Google Scholar]
- 29.Watt M.J., Miotto P.M., De Nardo W., Montgomery M.K. The liver as an endocrine organ-linking NAFLD and insulin resistance. Endocrine Reviews. 2019;40(5):1367–1393. doi: 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto O., Funaba M., Sekiyama K., Doi S., Shindo D., Satoh R. Activin E controls energy homeostasis in both Brown and white adipose tissues as a hepatokine. Cell Reports. 2018;25(5):1193–1203. doi: 10.1016/j.celrep.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Sugiyama M., Kikuchi A., Misu H., Igawa H., Ashihara M., Kushima Y. Inhibin βE (INHBE) is a possible insulin resistance-associated hepatokine identified by comprehensive gene expression analysis in human liver biopsy samples. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0194798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frayn K.N., Coppack S.W., Fielding B.A., Humphreys S.M. Coordinated regulation of hormone-sensitive lipase and lipoprotein lipase in human adipose tissue in vivo: implications for the control of fat storage and fat mobilization. Advances in Enzyme Regulation. 1995;35:163–178. doi: 10.1016/0065-2571(94)00011-q. [DOI] [PubMed] [Google Scholar]
- 33.Hato T., Tabata M., Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends in Cardiovascular Medicine. 2008;18(1):6–14. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Conklin D., Gilbertson D., Taft D.W., Maurer M.F., Whitmore T.E., Smith D.L. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics. 1999;62(3):477–482. doi: 10.1006/geno.1999.6041. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz Y., Ulukaya E., Atug O., Dolar E. Serum concentrations of human angiopoietin-like protein 3 in patients with nonalcoholic fatty liver disease: association with insulin resistance. European Journal of Gastroenterology and Hepatology. 2009;21(11):1247–1251. doi: 10.1097/MEG.0b013e32832b77ae. [DOI] [PubMed] [Google Scholar]
- 36.Robciuc M.R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Oorni K. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(7):1706–1713. doi: 10.1161/ATVBAHA.113.301397. [DOI] [PubMed] [Google Scholar]
- 37.Chi X., Britt E.C., Shows H.W., Hjelmaas A.J., Shetty S.K., Cushing E.M. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab. 2017;6(10):1137–1149. doi: 10.1016/j.molmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., McNutt M.C., Banfi S., Levin M.G., Holland W.L., Gusarova V. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proceedings of the National Academy of Sciences of the U S A. 2015;112(37):11630–11635. doi: 10.1073/pnas.1515374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haller J.F., Mintah I.J., Shihanian L.M., Stevis P., Buckler D., Alexa-Braun C.A. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. The Journal of Lipid Research. 2017;58(6):1166–1173. doi: 10.1194/jlr.M075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. Journal of Biological Chemistry. 2003;278(43):41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 41.Adam R.C., Mintah I.J., Alexa-Braun C.A., Shihanian L.M., Lee J.S., Banerjee P. Angiopoietin-like protein 3 (ANGPTL3) governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. The Journal of Lipid Research. 2020 doi: 10.1194/jlr.RA120000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimamura M., Matsuda M., Ando Y., Koishi R., Yasumo H., Furukawa H. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochemical and Biophysical Research Communications. 2004;322(3):1080–1085. doi: 10.1016/j.bbrc.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Smith U., Kahn B.B. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. Journal of Internal Medicine. 2016;280(5):465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgiadi A., Lichtenstein L., Degenhardt T., Boekschoten M.V., van Bilsen M., Desvergne B. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circulation Research. 2010;106(11):1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan R., Zhang T., Hernandez M., Gan F.X., Wright S.D., Waters M.G. Regulation of the angiopoietin-like protein 3 gene by LXR. The Journal of Lipid Research. 2003;44(1):136–143. doi: 10.1194/jlr.m200367-jlr200. [DOI] [PubMed] [Google Scholar]
- 46.Inukai K., Nakashima Y., Watanabe M., Kurihara S., Awata T., Katagiri H. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. Biochemical and Biophysical Research Communications. 2004;317(4):1075–1079. doi: 10.1016/j.bbrc.2004.03.151. [DOI] [PubMed] [Google Scholar]
- 47.Matsusue K., Miyoshi A., Yamano S., Gonzalez F.J. Ligand-activated PPARbeta efficiently represses the induction of LXR-dependent promoter activity through competition with RXR. Molecular and Cellular Endocrinology. 2006;256(1–2):23–33. doi: 10.1016/j.mce.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pramfalk C., Parini P., Gustafsson U., Sahlin S., Eriksson M. Effects of high-dose statin on the human hepatic expression of genes involved in carbohydrate and triglyceride metabolism. Journal of Internal Medicine. 2011;269(3):333–339. doi: 10.1111/j.1365-2796.2010.02305.x. [DOI] [PubMed] [Google Scholar]
- 49.Fugier C., Tousaint J.J., Prieur X., Plateroti M., Samarut J., Delerive P. The lipoprotein lipase inhibitor ANGPTL3 is negatively regulated by thyroid hormone. Journal of Biological Chemistry. 2006;281(17):11553–11559. doi: 10.1074/jbc.M512554200. [DOI] [PubMed] [Google Scholar]
- 50.Duntas L.H., Brenta G. A renewed focus on the association between thyroid hormones and lipid metabolism. Frontiers in Endocrinology. 2018;9:511. doi: 10.3389/fendo.2018.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romeo S., Yin W., Kozlitina J., Pennacchio L.A., Boerwinkle E., Hobbs H.H. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. Journal of Clinical Investigation. 2009;119(1):70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minicocci I., Montali A., Robciuc M.R., Quagliarini F., Censi V., Labbadia G. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. Journal of Clinical Endocrinology & Metabolism. 2012;97(7):E1266–E1275. doi: 10.1210/jc.2012-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musunuru K., Pirruccello J.P., Do R., Peloso G.M., Guiducci C., Sougnez C. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. New England Journal of Medicine. 2010;363(23):2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koster A., Chao Y.B., Mosior M., Ford A., Gonzalez-DeWhitt P.A., Hale J.E. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146(11):4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 55.Shimizugawa T., Ono M., Shimamura M., Yoshida K., Ando Y., Koishi R. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. Journal of Biological Chemistry. 2002;277(37):33742–33748. doi: 10.1074/jbc.M203215200. [DOI] [PubMed] [Google Scholar]
- 56.Gusarova V., Alexa C.A., Wang Y., Rafique A., Kim J.H., Buckler D. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. The Journal of Lipid Research. 2015;56(7):1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raal F.J., Rosenson R.S., Reeskamp L.F., Hovingh G.K., Kastelein J.J.P., Rubba P. Evinacumab for homozygous familial hypercholesterolemia. New England Journal of Medicine. 2020;383(8):711–720. doi: 10.1056/NEJMoa2004215. [DOI] [PubMed] [Google Scholar]
- 58.Hurt-Camejo E. ANGPTL3, PCSK9, and statin therapy drive remarkable reductions in hyperlipidemia and atherosclerosis in a mouse model. The Journal of Lipid Research. 2020;61(3):272–274. doi: 10.1194/jlr.C120000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dijk W., Heine M., Vergnes L., Boon M.R., Schaart G., Hesselink M.K. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. 2015;4 doi: 10.7554/eLife.08428. Elife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattijssen F., Alex S., Swarts H.J., Groen A.K., van Schothorst E.M., Kersten S. Angptl4 serves as an endogenous inhibitor of intestinal lipid digestion. Mol Metab. 2014;3(2):135–144. doi: 10.1016/j.molmet.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kovrov O., Kristensen K.K., Larsson E., Ploug M., Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. The Journal of Lipid Research. 2019;60(4):783–793. doi: 10.1194/jlr.M088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dijk W., Kersten S. Regulation of lipid metabolism by angiopoietin-like proteins. Current Opinion in Lipidology. 2016;27(3):249–256. doi: 10.1097/MOL.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 63.Oike Y., Yasunaga K., Suda T. Angiopoietin-related/angiopoietin-like proteins regulate angiogenesis. International Journal of Hematology. 2004;80(1):21–28. doi: 10.1532/ijh97.04034. [DOI] [PubMed] [Google Scholar]
- 64.Nakayama T., Hirakawa H., Shibata K., Abe K., Nagayasu T., Taguchi T. Expression of angiopoietin-like 4 in human gastric cancer: ANGPTL4 promotes venous invasion. Oncology Reports. 2010;24(3):599–606. doi: 10.3892/or_00000897. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y., Long J., Zhou Y. Angiopoietin-like 4 promotes melanoma cell invasion and survival through aldolase A. Oncol Lett. 2014;8(1):211–217. doi: 10.3892/ol.2014.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cushing E.M., Chi X., Sylvers K.L., Shetty S.K., Potthoff M.J., Davies B.S.J. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol Metab. 2017;6(8):809–818. doi: 10.1016/j.molmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H.F., Hesselink M.K. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(6):969–974. doi: 10.1161/ATVBAHA.108.182147. [DOI] [PubMed] [Google Scholar]
- 68.Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. Journal of Biological Chemistry. 2000;275(37):28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 69.Cinkajzlova A., Mraz M., Lacinova Z., Klouckova J., Kavalkova P., Kratochvilova H. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: the effect of weight reduction and realimentation. Nutrition & Diabetes. 2018;8(1):21. doi: 10.1038/s41387-018-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lichtenstein L., Berbee J.F., van Dijk S.J., van Dijk K.W., Bensadoun A., Kema I.P. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(11):2420–2427. doi: 10.1161/ATVBAHA.107.151894. [DOI] [PubMed] [Google Scholar]
- 71.Yoshida K., Shimizugawa T., Ono M., Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. The Journal of Lipid Research. 2002;43(11):1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 72.Fernández-Hernando C., Suárez Y. ANGPTL4: a multifunctional protein involved in metabolism and vascular homeostasis. Current Opinion in Hematology. 2020;27(3):206–213. doi: 10.1097/MOH.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu P., Goh Y.Y., Chin H.F., Kersten S., Tan N.S. Angiopoietin-like 4: a decade of research. Bioscience Reports. 2012;32(3):211–219. doi: 10.1042/BSR20110102. [DOI] [PubMed] [Google Scholar]
- 74.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. Journal of Biological Chemistry. 2006;281(2):934–944. doi: 10.1074/jbc.M506519200. [DOI] [PubMed] [Google Scholar]
- 75.Sukonina V., Lookene A., Olivecrona T., Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proceedings of the National Academy of Sciences of the U S A. 2006;103(46):17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Current Opinion in Lipidology. 2016;27(3):233–241. doi: 10.1097/MOL.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Gil A.M., Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients. 2020;12(6) doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu A., Lam M.C., Chan K.W., Wang Y., Zhang J., Hoo R.L. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proceedings of the National Academy of Sciences of the U S A. 2005;102(17):6086–6091. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies B.S.J. Can targeting ANGPTL proteins improve glucose tolerance? Diabetologia. 2018;61(6):1277–1281. doi: 10.1007/s00125-018-4604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desai U., Lee E.C., Chung K., Gao C., Gay J., Key B. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proceedings of the National Academy of Sciences of the U S A. 2007;104(28):11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mehta N., Qamar A., Qu L., Qasim A.N., Mehta N.N., Reilly M.P. Differential association of plasma angiopoietin-like proteins 3 and 4 with lipid and metabolic traits. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(5):1057–1063. doi: 10.1161/ATVBAHA.113.302802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barja-Fernandez S., Moreno-Navarrete J.M., Folgueira C., Xifra G., Sabater M., Castelao C. Plasma ANGPTL-4 is associated with obesity and glucose tolerance: cross-sectional and longitudinal findings. Molecular Nutrition & Food Research. 2018;62(10) doi: 10.1002/mnfr.201800060. [DOI] [PubMed] [Google Scholar]
- 83.Abu-Farha M., Al-Khairi I., Cherian P., Chandy B., Sriraman D., Alhubail A. Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. Lipids in Health and Disease. 2016;15(1):181. doi: 10.1186/s12944-016-0337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tjeerdema N., Georgiadi A., Jonker J.T., van Glabbeek M., Alizadeh Dehnavi R., Tamsma J.T. Inflammation increases plasma angiopoietin-like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2(1) doi: 10.1136/bmjdrc-2014-000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dewey F.E., Gusarova V., O'Dushlaine C., Gottesman O., Trejos J., Hunt C. Inactivating variants in ANGPTL4 and risk of coronary artery disease. New England Journal of Medicine. 2016;374(12):1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu D.J., Peloso G.M., Yu H., Butterworth A.S., Wang X., Mahajan A. Exome-wide association study of plasma lipids in >300,000 individuals. Nature Genetics. 2017;49(12):1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klarin D., Damrauer S.M., Cho K., Sun Y.V., Teslovich T.M., Honerlaw J. Genetics of blood lipids among ∼300,000 multi-ethnic participants of the Million Veteran Program. Nature Genetics. 2018;50(11):1514–1523. doi: 10.1038/s41588-018-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romeo S., Pennacchio L.A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H.H. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nature Genetics. 2007;39(4):513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gusarova V., O'Dushlaine C., Teslovich T.M., Benotti P.N., Mirshahi T., Gottesman O. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nature Communications. 2018;9(1):2252. doi: 10.1038/s41467-018-04611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruscica M., Zimetti F., Adorni M.P., Sirtori C.R., Lupo M.G., Ferri N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: new therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacological Research. 2020;153:104653. doi: 10.1016/j.phrs.2020.104653. [DOI] [PubMed] [Google Scholar]
- 91.Oike Y., Akao M., Yasunaga K., Yamauchi T., Morisada T., Ito Y. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Natura Med. 2005;11(4):400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 92.Kadomatsu T., Tabata M., Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. FEBS Journal. 2011;278(4):559–564. doi: 10.1111/j.1742-4658.2010.07979.x. [DOI] [PubMed] [Google Scholar]
- 93.Kitazawa M., Ohizumi Y., Oike Y., Hishinuma T., Hashimoto S. Angiopoietin-related growth factor suppresses gluconeogenesis through the Akt/forkhead box class O1-dependent pathway in hepatocytes. Journal of Pharmacology and Experimental Therapeutics. 2007;323(3):787–793. doi: 10.1124/jpet.107.127530. [DOI] [PubMed] [Google Scholar]
- 94.Qaddoumi M.G., Alanbaei M., Hammad M.M., Al Khairi I., Cherian P., Channanath A. Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes. Scientific Reports. 2020;10(1):6170. doi: 10.1038/s41598-020-63149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ebert T., Bachmann A., Lossner U., Kratzsch J., Bluher M., Stumvoll M. Serum levels of angiopoietin-related growth factor in diabetes mellitus and chronic hemodialysis. Metabolism. 2009;58(4):547–551. doi: 10.1016/j.metabol.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 96.Namkung J., Koh S.B., Kong I.D., Choi J.W., Yeh B.I. Serum levels of angiopoietin-related growth factor are increased in metabolic syndrome. Metabolism. 2011;60(4):564–568. doi: 10.1016/j.metabol.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Fan K.C., Wu H.T., Wei J.N., Chuang L.M., Hsu C.Y., Yen I.W. Serum angiopoietin-like protein 6, risk of type 2 diabetes, and response to hyperglycemia: a prospective cohort study. Journal of Clinical Endocrinology & Metabolism. 2020;105(5) doi: 10.1210/clinem/dgaa103. [DOI] [PubMed] [Google Scholar]
- 98.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E. Atypical angiopoietin-like protein that regulates ANGPTL3. Proceedings of the National Academy of Sciences of the U S A. 2012;109(48):19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nidhina Haridas P.A., Soronen J., Sadevirta S., Mysore R., Quagliarini F., Pasternack A. Regulation of angiopoietin-like proteins (ANGPTLs) 3 and 8 by insulin. Journal of Clinical Endocrinology & Metabolism. 2015;100(10):E1299–E1307. doi: 10.1210/jc.2015-1254. [DOI] [PubMed] [Google Scholar]
- 100.Dang F., Wu R., Wang P., Wu Y., Azam M.S., Xu Q. Fasting and feeding signals control the oscillatory expression of Angptl8 to modulate lipid metabolism. Scientific Reports. 2016;6:36926. doi: 10.1038/srep36926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang L., Yin R., Wang Z., Wang X., Zhang Y., Zhao D. Circulating Angptl3 and Angptl8 are increased in patients with hypothyroidism. BioMed Research International. 2019;2019:3814687. doi: 10.1155/2019/3814687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morinaga J., Zhao J., Endo M., Kadomatsu T., Miyata K., Sugizaki T. Association of circulating ANGPTL 3, 4, and 8 levels with medical status in a population undergoing routine medical checkups: a cross-sectional study. PloS One. 2018;13(3) doi: 10.1371/journal.pone.0193731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tuhan H., Abacı A., Anık A., Çatlı G., Küme T., Çalan G.Ö. Circulating betatrophin concentration is negatively correlated with insulin resistance in obese children and adolescents. Diabetes Research and Clinical Practice. 2016;114:37–42. doi: 10.1016/j.diabres.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Barja-Fernández S., Folgueira C., Seoane L.M., Casanueva F.F., Dieguez C., Castelao C. Circulating betatrophin levels are increased in anorexia and decreased in morbidly obese women. Journal of Clinical Endocrinology & Metabolism. 2015;100(9):E1188–E1196. doi: 10.1210/JC.2015-1595. [DOI] [PubMed] [Google Scholar]
- 105.Guo K., Lu J., Yu H., Zhao F., Pan P., Zhang L. Serum betatrophin concentrations are significantly increased in overweight but not in obese or type 2 diabetic individuals. Obesity. 2015;23(4):793–797. doi: 10.1002/oby.21038. [DOI] [PubMed] [Google Scholar]
- 106.Gómez-Ambrosi J., Pascual E., Catalán V., Rodríguez A., Ramírez B., Silva C. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2014;99(10):E2004–E2009. doi: 10.1210/jc.2014-1568. [DOI] [PubMed] [Google Scholar]
- 107.Luo M., Peng D. ANGPTL8: an important regulator in metabolic disorders. Frontiers in Endocrinology. 2018;9(169) doi: 10.3389/fendo.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang R., Yuan J., Zhang C., Wang L., Liu Y., Song L. Neuropeptide Y-positive neurons in the dorsomedial hypothalamus are involved in the anorexic effect of Angptl8. Frontiers in Molecular Neuroscience. 2018;11:451. doi: 10.3389/fnmol.2018.00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patrick Auberger L.F. vol. 58. Cell Press; 1989. Jean olivier contreres, gilles pages, ginette le cam, bernard rossi, alphonse le cam. (Characterization of a natural inhibitor of the insulin receptor tymsine kinase: cDNA cloning, purification, and anti-mitogenic activity). [DOI] [PubMed] [Google Scholar]
- 110.Mathews S.T., Chellam N., Srinivas P.R., Cintron V.J., Leon M.A., Goustin A.S. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Molecular and Cellular Endocrinology. 2000;164(1–2):87–98. doi: 10.1016/s0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 111.Rauth G., Pöschke O., Fink E., Eulitz M., Tippmer S., Kellerer M. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. European Journal of Biochemistry. 1992;204(2):523–529. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 112.Goustin A.S., Abou-Samra A.B. The "thrifty" gene encoding Ahsg/Fetuin-A meets the insulin receptor: insights into the mechanism of insulin resistance. Cellular Signalling. 2011;23(6):980–990. doi: 10.1016/j.cellsig.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Yilmaz Y., Yonal O., Kurt R., Ari F., Oral A.Y., Celikel C.A. Serum fetuin A/alpha2HS-glycoprotein levels in patients with non-alcoholic fatty liver disease: relation with liver fibrosis. Annals of Clinical Biochemistry. 2010;47(Pt 6):549–553. doi: 10.1258/acb.2010.010169. [DOI] [PubMed] [Google Scholar]
- 114.Bourebaba L., Marycz K. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. Journal of Clinical Medicine. 2019;8(12) doi: 10.3390/jcm8122033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haukeland J.W., Dahl T.B., Yndestad A., Gladhaug I.P., Loberg E.M., Haaland T. Fetuin A in nonalcoholic fatty liver disease: in vivo and in vitro studies. European Journal of Endocrinology. 2012;166(3):503–510. doi: 10.1530/EJE-11-0864. [DOI] [PubMed] [Google Scholar]
- 116.Reinehr T., Roth C.L. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. Journal of Clinical Endocrinology & Metabolism. 2008;93(11):4479–4485. doi: 10.1210/jc.2008-1505. [DOI] [PubMed] [Google Scholar]
- 117.Stefan N., Fritsche A., Weikert C., Boeing H., Joost H.G., Haring H.U. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57(10):2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan X., Wen S.W., Bestman P.L., Kaminga A.C., Acheampong K., Liu A. Fetuin-A in Metabolic syndrome: a systematic review and meta-analysis. PloS One. 2020;15(3) doi: 10.1371/journal.pone.0229776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mathews S.T., Singh G.P., Ranalletta M., Cintron V.J., Qiang X., Goustin A.S. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51(8):2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 120.Takata H., Ikeda Y., Suehiro T., Ishibashi A., Inoue M., Kumon Y. High glucose induces transactivation of the alpha2-HS glycoprotein gene through the ERK1/2 signaling pathway. Journal of Atherosclerosis and Thrombosis. 2009;16(4):448–456. doi: 10.5551/jat.no950. [DOI] [PubMed] [Google Scholar]
- 121.Ke Y., Xu C., Lin J., Li Y. Role of hepatokines in non-alcoholic fatty liver disease. J Transl Int Med. 2019;7(4):143–148. doi: 10.2478/jtim-2019-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pal D., Dasgupta S., Kundu R., Maitra S., Das G., Mukhopadhyay S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Natura Med. 2012;18(8):1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 123.Lee K.Y., Lee W., Jung S.H., Park J., Sim H., Choi Y.J. Hepatic upregulation of fetuin-A mediates acetaminophen-induced liver injury through activation of TLR4 in mice. Biochemical Pharmacology. 2019;166:46–55. doi: 10.1016/j.bcp.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 124.Dasgupta S., Bhattacharya S., Biswas A., Majumdar S.S., Mukhopadhyay S., Ray S. NF-kappaB mediates lipid-induced fetuin-A expression in hepatocytes that impairs adipocyte function effecting insulin resistance. Biochemical Journal. 2010;429(3):451–462. doi: 10.1042/BJ20100330. [DOI] [PubMed] [Google Scholar]
- 125.Hennige A.M., Staiger H., Wicke C., Machicao F., Fritsche A., Haring H.U. Fetuin-A induces cytokine expression and suppresses adiponectin production. PloS One. 2008;3(3):e1765. doi: 10.1371/journal.pone.0001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choi K.M., Han K.A., Ahn H.J., Lee S.Y., Hwang S.Y., Kim B.H. The effects of caloric restriction on fetuin-A and cardiovascular risk factors in rats and humans: a randomized controlled trial. Clinical Endocrinology. 2013;79(3):356–363. doi: 10.1111/cen.12076. [DOI] [PubMed] [Google Scholar]
- 127.Ochi A., Mori K., Emoto M., Nakatani S., Morioka T., Motoyama K. Direct inhibitory effects of pioglitazone on hepatic fetuin-A expression. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meex R.C., Hoy A.J., Morris A., Brown R.D., Lo J.C., Burke M. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metabolism. 2015;22(6):1078–1089. doi: 10.1016/j.cmet.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 129.Nishimura T., Nakatake Y., Konishi M., Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et Biophysica Acta. 2000;1492(1):203–206. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 130.Fon Tacer K., Bookout A.L., Ding X., Kurosu H., John G.B., Wang L. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Molecular Endocrinology. 2010;24(10):2050–2064. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 132.Xu J., Stanislaus S., Chinookoswong N., Lau Y.Y., Hager T., Patel J. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models--association with liver and adipose tissue effects. American Journal of Physiology. Endocrinology and Metabolism. 2009;297(5):E1105–E1114. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 133.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58(1):250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andersen B., Straarup E.M., Heppner K.M., Takahashi D.L., Raffaele V., Dissen G.A. FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. International Journal of Obesity. 2018;42(6):1151–1160. doi: 10.1038/s41366-018-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R., Galbreath E.J. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115(6):1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gaich G., Chien J.Y., Fu H., Glass L.C., Deeg M.A., Holland W.L. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metabolism. 2013;18(3):333–340. doi: 10.1016/j.cmet.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 137.von Holstein-Rathlou S., BonDurant L.D., Peltekian L., Naber M.C., Yin T.C., Claflin K.E. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metabolism. 2016;23(2):335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Talukdar S., Owen B.M., Song P., Hernandez G., Zhang Y., Zhou Y. FGF21 regulates sweet and alcohol preference. Cell Metabolism. 2016;23(2):344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanaka T., Ngwa J.S., van Rooij F.J., Zillikens M.C., Wojczynski M.K., Frazier-Wood A.C. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. American Journal of Clinical Nutrition. 2013;97(6):1395–1402. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schumann G., Liu C., O'Reilly P., Gao H., Song P., Xu B. KLB is associated with alcohol drinking, and its gene product beta-Klotho is necessary for FGF21 regulation of alcohol preference. Proceedings of the National Academy of Sciences of the U S A. 2016;113(50):14372–14377. doi: 10.1073/pnas.1611243113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.BonDurant L.D., Potthoff M.J. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annual Review of Nutrition. 2018;38:173–196. doi: 10.1146/annurev-nutr-071816-064800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hotta Y., Nakamura H., Konishi M., Murata Y., Takagi H., Matsumura S. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 143.Kurosu H., Choi M., Ogawa Y., Dickson A.S., Goetz R., Eliseenkova A.V. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. Journal of Biological Chemistry. 2007;282(37):26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suzuki M., Uehara Y., Motomura-Matsuzaka K., Oki J., Koyama Y., Kimura M. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Molecular Endocrinology. 2008;22(4):1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Adams A.C., Yang C., Coskun T., Cheng C.C., Gimeno R.E., Luo Y. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol Metab. 2012;2(1):31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yie J., Wang W., Deng L., Tam L.T., Stevens J., Chen M.M. Understanding the physical interactions in the FGF21/FGFR/beta-Klotho complex: structural requirements and implications in FGF21 signaling. Chemical Biology & Drug Design. 2012;79(4):398–410. doi: 10.1111/j.1747-0285.2012.01325.x. [DOI] [PubMed] [Google Scholar]
- 147.Yie J., Hecht R., Patel J., Stevens J., Wang W., Hawkins N. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Letters. 2009;583(1):19–24. doi: 10.1016/j.febslet.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 148.Lee S., Choi J., Mohanty J., Sousa L.P., Tome F., Pardon E. Structures of beta-klotho reveal a 'zip code'-like mechanism for endocrine FGF signalling. Nature. 2018;553(7689):501–505. doi: 10.1038/nature25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Micanovic R., Raches D.W., Dunbar J.D., Driver D.A., Bina H.A., Dickinson C.D. Different roles of N- and C- termini in the functional activity of FGF21. Journal of Cellular Physiology. 2009;219(2):227–234. doi: 10.1002/jcp.21675. [DOI] [PubMed] [Google Scholar]
- 150.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5(6):426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 151.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 152.Solon-Biet S.M., Cogger V.C., Pulpitel T., Heblinski M., Wahl D., McMahon A.C. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metabolism. 2016;24(4):555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Desai B.N., Singhal G., Watanabe M., Stevanovic D., Lundasen T., Fisher F.M. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. 2017;6(11):1395–1406. doi: 10.1016/j.molmet.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the U S A. 2009;106(26):10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C. FGF21 is an endocrine signal of protein restriction. Journal of Clinical Investigation. 2014;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maida A., Zota A., Sjoberg K.A., Schumacher J., Sijmonsma T.P., Pfenninger A. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. Journal of Clinical Investigation. 2016;126(9):3263–3278. doi: 10.1172/JCI85946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Sousa-Coelho A.L., Relat J., Hondares E., Perez-Marti A., Ribas F., Villarroya F. FGF21 mediates the lipid metabolism response to amino acid starvation. The Journal of Lipid Research. 2013;54(7):1786–1797. doi: 10.1194/jlr.M033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hill C.M., Laeger T., Dehner M., Albarado D.C., Clarke B., Wanders D. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Reports. 2019;27(10):2934–2947 e3. doi: 10.1016/j.celrep.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ozaki Y., Saito K., Nakazawa K., Konishi M., Itoh N., Hakuno F. Rapid increase in fibroblast growth factor 21 in protein malnutrition and its impact on growth and lipid metabolism - erratum. British Journal of Nutrition. 2015;114(9):1535–1536. doi: 10.1017/S0007114515003955. [DOI] [PubMed] [Google Scholar]
- 160.Pérez-Martí A., Garcia-Guasch M., Tresserra-Rimbau A., Carrilho-Do-Rosário A., Estruch R., Salas-Salvadó J. A low-protein diet induces body weight loss and browning of subcutaneous white adipose tissue through enhanced expression of hepatic fibroblast growth factor 21 (FGF21) Molecular Nutrition & Food Research. 2017;61(8) doi: 10.1002/mnfr.201600725. [DOI] [PubMed] [Google Scholar]
- 161.Laeger T., Albarado D.C., Burke S.J., Trosclair L., Hedgepeth J.W., Berthoud H.-R. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Reports. 2016;16(3):707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morrison C.D., Laeger T. Protein-dependent regulation of feeding and metabolism. Trends in Endocrinology and Metabolism: Trends in Endocrinology and Metabolism. 2015;26(5):256–262. doi: 10.1016/j.tem.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Flippo K.H., Jensen-Cody S.O., Claflin K.E., Potthoff M.J. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Scientific Reports. 2020;10(1):19521. doi: 10.1038/s41598-020-76593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gosby A.K., Conigrave A.D., Raubenheimer D., Simpson S.J. Protein leverage and energy intake. Obesity Reviews. 2014;15(3):183–191. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- 165.Hill C.M., Qualls-Creekmore E., Berthoud H.R., Soto P., Yu S., McDougal D.H. FGF21 and the physiological regulation of macronutrient preference. Endocrinology. 2020;161(3) doi: 10.1210/endocr/bqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Markan K.R., Naber M.C., Ameka M.K., Anderegg M.D., Mangelsdorf D.J., Kliewer S.A. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63(12):4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fisher F.M., Chui P.C., Nasser I.A., Popov Y., Cunniff J.C., Lundasen T. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147(5):1073–10783 e6. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singhal G., Kumar G., Chan S., Fisher F.M., Ma Y., Vardeh H.G. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol Metab. 2018;13:56–66. doi: 10.1016/j.molmet.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.BonDurant L.D., Ameka M., Naber M.C., Markan K.R., Idiga S.O., Acevedo M.R. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metabolism. 2017;25(4):935–944 e4. doi: 10.1016/j.cmet.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lan T., Morgan D.A., Rahmouni K., Sonoda J., Fu X., Burgess S.C. FGF19, FGF21, and an FGFR1/β-klotho-activating antibody act on the nervous system to regulate body weight and glycemia. Cell Metabolism. 2017;26(5):709–718. doi: 10.1016/j.cmet.2017.09.005. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ameka M., Markan K.R., Morgan D.A., BonDurant L.D., Idiga S.O., Naber M.C. Liver derived FGF21 maintains core body temperature during acute cold exposure. Scientific Reports. 2019;9(1):630. doi: 10.1038/s41598-018-37198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Owen B.M., Ding X., Morgan D.A., Coate K.C., Bookout A.L., Rahmouni K. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metabolism. 2014;20(4):670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jensen-Cody S.O., Flippo K.H., Claflin K.E., Yavuz Y., Sapouckey S.A., Walters G.C. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metabolism. 2020;32(2):273–286. doi: 10.1016/j.cmet.2020.06.008. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang X., Yeung D.C., Karpisek M., Stejskal D., Zhou Z.G., Liu F. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–1253. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 175.Berti L., Irmler M., Zdichavsky M., Meile T., Bohm A., Stefan N. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Mol Metab. 2015;4(7):519–527. doi: 10.1016/j.molmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mraz M., Bartlova M., Lacinova Z., Michalsky D., Kasalicky M., Haluzikova D. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clinical Endocrinology. 2009;71(3):369–375. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 177.Chen W.W., Li L., Yang G.Y., Li K., Qi X.Y., Zhu W. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes. 2008;116(1):65–68. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 178.Chavez A.O., Molina-Carrion M., Abdul-Ghani M.A., Folli F., Defronzo R.A., Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care. 2009;32(8):1542–1546. doi: 10.2337/dc09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dushay J., Chui P.C., Gopalakrishnan G.S., Varela-Rey M., Crawley M., Fisher F.M. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yilmaz Y., Eren F., Yonal O., Kurt R., Aktas B., Celikel C.A. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. European Journal of Clinical Investigation. 2010;40(10):887–892. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 181.de Kretser D.M., Hedger M.P., Loveland K.L., Phillips D.J. Inhibins, activins and follistatin in reproduction. Human Reproduction Update. 2002;8(6):529–541. doi: 10.1093/humupd/8.6.529. [DOI] [PubMed] [Google Scholar]
- 182.Rodino-Klapac L.R., Haidet A.M., Kota J., Handy C., Kaspar B.K., Mendell J.R. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle & Nerve. 2009;39(3):283–296. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shi L., Resaul J., Owen S., Ye L., Jiang W.G. Clinical and therapeutic implications of follistatin in solid tumours. CANCER GENOMICS and PROTEOMICS. 2016;13(6):425–435. doi: 10.21873/cgp.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao C., Qiao C., Tang R.H., Jiang J., Li J., Martin C.B. Overcoming insulin insufficiency by forced follistatin expression in beta-cells of db/db mice. Molecular Therapy. 2015;23(5):866–874. doi: 10.1038/mt.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sidis Y., Mukherjee A., Keutmann H., Delbaere A., Sadatsuki M., Schneyer A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147(7):3586–3597. doi: 10.1210/en.2006-0089. [DOI] [PubMed] [Google Scholar]
- 186.Brown M.L., Bonomi L., Ungerleider N., Zina J., Kimura F., Mukherjee A. Follistatin and follistatin like-3 differentially regulate adiposity and glucose homeostasis. Obesity. 2011;19(10):1940–1949. doi: 10.1038/oby.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee S.J., Lee Y.S., Zimmers T.A., Soleimani A., Matzuk M.M., Tsuchida K. Regulation of muscle mass by follistatin and activins. Molecular Endocrinology. 2010;24(10):1998–2008. doi: 10.1210/me.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hansen J.S., Plomgaard P. Circulating follistatin in relation to energy metabolism. Molecular and Cellular Endocrinology. 2016;433:87–93. doi: 10.1016/j.mce.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 189.Zhang Y.Q., Kanzaki M., Shibata H., Kojima I. Regulation of the expression of follistatin in rat hepatocytes. Biochimica et Biophysica Acta. 1997;1354(3):204–210. doi: 10.1016/s0167-4781(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 190.Hansen J.S., Rutti S., Arous C., Clemmesen J.O., Secher N.H., Drescher A. Circulating follistatin is liver-derived and regulated by the glucagon-to-insulin ratio. Journal of Clinical Endocrinology & Metabolism. 2016;101(2):550–560. doi: 10.1210/jc.2015-3668. [DOI] [PubMed] [Google Scholar]
- 191.Hansen J., Rinnov A., Krogh-Madsen R., Fischer C.P., Andreasen A.S., Berg R.M. Plasma follistatin is elevated in patients with type 2 diabetes: relationship to hyperglycemia, hyperinsulinemia, and systemic low-grade inflammation. Diabetes Metab Res Rev. 2013;29(6):463–472. doi: 10.1002/dmrr.2415. [DOI] [PubMed] [Google Scholar]
- 192.Hansen J.S., Pedersen B.K., Xu G., Lehmann R., Weigert C., Plomgaard P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 diabetes. Journal of Clinical Endocrinology & Metabolism. 2016;101(7):2816–2825. doi: 10.1210/jc.2016-1681. [DOI] [PubMed] [Google Scholar]
- 193.Yndestad A., Haukeland J.W., Dahl T.B., Bjøro K., Gladhaug I.P., Berge C. A complex role of activin A in non-alcoholic fatty liver disease. American Journal of Gastroenterology. 2009;104(9):2196–2205. doi: 10.1038/ajg.2009.318. [DOI] [PubMed] [Google Scholar]
- 194.Shimasaki S., Koga M., Esch F., Cooksey K., Mercado M., Koba A. Primary structure of the human follistatin precursor and its genomic organization. Proceedings of the National Academy of Sciences of the U S A. 1988;85(12):4218–4222. doi: 10.1073/pnas.85.12.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Schneyer A.L., Wang Q., Sidis Y., Sluss P.M. Differential distribution of follistatin isoforms: application of a new FS315-specific immunoassay. Journal of Clinical Endocrinology & Metabolism. 2004;89(10):5067–5075. doi: 10.1210/jc.2004-0162. [DOI] [PubMed] [Google Scholar]
- 196.Tao R., Wang C., Stohr O., Qiu W., Hu Y., Miao J. Inactivating hepatic follistatin alleviates hyperglycemia. Natura Med. 2018;24(7):1058–1069. doi: 10.1038/s41591-018-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Braga M., Reddy S.T., Vergnes L., Pervin S., Grijalva V., Stout D. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. The Journal of Lipid Research. 2014;55(3):375–384. doi: 10.1194/jlr.M039719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Singh R., Braga M., Reddy S.T., Lee S.-J., Parveen M., Grijalva V. Follistatin targets distinct pathways to promote Brown adipocyte characteristics in Brown and white adipose tissues. Endocrinology. 2017;158(5):1217–1230. doi: 10.1210/en.2016-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bauskin A.R., Brown D.A., Kuffner T., Johnen H., Luo X.W., Hunter M. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Research. 2006;66(10):4983–4986. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 200.Breit S.N., Johnen H., Cook A.D., Tsai V.W., Mohammad M.G., Kuffner T. The TGF-β superfamily cytokine, MIC-1/GDF15: a pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors. 2011;29(5):187–195. doi: 10.3109/08977194.2011.607137. [DOI] [PubMed] [Google Scholar]
- 201.Mullican S.E., Rangwala S.M. Uniting GDF15 and GFRAL: therapeutic opportunities in obesity and beyond. Trends in Endocrinology and Metabolism. 2018;29(8):560–570. doi: 10.1016/j.tem.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 202.Suriben R., Chen M., Higbee J., Oeffinger J., Ventura R., Li B. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Natura Med. 2020;26(8):1264–1270. doi: 10.1038/s41591-020-0945-x. [DOI] [PubMed] [Google Scholar]
- 203.Li P.X., Wong J., Ayed A., Ngo D., Brade A.M., Arrowsmith C. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. Journal of Biological Chemistry. 2000;275(26):20127–20135. doi: 10.1074/jbc.M909580199. [DOI] [PubMed] [Google Scholar]
- 204.Hsiao E.C., Koniaris L.G., Zimmers-Koniaris T., Sebald S.M., Huynh T.V., Lee S.J. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Molecular and Cellular Biology. 2000;20(10):3742–3751. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Baek S.J., Eling T. Growth differentiation factor 15 (GDF15): a survival protein with therapeutic potential in metabolic diseases. Pharmacology & Therapeutics. 2019;198:46–58. doi: 10.1016/j.pharmthera.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lockhart S.M., Saudek V., O'Rahilly S. GDF15: a hormone conveying somatic distress to the brain. Endocrine Reviews. 2020;41(4):610–642. doi: 10.1210/endrev/bnaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Baek S.J., Okazaki R., Lee S.H., Martinez J., Kim J.S., Yamaguchi K. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131(5):1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 208.Chrysovergis K., Wang X., Kosak J., Lee S.H., Kim J.S., Foley J.F. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. International Journal of Obesity. 2014;38(12):1555–1564. doi: 10.1038/ijo.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang X., Chrysovergis K., Kosak J., Kissling G., Streicker M., Moser G. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging. 2014;6(8):690–704. doi: 10.18632/aging.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chung H.K., Ryu D., Kim K.S., Chang J.Y., Kim Y.K., Yi H.S. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. The Journal of Cell Biology. 2017;216(1):149–165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Johnen H., Lin S., Kuffner T., Brown D.A., Tsai V.W., Bauskin A.R. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Natura Med. 2007;13(11):1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 212.Mullican S.E., Lin-Schmidt X., Chin C.N., Chavez J.A., Furman J.L., Armstrong A.A. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Natura Med. 2017;23(10):1150–1157. doi: 10.1038/nm.4392. [DOI] [PubMed] [Google Scholar]
- 213.Yang L., Chang C.C., Sun Z., Madsen D., Zhu H., Padkjaer S.B. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Natura Med. 2017;23(10):1158–1166. doi: 10.1038/nm.4394. [DOI] [PubMed] [Google Scholar]
- 214.Wischhusen J., Melero I., Fridman W.H. Growth/differentiation factor-15 (GDF-15): from biomarker to novel targetable immune checkpoint. Frontiers in Immunology. 2020;11:951. doi: 10.3389/fimmu.2020.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hsu J.Y., Crawley S., Chen M., Ayupova D.A., Lindhout D.A., Higbee J. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017;550(7675):255–259. doi: 10.1038/nature24042. [DOI] [PubMed] [Google Scholar]
- 216.Borner T., Shaulson E.D., Ghidewon M.Y., Barnett A.B., Horn C.C., Doyle R.P. GDF15 induces anorexia through nausea and emesis. Cell Metabolism. 2020;31(2):351–362. doi: 10.1016/j.cmet.2019.12.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wu H.T., Ou H.Y., Hung H.C., Su Y.C., Lu F.H., Wu J.S. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia. 2016;59(8):1732–1742. doi: 10.1007/s00125-016-3991-7. [DOI] [PubMed] [Google Scholar]
- 218.Gao M., Zhan Y.Q., Yu M., Ge C.H., Li C.Y., Zhang J.H. Hepassocin activates the EGFR/ERK cascade and induces proliferation of L02 cells through the Src-dependent pathway. Cellular Signalling. 2014;26(10):2161–2166. doi: 10.1016/j.cellsig.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 219.Demchev V., Malana G., Vangala D., Stoll J., Desai A., Kang H.W. Targeted deletion of fibrinogen like protein 1 reveals a novel role in energy substrate utilization. PloS One. 2013;8(3) doi: 10.1371/journal.pone.0058084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang R.L., Li C.H., Du Y.F., Cheng K.P., Lin C.H., Hu C.Y. Discovery of a role of the novel hepatokine, hepassocin, in obesity. BioFactors. 2020;46(1):100–105. doi: 10.1002/biof.1574. [DOI] [PubMed] [Google Scholar]
- 221.Wu H.T., Lu F.H., Ou H.Y., Su Y.C., Hung H.C., Wu J.S. The role of hepassocin in the development of non-alcoholic fatty liver disease. Journal of Hepatology. 2013;59(5):1065–1072. doi: 10.1016/j.jhep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 222.Moses A.C., Young S.C., Morrow L.A., O'Brien M., Clemmons D.R. Recombinant human insulin-like growth factor I increases insulin sensitivity and improves glycemic control in type II diabetes. Diabetes. 1996;45(1):91–100. doi: 10.2337/diab.45.1.91. [DOI] [PubMed] [Google Scholar]
- 223.Menu E., Kooijman R., Van Valckenborgh E., Asosingh K., Bakkus M., Van Camp B. Specific roles for the PI3K and the MEK-ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: study in the 5T33MM model. British Journal of Cancer. 2004;90(5):1076–1083. doi: 10.1038/sj.bjc.6601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rajkumar K., Krsek M., Dheen S.T., Murphy L.J. Impaired glucose homeostasis in insulin-like growth factor binding protein-1 transgenic mice. Journal of Clinical Investigation. 1996;98(8):1818–1825. doi: 10.1172/JCI118982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jones J.I., Clemmons D.R. Insulin-like growth factors and their binding proteins: biological actions∗. Endocrine Reviews. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 226.Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 227.Van Wyk J.J., Smith E.P. Insulin-like growth factors and skeletal growth: possibilities for therapeutic interventions. Journal of Clinical Endocrinology & Metabolism. 1999;84(12):4349–4354. doi: 10.1210/jcem.84.12.6201. [DOI] [PubMed] [Google Scholar]
- 228.Fernandez A.M., Kim J.K., Yakar S., Dupont J., Hernandez-Sanchez C., Castle A.L. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes & Development. 2001;15(15):1926–1934. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Isley W.L., Underwood L.E., Clemmons D.R. Dietary components that regulate serum somatomedin-C concentrations in humans. Journal of Clinical Investigation. 1983;71(2):175–182. doi: 10.1172/JCI110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Clemmons D.R. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. Journal of Clinical Investigation. 2004;113(1):25–27. doi: 10.1172/JCI200420660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sandhu M.S., Heald A.H., Gibson J.M., Cruickshank J.K., Dunger D.B., Wareham N.J. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. The Lancet. 2002;359(9319):1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 232.Succurro E., Andreozzi F., Marini M.A., Lauro R., Hribal M.L., Perticone F. Low plasma insulin-like growth factor-1 levels are associated with reduced insulin sensitivity and increased insulin secretion in nondiabetic subjects. Nutrition, Metabolism, and Cardiovascular Diseases. 2009;19(10):713–719. doi: 10.1016/j.numecd.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 233.Juul A., Scheike T., Davidsen M., Gyllenborg J., Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 234.Laron Z., Avitzur Y., Klinger B. Carbohydrate metabolism in primary growth hormone resistance (Laron syndrome) before and during insulin-like growth factor-I treatment. Metabolism. 1995;44(10 Suppl 4):113–118. doi: 10.1016/0026-0495(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 235.Laron Z. The essential role of IGF-I: lessons from the long-term study and treatment of children and adults with Laron syndrome. Journal of Clinical Endocrinology & Metabolism. 1999;84(12):4397–4404. doi: 10.1210/jcem.84.12.6255. [DOI] [PubMed] [Google Scholar]
- 236.Hong H., Cui Z.Z., Zhu L., Fu S.P., Rossi M., Cui Y.H. Central IGF1 improves glucose tolerance and insulin sensitivity in mice. Nutrition & Diabetes. 2017;7(12):2. doi: 10.1038/s41387-017-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Arturi F., Succurro E., Procopio C., Pedace E., Mannino G.C., Lugara M. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. Journal of Clinical Endocrinology & Metabolism. 2011;96(10):E1640–E1644. doi: 10.1210/jc.2011-1227. [DOI] [PubMed] [Google Scholar]
- 238.Telgenkamp I., Kusters Y., Schalkwijk C.G., Houben A., Kooi M.E., Lindeboom L. Contribution of liver fat to weight loss-induced changes in serum hepatokines: a randomized controlled trial. Journal of Clinical Endocrinology & Metabolism. 2019;104(7):2719–2727. doi: 10.1210/jc.2018-02378. [DOI] [PubMed] [Google Scholar]
- 239.Lan F., Misu H., Chikamoto K., Takayama H., Kikuchi A., Mohri K. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes. 2014;63(5):1649–1664. doi: 10.2337/db13-0728. [DOI] [PubMed] [Google Scholar]
- 240.Jung T.W., Yoo H.J., Choi K.M. Implication of hepatokines in metabolic disorders and cardiovascular diseases. BBA Clin. 2016;5:108–113. doi: 10.1016/j.bbacli.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Segawa Y., Itokazu Y., Inoue N., Saito T., Suzuki K. Possible changes in expression of chemotaxin LECT2 mRNA in mouse liver after concanavalin A-induced hepatic injury. Biological and Pharmaceutical Bulletin. 2001;24(4):425–428. doi: 10.1248/bpb.24.425. [DOI] [PubMed] [Google Scholar]
- 242.Yamagoe S., Yamakawa Y., Matsuo Y., Minowada J., Mizuno S., Suzuki K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunology Letters. 1996;52(1):9–13. doi: 10.1016/0165-2478(96)02572-2. [DOI] [PubMed] [Google Scholar]
- 243.Ovejero C., Cavard C., Périanin A., Hakvoort T., Vermeulen J., Godard C. Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology. 2004;40(1):167–176. doi: 10.1002/hep.20286. [DOI] [PubMed] [Google Scholar]
- 244.Lu X.J., Chen J., Yu C.H., Shi Y.H., He Y.Q., Zhang R.C. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. Journal of Experimental Medicine. 2013;210(1):5–13. doi: 10.1084/jem.20121466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Koshimizu Y., Ohtomi M. Regulation of neurite extension by expression of LECT2 and neurotrophins based on findings in LECT2-knockout mice. Brain Research. 2010;1311:1–11. doi: 10.1016/j.brainres.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 246.Slowik V., Apte U. Leukocyte cell-derived chemotaxin-2: it's role in pathophysiology and future in clinical medicine. Clin Transl Sci. 2017;10(4):249–259. doi: 10.1111/cts.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Nagai H., Hamada T., Uchida T., Yamagoe S., Suzuki K. Systemic expression of a newly recognized protein, LECT2, in the human body. Pathology International. 1998;48(11):882–886. doi: 10.1111/j.1440-1827.1998.tb03855.x. [DOI] [PubMed] [Google Scholar]
- 248.Hwang H.J., Jung T.W., Hong H.C., Seo J.A., Kim S.G., Kim N.H. LECT2 induces atherosclerotic inflammatory reaction via CD209 receptor-mediated JNK phosphorylation in human endothelial cells. Metabolism. 2015;64(9):1175–1182. doi: 10.1016/j.metabol.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 249.Hwang H.J., Jung T.W., Kim B.H., Hong H.C., Seo J.A., Kim S.G. A dipeptidyl peptidase-IV inhibitor improves hepatic steatosis and insulin resistance by AMPK-dependent and JNK-dependent inhibition of LECT2 expression. Biochemical Pharmacology. 2015;98(1):157–166. doi: 10.1016/j.bcp.2015.08.098. [DOI] [PubMed] [Google Scholar]
- 250.Jung T.W., Chung Y.H., Kim H.C., Abd El-Aty A.M., Jeong J.H. LECT2 promotes inflammation and insulin resistance in adipocytes via P38 pathways. Journal of Molecular Endocrinology. 2018;61(1):37–45. doi: 10.1530/JME-17-0267. [DOI] [PubMed] [Google Scholar]
- 251.Willis S.A., Sargeant J.A., Yates T., Takamura T., Takayama H., Gupta V. Acute hyperenergetic, high-fat feeding increases circulating FGF21, LECT2, and fetuin-A in healthy men. Journal of Nutrition. 2020;150(5):1076–1085. doi: 10.1093/jn/nxz333. [DOI] [PubMed] [Google Scholar]
- 252.Grzyb J., Latowski D., Strzałka K. Lipocalins - a family portrait. Journal of Plant Physiology. 2006;163(9):895–915. doi: 10.1016/j.jplph.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 253.Flower D.R. The lipocalin protein family: structure and function. Biochemical Journal. 1996;318(Pt 1):1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhou Y., Rui L. Lipocalin 13 regulation of glucose and lipid metabolism in obesity. Vitamins & Hormones. 2013;91:369–383. doi: 10.1016/B978-0-12-407766-9.00015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hui X., Zhu W., Wang Y., Lam K.S., Zhang J., Wu D. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. Journal of Biological Chemistry. 2009;284(21):14050–14057. doi: 10.1074/jbc.M109.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kotnik P., Fischer-Posovszky P., Wabitsch M. RBP4: a controversial adipokine. European Journal of Endocrinology. 2011;165(5):703–711. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- 257.Ekim Üstünel B., Friedrich K., Maida A., Wang X., Krones-Herzig A., Seibert O. Control of diabetic hyperglycaemia and insulin resistance through TSC22D4. Nature Communications. 2016;7 doi: 10.1038/ncomms13267. 13267-13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cho K.W., Zhou Y., Sheng L., Rui L. Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Molecular and Cellular Biology. 2011;31(3):450–457. doi: 10.1128/MCB.00459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sheng L., Cho K.W., Zhou Y., Shen H., Rui L. Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid β-oxidation. Journal of Biological Chemistry. 2011;286(44):38128–38135. doi: 10.1074/jbc.M111.256677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Misu H., Takamura T., Takayama H., Hayashi H., Matsuzawa-Nagata N., Kurita S. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metabolism. 2010;12(5):483–495. doi: 10.1016/j.cmet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 261.Misu H., Ishikura K., Kurita S., Takeshita Y., Ota T., Saito Y. Inverse correlation between serum levels of selenoprotein P and adiponectin in patients with type 2 diabetes. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0034952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hariharan S., Dharmaraj S. Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacology. 2020;28(3):667–695. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Misu H. Identification of hepatokines involved in pathology of type 2 diabetes and obesity. Endocrine Journal. 2019;66(8):659–662. doi: 10.1507/endocrj.EJ19-0255. [DOI] [PubMed] [Google Scholar]
- 264.Yang S.J., Hwang S.Y., Choi H.Y., Yoo H.J., Seo J.A., Kim S.G. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. Journal of Clinical Endocrinology & Metabolism. 2011;96(8):E1325–E1329. doi: 10.1210/jc.2011-0620. [DOI] [PubMed] [Google Scholar]
- 265.Choi H.Y., Hwang S.Y., Lee C.H., Hong H.C., Yang S.J., Yoo H.J. Increased selenoprotein p levels in subjects with visceral obesity and nonalcoholic Fatty liver disease. Diabetes & Metabolism J. 2013;37(1):63–71. doi: 10.4093/dmj.2013.37.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Montgomery M.K., Bayliss J., Devereux C., Bezawork-Geleta A., Roberts D., Huang C. SMOC1 is a glucose-responsive hepatokine and therapeutic target for glycemic control. Science Translational Medicine. 2020;12(559) doi: 10.1126/scitranslmed.aaz8048. [DOI] [PubMed] [Google Scholar]
- 267.Awwad K., Hu J., Shi L., Mangels N., Abdel Malik R., Zippel N. Role of secreted modular calcium-binding protein 1 (SMOC1) in transforming growth factor β signalling and angiogenesis. Cardiovascular Research. 2015;106(2):284–294. doi: 10.1093/cvr/cvv098. [DOI] [PubMed] [Google Scholar]
- 268.Wang Y., Wu X. SMOC1 silencing suppresses the angiotensin II-induced myocardial fibrosis of mouse myocardial fibroblasts via affecting the BMP2/Smad pathway. Oncol Lett. 2018;16(3):2903–2910. doi: 10.3892/ol.2018.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mouchiroud M., Camire E., Aldow M., Caron A., Jubinville E., Turcotte L. The hepatokine Tsukushi is released in response to NAFLD and impacts cholesterol homeostasis. JCI Insight. 2019;4(15) doi: 10.1172/jci.insight.129492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Xiong X., Wang Q., Wang S., Zhang J., Liu T., Guo L. Mapping the molecular signatures of diet-induced NASH and its regulation by the hepatokine Tsukushi. Mol Metab. 2019;20:128–137. doi: 10.1016/j.molmet.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang Q., Sharma V.P., Shen H., Xiao Y., Zhu Q., Xiong X. The hepatokine Tsukushi gates energy expenditure via brown fat sympathetic innervation. Nat Metab. 2019;1(2):251–260. doi: 10.1038/s42255-018-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mouchiroud M., Camire E., Aldow M., Caron A., Jubinville E., Turcotte L. The Hepatokine TSK does not affect brown fat thermogenic capacity, body weight gain, and glucose homeostasis. Mol Metab. 2019;30:184–191. doi: 10.1016/j.molmet.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]