Abstract
On March 11, 2020 the World Health Organization (WHO) declared the state of global pandemic caused by the new SARS-CoV-2 (COVID-19). To date, no antivirals directed against SARS-CoV-2 or effective vaccines to combat the viral infection are available. Severe acute respiratory syndrome caused by SARS-CoV-2 is treated empirically with antivirals, anti-inflammatory, anticoagulants. The approval of an effective vaccine still takes time. In this state, it may be useful to find new therapeutic solutions from drugs already on the market. Recent hypotheses suggest that the use of AT-1 receptor antagonists (ARB) in combination with neprilisin inhibitors (NEPi) could indirectly provide clinical benefits to patients with SARS-CoV-2 and cardiac involvement. In this article we investigate and describe a possible innovative pharmacological approach for the treatment of the most severe stages of COVID-19 infection.
Keywords: Cardiology, Covid-19, Cytokine, Neprilisin, Sacubitril, Sars-CoV-2
Introduction
Clinical aspects SARS-CoV-2 (COVID-19) infection
A viral epidemic caused by a new coronavirus SARS-CoV-2 (COVID-19) began in Wuhan (China) in November 2019. The epidemic quickly turned into a global pandemic in March 2020 [1]. Knowledge of this viral infection is evolving rapidly, to date there are no direct antivirals or effective vaccines and therapeutic treatments are on empirical basis, antiviral, immunomodulants, antiflammatory, anticoagulants [2]. At the time of writing of this article, 1.21 Mln deaths and 47.4 Mln infected people have been reported [3]. SARS-CoV-2 is a family of RNA viruses that can infect humans and cause serious respiratory tract infections that can be fatal in some cases. Studies have shown that SARS-CoV-2 has about 80% of the SARS-CoV like genome, responsible for the 2003 outbreak [4]. SARS-CoV-2 penetrates cells using the S protein through the angiotensin 2 conversion enzyme receptor (ACE-2) on the cell surface, which is widely present in the epithelial cells of the respiratory mucosa [5]. ACE-2 is also a conversion enzyme with a key role in the renin-angiotensin (RAS) system. Clinical experts and scientists have described SARS-CoV-2 infection in three phases: the first asymptomatic or slightly symptomatic, the second moderately severe characterized by a pulmonary inflammatory state, the third very severe phase characterized by a generalized inflammatory state affecting all tissues causing multi-organ dysfunction [6]. In the most severe stages of infection, COVID-19 lung lesions are characterized by diffuse alveolar damage with irregular inflammatory cellular infiltration consisting of monocytes, macrophages and lymphocytes infiltrating the lung tissue and the presence of intravascular thrombosis [7]. Severe inflammatory pulmonary infiltration prevents the exchange of alveolar gases, in addition, in more serious cases other organs may be damaged such as the heart or liver [8], [9]. Systemic inflammation in the most severe stages of infection can cause cardiac damage such as pericarditis, acute coronary syndrome, electrophysiological disorders and the appearance of arrhythmias. These aspects are further confirmed by studies showing that patients with a history of cardiovascular disease are at increased risk of COVID-19 complications [10]. In fact, in a recent study [11] it was found that 77% of deceased patients developed acute myocardial damage [12]. Some molecular and pathophysiological bases have been hypothesized, one of which is that the phenomenon of “cytokinic storm” [13], [14] that occurs in the most severe stages of COVID-19 infection causes myocarditis which is the cause of acute heart failure, TNF-α and some other pro-inflammatory cytokines are able to induce typical cellular modifications of the decompensated heart, such as down-regulation of the sarcoplasmic ATPasic calcium pump [15], or decoupling of beta-adrenergic receptors from activation of cyclic intracellular AMP [16] or death of cardiac cells.
Cytokinic storm and heart failure
The most severe stages of COVID-19 infection are characterized by a hyperinflammatory state caused by a cytokinic storm. The term indicates a massive and sudden release of cytokines that generate an uncontrolled and generalized inflammatory response [17], [18], [19] causing organ damage. The data clearly indicate an uncontrolled and sudden release by immuno effector cells of large amounts of proinflammatory cytokines such as IFNα, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGF-β [20] and chemokines such as CXCL10, CXCL8, CXCL9 [21], [22], [23], [24]. An increase in pro-inflammatory cytokines in particular may be responsible for cardiac damage. Several studies show that TNF-α plays a central role in myocardial contractility depression through various time-dependent mechanisms. The cardiodepressant effect of TNF-α is the consequence of a signaling dependent on nitric oxide synthase (NOS), a high concentration of nitric oxide (NO) leads to [25] an inotropic negative effect [26] and a profound systolic and diastolic dysfunction [27]. TNF-a also induces apoptosis in cardiac myocytes [28], which contributes to the thinning of the left ventricular wall [29], [30]. At molecular level, sustained overexpression of TNF-a activates both intrinsic and extrinsic apoptotic pathways and leads to progressive loss of antiapoptotic proteins [31]. IL-6 is a powerful mediator of myocardial depression, which in turn improves the cardiodepressant effects of TNF-a and IL-1 [32]. The inotropic negative effect of IL-6 is the result of JAK2/STAT3 mediated activation of iNOS [33]. IL-1 also produces a prolonged decrease in myocardial contractility [34] Finally IL-18 stimulates proinflammatory cytokines with known cardiodepressant effects, i.e., TNF-a, IL-1a, IL-1b, IL-6, and also IL-18 has been shown to induce the synthesis of NO, which mediates myocardial dysfunction. In addition, cardiac damage induced by COVID-19 may further intensify a local inflammatory reaction and excessive production of reactive oxygen species (ROS). Finally, through different mechanisms of action, the proinflammatory cytokines described above mediate contractile dysfunction and myocytic cardiac apoptosis with cardiac damage (Fig. 1, Fig. 2 ) [35], [36].
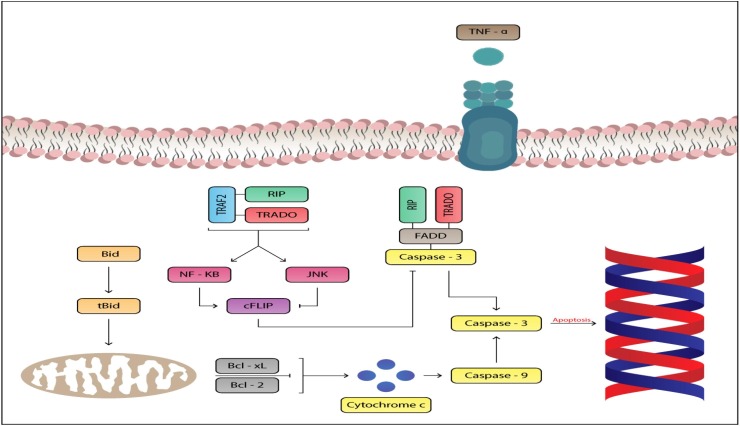
Fig. 1.
The proinflammatory cytokine TNF-alpha can induce apoptosis of cardiac myocytics inducing cardiac damage.
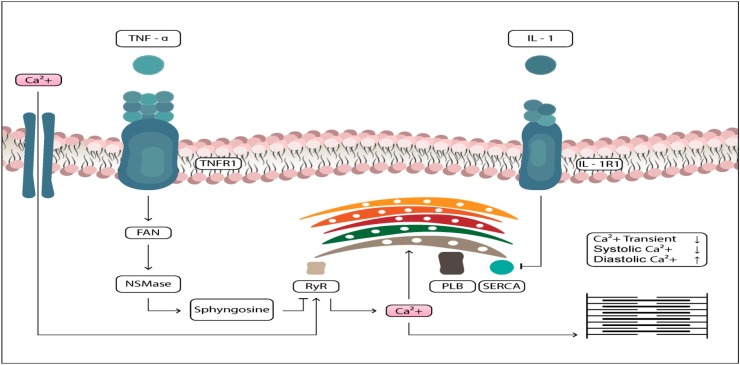
Fig. 2.
Pro-inflammatory cytokine IL-1 mediates inotropic negative effect and contractile cardiac dysfunction.
Natriuretic peptide system, biological effects
Natriuretic peptides are a family of structurally related hormonal factors. Atrial natriuretic peptide (ANP) and type B natriuretic peptide (BNP) are secreted by the atria and cardiac ventricles. Type C natriuretic peptide (CNP) is the most highly expressed natriuretic peptide in the brain, but is also highly expressed in chondrocytes and endothelial cells. Neutral neprilisin endopeptidase (NEP) is the enzyme that metabolizes natriuretic peptides. Natriuretic peptides mediate different physiological effects through interaction with specific guanylyl cyclase receptors (GC) that stimulate intracellular cGMP production. The main physiological effects are natriuresis / diuresis and peripheral vasodilation, inhibition of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (SNS). Recent evidence associates natriuretic peptides with other important functions, in particular, some studies have demonstrated an antifibrotic and anti-inflammatory action. The natriuretic peptide type C (CNP), a member of the family of natriuretic peptides, through selective binding to the transmembrane receptor guanylyl cyclase (GC)-B, mediates different biological effects in various organs [37]. CNP is expressed in a wide variety of tissues, such as vascular endothelium, heart, bones and adrenal glands [38], [39], [40], [41]. CNP plays an important role in the regulation of local vascular tone and has been shown to have mainly cardioprotective, antihypertrophic [42] and antifibrotic [43] effects. Recently, CNP has been shown to have protective effects against inflammatory and fibrotic reactions [44], [45]. Tests in vivo have revealed that CNP attenuates acute lipopolysaccharid-induced lung lesions (LPS) [46]. CNP also regulates the secretion of inflammatory cytokines [47], [48]. In the inflammatory phase, expression levels of various chemokines, cytokines and growth factors are high and these mediators exert their profibrotic activity through the activation and proliferation of fibroblasts [49]. Considering the pathophysiological importance of fibroblast activation in pulmonary fibrosis [50], and the above mentioned biological effects, it is suggested that there is a direct effect on pulmonary fibroblasts by natriuretic peptides. These insights suggest the use of therapeutic agents that increase the concentration of these peptides in the most severe stages of COVID-19 infection when a fibrotic pulmonary state and a cardiac fibrotic tissue is present. In association with evidence of antihyperproliferative effects, the studies also show direct antifibrotic effects mediated by the action of natriuretic peptides. In particular, some studies associate the peptide BNP with an important inhibitory effect on NALP3 the activation of inflammation, which is related to the BNP-induced reduction of NF-kB and ERK1/2 activation. The data indicate a powerful anti-inflammatory and immunomodulatory role for this peptide [51]. These mediated effects described above suggest that natriuretic peptides may play an important role in COVID-19 infection.
NT-proBNP in patients with severe COVID-19
BNP is synthesized as a prehormone (proBNP), upon release into the bloodstream it is divided in equal proportions into biologically active BNP and biologically inactive NT-proBNP. Stress and myocardial damage are the main release stimuli for BNP and NT-proBNP, studies have shown that increased cytokines and an inflammatory state are important additional factors inducing hormone secretion [52]. BNP is degraded by plasma through endopeptidase neprilisin (NEP), NT-proBNP is excreted primarily by renal excretion. BNP and NT-proBNP are important biomarkers for the evaluation of cardiac function [53], [54]. As described above, cardiac lesions are a common condition among patients hospitalized with COVID-19. A recent study has shown that the NT-proBNP marker has increased significantly in more severe cases of COVID-19, suggesting a relationship between high plasma levels of NT-proBNP, cardiac damage, and risk of death in patients with severe COVID-19. The explanation for the increase in NT-proBNP in severe COVID-19 is probably due to cardiac complications resulting from up-regulation of the sympathetic and angiotensin system (RAS), cytokinic cascade and systemic inflammation. In particular, cytokine storm [55], [56] could probably play an important role in cardiac damage [57] and the increase of NT-proBNP. In addition, RAS overactivation with reduced ACE-2 concentration, as evidenced in the most severe stages of COVID-19 infection, may lead to increased synthesis of Ang II with proinflammatory and profibrotic effects (mediated by AT-1 receptors) that facilitates the secretion of NT-proBNP [58], [59]. Pending well-structured and thorough studies, to assess whether the NT-proBNP marker can be a useful diagnostic test to assess the severity of COVID-19 infection, all the considerations expressed suggest a therapeutic pharmacological solution with the action of increasing the concentration of circulating natriuretic peptides, decrease the concentration of NT-proBNP, increase RAS via ACE-2 axis with increased synthesis of Ang 1–7 and Ang 1–9 with antifibrotic and anti-inflammatory effects, and decrease the effects of Ang II on the AT-1 receptor [60], [61], [62], [63].
The Hypotheses/theory
Potential role of Sacubitril/valsartan
The Sacubitril/valsartan combination is the first of a new class of drugs with therapeutic indication for the treatment of symptomatic chronic heart failure with reduced ejection fraction [64]. Sacubitril is a neprilisin inhibitor (NEPi), valsartan an angiotensin II receptor antagonist (ARB). Based on the above considerations, the association sacubitril/valsartan could be an important therapeutic solution to combat COVID-19 infection and reduce cardiac induced damage. The use of the sacubitrile/valsartan association could be of clinical benefit for several reasons, in particular the antagonism on the AT-1 receptor mediated by valsartan would lead to increased AT-2 receptor occupation by Ang II with antifibrotic, anti-inflammatory, antihyperproliferative and vasodilator effects with potential benefits on both lung lesions caused by fibrotic tissue and cardiac damage caused by COVID-19. In addition, the actions of Ang-II on the AT-1 receptor, which mediates vasoconstrictive, profibrotic and hyperproliferative effects, are blocked.
Finally, angiotensin II can cause increased inflammation through the production of IL-6, TNF-α and other inflammatory cytokines mediated by AT-1. [65], [66], [67] It is known that in the more severe stages of COVID-19 infection there is a decrease in ACE-2 which plays a protective role. ACE-2 synthesizes Ang 1–7 and Ang 1–9 with known anti-inflammatory, vasodilator, antifibrotic and antihyperproliferative effects [68], [69], [70]. The antagonism on AT-1 receptors by valsartan leads to a compensatory increase of ACE-2 and a higher stimulation of MAS receptors [71]. Finally, after ARB administration the response to hypertrophic growth induced by TNF-a is significantly attenuated [72] (Fig. 3 ). The beneficial effects of NEPi are attributable to the decrease in the degradation of natriuretic peptides. Natriuretic peptides cause vasodilation by stimulating the guanylate cyclase receptor to produce cGMP. In addition, sacubitrile administration is known to decrease NT-proBNP, which in severe cases COVID-19 is increased. In patients with COVID-19, with and without symptoms attributable to pneumonia, there is evidence of a significant increase in NT-proBNP, regardless of left ventricular dysfunction. Indeed, studies show that NT-proBNP levels are also the result of acute renal lesions and pro-inflammatory molecules such as interleukin-1 and C-reactive protein [73], In addition, natriuretic peptides act to suppress hyperactivation of the sympathetic system and decrease endothelin secretion. In addition, as mentioned above, natriuretic peptides also exert anti-inflammatory, antifibrotic and antihypertrophic effects. In particular, some evidence shows direct anti-inflammatory effects mediated. In particular, some studies associate the BNP peptide with an important inhibitory effect on the activation of inflammatory NALP3, which is related to the reduction of NF-kB and ERK1/2 activation induced by BNP [74]. In addition, for this class of drugs acting on RAS, there is a potential indirect protection against SARS-CoV-2. In fact, patients with cardiovascular diseases are at high risk of pneumonia, studies show that the use of drugs that block RAS decreases this risk [75].
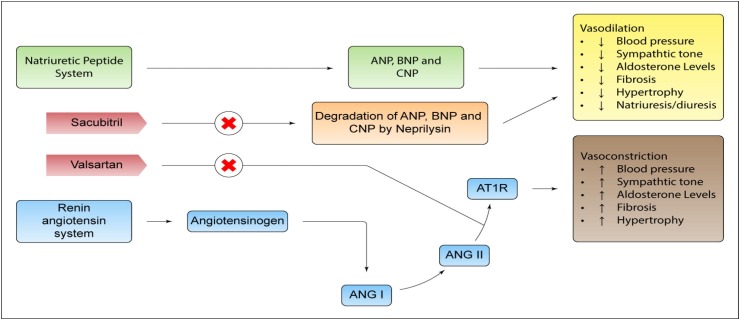
Fig. 3.
Mechanism of action: Sacubitril/valsartan has the mechanism of action of neprilisin inhibitor and angiotensin II receptor blocker type-1 (AT1). The complementary cardiovascular benefits of sacubitril/valsartan are attributed to the increase in neprilisin-degraded peptides and the simultaneous inhibition of the effects of angiotensin II. Natriuretic peptides exert their effects through the activation of membrane bound receptors coupled to the enzyme guanylyl cyclase, causing an increase in concentrations of the second messenger, cyclic guanosine monophosphate (cGMP), which can lead to vasodilation, natriuresis and diuresis, increased glomerular filtration rate and renal blood flow, inhibition of renin and aldosterone release, reduction of sympathetic activity and antihypertrophic/antifibrotic effects.
Conclusions
In severe COVID-19 patients, in addition to lung damage, there may be significant cardiac involvement, which is responsible for worsening the clinical condition of the host. The main cardiac manifestations can be edema, pericarditis, myocarditis, cardiac fibrosis, and impairment of contractile function and cardiac electrophysiology. Many pharmacological agents, when used appropriately, may be helpful in preserving cardiac homeostasis or reducing induced COVID-1 cardiac damage by decreasing mortality. Based on the evidence described and in relation to the hypotheses suggested by us, the use of the sacubityl/valsartan association in patients with COVID-19, especially in the most severe cases with induced cardiac damage, could be of therapeutic benefit, with cardioprotective, anti-inflammatory and antifibrotic effects that can also combat lung damage, through an increase in the natriuretic peptide system and a decrease in the effects of AT-1 receptor-mediated Ang-II. Well-structured clinical studies are needed to confirm these hypotheses.
Founds
None
Copyright
The authors certify that the manuscript is original, never submitted to other journal for publication before. All authors contributed equally to the manuscript and had the opportunity to revise and approve the final text.
Declaration of competing interest
None of the Authors have conflicts of interest to disclose.
Acknowledgements
We would like to thank Dr. Daniel Bittencourt, graphic designer, for creating new and unpublished images for our article.
References
- 1.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G., et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara F., Porta R., D'Aiuto V., Vitiello A. Remdesivir and COVID-19. Ir J Med Sci. 2020 Oct;17:1–2. doi: 10.1007/s11845-020-02401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World health organization (WHO) https://www.who.int/emergencies/diseases/novel coronavirus2019/situation-reports (Situation Reports July 2020).
- 4.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus. 2020 Oct In: StatPearls [Internet]. [PubMed]
- 5.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian S., Xiong Y., Liu H., Niu L.I., Guo J., Liao M., et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. Erratum in: Am J Clin Pathol. 2020 May 5;153(6):852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhanm, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitiello A., Ferrara F., Pelliccia C., Granata G., La Porta R. Cytokine storm and colchicine potential role in fighting SARS-CoV-2 pneumonia. Italian J Med. 2020;14(2):88–94. [Google Scholar]
- 14.Ferrara F., Granata G., Pelliccia C., La Porta R., Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2: Anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur J Clin Pharmacol. 2020;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McTiernan C.F., Lemster B.H., Frye C., Brooks S., Combes A., Feldman A.M. Interleukin-1 beta inhibits phospholamban gene expression in cultured cardiomyocytes. Circ Res. 1997;81(4):493–503. doi: 10.1161/01.res.81.4.493. [DOI] [PubMed] [Google Scholar]
- 16.Gulick T., Chung M.K., Pieper S.J., Lange L.G., Schreiner G.F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci USA. 1989;86(17):6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017 Jul;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133(1):13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams A.E., Chambers R.C. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306(3):L217–L230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar A., Brar R., Wang P., Dee L., Skorupa G., Khadour F., et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 1999;276(1):R265–R276. doi: 10.1152/ajpregu.1999.276.1.R265. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A., Paladugu B., Mensing J., Kumar A., Parrillo J.E. Nitric oxide-dependent and -independent mechanisms are involved in TNF-alpha -induced depression of cardiac myocyte contractility. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1900–R1906. doi: 10.1152/ajpregu.00146.2006. [DOI] [PubMed] [Google Scholar]
- 27.Elahi M., Asopa S., Matata B. NO-cGMP and TNF-alpha counter regulatory system in blood: understanding the mechanisms leading to myocardial dysfunction and failure. Biochim Biophys Acta. 2007;1772(1):5–14. doi: 10.1016/j.bbadis.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Haudek S.B., Taffet G.E., Schneider M.D., Mann D.L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Invest. 2007;117(9):2692–2701. doi: 10.1172/JCI29134DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozkurt B., Kribbs S.B., Clubb F.J., Jr, Michael L.H., Didenko V.V., Hornsby P.J., et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97(14):1382–1391. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 30.Kubota T., McTiernan C.F., Frye C.S., Slawson S.E., Lemster B.H., Koretsky A.P., et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81(4):627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 31.Haudek S.B., Taffet G.E., Schneider M.D., Mann D.L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117(9):2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maass D.L., White J., Horton J.W. IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock. 2002 Oct;18(4):360–366. doi: 10.1097/00024382-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Yu X., Kennedy R.H., Liu S.J. JAK2/STAT3, Not ERK1/2, Mediates Interleukin-6-induced Activation of Inducible Nitric-oxide Synthase and Decrease in Contractility of Adult Ventricular Myocytes. J. Biol. Chem. 2003;278(18):16304–16309. doi: 10.1074/jbc.M212321200. [DOI] [PubMed] [Google Scholar]
- 34.Prabhu S.D. Cytokine-Induced Modulation of Cardiac Function. Circ Res. 2004;95(12):1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 35.Boccellino M., Donniacuo M., Bruno F., Rinaldi B., Quagliuolo L., Ambruosi M., Pace S., De Rosa M., Olgaç A., Banoglu E., Alessio N., Massa A., Kahn H., Werz O., Fiorentino A., Filosa R. Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur J Med Chem. 2019;180:637–647. doi: 10.1016/j.ejmech.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 36.Toldo S., Boccellino M., Rinaldi B., Seropian I.M., Mezzaroma E., Severino A., Quagliuolo L., Van Tassell B.W., Marfella R., Paolisso G., Rossi F., Natarajan R., Voelkel N., Abbate A., Crea F., Baldi A. Altered Oxido-Reductive State in the Diabetic Heart: Loss of Cardioprotection due to Protein Disulfide Isomerase. Mol Med. 2011;17(9-10):1012–1021. doi: 10.2119/molmed.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Ry S. C-type natriuretic peptide: A new cardiac mediator. Peptides. 2013;40:93–98. doi: 10.1016/j.peptides.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Leuranguer V., Vanhoutte P.M., Verbeuren T., Félétou M. C-type natriuretic peptide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Br J Pharmacol. 2008 Jan;153(1):57–65. doi: 10.1038/sj.bjp.0707476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Ry S., Cabiati M., Vozzi F., Battolla B., Caselli C., Forini F., Segnani C., Prescimone T., Giannessi D., Mattii L. Expression of C-type natriuretic peptide and its receptor NPR-B in cardiomyocytes. Peptides. 2011;32(8):1713–1718. doi: 10.1016/j.peptides.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Suda M., Tanaka K., Fukushima M., Natsui K., Yasoda A., Komatsu Y., Ogawa Y., Itoh H., Nakao K. C-Type Natriuretic Peptide as an Autocrine/Paracrine Regulator of Osteoblast. Biochem Biophys Res Commun. 1996;223(1):1–6. doi: 10.1006/bbrc.1996.0836. [DOI] [PubMed] [Google Scholar]
- 41.K. Totsune K. Takahashi O. Murakami F. Satoh M. Sone M. Ohneda Y. Miura T. Mouri Immunoreactive brain natriuretic peptide in human adrenal glands and adrenal tumors 135 3 1996 352 356 10.1530/eje.0.1350352. [DOI] [PubMed]
- 42.Obata H., Yanagawa B., Tanaka K., Ohnishi S., Kataoka M., Miyahara Y., Ishibashi-Ueda H., Kodama M., Aizawa Y., Kangawa K., Nagaya N. CNP infusion attenuates cardiac dysfunction and inflammation in myocarditis. Biochem Biophys Res Commun. 2007;356(1):60–66. doi: 10.1016/j.bbrc.2007.02.085. [DOI] [PubMed] [Google Scholar]
- 43.Soeki T., Kishimoto I., Okumura H., Tokudome T., Horio T., Mori K., Kangawa K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2005;45(4):608–616. doi: 10.1016/j.jacc.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 44.Bükülmez H., Khan F., Bartels C.F., Murakami S., Ortiz‐Lopez A., Sattar A., Haqqi T.M., Warman M.L. Protective Effects of C‐Type Natriuretic Peptide on Linear Growth and Articular Cartilage Integrity in a Mouse Model of Inflammatory Arthritis. Arthritis & Rheumatology. 2014;66(1):78–89. doi: 10.1002/art.38199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peake N.J., Pavlov A.M., D’Souza A., Pingguan-Murphy B., Sukhorukov G.B., Hobbs A.J., Chowdhury T.T. Controlled Release of C-Type Natriuretic Peptide by Microencapsulation Dampens Proinflammatory Effects Induced by IL-1β in Cartilage Explants. Biomacromolecules. 2015;16(2):524–531. doi: 10.1021/bm501575w. [DOI] [PubMed] [Google Scholar]
- 46.Kimura T., Nojiri T., Hosoda H., Ishikane S., Shintani Y., Inoue M., Miyazato M., Okumura M., Kangawa K. C-type natriuretic peptide attenuates lipopolysaccharide-induced acute lung injury in mice. J Surg Res. 2015;194(2):631–637. doi: 10.1016/j.jss.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 47.T. Horio T. Tokudome T. Maki F. Yoshihara S.-I. Suga T. Nishikimi M. Kojima Y. Kawano K. Kangawa Gene Expression, Secretion, and Autocrine Action of C-Type Natriuretic Peptide in Cultured Adult Rat Cardiac Fibroblasts 144 6 2003 2279 2284 10.1210/en.2003-0128. [DOI] [PubMed]
- 48.ZHI.-QIANG. LI YING.-LONG. LIU GANG LI BIN LI YANG LIU XIAO.-FENG. LI AI.-JUN. LIU Inhibitory effects of C-type natriuretic peptide on the differentiation of cardiac fibroblasts, and secretion of monocyte chemoattractant protein-1 and plasminogen activator inhibitor-1 11 1 2015 159 165 10.3892/mmr.2014.2763. [DOI] [PMC free article] [PubMed]
- 49.Uchida M., Shiraishi H., Ohta S., Arima K., Taniguchi K., Suzuki S., Okamoto M., Ahlfeld S.K., Ohshima K., Kato S., Toda S., Sagara H., Aizawa H., Hoshino T., Conway S.J., Hayashi S., Izuhara K. Periostin, a Matricellular Protein, Plays a Role in the Induction of Chemokines in Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2012;46(5):677–686. doi: 10.1165/rcmb.2011-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura T., Nojiri T., Hino J., Hosoda H., Miura K., Shintani Y., Inoue M., Zenitani M., Takabatake H., Miyazato M., Okumura M., Kangawa K. Erratum to: C-type natriuretic peptide ameliorates pulmonary fibrosis by acting on lung fibroblasts in mice. Respir Res. 2016;17(1) doi: 10.1186/s12931-016-0429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mezzasoma L., Antognelli C., Talesa V.N. A Novel Role for Brain Natriuretic Peptide: Inhibition of IL-1β Secretion via Downregulation of NF-kB/Erk 1/2 and NALP3/ASC/Caspase-1 Activation in Human THP-1 Monocyte. Mediators Inflamm. 2017;2017:5858315. doi: 10.1155/2017/5858315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Lemos J.A., McGuire D.K., Drazner M.H. B-type natriuretic peptide in cardiovascular disease. The Lancet. 2003;362(9380):316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 53.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Restrepo M.I., Reyes L.F. Pneumonia as a cardiovascular disease: Pneumonia-related cardiac damage. Respirology. 2018;23(3):250–259. doi: 10.1111/resp.13233. [DOI] [PubMed] [Google Scholar]
- 56.Chen C, Zhang XR, Ju ZY, He WF. Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019]. Zhonghua Shao Shang Za Zhi. 2020 Jun 20;36(6):471-475. Chinese. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed]
- 57.Vitiello A., Ferrara F. Pharmacological agents to therapeutic treatment of cardiac injury caused by Covid-19. Life Sci. 2020;262:118510. doi: 10.1016/j.lfs.2020.118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei ZY, Qian HY. [Myocardial injury in patients with COVID-19 pneumonia]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020 Mar 2;48(0):E006. Chinese. doi: 10.3760/cma.j.issn.cn112148-20200220-00106. [DOI] [PubMed]
- 59.Weber M., Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92(6):843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M et al. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7).
- 62.Patel V.B., Zhong J.-C., Grant M.B., Oudit G.Y. Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure. Circ Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao L., Jiang D., Wen X.S., Cheng X.C., Sun M., et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21(1) doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hubers S.A., Brown N.J. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation. 2016;133(11):1115–1124. doi: 10.1161/CIRCULATIONAHA.115.018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Recinos A., III, LeJeune W.S., Sun H., Lee C.Y., Tieu B.C., Lu M., et al. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194(1):125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto S., Yancey P.G., Zuo Y., Ma L.J., Kaseda R., Fogo A.B., et al. Macrophage Polarization by Angiotensin II-Type 1 Receptor Aggravates Renal Injury-Acceleration of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(12):2856–2864. doi: 10.1161/ATVBAHA.111.237198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vitiello A., Ferrara F. Correlation between renin-angiotensin system and Severe Acute Respiratory Syndrome Coronavirus 2 infection: What do we know? Eur J Pharmacol. 2020;883:173373. doi: 10.1016/j.ejphar.2020.173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mariarosaria Boccellino Marina Di Domenico Maria Donniacuo Giuseppe Bitti Giulia Gritti Pasqualina Ambrosio Lucio Quagliuolo Barbara Rinaldi Elena Cavarretta AT1-receptor blockade: Protective effects of irbesartan in cardiomyocytes under hypoxic stress PLoS ONE 13 10 e0202297 10.1371/journal.pone.0202297.g003. [DOI] [PMC free article] [PubMed]
- 69.Ferrara F. Antirheumatic in SARS-CoV-2: benefit or risk? Italian Journal of Medicine. 2020;14(2):114–115. doi: 10.4081/itjm.2020.1290. [DOI] [Google Scholar]
- 70.Meng Y., Yu C.-H., Li W., Li T., Luo W., Huang S., Wu P.-S., Cai S.-X., Li X. Angiotensin-Converting Enzyme 2/Angiotensin-(1-7)/Mas Axis Protects against Lung Fibrosis by Inhibiting the MAPK/NF-κB Pathway. Am J Respir Cell Mol Biol. 2014;50(4):723–736. doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- 71.Vitiello A., Ferrara F. Therapeutic Strategies for SARS-CoV-2 acting on ACE-2. Eur J Pharm Sci. 2021;156:105579. doi: 10.1016/j.ejps.2020.105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flesch M., Höper A., Dell'Italia L., Evans K., Bond R., Peshock R., et al. Activation and functional significance of the renin-angiotensin system in mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2003 Aug 5;108(5):598–604. doi: 10.1161/01.CIR.0000081768.13378. [DOI] [PubMed] [Google Scholar]
- 73.Domenico Acanfora Marco Matteo Ciccone Pietro Scicchitano Chiara Acanfora Gerardo Casucci Sacubitril/valsartan in COVID-19 patients: the need for trials 6 4 2020 2020 253 254 10.1093/ehjcvp/pvaa044. [DOI] [PMC free article] [PubMed]
- 74.Antonio Vitiello Raffaele La Porta Francesco Ferrara Sacubitril, valsartan and SARS-CoV-2 bmjebm-2020-111497 10.1136/bmjebm-2020-111497. [DOI] [PubMed]
- 75.Henry C., Zaizafoun M., Stock E., Ghamande S., Arroliga A.C., White H.D. Impact of angiotensin-converting enzyme inhibitors and statins on viral pneumonia. Baylor University Medical Center Proceedings. 2018;31(4):419–423. doi: 10.1080/08998280.2018.1499293. [DOI] [PMC free article] [PubMed] [Google Scholar]