Abstract
Background and Objectives
Fatigue is a common complaint and shares many risk factors with falls, yet the independent contribution of fatigue on fall risk is unclear. This study’s primary aim was to assess the association between fatigue and prospective fall risk in 5642 men aged 64–100 enrolled in the Osteoporotic Fractures in Men Study (MrOS). The secondary aim was to examine the association between fatigue and recurrent fall risk.
Research Design and Methods
Fatigue was measured at baseline using the Medical Outcomes Study (short form) single-item question “During the past four weeks, how much of the time did you feel energetic?” Responses were then classified: higher fatigue = “none,” “a little,” or “some” of the time and lower fatigue = “a good bit,” “most,” or “all” of the time. We assessed falls using triannual questionnaires. Fall risk was examined prospectively over 3 years; recurrent falling was defined as at least 2 falls within the first year. Generalized estimating equations and multinomial logistic regression modeled prospective and recurrent fall risk as a function of baseline fatigue status, adjusted for demographics, medications, physical activity, and gait speed.
Results
Men with higher (26%) versus lower baseline fatigue were older (75.1 ± 6.2 vs 73.2 ± 5.7 years), 24% less active, and had worse physical function (gait speed = 1.09 ± 0.24 vs 1.24 ± 0.21 m/s), all p < .0001. Within 1 year, 25.4% (n = 1409) had fallen at least once, of which 47.4% (n = 668) were recurrent fallers. Men with higher versus lower fatigue had 25% increased fall risk (relative risk = 1.25, 95% CI: 1.14–1.36) over 3 years follow-up, but had 50% increased odds of recurrent falling (odds ratio = 1.50, 95% CI: 1.22–1.85) within the first year.
Discussion and Implications
Fatigue is an important risk factor of falling independent of established risk factors. Reductions in fatigue (ie, increased energy) may lessen the burden of falls in older men and provide a novel avenue for fall risk intervention.
Keywords: Epidemiology, Fatigability, Gait speed, Recurrent falls, Risk factors
Translational Significance: Fatigue is an underappreciated and undertreated complaint common among older adults associated with multiple health conditions and physical functional decline. This work highlights higher fatigue as an important and significant independent risk factor for prospective and recurrent falls. A novel translational finding was that the combined effect of slower gait speed and higher fatigue increased recurrent fall risk among older men. Identifying those with higher fatigue may provide a novel avenue for interventions targeting older adults at increased fall risk.
Background and Objectives
Falls are a significant cause of disability and mortality in older adults (1–4). Fall injuries are among the most expensive medical conditions to treat, with costs totaling more than $31 billion to Medicare and more than $50 billion in medical costs for fatal and nonfatal fall injuries in 2015 (5–8). Although women report falling more frequently than men, both older men and women have a high incidence of falls (9,10). Worse physical function, slower gait speed, executive dysfunction, and chronic disease burden are all strongly associated with increased fall risk (11–14).
Fatigue is commonly regarded as “a subjective lack of physical and/or mental energy that is perceived by the individual to interfere with usual or desired activities” (15,16). Fatigue is a frequently reported symptom among older adults with and without disease and shares several fall risk factors, including slower gait speed, worse physical function, and greater disability (17–19). However, the prospective association between fatigue and prospective falls has not yet been determined. One previous study found that walking-induced fatigue contributed to increased step width variability and reduced minimum foot clearance, 2 gait-related factors known to increase tripping risks (20). In another study, fatigue severity was positively associated with fall frequency, and older adults with more severe fatigue had a higher rate of falls compared to those reporting milder fatigue (21). While there is some indication that fatigue likely increases fall risk, these studies are few, as only Kamitani et al. (21) examined the prospective relationship between fatigue and fall risk.
In this investigation, we examined the prospective association between fatigue (ie, less vs more energy) and fall risk in older men. As a secondary aim, we examined the prospective association between fatigue and recurrent fall risk. We hypothesized that men with higher fatigue will have an increased risk of both falling and recurrent falls compared to men with lower fatigue.
Research Design and Methods
Study Participants
The Osteoporotic Fractures in Men Study (MrOS) is a multicenter, prospective cohort study designed to examine osteoporosis, fracture, and prostate cancer risk factors in older men (22,23). MrOS enrolled 5994 community-dwelling, ambulatory men from 6 sites in the United States (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; San Diego, CA). Baseline visits were conducted from March 2000 to April 2002. To be eligible, participants needed to be aged 65 years or older, able to walk without assistance, have no history of bilateral hip replacements, able to provide self-reported data, be absent of a medical condition that would result in imminent death (judgment made by the investigator), and able to understand and sign an informed consent. Further details on recruitment can be found elsewhere (22,23). The institutional review board from each site approved the study protocol, and written informed consent was obtained from all participants.
Outcome Measure: Falls Assessment
Falls were defined as unintentionally coming to rest on the ground or another lower level not resulting from a major health event (eg, stroke) or external hazard (eg, vehicular accident) (24). Information on falls was collected from triannual follow-up questionnaires that were mailed to participants every March, July, and November. Response rate exceeded 95%. The questionnaire assessed whether the participant had fallen in the previous 4 months, and if the participant indicated a fall, queried how many times they fell. Falls were prospectively assessed over 3 years for a total of 9 triannual questionnaires. The follow-up time was truncated in order to not extend too far from the exposure variable measurement because fatigue levels likely change over time. As a secondary analysis, recurrent falling was defined as having 2 or more falls within 1 year of follow-up after baseline. Recurrent falling was assessed over 1 year or 3 triannual questionnaires, as most published studies have defined people who had recurrent falls as “persons who fell at least twice within 1 year” (25).
Predictor Variable: Fatigue
A single-item question from the Medical Outcomes Study (short form) asked participants at baseline “During the past four weeks, how much of the time did you have a lot of energy?” (26,27). Participants who answered feeling energetic “none,” “a little,” or “some” of the time were categorized as having higher fatigue (ie, less energy), while participants who responded feeling energetic “a good bit,” “most,” or “all” of the time were categorized as having lower fatigue (ie, more energy). In addition, we also evaluated the full range of responses to evaluate fatigue severity. This question has been used previously as a marker of fatigue in other studies (21,26–28).
Potential Covariates
An in-person clinical examination included anthropometric and physical function measures (22,23). A trained examiner used standard equipment to measure the height (stadiometer) and weight (digital or balance beam scale) to calculate body mass index (weight [kg]/height [m2]). Gait speed (ie, mobility) was measured using a timed completion of a 6-m course performed at the participant’s usual walking speed, with the faster of the 2 walks used for these analyses (29). Grip strength was assessed in both hands using a Jamar handheld dynamometer, and the maximum score for either hand was included as a covariate (30).
Self-administered questionnaires were used to ascertain education level, lifestyle, medical history, and medication use (22,23). Self-reported health was measured using the question “Compared to other people your own age, how would you rate your overall health?” on a 5-point Likert scale, with higher scores indicating better health. Medical history questions were self-reported and phrased: “Has a doctor or other health care provider ever told you that you had X disease” (ie, diabetes, cancer, hypertension, heart attack, congestive heart failure, and stroke). Participants brought current prescription and nonprescription medications to the baseline clinic visit, and study staff recorded the name and dose of all medications. Medications were entered into an electronic database, and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (31). Trouble with dizziness was self-reported and phrased in the following manner: “Do you sometimes have trouble with dizziness?”
Physical activity was measured using the Physical Activity Scale for the Elderly, a 12-item questionnaire designed to assess leisure, household, and occupational activities in which older adults commonly engage (32). Functional impairment was assessed by a questionnaire that asked about difficulty walking 2–3 blocks on level ground, climbing 10 steps, preparing meals, doing heavy housework, and shopping for groceries or clothing (33,34). Conditions that may prevent standing or stepping were self-reported and phrased in the following manner: “Do you have any problems from recent surgery, injury or other health conditions that might prevent you from standing straight up from a chair or walking quickly?”
Statistical Analyses
We compared baseline characteristics of men with higher versus lower fatigue, using t test or Wilcoxon Mann–Whitney U test for continuous variables and chi-square tests for categorical variables. Generalized estimating equations for repeated measures data with Poisson distribution were used to model prospective falls over 3 years of self-reported falls every 4 months as a function of baseline fatigue status; fall risk ratios (relative risk [RR]) and 95% confidence intervals were estimated from the generalized estimating equation models. A first-order autoregressive correlation structure was used to account for the correlated responses from the same participants (14,35). Multinomial logistic regression (0, 1, ≥2 falls) was used to model the odds of recurrent falling after 1 year of follow-up. All analyses were first adjusted for age and clinic site, then adjusted for correlates of fatigue and fall risk, but not factors that precede fatigue. Although self-reported health conditions were significant when comparing men with higher versus lower fatigue, they did not significantly contribute to the model. Final models were adjusted for site, age, body mass index, number of medications, total physical activity score, dizziness, any functional impairment, self-reported health rating, self-reported conditions preventing standing or stepping, gait speed, and maximum grip strength. Furthermore, in the final model, we examined the interaction between gait speed and fatigue on fall and recurrent fall risk. Additional adjustment for fall history was included to determine whether the association between fatigue and fall risk was independent of fall history. If an interaction with fall history and fatigue was found, we further examined these results stratified by fall history. We repeated these analyses by replacing our dichotomous predictor with a variable using the full range of responses to examine fatigue severity.
A total of 5642 men were included in the final analytic sample, as we excluded 352 men due to missing data for covariates of interest (N = 1 fatigue, N = 344 medications, and N = 7 functional impairment). We performed all analyses using SAS 9.4 software (SAS Institute Inc. Cary, NC). Statistical significance was considered at p value less than .05.
Results
Participant Characteristics by Fatigue Status
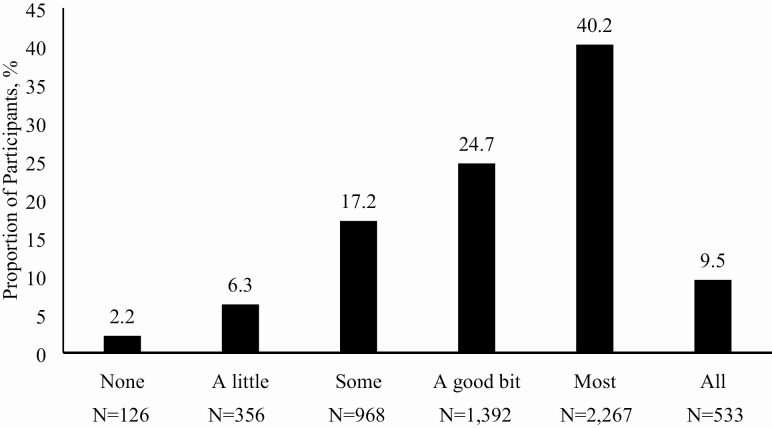
The mean age of the men at baseline was 73.7 (SD = 5.9, range: 64–100 years). The distribution of fatigue scores is shown in Figure 1; nearly 26% of men were categorized as having higher fatigue. Men with higher fatigue differed from those with lower fatigue on most characteristics examined, except race (Table 1). Further descriptive characteristics comparing men with higher fatigue versus lower fatigue can be found in Table 1.
Figure 1.
Distribution of participant responses (ie, fatigue severity) to the question “During the past 4 weeks, how much of the time did you have a lot of energy?” in the Osteoporotic Fractures in Men Study (MrOS). Higher fatigue (ie, less energy) was categorized as those who answered the question with “none”, “a little” or “some” of the time.
Table 1.
Baseline Characteristics of Participants in the Osteoporotic Fractures in Men Study (MrOS) Stratified by Fatigue Status (N = 5642)
| Higher Fatigue* | Lower Fatigue | |
|---|---|---|
| n = 1450 | n = 4192 | |
| Characteristics | Mean ± SD or n (%) | Mean ± SD or n (%) |
| Demographics and anthropometrics | ||
| Age, years | 75.0 ± 6.2 | 73.2 ± 5.7† |
| Race/ethnicity | ||
| White | 1306 (90.1) | 3725 (88.9) |
| Non-White | 144 (9.9) | 467 (11.1) |
| Education | ||
| Less than high school | 136 (9.4) | 232 (5.5) |
| High school | 277 (19.1) | 703 (16.8) |
| College or graduate school | 1037 (71.5) | 3257 (77.7) |
| Body mass index, kg/m2 | 28.0 ± 4.3 | 27.2 ± 3.7 |
| Physical activity and function | ||
| Physical Activity Scale for the Elderly total score | 118.5 ± 61.7 | 155.6 ± 68.1 |
| Any functional impairment | 668 (46.1) | 512 (12.2) |
| Physical performance measures | ||
| Gait speed, m/s | 1.09 ± 0.24 | 1.24 ± 0.21 |
| Grip strength, kg | 39.2 ± 8.4 | 42.4 ± 8.4 |
| Self-reported any conditions preventing standing/stepping | 180 (12.4) | 155 (3.7) |
| Medical history and medications | ||
| Self-reported good or excellent health rating | 546 (37.7) | 272 (6.5) |
| Self-reported doctor/health care provider diagnosed | ||
| Diabetes | 242 (16.7) | 371 (8.9) |
| Any cancer | 480 (33.1) | 1165 (27.8) |
| Nonskin cancer | 322 (22.2) | 708 (16.9) |
| Hypertension | 762 (52.6) | 1698 (40.5) |
| Heart attack | 293 (20.2) | 503 (12.0) |
| Congestive heart failure | 136 (9.4) | 161 (3.8) |
| Stroke | 138 (9.5) | 179 (4.3) |
| Benzodiazepine use | 96 (6.6) | 108 (2.6) |
| Antidepressant use | 162 (11.2) | 190 (4.5) |
| Nonsteroidal anti-inflammatory drug use | 305 (21.0) | 583 (13.9) |
| Total number of medications used | 5.5 ± 4.0 | 3.8 ± 3.4 |
| Previous history of falling (12 months prior baseline) | 418 (28.8) | 782 (18.7) |
| Self-reported trouble with dizziness | 584 (40.3) | 859 (20.5) |
*Based on the question “During the past 4 weeks, how much of the time did you have a lot of energy?” Higher fatigue (ie, less energy) = “none,” “a little,” or “some” of the time and lower fatigue (ie, more energy) = “a good bit,” “most,” or “all” of the time.
†All comparisons between fatigue status were significant at p < .0001 except race (p = .20) and any cancer (p = .0001).
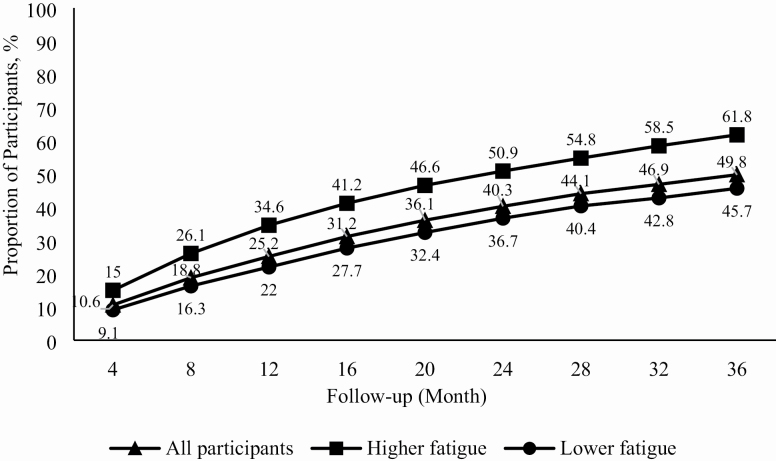
After 1 year of follow-up, 34.6% of those in MrOS with higher fatigue reported having a fall compared to 22% with lower fatigue (Figure 2). Over the 3-year follow-up period, the proportion of participants who fell increased across both fatigue groups; however, the proportion reporting a fall was consistently higher at each 4-month period in those with higher baseline fatigue. After 3 years of follow-up, 61.8% with higher baseline fatigue reported having a fall compared to 45.7% with lower fatigue. Within 1 year of follow-up, 25.4% (n = 1409) of the sample had fallen at least once, of which 47.4% (n = 668) were recurrent fallers.
Figure 2.
Cumulative frequency of falls stratified by baseline fatigue status over a 3-year follow-up in the Osteoporotic Fractures in Men Study (MrOS).
Risk of Prospective Falls and Recurrent Falling in Those With Higher Baseline Fatigue
Those with higher baseline fatigue had a 62% increased risk (p < .0001) of a fall adjusted for age and clinic site compared to those with lower baseline fatigue (Table 2 and Supplementary Table 1). Having higher baseline fatigue was associated with an increased risk of a fall by 25% (p < .0001) in the final model adjusted for all covariates (Table 2 and Supplementary Table 1). Furthermore, there was no interaction between gait speed and fatigue (p = .34).
Table 2.
Risk of Prospective Falls and Recurrent Falls in Men by Fatigue Status From the Osteoporotic Fractures in Men Study (MrOS)
| Three-Year Prospective Fall Risk | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age and site adjusted | Covariate adjusted* | |||||||
| Predictor Variable | RR (95% CI) | p value | RR (95% CI) | p value | ||||
| Higher fatigue† | 1.62 (1.50–1.75) | <.0001 | 1.25 (1.14–1.36) | <.0001 | ||||
| One-Year Recurrent Fall Risk | ||||||||
| Age and site adjusted | Covariate adjusted* | |||||||
| 0 vs 1 fall | 0 vs 2 falls | 0 vs 1 fall | 0 vs 2 falls | |||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Higher fatigue† | 1.41 (1.18–1.68) | .002 | 2.30 (1.93–2.74) | <.0001 | 1.21 (0.98–1.48) | .07 | 1.50 (1.22–1.85) | .0001 |
Notes: BMI = body mass index; CI = confidence interval; OR = odds ratio; RR = relative risk.
*Adjusted for age, clinical site, BMI, Physical Activity Scale for the Elderly total score, gait speed, maximum grip strength, number of medications, self-reported dizziness, any functional impairment, self-reported health rating, and self-reported conditions preventing standing or stepping.
†Based on the question “During the past 4 weeks, how much of the time did you have a lot of energy?” Higher fatigue (ie, less energy) = “none,” “a little,” or “some” of the time and lower fatigue (ie, more energy) = “a good bit,” “most,” or “all” of the time.
Additional adjustment for previous fall history attenuated the relationship but remained significant (RR: 1.22, 95% CI: 1.12–1.33). Additionally, there was a significant interaction between fatigue and fall history, and after stratification, having higher baseline fatigue was more strongly associated with increased fall risk in those without a previous fall history (RR: 1.26, 95% CI: 1.12–1.42) compared to those with a previous fall history (RR: 1.18, 95% CI: 1.03–1.34).
Having higher baseline fatigue was associated with increased odds of recurrent falling by 2.3-fold compared to those with lower fatigue, after age and clinical site adjustment, p < .0001 (Table 2 and Supplementary Table 2). In the fully adjusted model, having higher fatigue increased the odds of recurrent falling by 50%, p = .0001 (Table 2 and Supplementary Table 2).
There was a significant interaction between gait speed and fatigue for recurrent falls (0 vs 1 fall: β coefficient = −0.89 ± 0.41, p = .03; 0 vs 2 falls: β coefficient = −0.95 ± 0.41, p = .02), regardless of adjustment for previous fall history. In the fully adjusted model, additional adjustment for previous fall history attenuated the relationship between fatigue and recurrent fall risk, but it remained significant (odds ratio: 1.48, 95% CI: 1.19–1.83). There was no significant interaction between fatigue and fall history for recurrent falling (0 vs 1 fall p = .99, 0 vs 2 falls p = .70).
There was a dose–response relationship between fatigue severity and fall and recurrent fall risk (Supplementary Table 3).
Discussion and Implications
This study extended previous work on fall risk factors by examining the prospective association between fatigue and fall and recurrent fall risk in a large cohort of older men aged 64–100 years (14,36–40). We found that higher baseline fatigue (ie, less energy), which was prevalent in 26% of participants, was associated with a 25% increased risk of having at least one fall during a 4-month period over 3 years and 50% greater odds of recurrent falls within 1 year compared to those with lower fatigue.
While prevalence rates of self-reported fatigue vary greatly depending on the assessment tool (41–44), the prevalence of higher fatigue in MrOS is similar to other studies that have examined fatigue using a single-item question. In the Jerusalem Longitudinal Cohort Study, the prevalence of fatigue among similarly aged older adults was 29% (41), comparable to the 26% of men in MrOS. Similar to the Jerusalem Longitudinal Cohort Study (41), our investigation found that higher fatigue was associated with older age, indicating a need for earlier intervention to reduce the future burden of fatigue on falls and other health outcomes.
Fatigue and falls share several risk factors. Fatigue was associated with worse physical function and greater disability in our study and the InChianti Study (18), and worse physical function and worse gait characteristics (ie, stride frequency, stride lengths, center-of-mass lateral sway, and ankle plantarflexion and hip extension during push-off) have been found to increase fall risk (12,45). Walking-induced fatigue has also been shown to increase factors associated with tripping (ie, increasing step width variability and reducing minimum foot clearance) (20), which may be one pathway for fatigue to increase fall risk. Our findings support this novel pathway for recurrent fallers (eg, high-risk individuals) only, as we identified a significant interaction between gait speed and fatigue, indicating that those with slower gait and higher fatigue were at even greater risk of recurrent falls. Fatigue may contribute to the inability to maintain postural control, as both cognitive and physical fatigue have been found to decrease postural control in young adults (46), and is also associated with increased fall risk, particularly injurious fall risk (47,48).
In this study, men with higher baseline fatigue had significantly lower levels of physical activity, which is consistent with the literature (49–51). It has been proposed that fatigue and energy may be separate but related states (51,52). Fatigue has been conceptualized as one’s perception of having reduced capacity to complete activities/tasks, while energy is defined as the subjective capacity to complete the physical activity and is associated with energy expenditure (53,54). Physical activity interventions have been shown to improve fatigue in diseased and frail populations with high fatigue (55–58) and also are beneficial in preventing falls in older adults at increased fall risk (59). Thus, utilizing lack of energy as the definition of having higher fatigue supports the underlying mechanisms for how physical activity interventions may be beneficial to reduce fall risk by lessening fatigue (ie, increasing energy levels).
One of the major strengths of this study is that MrOS is a very large cohort of community-dwelling older men who were not selected for specific ailments. These men were prospectively followed using triannual questionnaires, which may have reduced recall bias. Other strengths include the ability to adjust for many fall- and fatigue-related covariates to limit confounding. A limitation of this work is that fatigue status was only assessed at baseline and could vary depending on circumstances such as mood, self-motivation, season, and health (60). Single-item questions assessing fatigue are also less sensitive given that older adults may modify activities (ie, slow down or self-pace) to maintain fatigue within an acceptable range (43,61,62). Nonetheless, single-item fatigue questions have been sensitive enough to differentiate between fatigued and nonfatigued individuals (18,41). Another limitation is that we were unable to examine the impact of sleep, cognitive, or mental health on the relationship between fatigue and falls. Detailed sleep, cognitive, and mental health measures were added later in MrOS and will be an area of future examination. The findings may not be generalizable to population groups other than community-dwelling older men. It is important to note that these data were collected in the early 2000s; however, the definition of falls and recurrent falling and the methods to ascertain information on falls in MrOS have remained unchanged. Nonetheless, it is plausible that our findings may be less generalizable to a contemporary population. Another minor limitation is the use of self-reported fall events, which could have been affected by recall bias whereas an interviewer could clarify any questions to ascertain reliable fall data (63); however, the prospective ascertainment of falls may minimize underreporting (64). We also did not collect information about the timing and circumstances of the falls.
Fatigue is an underappreciated and undertreated complaint common among older adults associated with multiple comorbid disorders and functional decline. We found that fatigue was significantly associated with increased fall risk by 25%, after adjustment for several covariates, including previous fall history. Future studies should include a more sensitive measure of fatigue, namely perceived fatigability (15,62,65), as well as include women, as they have a greater prevalence of fatigability (65). Measuring fatigability enables us to describe an older adults’ susceptibility to experiencing fatigue in the context of a quantifiable demand at a fixed intensity and duration (61,62). Fatigue appears to be an important risk factor that may provide a novel avenue to identify older adults at increased fall risk for appropriate intervention.
Supplementary Material
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. S.W.R. was supported as a Graduate Student Researcher by the National Institute on Aging (U01 AG023712, U01 AG023744, U01AG023746, U01AG023749, and U01AG023755).
Conflict of Interest
None declared.
References
- 1. Rubenstein LZ Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084 [DOI] [PubMed] [Google Scholar]
- 2. Vennu V, Bindawas SM. Relationship between falls, knee osteoarthritis, and health-related quality of life: data from the Osteoarthritis Initiative study. Clin Interv Aging. 2014;9:793–800. doi: 10.2147/CIA.S62207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stenhagen M, Ekström H, Nordell E, Elmståhl S. Accidental falls, health-related quality of life and life satisfaction: a prospective study of the general elderly population. Arch Gerontol Geriatr. 2014;58:95–100. doi: 10.1016/j.archger.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 4. Roe B, Howell F, Riniotis K, Beech R, Crome P, Ong BN. Older people and falls: health status, quality of life, lifestyle, care networks, prevention and views on service use following a recent fall. J Clin Nurs. 2009;18:2261–2272. doi: 10.1111/j.1365-2702.2008.02747.x [DOI] [PubMed] [Google Scholar]
- 5. Carroll NV, Slattum PW, Cox FM. The cost of falls among the community-dwelling elderly. J Manag Care Pharm. 2005;11:307–316. doi: 10.18553/jmcp.2005.11.4.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996–2013. JAMA. 2016;316(24):2627–2646. doi: 10.1001/jama.2016.16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burns ER, Stevens JA, Lee R. The direct costs of fatal and non-fatal falls among older adults—United States. J Safety Res. 2016;58:99–103. doi: 10.1016/j.jsr.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66:693–698. doi: 10.1111/jgs.15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gale CR, Cooper C, Aihie Sayer A. Prevalence and risk factors for falls in older men and women: the English longitudinal study of ageing. Age Ageing. 2016;45:789–794. doi: 10.1093/ageing/afw129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens JA, Sogolow ED. Gender differences for non-fatal unintentional fall related injuries among older adults. Inj Prev. 2005;11:115–119. doi: 10.1136/ip.2004.005835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ward RE, Leveille SG, Beauchamp MK, et al. Functional performance as a predictor of injurious falls in older adults. J Am Geriatr Soc. 2015;63:315–320. doi: 10.1111/jgs.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Rekeneire N, Visser M, Peila R, et al. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:841–846. doi: 10.1046/j.1365-2389.2003.51267.x [DOI] [PubMed] [Google Scholar]
- 13. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 14. Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165:696–703. doi: 10.1093/aje/kwk050 [DOI] [PubMed] [Google Scholar]
- 15. Eldadah BA Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi: 10.1016/j.pmrj.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 16. Zengarini E, Ruggiero C, Pérez-Zepeda MU, et al. Fatigue: relevance and implications in the aging population. Exp Gerontol. 2015;70:78–83. doi: 10.1016/j.exger.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 17. Mänty M, de Leon CFM, Rantanen T, et al. Mobility-related fatigue, walking speed, and muscle strength in older people. J Gerontol A Biol Sci Med Sci. 2012;67(5):523–529. doi: 10.1093/gerona/glr183 [DOI] [PubMed] [Google Scholar]
- 18. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morrison S, Colberg SR, Parson HK, et al. Walking-induced fatigue leads to increased falls risk in older adults. J Am Med Dir Assoc. 2016;17:402–409. doi: 10.1016/j.jamda.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagano H, James L, Sparrow WA, Begg RK. Effects of walking-induced fatigue on gait function and tripping risks in older adults. J Neuroeng Rehabil. 2014;11:155. doi: 10.1186/1743-0003-11-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamitani T, Yamamoto Y, Kurita N, et al. Longitudinal association between subjective fatigue and future falls in community-dwelling older adults: the Locomotive Syndrome and Health Outcomes in the Aizu Cohort Study (LOHAS). J Aging Health. 2019;31:67–84. doi: 10.1177/0898264317721825 [DOI] [PubMed] [Google Scholar]
- 22. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 23. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 24. Lamb SE, Jørstad-Stein EC, Hauer K, Becker C; Prevention of Falls Network Europe and Outcomes Consensus Group Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x [DOI] [PubMed] [Google Scholar]
- 25. Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658–668. doi: 10.1097/EDE.0b013e3181e89905 [DOI] [PubMed] [Google Scholar]
- 26. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 27. Neuberger GB Measures of fatigue: the fatigue questionnaire, fatigue severity scale, multidimensional assessment of fatigue scale, and short form-36 vitality (Energy/Fatigue) subscale of the short form health survey. Arthritis Care and Research. 2003;49(5S):S175–S183. doi: 10.1002/art.11405 [DOI] [Google Scholar]
- 28. Cawthon PM, Ensrud KE, Laughlin GA, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2009;94:3806–3815. doi: 10.1210/jc.2009-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Härkönen R, Harju R, Alaranta H. Accuracy of the Jamar dynamometer. J Hand Ther. 1993;6:259–262. doi: 10.1016/s0894-1130(12)80326-7 [DOI] [PubMed] [Google Scholar]
- 31. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664 [DOI] [PubMed] [Google Scholar]
- 32. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 33. Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987;(21):1–115. https://www.cdc.gov/nchs/data/series/sr_01/sr01_021.pdf [PubMed] [Google Scholar]
- 34. Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107 [DOI] [PubMed] [Google Scholar]
- 35. Ballinger GA Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7(2):127–150. doi: 10.1177/1094428104263672 [DOI] [Google Scholar]
- 36. Cauley JA, Harrison SL, Cawthon PM, et al. Objective measures of physical activity, fractures and falls: the osteoporotic fractures in men study. J Am Geriatr Soc. 2013;61:1080–1088. doi: 10.1111/jgs.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karlsson MK, Ribom E, Nilsson JÅ, et al. Inferior physical performance tests in 10,998 men in the MrOS study is associated with recurrent falls. Age Ageing. 2012;41:740–746. doi: 10.1093/ageing/afs104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hooker ER, Shrestha S, Lee CG, et al. ; Osteoporotic Fractures in Men (MrOS) Study Obesity and falls in a prospective study of older men: the osteoporotic fractures in men study. J Aging Health. 2017;29:1235–1250. doi: 10.1177/0898264316660412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marshall LM, Litwack-Harrison S, Makris UE, et al. A prospective study of back pain and risk of falls among older community-dwelling men. J Gerontol A Biol Sci Med Sci. 2017;72(9):1264–1269. doi: 10.1093/gerona/glw227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munch T, Harrison SL, Barrett-Connor E, et al. Pain and falls and fractures in community-dwelling older men. Age Ageing. 2015;44:973–1269. doi: 10.1093/ageing/afv125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:887–895. doi: 10.1093/gerona/glq064 [DOI] [PubMed] [Google Scholar]
- 42. Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. doi: 10.1111/j.1532-5415.2000.tb04702.x [DOI] [PubMed] [Google Scholar]
- 43. Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58:967–975. doi: 10.1111/j.1532-5415.2010.02811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng H, Hale L, Friedberg F. Prevalence and predictors of fatigue in middle-aged and older adults: evidence from the health and retirement study. J Am Geriatr Soc. 2010;58:2033–2034. doi: 10.1111/j.1532-5415.2010.03088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86:1501–1510. doi: 10.2522/ptj.20050387 [DOI] [PubMed] [Google Scholar]
- 46. Beurskens R, Haeger M, Kliegl R, Roecker K, Granacher U. Postural control in dual-task situations: does whole-body fatigue matter? PLoS One. 2016;11:e0147392. doi: 10.1371/journal.pone.0147392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El-Khoury F, Cassou B, Latouche A, Aegerter P, Charles MA, Dargent-Molina P. Effectiveness of two year balance training programme on prevention of fall induced injuries in at risk women aged 75–85 living in community: Ossébo randomised controlled trial. BMJ. 2015;351:h3830. doi: 10.1136/bmj.h3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kerber KA, Enrietto JA, Jacobson KM, Baloh RW. Disequilibrium in older people: a prospective study. Neurology. 1998;51:574–580. doi: 10.1212/wnl.51.2.574 [DOI] [PubMed] [Google Scholar]
- 49. Zaslavsky O, Su Y, Rillamas-Sun E, Roopsawang I, LaCroix AZ. Accelerometer-measured physical activity levels and fatigue in older women. J Aging Phys Act. 2020 April 16:1–7. doi: 10.1123/japa.2019-0308 [DOI] [PubMed] [Google Scholar]
- 50. Engberg I, Segerstedt J, Waller G, Wennberg P, Eliasson M. Fatigue in the general population—associations to age, sex, socioeconomic status, physical activity, sitting time and self-rated health: the northern Sweden MONICA study 2014. BMC Public Health. 2017;17(1):654. doi: 10.1186/s12889-017-4623-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puetz TW Physical activity and feelings of energy and fatigue: epidemiological evidence. Sports Med. 2006;36:767–780. doi: 10.2165/00007256-200636090-00004 [DOI] [PubMed] [Google Scholar]
- 52. Loy BD, Cameron MH, O’Connor PJ. Perceived fatigue and energy are independent unipolar states: supporting evidence. Med Hypotheses. 2018;113:46–51. doi: 10.1016/j.mehy.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O’Connor PJ Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res. 2004;57(5):435–441. doi: 10.1016/j.jpsychores.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 54. Tian Q, Glynn NW, Ehrenkranz RC, Sprague BN, Rosso AL, Rosano C. Perception of energy and objective measures of physical activity in older adults. J Am Geriatr Soc. 2020;68:1876–1878. doi: 10.1111/jgs.16577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weinstein AA, Chin LM, Keyser RE, et al. Effect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med. 2013;107:778–784. doi: 10.1016/j.rmed.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tomlinson D, Diorio C, Beyene J, Sung L. Effect of exercise on cancer-related fatigue: a meta-analysis. Am J Phys Med Rehabil. 2014;93:675–686. doi: 10.1097/PHM.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 57. Salmon VE, Hewlett S, Walsh NE, Kirwan JR, Cramp F. Physical activity interventions for fatigue in rheumatoid arthritis: a systematic review. Phys Ther Rev. 2017;22(1–2):12–22. doi: 10.1080/10833196.2016.1277454 [DOI] [Google Scholar]
- 58. Heine M, van de Port I, Rietberg MB, van Wegen EEH, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2015. September 11; (9):CD009956. doi: 10.1002/14651858.CD009956.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. US Preventive Services Task Force, Grossman DC, Curry SJ, et al. Interventions to prevent falls in community-dwelling older adults: US preventive services task force recommendation statement. JAMA. 2018;319(16):1696–1704. doi: 10.1001/jama.2018.3097 [DOI] [PubMed] [Google Scholar]
- 60. O’Connor PJ, Puetz TW. Chronic physical activity and feelings of energy and fatigue. Med Sci Sports Exerc. 2005;37:299–305. doi: 10.1249/01.mss.0000152802.89770.cf [DOI] [PubMed] [Google Scholar]
- 61. Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability Scale for older adults: development and validation. J Am Geriatr Soc. 2015;63:130–135. doi: 10.1111/jgs.13191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simonsick EM, Schrack JA, Santanasto AJ, Studenski SA, Ferrucci L, Glynn NW. Pittsburgh Fatigability Scale: one-page predictor of mobility decline in mobility-intact older adults. J Am Geriatr Soc. 2018;66:2092–2096. doi: 10.1111/jgs.15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cummings SR, Nevitt MC, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. J Am Geriatr Soc. 1988;36:613–616. doi: 10.1111/j.1532-5415.1988.tb06155.x [DOI] [PubMed] [Google Scholar]
- 64. Hannan MT, Gagnon MM, Aneja J, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am J Epidemiol. 2010;171:1031–1036. doi: 10.1093/aje/kwq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. LaSorda KR, Gmelin T, Kuipers AL, et al. Epidemiology of perceived physical fatigability in older adults: the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2020;75(9):e81–e88. doi: 10.1093/gerona/glz288 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.