Abstract
Objective
The treatment of infective endocarditis (IE) has become more complex with the current myriad healthcare-associated factors and the regional differences in causative organisms. We aimed to investigate the overall trends, microbiological features, and outcomes of IE in South Korea.
Methods
A 12-year retrospective cohort study was performed. Poisson regression was used to estimate the time trends of IE incidence and mortality rate. Risk factors for in-hospital mortality were identified with multivariable logistic regression, and model comparison was performed to evaluate the predictive performance of notable risk factors. Kaplan-Meier survival analysis and Cox regression were performed to assess long-term prognosis.
Results
We included 419 patients with IE, the incidence of which showed an increasing trend (relative risk 1.06, p=0.005), whereas mortality demonstrated a decreasing trend (incidence rate ratio 0.93, p=0.020). The in-hospital mortality rate was 14.6%. On multivariable logistic regression analysis, aortic valve endocarditis (OR 3.18, p=0.001), IE caused by Staphylococcus aureus (OR 2.32, p=0.026), neurological complications (OR 1.98, p=0.031), high Sequential Organ Failure Assessment score (OR 1.22, p=0.023) and high Charlson Comorbidity Index (OR 1.11, p=0.019) were predictors of in-hospital mortality. Surgical intervention for IE was a protective factor against in-hospital mortality (OR 0.25, p<0.001) and was associated with improved long-term prognosis compared with medical treatment only (p<0.001).
Conclusions
The incidence of IE is increasing in South Korea. Although the mortality rate has slightly decreased, it remains high. Surgery has a protective effect with respect to both in-hospital mortality and long-term prognosis in patients with IE.
Keywords: endocarditis, valve disease surgery
Introduction
Despite advances in diagnostics and therapeutics, infective endocarditis (IE) remains associated with high morbidity and mortality.1 The epidemiology of IE varies and depends on multiple hosts and microbiological factors.2 3 Moreover, IE treatment has become more complex with the emergence of various healthcare-associated factors and regional differences in causative organisms.4 The incidence of IE is 2–8 per 100 000 person-years and has been reported to be increasing.5 6 The in-hospital mortality rate of IE has not shown significant improvement and, in fact, an increasing trend in mortality has been reported.7 8
To evaluate the disease burden caused by IE, it is important to identify the trends in its incidence and mortality; investigate its microbiological characteristics, clinical features and treatment outcomes; and collect region-specific data while considering the regional differences in the patients’ medical background, microbiological distribution and resistance.9 Therefore, this study aimed to evaluate the incidence-related and mortality-related trends, and the clinical and microbiological characteristics and treatment outcomes of IE in South Korea.
Patients and methods
Study population
We retrospectively analysed adult patients with IE admitted to Severance Hospital, a large tertiary care teaching hospital with 2400 beds in South Korea, from November 2005 to August 2017. IE was defined as definite or possible according to the modified Duke criteria, and both types were included in the study.10 Patients admitted for suspicion of IE were managed by a multidisciplinary team including cardiologists, cardiovascular surgeons and infectious disease specialists. Surgery was considered according to the American Heart Association guidelines and South Korea’s national guidelines.4 11 These guidelines indicate surgery for uncontrolled heart failure or cardiogenic shock, paravalvular abscess, uncontrolled infection and vegetation of >10 mm with systemic embolisation. Surgery was determined according to the agreement of cardiologists and cardiovascular surgeons, as well as the advice of infectious disease specialists, if necessary. Transoesophageal echocardiography was performed in most patients, including those with negative transthoracic echocardiography findings. Follow-up visits to the outpatient clinic were made at 1 week and at 1, 3, 6 and 12 months after discharge. At each visit, the patients were checked for evidence of heart failure and relapse of IE through a system review and physical examination. Further, if anticoagulation therapy was performed after valve replacement, the prothrombin time was determined to ensure that the proper dose of anticoagulation had been used. At 6 months after discharge, follow-up echocardiography was performed to evaluate valvular and ventricular functions. Subsequently, follow-up visits to the outpatient clinic were made every 6 months.
Variables and definitions
Nosocomial infection was defined as an infection that occurred >48 hour after hospitalisation with no evidence of infection at admission. Nosocomial infection was also diagnosed if IE occurred within 60 days after hospital discharge when a high-risk procedure for bacteraemia was performed or when any predisposing factor for IE was present during hospitalisation, including dental manipulation, gastrointestinal manipulation, gynaecology procedures, urological manipulation and invasive intravascular techniques (intravascular device implantation, pacemaker insertion and cardiac catheterisation).12–14 Among comorbidities, cardiac devices were defined as implantable pacemakers or defibrillators,15 and the Charlson Comorbidity Index was used to categorise patients according to overall comorbidity at hospital admission.16 Among clinical symptoms and signs at diagnosis, neurological complications included ischaemic or haemorrhagic stroke, cerebral abscess and intracranial mycotic aneurysm, including middle cerebral artery aneurysm with or without cerebral haemorrhage. Complications were diagnosed according to clinical, CT or MRI findings.17 Peripheral embolic complications included pulmonary embolism, coronary embolism, splenic infarct or abscess, and peripheral limb embolisation.17 Signs of peripheral vasculitis included Roth spots on retinal examination, subconjunctival haemorrhage, Osler nodes and Janeway lesions. The Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE)-II scores were used to stratify the disease severity, and EuroSCORE II was used to calculate the risk of death in patients undergoing surgery.18 Causative microorganisms were defined as those present in blood or tissue samples (valve and/or vegetation).
Statistical analysis
Poisson log-linear regression was used to estimate the time trends in IE incidence (relative risk (RR)) and mortality rate (incidence rate ratio (IRR)). To analyse long-term survival, we used mortality data obtained from the Ministry of the Interior and Safety of South Korea, which collects death information of all Korean citizens. Comparisons were made between patients who died in the hospital and those who survived. The Mann-Whitney U-test was used to compare continuous variables, and χ2 or Fisher’s exact test was used for categorical variables. The final logistic regression model predicting in-hospital mortality was constructed based on clinical significance among risk factors with p<0.05 in univariate analysis after checking for multicollinearity and interaction effects. Multicollinearity was defined as a variance inflation factor of <5. ORs and 95% CIs were calculated from this analysis. The predicted performance of the final model was evaluated using areas under the curve (AUCs), Hosmer-Lemeshow test and calibration plots. To verify the efficiency of our model (free from overfitting, with high predictive performance), we compared the AUCs of our models with that of high-predictive performance models selected based on bidirectional elimination selection with Akaike’s information criterion. The DeLong method was used to compare the AUCs of the models.19 Kaplan-Meier survival analysis and Cox regression were performed to assess long-term prognosis. A p value of <0.05 was considered statistically significant. Poisson regression analyses for trend tests were performed using SAS V.9.4. All other statistical analyses were performed using R V.3.4.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
This study was performed without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or to interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Study population and characteristics
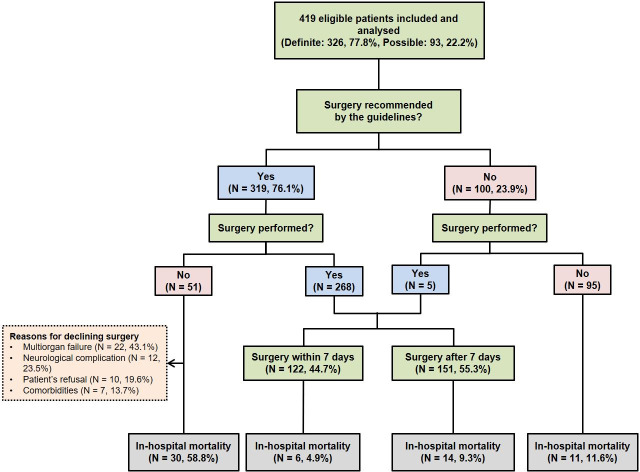
A total of 419 patients who met the inclusion criteria were included (figure 1). The median age was 56 years, with 275 male patients (65.6%). Ninety-one patients (21.7%) had nosocomial infection. Most patients (74%) had fever, and 129 (30.8%) had neurological complications. The in-hospital, 30-day and 1-year mortality rates were 14.6%, 8.8% and 17.4%, respectively (table 1).
Figure 1.
Flow of patients suspected of having infective endocarditis during the study period.
Table 1.
Clinical characteristics and outcomes of patients with IE
| Total (N=419) | |
| Demographics | |
| Age (years) | 56 (43–68) |
| Age ≥65 years | 138 (32.9) |
| Male sex | 275 (65.6) |
| Nosocomial infection | 91 (21.7) |
| Comorbidities | |
| Previous valve surgery | 83 (19.8) |
| Diabetes | 77 (18.4) |
| Antibiotic treatment within 30 days | 74 (17.7) |
| Cancer | 54 (12.9) |
| Renal disease | 43 (10.3) |
| Central venous catheter access | 32 (7.6) |
| Congestive heart failure | 29 (6.9) |
| Liver disease | 27 (6.5) |
| Haemodialysis | 23 (5.5) |
| Previous IE | 21 (5.0) |
| Cardiac device | 19 (4.5) |
| Immunosuppressive therapy | 18 (4.3) |
| Connective tissue disease | 15 (3.6) |
| Chemotherapy within 30 days | 15 (3.6) |
| Charlson Comorbidity Index | 2 (0–4) |
| Clinical symptoms and signs | |
| Fever | 310 (74.0) |
| Sepsis, including septic shock | 294 (70.2) |
| Left ventricular dysfunction | 141 (33.7) |
| Neurological complications | 129 (30.8) |
| Peripheral embolic complications | 35 (8.4) |
| Signs of peripheral vasculitis | 9 (2.1) |
| Outcomes | |
| Acute renal failure | 62 (14.8) |
| New-onset heart failure | 58 (13.8) |
| New-onset conduction abnormality | 32 (7.6) |
| In-hospital mortality | 61 (14.6) |
| 30-day mortality | 37 (8.8) |
| 1-year mortality | 73 (17.4) |
Data are presented as median (IQR) or number (%) of patients.
IE, infective endocarditis.
The mitral valve was the most commonly affected valve (61.3%) followed by the aortic valve (43.2%), and 70 patients (16.7%) showed simultaneous involvement of more than two valves. Sixty-three patients (15.0%) developed IE related to prosthetic valves, and 68 patients (16.2%) had accompanying paravalvular complications. Inflammatory markers such as C reactive protein were elevated (table 2).
Table 2.
Echocardiographic and laboratory findings in patients with infective endocarditis
| Total (N=419) | |
| Affected valve | |
| Mitral valve | 257 (61.3) |
| Aortic valve | 181 (43.2) |
| Tricuspid valve | 34 (8.1) |
| Pulmonary valve | 16 (3.8) |
| Multiple valves | 70 (16.7) |
| Prosthetic valve | 63 (15.0) |
| Paravalvular complications | 68 (16.2) |
| Associated ventricular septal defect | 11 (2.7) |
| Vegetation size (cm) | 1.1 (0.7–1.6) |
| Preoperative laboratory findings (normal range) | |
| White blood cell count, ×103 (4.0–10.8) | 9.54 (6.92–12.95) |
| Segmented neutrophil (%) (39.0–74.0) | 79.4 (70.3–86.5) |
| Platelet count, 103/μL (150.0–400.0) | 202 (133–280) |
| Erythrocyte sedimentation rate (mm/hour) (0.0–15.0) | 61 (36–83) |
| C reactive protein (mg/L) (0.0–8.0) | 46.8 (10.3–103.0) |
| Procalcitonin, ng/mL (0.00–0.50) | 0.35 (0.16–1.13) |
| Severity scales | |
| SOFA score | 1 (1–3) |
| APACHE-II score | 6 (4–9) |
Data are presented as median (IQR) or number (%) of patients. Normal range refers to the hospital criteria.
APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment.
Incidence and mortality trends of IE
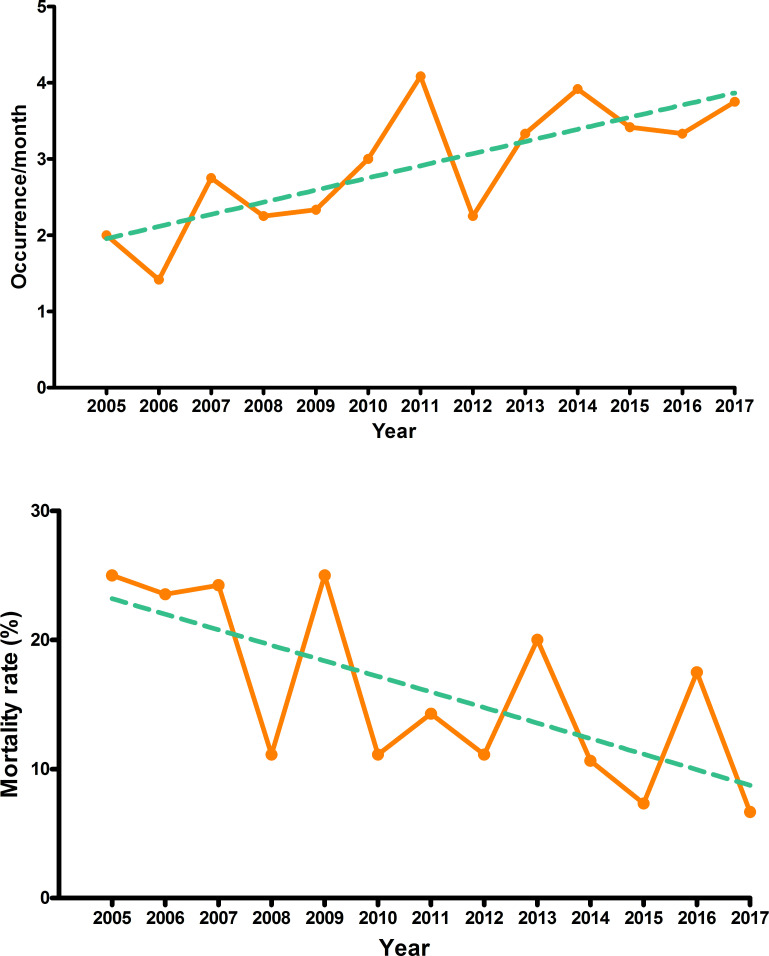
The monthly incidence rate of IE progressively increased from 2.0 in 2005 to 3.8 in 2017. An overall significant increase over time was observed (RR 1.06, 95% CI 1.02 to 1.10, p=0.005). The proportion of patients admitted with IE among all inpatients of the cardiovascular surgery division of our hospital also exhibited an increasing trend over time (IRR 1.03, 95% CI 1.01 to 1.06, p=0.042). The mortality rate showed a statistically significant, gradually declining trend (IRR 0.93, 95% CI 0.88 to 0.99, p=0.020) (figure 2).
Figure 2.
Trends in the incidence and mortality rate of infective endocarditis according to calendar year in Poisson log-linear regression. Trends are depicted as green dashed lines.
Changes in causative microorganisms with calendar year
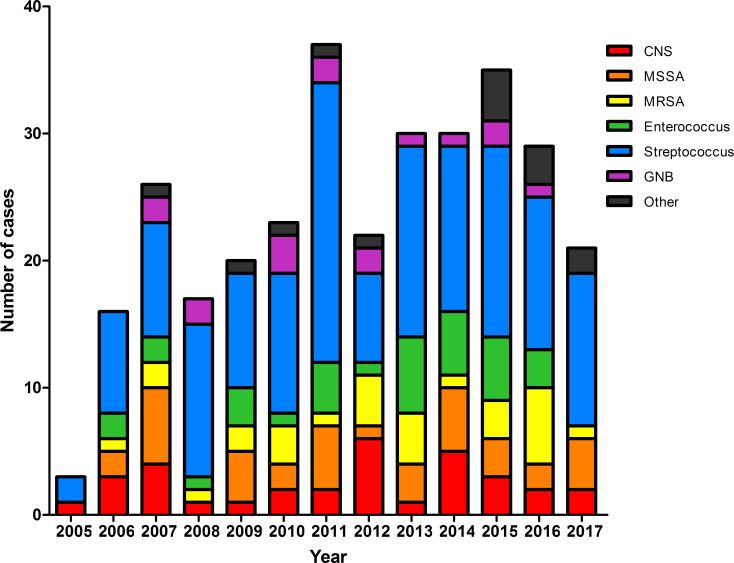
Among the 419 patients, causative microorganisms of IE were identified in 309 (73.7%). Streptococcus species were the most common species identified (35.1%), followed by Staphylococcus aureus (15.8%), Enterococcus species (8.6%) and coagulase-negative staphylococci (7.6%) (online supplemental table S1). Causative microorganisms were identified in 61 of 91 patients with nosocomial infection. Among these cases, S. aureus was the most commonly identified microorganism (19 cases), followed by Streptococcus species (14 cases). Streptococcus species were consistently identified most frequently throughout the study period, and no specific trend was identified for other isolates (figure 3).
Figure 3.
Distribution of microorganisms causing infective endocarditis according to calendar year. CNS, coagulase-negative staphylococci; GNB, Gram-negative bacillus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
heartjnl-2020-317265supp001.pdf (124KB, pdf)
Results of patients who underwent surgery
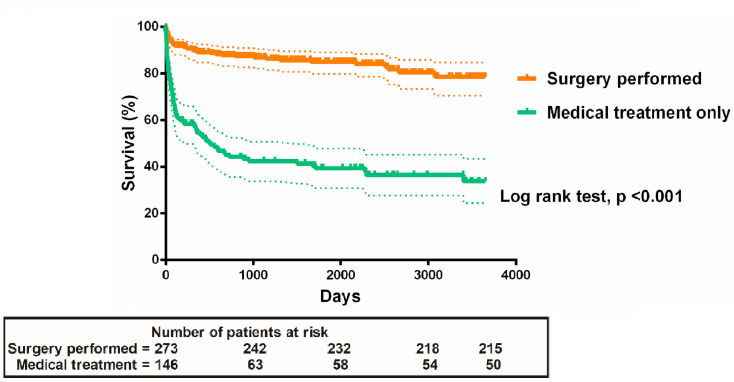
Among the 419 patients, 273 (65.2%) underwent surgery for IE. No difference was noted in the proportion of patients undergoing surgery over time (IRR 1.01, 95% CI 0.97 to 1.05, p=0.688) (online supplemental figure S1). Patients who underwent surgery had in-hospital, 30-day and 1-year mortality rates of 7.3%, 3.7% and 7.7%, respectively (table 3). Kaplan-Meier curves showed that patients who underwent surgery had a significantly higher long-term survival rate than those who received medical treatment only (p<0.001, log-rank test) (figure 4). The effects of surgery on long-term prognosis were robust after adjustment for potential confounders with Cox regression (HR 0.20, 95% CI 0.11 to 0.34, p<0.001). The mortality rate tended to be lower after 2012 than before, although the difference was not significant (5.1% vs 9.5%, p=0.169). In contrast, the median time between diagnosis and surgery was significantly shorter after 2012. Thus, the number of patients who underwent surgery within 7 days after diagnosis was significantly higher after 2012 than before (60.3% vs 29.2%, p<0.001) (table 4). Among the 101 patients who underwent surgery to prevent embolism, systemic embolic events (five in the middle cerebral artery, three in the spleen and one in the kidney) occurred significantly less frequently in the early surgery group than in the late surgery group during hospitalisation (2.1%, 1/48, vs 15.1%, 8/53; p=0.033).
Table 3.
Indications, timing and outcomes in 273 patients who underwent surgery for infective endocarditis
| Total (N=273) | |
| Surgical indications | |
| Congestive heart failure | 217 (79.5) |
| Prevention of embolism | 101 (37.0) |
| Paravalvular complications | 58 (21.2) |
| Uncontrolled infections | 19 (7.0) |
| Pacemaker infections | 7 (2.6) |
| Valve locations | |
| Mitral valve | 174 (63.7) |
| Aortic valve | 131 (48) |
| Tricuspid valve | 17 (6.2) |
| Pulmonary valve | 10 (3.7) |
| Multiple valves | 56 (20.5) |
| Timing of surgery | |
| Surgery within 24 hours | 12 (4.4) |
| Surgery within 2–7 days | 110 (40.3) |
| Surgery at >7 days | 151 (55.3) |
| EuroSCORE II (%) | 6.2 (4.7–9.3) |
| Replaced valve | |
| Mechanical valve | 184 (67.4) |
| Bioprosthetic valve | 68 (24.9) |
| Bentall operation | 5 (1.8) |
| Homograft | 3 (1.1) |
| Valve repair only | 18 (6.6) |
| Outcomes | |
| New-onset heart failure | 32 (11.7) |
| Acute renal failure | 29 (10.6) |
| New-onset conduction abnormality | 27 (9.9) |
| In-hospital mortality | 20 (7.3) |
| 30-day mortality | 10 (3.7) |
| 1-year mortality | 21 (7.7) |
Data are presented as median (IQR) or number (%) of patients.
Figure 4.
Kaplan-Meier curves of the long-term survival rates of patients with infective endocarditis who underwent surgery versus those who underwent medical treatment only.
Table 4.
Changes in the timing of surgery according to calendar year
| Total | 2005–2012 | 2013–2017 | P value | |
| No. of patients who underwent surgery | 273 (100.0) | 137 (100.0) | 136 (100.0) | |
| Surgery within 7 days | 122 (44.7) | 40 (29.2) | 82 (60.3) | <0.001* |
| Days from diagnosis to surgery | 8.0 (4.0–17.0) | 11.0 (5.0–22.5) | 6.0 (4.0–11.0) | <0.001† |
| In-hospital mortality | 20 (7.3) | 13 (9.5) | 7 (5.1) | 0.169* |
Data are presented as a median (IQR) or number (%) of patients.
*χ2 test.
†Mann-Whitney U-test.
heartjnl-2020-317265supp002.pdf (37.1KB, pdf)
heartjnl-2020-317265supp003.pdf (18.4KB, pdf)
Risk factors for in-hospital mortality
Sixty-one of the 419 patients (14.6%) died during the hospital stay. On univariate analysis, various variables were identified as risk factors for mortality (online supplemental table S2). After checking for multicollinearity, several factors were selected as variables for multivariable logistic regression analysis based on clinical significance. To verify the predictive performance of the selected logistic regression model, receiver operating characteristics curves were generated (AUC=0.83). Using the DeLong method, we found the model to be non-inferior to other possible models selected with bidirectional elimination selection using Akaike’s information criterion. The model was also identified as appropriate (p=0.118) with the Hosmer-Lemeshow test. Multivariable logistic regression analysis indicated that aortic valve endocarditis (OR 3.18, 95% CI 1.63 to 6.19, p=0.001), S. aureus infection (OR 2.32, 95% CI 1.11 to 4.85, p=0.026), neurological complications (OR 1.98, 95% CI 1.07 to 3.69, p=0.031), high SOFA score (OR 1.22, 95% CI 1.03 to 1.45, p=0.023) and high Charlson Comorbidity Index (OR 1.11, 95% CI 1.02 to 1.22, p=0.019) were independent risk factors for in-hospital mortality from IE. The protective effect of surgery against in-hospital mortality remained robust (OR 0.25, 95% CI 0.13 to 0.50, p<0.001) after adjusting for other variables (online supplemental table S3).
Discussion
Studies have identified a stable incidence of IE, although most have reported an increasing trend over time.5 7 We found an increasing trend during each calendar year of the study period. The increasing trend was also identified when hospital size was considered according to inpatient ratio. This is likely related to an increase in the sizes of high-risk populations comprising individuals with older age, diabetes and haemodialysis therapy.20 Actually, in this study, the median patient age tended to increase over time, although not statistically significant (IRR 1.01, 95% CI 0.99 to 1.03, p=0.570) (online supplemental figure S2). Moreover, the number of invasive procedures, including spinal surgery, which could lead to transient bacteraemia, has markedly increased over time.21
heartjnl-2020-317265supp004.pdf (18.9KB, pdf)
Streptococcus species have continued to be the major causative organisms of IE. The frequency of isolation of these species did not decrease, but rather increased, over the calendar years studied. Because Streptococcus species are the major microorganisms contributing to IE development, which is preventable with antibiotic prophylaxis, it is possible that antibiotic prophylaxis for IE was not effectively performed during the study period. In fact, according to South Korea’s national guidelines, antibiotic prophylaxis for IE was performed in patients with the highest risk of an adverse outcome of IE.11 Antibiotic prophylaxis was recommended in patients with a high risk of bacteraemia during dental procedures, which may involve the manipulation of the gingival or periapical region of teeth or perforation of the oral mucosa (including scaling and root canal procedures). However, Ki et al reported that the prescription rate of prophylactic antibiotics for IE was only 14.1%, which may explain the sustained development of IE caused by Streptococcus species.22 The high incidence of IE caused by Streptococcus may explain why patients with IE in South Korea are younger than those in Western countries.
A study from the UK showed an increase in the incidence of IE with a decrease in prophylactic antibiotic use, after the National Institute for Health and Care Excellence (NICE) guidelines were modified in 2008.1 However, in South Korea, the national guidelines for IE were revised in 2011 and did not include all of the NICE guidelines.11 Further, the preguideline revision era was shorter than the postguideline revision era during our study period, and the prescription rate of prophylactic antibiotics for IE was low. Therefore, we did not observe any significant change in the incidence and microbiological profile of IE between the two periods.
The overall in-hospital mortality rate of patients with IE was 14.6%, which is lower than that in a study performed in Japan (with a similar racial background to South Korea).23 This may be because of the younger patient age and the lower proportion of infections caused by S. aureus in our study. Meanwhile, the mortality rate declined over each calendar year. To understand the decline in the in-hospital mortality rate over the calendar years, factors affecting mortality need to be considered. Although the number of resistant bacterial strains has increased, the proportion of resistant strains, such as methicillin-resistant S. aureus, did not significantly increase during the study period. Streptococcus species remained the most frequently identified microorganisms, which might have contributed to the favourable outcomes. Moreover, early surgery has been reported to improve the prognosis of IE.24 Thus, in recent years, surgery for IE has been performed earlier, which likely facilitated the gradual decline in the in-hospital mortality rate of IE in South Korea.
In this study, there were patients (n=29) who met the surgical criteria but did not undergo surgery because of multiorgan failure and comorbidities. Therefore, although surgical intervention reduces the in-hospital mortality rate of IE, there is a potential for bias because the severity of IE in patients who underwent surgery might be less than that in patients who received medical treatment only. To clarify this, we used the Charlson Comorbidity Index and SOFA score as variables in multivariable logistic regression analysis to adjust for the patients’ medical background and disease severity. We also assessed the interaction effects between surgical intervention and the Charlson Comorbidity Index and SOFA score. No interaction between these factors was identified. This result is consistent with previous reports that surgery in IE is associated with a good prognosis.25 26 Our findings on the impact of early surgery to prevent embolism are consistent with those of previous studies and support the guideline recommendations.4 27
To identify factors affecting the in-hospital mortality rate of IE, we selected variables for multivariable logistic regression analysis (based on clinical significance) from among the significant variables in univariate analyses. The logistic regression model including these variables was compared with two logistic regression models selected using bidirectional elimination selection to verify the predictive performance. Our model not only was non-inferior to the other two models in predictive performance but also was free of problems of overfitting. Therefore, we concluded that the model is appropriate for predicting in-hospital mortality from IE and that the selected variables in the model are significant. This rigorous statistical process strengthens our conclusions.
Patients with aortic valve endocarditis had a poor prognosis. Conversely, Kaartama et al 28 reported that patients with aortic valve endocarditis had better short-term and long-term survival than those with mitral valve endocarditis. In their study, the mean patient age and the frequency of S. aureus infection were higher in patients with mitral valve endocarditis than in those with aortic valve endocarditis. Further, surgery was delayed in patients with mitral valve endocarditis. However, in our study, patients with aortic valve endocarditis were significantly older than those without (mean age 58.4 vs 53.6 years, p=0.009). Moreover, in patients with aortic valve endocarditis, the median SOFA (2.0 vs 1.0, p<0.001) and APACHE-II (7.0 vs 6.0, p=0.014) scores were significantly higher than in those without aortic valve endocarditis. Additionally, there was no difference in the frequency of S. aureus infection (14.7% vs 16.7%, p=0.640) or in the proportions of patients who underwent surgery within 7 days after the IE diagnosis (63.8% vs 61.5%, p=0.682) between patients with and without aortic valve endocarditis. This suggests that aortic valve endocarditis may not confer a good prognosis and that clinical features should be considered in assessing the prognosis of IE.
Because many variables affect the in-hospital mortality rate of patients with IE, we used the Charlson Comorbidity Index to represent comorbidities, including age, and the SOFA score to indicate disease severity. Both variables were identified as independent risk factors for in-hospital mortality in patients with IE. The other independent risk factors for in-hospital mortality in patients with IE were S. aureus infection and neurological complications. These findings are consistent with those of previous studies.29 30
This study had some limitations. First, it was a non-randomised retrospective study and certain clinical variables might have been missed. Although we used rigorous statistical analysis to adjust for host-related factors and disease severity, unmeasured confounders could have affected the outcomes. Second, because there are very few intravenous drug users in South Korea, the effect of intravenous drug use on IE could not be evaluated. Third, this was performed at a single centre, which limits the extrapolation of our results to the overall trends of IE in South Korea. Moreover, the incidence, microbiological profile and severity of disease may have been biassed, as this study was performed in a single country. However, our results are still meaningful because data on IE trends in Asia are currently lacking. Further nationwide population-based studies and multinational cohort studies are needed to validate our findings.
In conclusion, the incidence of IE has increased over time and the mortality rate has slightly declined but remains high. Streptococcus species were the most common causative organisms of IE throughout the study period. Aortic valve endocarditis, IE caused by S. aureus, high SOFA score, high Charlson Comorbidity Index and presence of neurological complications were independent risk factors for in-hospital mortality in patients with IE, whereas surgical intervention for IE was associated with an improved prognosis. Further nationwide studies on the trends, causative organisms and risk factors of IE are needed.
Key messages.
What is already known on this subject?
Studies have reported that the incidence of infective endocarditis (IE) is increasing, and its mortality rate has not shown significant improvement. Although many variables were reported to be related to in-hospital mortality in patients with IE, the study results differed according to the statistical method. In particular, the effects of surgical treatment on long-term prognosis were not conclusive.
What might this study add?
This study shows that the incidence of IE is increasing and that, although the in-hospital mortality rate has slightly decreased, it remains high in South Korea. Data about the causative microorganisms of IE and factors affecting in-hospital mortality, determined using rigorous statistical methods, are also provided. Finally, this study reveals that surgical intervention has a protective effect with respect to both in-hospital mortality and long-term mortality.
How might this impact on clinical practice?
Given the increasing incidence and high mortality rate, the disease burden of IE is significant. The fact that Streptococcus species are still the main causative microorganisms means that antibiotic prophylaxis for IE should be improved. Because the protective effect of surgical intervention against in-hospital mortality and long-term mortality was identified, a more active approach to surgical treatment is needed.
heartjnl-2020-317265supp005.pdf (293.1KB, pdf)
Acknowledgments
We extend our gratitude to the staff of the Biostatistics Collaboration Unit and the medical illustrator at Yonsei University College of Medicine.
Footnotes
Presented at: This study was accepted and presented in the form of a poster presentation at the IDWeek 2019 Annual Meeting, Washington, District of Columbia, 2–6 October 2019. Our abstract presented at IDWeek 2019 can be found at Open Forum Infectious Diseases, Volume 6, Issue Supplement 2, October 2019, pages S100–S101.
Contributors: NSK and SHL contributed to the conception and design of the study. JHK, HJL and SL contributed to data acquisition and analysis. JHK, NSK and SHL performed statistical analysis of the data. JHK wrote the first draft. NSK, SHL, SL, JYC and J-SY contributed to the review and editing of the manuscript. NSK and SHL are responsible for the overall content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the institutional review board of Yonsei University Health System Clinical Trial Center (4-2018-0248).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000-13: a secular trend, interrupted time-series analysis. Lancet 2015;385:1219–28. 10.1016/S0140-6736(14)62007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferraris L, Milazzo L, Rimoldi SG, et al. Epidemiological trends of infective endocarditis in a single center in Italy between 2003-2015. Infect Dis 2018;50:749–56. 10.1080/23744235.2018.1472806 [DOI] [PubMed] [Google Scholar]
- 3. Vogkou CT, Vlachogiannis NI, Palaiodimos L, et al. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis 2016;35:1227–45. 10.1007/s10096-016-2660-6 [DOI] [PubMed] [Google Scholar]
- 4. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart association. Circulation 2015;132:1435–86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 5. Ahtela E, Oksi J, Porela P, et al. Trends in occurrence and 30-day mortality of infective endocarditis in adults: population-based registry study in Finland. BMJ Open 2019;9:e026811. 10.1136/bmjopen-2018-026811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olmos C, Vilacosta I, Fernández-Pérez C, et al. The Evolving Nature of Infective Endocarditis in Spain: A Population-Based Study (2003 to 2014). J Am Coll Cardiol 2017;70:2795–804. 10.1016/j.jacc.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 7. Keller K, von Bardeleben RS, Ostad MA, et al. Temporal trends in the prevalence of infective endocarditis in Germany between 2005 and 2014. Am J Cardiol 2017;119:317–22. 10.1016/j.amjcard.2016.09.035 [DOI] [PubMed] [Google Scholar]
- 8. Cresti A, Chiavarelli M, Scalese M, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther 2017;7:27–35. 10.21037/cdt.2016.08.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holland TL, Baddour LM, Bayer AS, et al. Infective endocarditis. Nat Rev Dis Primers 2016;2:16059. 10.1038/nrdp.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 11. The Korean Society for Thoracic and Cardiovascular Surgery Clinical guideline for the diagnosis and treatment of cardiovascular infections. Infect Chemother 2011;43:129–77. 10.3947/ic.2011.43.2.129 [DOI] [Google Scholar]
- 12. Chirio D, Le Marechal M, Moceri P, et al. Factors associated with unfavorable outcome in a multicenter audit of 100 infective endocarditis. Eur J Clin Microbiol Infect Dis 2019;38:109–15. 10.1007/s10096-018-3401-9 [DOI] [PubMed] [Google Scholar]
- 13. Martín-Dávila P, Fortún J, Navas E, et al. Nosocomial endocarditis in a tertiary Hospital: an increasing trend in native valve cases. Chest 2005;128:772–9. 10.1378/chest.128.2.772 [DOI] [PubMed] [Google Scholar]
- 14. Hwang J-W, Park SW, Cho EJ, et al. Risk factors for poor prognosis in nosocomial infective endocarditis. Korean J Intern Med 2018;33:102–12. 10.3904/kjim.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sy RW, Chawantanpipat C, Richmond DR, et al. Development and validation of a time-dependent risk model for predicting mortality in infective endocarditis. Eur Heart J 2011;32:2016–26. 10.1093/eurheartj/ehp085 [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 17. Hsu C-N, Wang J-Y, Tseng C-D, et al. Clinical features and predictors for mortality in patients with infective endocarditis at a university hospital in Taiwan from 1995 to 2003. Epidemiol Infect 2006;134:589–97. 10.1017/S0950268805005224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nashef SAM, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:734–44. discussion 44-5. 10.1093/ejcts/ezs043 [DOI] [PubMed] [Google Scholar]
- 19. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 20. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol 2002;31:776–81. 10.1093/ije/31.4.776 [DOI] [PubMed] [Google Scholar]
- 21. Kim T-Y, Jang S, Park C-M, et al. Trends of incidence, mortality, and future projection of spinal fractures in Korea using nationwide claims data. J Korean Med Sci 2016;31:801–5. 10.3346/jkms.2016.31.5.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. HK K, Kim SH, Sohn KM, et al. Current status of prophylaxis for endocarditis. Korean Circ J 2005;35:328–34. [Google Scholar]
- 23. Hase R, Otsuka Y, Yoshida K, et al. Profile of infective endocarditis at a tertiary-care hospital in Japan over a 14-year period: characteristics, outcome and predictors for in-hospital mortality. Int J Infect Dis 2015;33:62–6. 10.1016/j.ijid.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 24. Kang D-H, Kim Y-J, Kim S-H, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012;366:2466–73. 10.1056/NEJMoa1112843 [DOI] [PubMed] [Google Scholar]
- 25. Kiefer T, Park L, Tribouilloy C, et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA 2011;306:2239–47. 10.1001/jama.2011.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010;121:1005–13. 10.1161/CIRCULATIONAHA.109.864488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim D-H, Kang D-H, Lee M-Z, et al. Impact of early surgery on embolic events in patients with infective endocarditis. Circulation 2010;122:S17–22. 10.1161/CIRCULATIONAHA.109.927665 [DOI] [PubMed] [Google Scholar]
- 28. Kaartama T, Nozohoor S, Johansson M, et al. Difference in outcome following surgery for native aortic and mitral valve infective endocarditis. Thorac Cardiovasc Surg 2019;67:652–8. 10.1055/s-0038-1676127 [DOI] [PubMed] [Google Scholar]
- 29. García-Cabrera E, Fernández-Hidalgo N, Almirante B, et al. Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013;127:2272–84. 10.1161/CIRCULATIONAHA.112.000813 [DOI] [PubMed] [Google Scholar]
- 30. Olmos C, Vilacosta I, Habib G, et al. Risk score for cardiac surgery in active left-sided infective endocarditis. Heart 2017;103:1435–42. 10.1136/heartjnl-2016-311093 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-317265supp001.pdf (124KB, pdf)
heartjnl-2020-317265supp002.pdf (37.1KB, pdf)
heartjnl-2020-317265supp003.pdf (18.4KB, pdf)
heartjnl-2020-317265supp004.pdf (18.9KB, pdf)
heartjnl-2020-317265supp005.pdf (293.1KB, pdf)