Abstract
With the COVID-19 pandemic now ongoing for close to a year, people all over the world are still waiting for a vaccine to become available. The initial focus of accelerated global research and development efforts to bring a vaccine to market as soon as possible was on novel platform technologies that promised speed but had limited history in the clinic. In contrast, recombinant protein vaccines, with numerous examples in the clinic for many years, missed out on the early wave of investments from government and industry. Emerging data are now surfacing suggesting that recombinant protein vaccines indeed might offer an advantage or complement to the nucleic acid or viral vector vaccines that will likely reach the clinic faster. Here, we summarize the current public information on the nature and on the development status of recombinant subunit antigens and adjuvants targeting SARS-CoV-2 infections.
Keywords: SARS-CoV-2, COVID-19, Adjuvants, Vaccine production, Vaccine delivery, Clinical trials, Neutralizing antibodies, Th1
Abbreviations: aa, Amino acid; ACE-2, Angiotensin-converting enzyme 2; ADE, Antibody-dependent enhancement; Alum, Aluminum hydroxide; APC, Antigen-presenting cell; cGMP, Current good manufacturing practices; CoV, Coronavirus; COVID, Coronavirus disease 2019; FDA, US Federal Drug Administration; HHS, US Department of Health and Human Services; MERS, Middle East respiratory syndrome; MNA, Micro-needle array; NTD, N-terminal domain; OWS, Operation Warp Speed; PTM, Post-translational modification; RBD, Receptor binding domain; RBM, Receptor binding motif; RSV, Respiratory syncytial virus; S-protein, Spike protein (S1/S2); SARS, Severe acute respiratory syndrome; TMPRSS2, Transmembrane protease serine 2; WHO, World Health Organization
Graphical abstract
1. Introduction
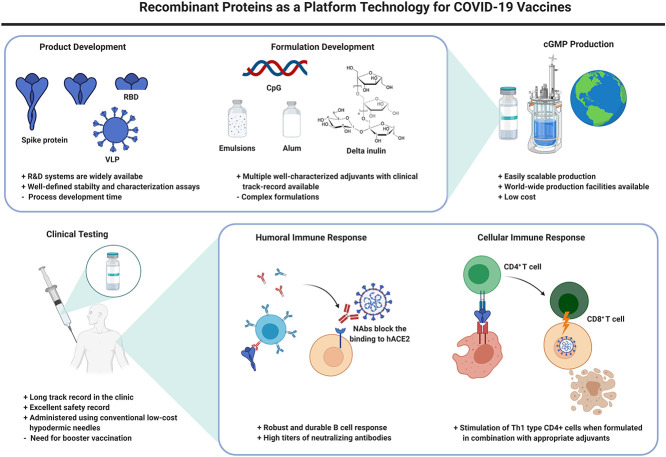
More than a year into the COVID-19 pandemic and in light of unprecedented worldwide efforts to develop countermeasures, the first generation of vaccines have now reached the clinic. Russia [1] and China [2] were the first to start mass vaccination campaigns, and are now followed by mRNA vaccines recently authorized for use in Europe [3] and the Americas [4]. As of December 8, 2020, the WHO lists 52 candidates in clinical evaluation and 162 in pre-clinical testing [5]. With this never-before-seen acceleration of research efforts, some of the front-runner platform technologies in this vaccine race have not previously been in the clinic, such as DNA or mRNA-based vaccines. More traditionally produced vaccines such as those based on recombinantly produced subunit proteins are lagging; nonetheless, there are currently 16 vaccines based on recombinant protein antigens in the clinic (Table 1 ), and 56 in pre-clinical testing. Arguably, the fact that this type of vaccine is lagging may not necessarily be a reflection of their validity or promise, but has multiple reasons, including possibly the way the initial funding was directed. Here we will provide a review of the status of the most advanced recombinant protein vaccines for COVID-19. While other candidates undoubtedly have high scientific merit, we intentionally limited the scope of this review to allow a focus on those vaccines that will likely make the strongest impact in the short term.
Table 1.
Select recombinant protein vaccine candidates in clinical trials for COVID-19 as of December 8, 2020 [5]
| Antigen | Vaccine developer | Platform/technology | Adjuvants | Most advanced clinical stage | References |
|---|---|---|---|---|---|
| Full-length S-protein based vaccines | |||||
| Trimer | Novavax | Insect cells | Matrix M | Phase 3 | [[6], [7], [8]] |
| S-protein | Sanofi Pasteur/GSK | Insect cells | 2 different adjuvants (likely variants of AS03) | Phase 1 (to be repeated) | [9] |
| SCB-2019 trimer | Clover Biopharmaceuticals Inc./GSK/Dynavax | CHO cells | Alum+CpG 1018 or AS03 | Phase 1 | [10,11] |
| S-2P (MVC-COV1901) | Medigen Vaccine Biologics Corporation/NIAID/Dynavax | CHO cells | Alum+CpG1018 | Phase 1 | [12,13] |
| Covax-19 | Vaxine Pty Ltd/Medytox | Insect cells | AdvaxCpG55.2 | Phase 1 | [14,15] |
| RBD-based vaccines | |||||
| AdimrSC-2f | Adimmune | Baculovirus/Sf9 | Alum | Phase 1 | [16] |
| SARS-CoV-2-RBDN1C1 | Biological E/BCM | Yeast | Alum+CpG | Phase 1-2 | [[17], [18], [19]] |
| FINLAY-FR-1/2 | Instituto Finlay de Vacunas, Cuba | Phase 1 | [20,21] | ||
| KBP-201 | Kentucky Bioprocessing, Inc | Plants | Phase 1-2 | [22] | |
| RBD Dimer | Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | CHO Cells | Aluminum preparation | Phase 3 | [23,24] |
| RBD | West China Hospital, Sichuan University P | Insect Cells | Alum | Phase 2 | [[25], [26], [27]] |
| Multi-epitope vaccines | |||||
| Multitope Peptide-based Vaccine (MPV) | COVAXX | Peptides | CpG and alum (AdjuPhos®) | Phase 1 | [28,29] |
| EpiVacCoron | Vektor Laboratories, Russia | Chemical synthesis | Alum | Phase 1 | [30] |
| CoVac-1 | University Hospital Tübingen | Peptides | Montanide ISA51 | Phase 1 | [31,32] |
2. The spike protein as a vaccine antigen candidate
The ~29.8 kb SARS-CoV-2 genome contains 14 open-reading frames encoding 27 proteins, including the four major structural proteins, E, envelope protein, M, matrix protein, N, nucleocapsid protein, and S, the spike protein [33]. Among these, the immunodominant trimeric S protein is the primary source of all major vaccine antigen targets to date. Other proteins have received considerably less attention as vaccine antigen candidates for various reasons. For instance, while the abundant SARS-CoV-2 N-protein is used in virus diagnostics [[34], [35], [36]], it is not included in most COVID-19 vaccine candidates because its SARS-CoV homolog was shown to increase the number of eosinophils within inflammatory infiltrates upon vaccination and subsequent challenge [37]. The S-protein is made up of two subunits, S1 and S2 that fulfill multiple functions related to the initial binding of the virus to its angiotensin-converting enzyme 2 (ACE-2) cell surface receptor and the subsequent endosome mediated entry of the virus into the host cell [38]. In the S-protein trimer, three S1 subunits sit on top of a stem of three S2 subunits. Within S1, a distinct receptor-binding domain (RBD, residues 331-524) and within it, a distinct receptor-binding motif (RBM), is responsible for the initial docking to ACE-2 [39]. Despite each S1 domain having its own functional RBD domain, it appears though that only one at a time is active, folded into the exposed confirmation, while the other two are hidden from the immune system within the trimer [40]. Moreover, there does not appear to be any cooperativity between the three RBDs within the S1 trimer when it comes to ACE-2 binding. Upon RBD/ACE-2 binding and catalyzed by a host protease, transmembrane protease serine 2 (TMPRSS2), S is then cleaved, allowing the S2-fusion peptide to facilitate cell entry. While this process, in general, is similar to what is observed in SARS-CoV, SARS-CoV-2 is distinguished by the presence of a unique furin cleavage site proximal to the S1/S2 junction that might facilitate cell entry and thus may be responsible for the increased virulence of SARS-CoV-2 over SARS-CoV [41].
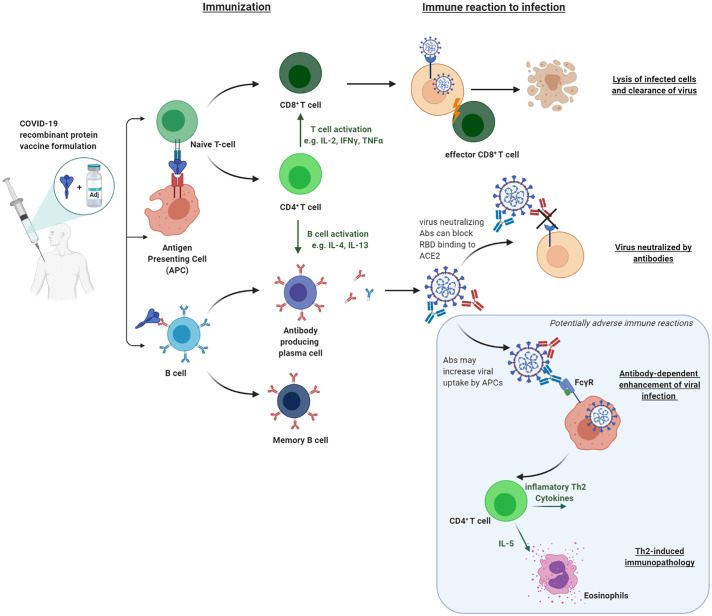
SARS-CoV-2 shares extensive sequence homology, as well as structural and functional homologies with prior coronaviruses, namely SARS-CoV, but also MERS-CoV, the causative agent of Middle East Respiratory Syndrome. Early on in the pandemic, it was shown that anti-SARS S-protein antibodies were also capable of inhibiting the binding of SARS-CoV-2 to ACE-2. These observations concentrated vaccine development on antigens derived from the spike protein [42]. While some groups focus on the whole S1 subunit as their primary vaccine antigen candidate, others are using the RBD as their main vaccine antigen. A reason for the focus on the RBD lies in observations with the homologous SARS-CoV S-protein vaccine in mice, made by Drs. Jiang and Tseng [43], who observed lung pathology in mice with the full-length S-protein as their vaccine antigen, but not with the RBD. As a possible underlying cause for this observation, antibody-dependent enhancement (ADE) is considered as a possible contributing factor. In ADE, antibodies present in vaccinated individuals facilitate the entry of virus particles into the host cell through an additional mechanism using the Fc receptor II (Fig. 1 ). In particular, non-neutralizing antibodies that do not interfere with the binding of the RBD to ACE-2 might thus increase the risk of ADE. Thus, reducing the size of the antigen to limit exposure to non-neutralizing epitopes might reduce the risk of undesired immunopathology. Notably, though, the majority of ADE data almost exclusively stems from experiments in mice and has not been unequivocally reproduced in, for example, Rhesus models for either SARS-CoV or SARS-CoV-2.
Fig. 1.
Overview of immune reactions triggered by recombinant protein vaccines and their role in protecting against COVID-19
2.1. Full-length S-protein based vaccines
The COVID-19 vaccines currently in the clinic, including the recombinant protein vaccines, use various versions of the S-protein as their vaccine antigen component. The NVX-CoV2372 trimeric nanoparticle produced by Novavax is made from the full-length S-protein (GenBank accession number, MN908947; nucleotides 21563–25384). One mutation, 682-QQAQ-685, was introduced at the S1/S2 junction to increase protease resistance, and two other mutations, K986P and V987P, were added to increase the stability of the recombinantly produced vaccine antigen [6]. [[44], [45], [46]] In an approach to increase the stability of the prefusion S-protein antigen (residues 1-1208), Medigen, with support from the NIAID, mutated the furin recognition site at the S1/S2 junction (682-RRAR-685 to GSAS) and exchanged amino acid residues K986 and V987 near the top of the central S-2P helix with two proline residues. The same mutations had also been inserted into S-2 by Wrapp et al. [47] to allow the determination of the SARS-CoV-2 structure by cryo-EM and had previously been used with Medigen’s MERS-CoV vaccine antigen [48]. In addition, a C-terminal T4 fibritin trimerization domain, an HRV3C cleavage site, an octa-histidine tag as well as a Twin-Strep96 tag were added to the wild-type sequence [49].
A recombinantly produced homotrimer of the full-length S-protein also serves as the antigen in Clover Biopharmaceuticals’ S-Trimer vaccine [11]. Using the company’s Trimer-tag platform, originally developed for cancer therapeutics, Clover has genetically fused the SARS-COV-2 S-protein (aa residues 1-1211) to human C-propeptide of alpha1(I) collagen. The fusion protein self-trimerizes and, as an added advantage, aids purification via affinity chromatography using a collagen-receptor-derived resin [50].
For Vaxine’s COVAX-19 candidate [[51], [52], [53], [54], [55], [56], [57], [58]], there is a significant amount of information about the adjuvant component of the vaccine, but details of the S-protein derived antigen [15] have not been published yet.
What the public is also reminded of now that the first front-runner clinical trials are completed though is that a clinical trial does not guarantee a successful product. At least two of the leading candidates, the University of Queensland’s Sclamp project [59] as well as Sanofi/GSK’s baculovirus produced S-protein vaccine [9] have been marred by unexpected results in Phase 1, leading to a halt or a significant delay of the project. This further reiterates the need to continue all efforts to bring additional vaccine candidates forward even if the first products have already been authorized.
2.2. RBD-based vaccines
Among those entities that focus on the RBD of the S-protein, Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd., is developing an RBD-dimer produced in mammalian cells as their vaccine antigen. In addition to expressing an RBD monomer (aa residues 319-541), two copies of the RBD-encoding gene fragment (aa residues 319-537) were cloned in tandem, leading to the expression of a 60 kDa homodimer. Based on published reports, this dimerization increased the stability of the vaccine antigen, not just for SARS-CoV-2, but also in similar SARS-CoV and MERS-CoV constructs [60]. A slightly longer RBD (aa residues 319-545) is used in the vaccine candidate from West China Hospital [25]. After the alum-adjuvanted vaccine had shown protection in non-human primates, it is now in Phase 2 clinical trials [26]. Baylor College of Medicine in collaboration with Biological E is using an antigen comprising residues 331-549 of the RBD with mutations introduced to reduce glycosylation and aggregation [18,19]. Adjuvanted with Alum and CpG, this vaccine candidate is currently in Phase 1-2 clinical trials in India [17].
For some of the vaccine development efforts, for various reasons, little public information about the nature of the vaccine antigen is available. For example, while it is known that Cuba’s Soberana 01 vaccine is based on the RBD antigen, additional details have not yet been widely published, although original news reports suggest that a combination with the proven outer membrane vesicle platform of the Cuban meningococcus B vaccine was planned [21]. AdimrSC-2f is a vaccine candidate developed by Adimmune, with the RBD antigen expressed in insect cells. The vaccine is currently in a Phase 1 clinical trial with or without aluminum as the adjuvant [16].
2.3. Multi-epitope vaccines
Many vaccine candidates in the literature employ neither the native virus's full-length S-protein or its RBD as their antigen but instead are engineered multi-epitope vaccines synthesized from peptides. Among the most advanced candidates are COVAXX’s COVID-19 vaccine, made from epitopes of the RBD, the S2 protein, as well as other SARS-CoV-2 proteins, such as membrane and nucleoprotein regions. In guinea pigs, the company reports seeing neutralizing antibody titers that exceed those in human convalescent serum by a factor of 400 [61]. Also using peptides, and based on studies with convalescent sera, Tübingen University is advancing a multi-peptide vaccine made from HLA class I and HLA-DR T-cell epitopes of the S-protein as a potential COVID-19 vaccine to induce broad T-cell immunity [32], and Vektor Labs’ (Russia) EpiVacCorona vaccine is also reportedly composed of chemically synthesized peptides of SARS-CoV-2 epitopes, conjugated to a recombinant carrier protein and adsorbed on aluminum hydroxide [28]. It should have recently completed Phase 1 trials, with no results published yet.
It will be interesting to see how the ongoing studies shift the focus between the full-length S-protein based and the RBD vaccines. The main argument in favor of the S-trimer is certainly the ambition to maintain the nature of the vaccine antigen as close to the natural confirmation as possible, while the interest in the RBD alone likely stems from concerns over adverse immune reactions triggered by full-length spike protein in SARS-CoV and also in RSV [62].
3. Protein production technologies
Over the last decades, recombinant protein technology has become efficient, relatively inexpensive, and widely available, allowing for cost-effective production of recombinant proteins in microbial and other expression host systems [63,64]. Among other advantages, since recombinant protein vaccines are non-replicating and lack any of the infectious components of an, albeit attenuated, viral particle, the vaccines are considered a safer approach compared to vaccines derived from live viruses. The technology has been tested widely and in general, these vaccines produce only very mild side-effects [65,66]. Consequently, multiple recombinant protein vaccines are now in clinical use worldwide [67].
3.1. Escherichia coli
For the production of recombinant proteins, a variety of expression platforms are now available, including microbial systems, such as Escherichia coli and various yeasts, as well as insect cells, mammalian cells, and even plants. Certainly, for non-industrial research purposes, E. coli is the most widely used system for recombinant protein production due to its rapid growth and general cost-effectivity, as well as the availability of the widest range of molecular manipulation tools. Several vaccine antigens have been produced in E. coli, including, in 1998, an FDA approved Lyme disease vaccine, which contained the recombinantly-expressed outer surface lipoprotein, OspA, from Borrelia burgdorferi. While this particular vaccine was withdrawn from the market in 2002 due to concerns over adverse side effects [68], an improved version, VLA15, likewise produced in E. coli, is now in a Phase 3 clinical trial [69,70]. Other examples of E. coli produced antigens include vaccines against meningococcal serogroup B infections; Trumenba®, developed by Pfizer, uses two variants of the meningococcal factor H-binding protein (fHBP) as antigens [71,72], while Bexsero®, developed by GSK, uses three immunogenic meningococcal antigens (fHbp, NadA, and NHBA) synthesized in E. coli [73]. These two vaccines were approved by the FDA in 2014 and 2017, respectively.
However, E. coli expression systems do not typically provide post-translational modifications (PTMs), such as glycosylation, which can affect the nature of the immune response and consequently, the functionality of the vaccine. PTMs also affect protein characteristics such as solubility and stability, and therefore it is critical to confirm correct folding and disulfide bond formation. In the case of SARS-CoV-2, depending on the product, the length of the vaccine antigen component ranges from ~200 to ~1,300 amino acids with 4-12 potential disulfide bonds [40]. Due to this complexity, it is difficult to produce these antigens properly folded in E. coli, and other production platforms are favored.
3.2. Yeasts
Yeasts are another well-known microbial expression platform. Similar to E. coli, they grow rapidly and are easy to manipulate genetically. Unlike E. coli, yeasts can secrete recombinant proteins extracellularly, which makes the downstream purification process simpler and less costly. The inclusion of certain PTMs in this eukaryotic expression system also often facilitates proper folding of the recombinant protein [74]. Several currently licensed hepatitis B vaccines, such as Engerix-B®, Recombivax HB, and HEPLISAV-B, use recombinant hepatitis B surface antigens (HBsAg) synthesized in yeast [75]. Another licensed vaccine, Gardasil®, uses the major capsid protein, L1, from four human papillomaviruses (HPV L1) as its antigens [76]. While N-linked glycosylation in yeast resembles that in higher eukaryotes, a more controlled and humanlike N-glycosylation can be achieved in specialized, genetically engineered strains of the fungus [77].
For the production of the SARS-CoV-2 spike protein, it was discovered that the epitopes which are likely to trigger a potent neutralizing antibody response, are located in the N-terminal domain (NTD, residues 1-290 of S protein) and in the RBD (residues 306-577) of the spike protein [47], where the most potent ones could block ACE-2 binding [78]. With respect to the NTD, there are eight potential N-glycosylation sites within this region [40], making it likely that different glycosylation of a potential recombinant vaccine antigen will affect the ability to trigger neutralizing antibodies within this region. However, no N-glycosylation sites are within or proximal to the ACE-2 binding site, making glycosylation much less of a concern when expressing the antigen in yeast. A yeast-expressed RBD antigen (Residues 332-549 of the spike protein) is currently being pursued by Texas Children’s Center for Vaccine Development at Baylor College of Medicine (TCH-CVD) in partnership with Biological E [18,19,79]. Formulated with CpG as an adjuvant, it entered a Phase I/II clinical trial in India in November 2020 [80]. This follows the prior production of a recombinant SARS-CoV RBD antigen in the same system; formulated with alum, this antigen-induced high neutralizing antibody titers and 100% protection in mice after viral challenge [81,82].
3.3. Mammalian cell culture expression systems
Most current COVID-19 recombinant protein vaccine candidates are expressed in mammalian cell culture-based expression systems (Table 1) that have been used to produce various biopharmaceuticals in recent years, including enzymes, antibodies, and vaccine antigens. Though more costly, mammalian systems are appreciated for their ability to express glycoproteins with their native structures and PTMs, and thus constitute the majority of the recently approved recombinant biologics [83]. A successful example for this class of vaccines is Shingrix®, the herpes zoster vaccine manufactured by GSK, which uses Chinese Hamster Ovary (CHO) cells to produce recombinant glycoprotein E from the virus as its antigen [84].
3.4. Insect cells
COVID-19 subunit vaccine candidates, like those from Novavax, Sanofi and Adimmune are produced in a system that uses a baculovirus vector and insect cells as hosts. This system was first developed in 1983 [85] and has since been used for several recombinant proteins [86,87]. Currently, there are two licensed vaccines in the USA, utilizing insect cell-expressed antigens; Cervarix®, an HPV vaccine that uses the recombinant HPV L1 antigen [60], and Flublok®, an influenza vaccine using a recombinant trivalent hemagglutinin antigen [75]. When compared to E. coli or yeast, the required growth medium is more costly and the cell growth rate is slower, but insect cells can reach higher densities in a shorter period when compared to mammalian cells [88,89]. Additionally, like mammalian cells, insect-cell expressed recombinant proteins are usually well-folded, soluble, and often contain the desired PTMs. However, even though this system does not cause hyperglycosylation, N-glycosylation by baculovirus-infected insect cells is not equivalent to those of higher eukaryotes [90], and thus, if sophisticated glycans are required to maintain the function of a recombinant protein, this system may not be the optimal option.
In addition to traditional vaccine manufacturing platforms, alternative expression systems are also being used to produce vaccine antigens. Kentucky BioProcessing and other tobacco growers, for example, are employing tobacco plants to express SARS-CoV-2 vaccine antigens [91]. While the manufacturing of recombinant proteins in tobacco is a proven technology [[92], [93], [94], [95]], controlling cost at the pandemic scale might reserve this expression system to those with access to the necessary capacity.
Generally speaking, for any expression system, production cost will vary depending on the production yield, but based on the general cost comparison analyzed by Owczark et al. [96], and the example retail pricing for a few biopharmaceuticals [64], E. coli is the least expensive choice for protein production, and while mammalian cells are the most expensive option, the production cost for insect cells and yeasts is generally somewhere in between.
4. Adjuvants
Recombinant proteins by themselves generally elicit only a weak immune response, unless they are assembled into larger particles [97]. To augment the immune response and allow for antigen dose sparing, most protein-based COVID-19 vaccines are formulated in combination with adjuvants (Table 2 ). The addition of these immunostimulants can trigger specific cell receptors and induce an innate immune response at the site of injection and in the draining lymph nodes. The innate immune response to the adjuvants then further activates the adaptive immune system by mobilizing antigen-presenting cells (APCs), thus improving antigen presentation to CD4 T helper cells. Depending on the phenotype, the activated T helper cell will stimulate the proliferation of antigen-specific antibody-producing B cells or CD8+ T cells (Fig. 1).
Table 2.
List of adjuvants used in recombinant protein COVID-19 vaccine candidates currently tested in the clinic.
| Name | Components | Receptor/pathway | Disease target tested in the clinic |
|---|---|---|---|
| Aluma | Aluminum salts (aluminum hydroxide, aluminum phosphate) |
NLRP3 uric acid, DNA | Anthraxa, Diphtheriaa, Tetanusa, Pneumococcusa, hepatitis Aa Hepatitis Ba, Japanese Encephalitisa, Meningococcal Ba and Ca, human papillomavirusa, SARS, COVID-19 |
| MF59a, AS03a | Oil-in-water emulsion squalene oil plus surfactants |
MyD88, ASC, ATP | Influenzaa, COVID-19 |
| CpG 1018a | Synthetic DNA alone or formulated with Alum | TLR9 | Hepatitis Ba, Malaria, Influenza, Anthrax, Cancer, COVID-19 |
| Matrix M/IscoMatrix | Saponin | Unknown | Hepatitis C, Influenza, HSV, human papillomavirus, Malaria, Cancer, COVID-19 |
| Advax | polysaccharide particle made from delta inulin | Unknown | HIV, Influenza, Hepatitis B, COVID-19 |
Adjuvants in licensed vaccines in the USA.
To protect against COVID-19, high levels of neutralizing antibodies to the spike protein of SARS-CoV-2 are essential. However, similarly to antibody levels in patients that have recovered from SARS-CoV, SARS-CoV-2 antibody responses seem to wane rapidly within months after infection. In addition, while less severe cases of SARS were associated with accelerated induction of a Th1-type immune response, Th2 cell responses have been associated with enhancement of lung disease following infection in mice parenterally vaccinated with inactivated SARS-CoV viral vaccines. Therefore, the FDA specifically stated in their guidelines to the industry from earlier this year that COVID-19 vaccine candidates should preferably elicit a strong Th1-skewed CD4 T cell response, in addition to the induction of high levels of neutralizing antibodies [98].
We here provide an overview of the vaccine adjuvants that have been formulated in reported COVID-19 protein vaccine candidates.
4.1. Aluminum hydroxide (alum)
Semi-crystalline suspensions of aluminum are the most commonly used adjuvants in vaccine development worldwide [99]. The aluminum salts have a high binding capacity and typically will adsorb the antigens on their surface. Although hundreds of millions of people have been vaccinated with aluminum-based vaccines, there is still discussion on the exact mechanism of action. The most widely accepted explanations include a possible depot effect, enhancement of phagocytosis of the antigen, and activation of the pro-inflammatory NLRP3 pathway [100]. Aluminum-based formulations generally induce a strong humoral immune response in combination with the secretion of Th2-biased cytokines (e.g., IL-4, IL-6, IL-10). While some studies found that candidate SARS-CoV vaccines formulated with aluminum induced specific Th2-biased responses and possibly induced lung eosinophilic immunopathology in mice [101], other studies found no direct evidence linking aluminum to enhanced eosinophilia [82]. The true cause of the undesired immune response remains under discussion [62,102]. Nonetheless, since the FDA guided the industry toward a Th1 immune response, most COVID-19 recombinant protein vaccine formulations that are formulated with aluminum hydroxide (alum) include a second adjuvant, such as CpG, in order to balance the immune response and also stimulate proliferation of Th1 type CD4(+) cells. Alum has an excellent safety record and can be produced at a relatively low cost, making it an ideal COVID-19 vaccine adjuvant for global health [102,103].
4.2. MF59
MF59® is an oil-in-water emulsion developed by Novartis. The adjuvant contains squalene oil and two surfactants, Tween-80 and Span-85, emulsified in a citric acid buffer [104]. MF59 has been deemed safe and is well-tolerated in humans and MF59-adjuvanted vaccines have been approved for pandemic and seasonal influenza in over 38 countries worldwide [105]. For example, Fluad®, an MF59-adjuvanted seasonal influenza vaccine, has been licensed since 1997. However, in the United States, FLUAD and FLUAD Quadrivalent are licensed only for persons over the age of 65 years [106]. MF59 was also added to vaccines against the pandemic flu strain H1N1 (Focetria® and Celtura®). Within oil-in-water formulations, the antigen remains typically in the water phase and does not interact with the oil droplets. It provides neither direct transport nor depot effect for the antigen. Antigens and MF59 are taken up by neutrophils and monocytes, and later followed by dendritic cells (DCs) and B cells, and moved to draining lymph nodes [107]. MF59 effects the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and stimulates IL-4 and Stat-6 signaling, while being independent of any type-1 interferon or inflammasome pathways. The emulsion has further been shown to significantly increase the IL-5 and IL-6 levels [108]. MF59 has been selected as a COVID-19 vaccine adjuvant because it was proven to induce fast priming of antigen-specific CD4(+) T-cell responses, and to induce strong and long-lasting memory T- and B-cell responses, and to overall broaden the immune response against the vaccine antigens [109].
4.3. CpG
CpG adjuvants are synthetic DNA sequences containing unmethylated CpG sequences. These oligonucleotides are potent stimulators of the innate immune system through activation of Toll-like receptor-9. TLR9 agonists directly induce the activation and maturation of plasmacytoid dendritic cells and enhance differentiation of B cells into antibody-secreting plasma cells [110]. As a vaccine adjuvant, CpG augments the induction of vaccine-specific cellular and humoral responses. Dynavax Technologies has developed a short CpG-containing oligonucleotide sequence named CpG 1018 and progressed it through clinical testing as an adjuvant for immunization against hepatitis B virus (HEPLISAV-B) [111]. The immunostimulatory effects of CpG are optimized by keeping the oligonucleotide and the vaccine antigen in close proximity. Driven by electrostatic interaction, CpG 1018 binds well to aluminum hydroxide and is therefore well-suited for co-formulation with aluminum-based subunit vaccines [11].
4.4. AS03
AS03 is a squalene-based oil-in-water emulsion produced by GSK. It has been tested extensively in the clinic and is used for the H1N1 pandemic flu vaccine Pandemrix [112]. It is also in Arepanrix and the new Q-pan for H5N1 influenza [113]. Similar to MF59, AS03 can induce proinflammatory cytokines and chemokines, including CXCL10, but independently of type-1 interferon. This pro-inflammatory response is associated with improved recruitment, activation, and maturation of antigen-presenting cells at the injection site [114].
4.5. Matrix-M
Novavax’s proprietary Matrix-M adjuvant consists of two individually nanosized particles, made with a different saponin fraction (Fraction-A and Fraction-C). The saponin particles are stabilized with cholesterol and phospholipid [115]. As a part of different vaccine formulations, Matrix-M has been proven to augment both Th1 and Th2 type responses, inducing high levels of neutralizing antibodies, and enhancing immune cell trafficking [116,117]. Based on clinical data from over a dozen studies Matrix-M is considered safe and potent [[118], [119], [120], [121], [122]], however, it has not yet been part of a commercially available vaccine.
4.6. Advax
Advax made by Vaxine (Australia) is a microcrystalline polysaccharide particle composed of delta inulin [55]. In published studies covering many years of research, delta inulin has been shown to provide a robust humoral and cellular immune response when formulated with recombinant vaccine antigens. Advax adjuvant has recently also successfully been tested in several human trials including vaccine studies to prevent seasonal and pandemic influenza, hepatitis B, and hyperallergic reactions to insect venom [123]. Compared to the controls, the Advax adjuvant [56,123] seems to improve antibody and T-cell responses, while being safe and well-tolerated [54]. It should however be noted that Advax is still a relatively new adjuvant, which has only been tested on small groups of patients and has not yet been part of a marketed vaccine.
4.7. Assessment of the adjuvant landscape for COVID-19 vaccines and outlook for novel adjuvant systems
Potential vaccines against the coronavirus should induce a strong humoral response with high neutralizing antibody titers, in combination with a Th1-type cellular response [124]. There has also been discussion on the role of Th17 induced by an IL-6 inflammatory response, as this seems to contribute to severe lung pathology and mortality [125]. Except for alum, all adjuvants listed above have the potential to induce an antigen-specific, safe, and adequate Th1-type immune response when added to a SARS-CoV-2 recombinant vaccine formulation. Aluminum hydroxide still has value as part of a vaccine formulation, as it is known to promote a stronger humoral response; it will, however, require an additional immunostimulant to skew the cellular response towards Th1.
In addition to the adjuvant combinations currently evaluated for COVID-19, other experimental adjuvants might be beneficial for a vaccine against SARS-CoV-2 based on earlier coronavirus research studies. For a detailed overview of adjuvants for coronaviruses, we refer to recent reviews by Liang [120] and Gupta [121].
Aside from the functional aspect of the adjuvants, production capacity and costs are of key importance to guarantee vaccine availability to citizens of all nations, independent of their status or wealth. From that perspective, protein-based adjuvants may offer a significant advantage. They can be made using the same technologies used to produce the vaccine antigen (Section 3) and can easily be scaled up at a low cost. One example is rOv-ASP-1, a helminth-derived molecule that has been shown to induce potent Th1-type immune responses when added to influenza, helminth, and rabies vaccines [[126], [127], [128], [129]]. Another example is dmLT or LT(R192G/L211A), an ADP-ribosylating enterotoxin from Escherichia coli. What distinguishes dmLT is that it enhances antigen-specific IgA antibodies and long-lasting memory to co-administered antigens, in addition to an increase in Th1, Th2, Th17, cytotoxic T lymphocytes (CTLs), and antigen-specific antibodies [130,131]. Secretory IgA can act as an immune barrier and neutralize SARS-CoV-2 before it reaches and binds to epithelial cells [132]. Therefore, dmLT may be an excellent choice to add to any mucosal or parental vaccine targeting SARS-CoV-2 RBD.
5. Vaccine delivery
5.1. Parenteral vaccination
COVID-19 subunit vaccine candidates currently at an advanced clinical stage of development are being administered either by intramuscular (i.m.) or subcutaneous (s.c.) injection and while some novel vaccine platforms require specialized administration equipment (e.g., electroporation devices), protein-based vaccines can be administered using conventional low-cost hypodermic needles. Intradermal (i.d.) immunizations might be able to generate a stronger immune response [133] since the dermis contains higher numbers of dendritic cells, which will facilitate the uptake of antigens. Local inflammation in the dermis induces the maturation of the dendritic cells and stimulates migration into draining lymph nodes [134]. However, i.d. needle injections are technically complex and allow for only small volumes to be administered. Therefore, alternative delivery systems for i.d. injection of recombinant protein subunits are being developed. For example, Kim et al. have published on an intradermal system [135] comprising of two antigens derived from SARS-CoV-2 spike proteins, rSARS-CoV-2 S1, and rSARS-CoV-2-S1fRS09 that are delivered using a Micro-Needle Array (MNA) technology. This vaccine triggered substantial antigen-specific antibodies in mice when dosing low amounts of antigen.
5.2. Mucosal vaccination
Wang et al. [136] have conceived a strategy to produce an oral vaccine based on the SARS-CoV-2 spike protein. Oral vaccines promise to be particularly suitable for low-and middle-income countries since they can be administered without trained personnel and can be transported and stored without requiring a cold chain. In addition, the vaccine designed in this study in the benign probiotic bacterium Lactobacillus plantarum is expected to specifically trigger an enhanced mucosal immune response, desirable for preventing viral respiratory infections such as COVID-19. In their study, the authors cloned the full-length spike protein from strain Wuhan-Hu-1 and confirmed its expression on the bacterial cell surface by Western Blotting. While further evaluations of safety, immunogenicity, and functionality of the vaccine candidate are pending, the authors have shown that that the functionalized bacteria displaying the spike protein were stable in a high temperature, low pH environment as found in the digestive system.
In addition, a first Phase 1 clinical trial of an oral COVID-19 vaccine tablet, containing an adenovirus vector expressing the spike protein was started by Vaxart Inc. on October 13, 2020 [137,138]. Merck, another major player in the vaccine realm, also reports that it is looking at testing an oral COVID-19 vaccine in the clinic [139].
Intranasal vaccination for COVID-19 has also been investigated by many groups, mostly with live attenuated flu viruses that are genetically modified to express the spike protein. These viral mimickers can infect cells in the mucosal layer of the nose through the ACE-2 receptors and induce protection by producing high levels of both mucosal and systemic antibodies as well as by cell-mediated immunity. Approval was granted in China on September 9, 2020, to Hong Kong University, Xiamen University, and Wantai Biological Pharmacy Enterprise Co. Ltd, to initiate the first intranasal Phase I clinical trial for COVID-19 [140,141]. Elsewhere, Coroflu (University of Wisconsin-Madison, FluGen, Bharat Biotech) and Altimmune are developing intranasal COVID-19 vaccine candidates using similar viral platforms, however, to date, no data has been published on any recombinant protein nasal vaccine [142,143].
5.3. Outlook for alternative vaccine delivery systems
While the pandemic has certainly catalyzed a technology boost, advanced drug delivery platforms will need additional funding and time for research and clinical testing before they can be considered for mass vaccination. Undoubtedly, novel drug delivery systems promise advantages in the future; for example, controlled-release nanotechnology to mimic repeated immunizations may allow for single-dose administration of a vaccine [144]. Based on current clinical data, most of the COVID-19 vaccine candidates will require a boost injection to increase the cell-mediated immune response, induce memory cells, and sustain high titers of neutralizing antibodies. This doubles the need for vaccine supply and it involves detailed patient follow up and logistical oversight. A dose release mechanism can allow for fine manipulation of antigen delivery rate and presentation as well as host immune cell response. Currently, multiple novel biomaterials-based solutions are being evaluated including nanoparticles, liposomes, scaffolds, and microneedles [144,145].
Alternative vaccine delivery systems may in the future also be able to support more desirable routes of administration that may improve the immune response and offer significant dose sparing. Delivery through less invasive routes offers also reduces the need for specifically trained medical personnel and possibly even allows for self-administration. Vaccine hesitancy caused by fear of needles may also be lowered [146].
Alternative delivery vehicles can further have a positive impact on vaccine stability. Aluminum salt adjuvants, for instance, can either stabilize or destabilize proteins, depending on their interaction with the protein [147,148]. In another example, Chitosan–alginate nanoparticles have been found to stabilize proteins [149].
Another new approach, virus-like particles, represents an innovative delivery technology to further boost the immune response. For example, virosome-based vaccines cause an enhanced interaction between the spike protein antigen and the immune system, potentially making them more efficient in protecting the elderly and other at-risk populations. Virosomes are made from reconstituted influenza virus envelope proteins that retain the cell binding and membrane fusion properties of the native virus [150,151]. The haemagglutinin proteins on the virosomal surface mediate increased interaction of the antigen with immunoglobulin receptors on B cells, stimulating stronger antibody responses. In addition, virosomes also mediate more efficient interaction with antigen-presenting cells, triggering enhanced activation of T lymphocytes. SARS-CoV-2 virosome-based vaccines are currently being evaluated in pre-clinical studies [152].
6. Status of COVID-19 vaccine development
According to the clinical trials database (clinicaltrials.gov) and the WHO, there are currently more than ten subunit vaccine candidates in clinical trials and over 50 at the pre-clinical stage. In this review, we will briefly summarize selected information about some of those pre-clinical and clinical recombinant protein vaccines that appear to be most advanced. We note that this is a fast-moving field, so it is understood that by the time this review is published, new data will likely have been made available, including from groups that have yet to show results.
6.1. Clinical stage
6.1.1. Novavax
In its Phase 1/2 study, Novavax’s NVX-CoV2373 vaccine, formulated with Matrix-M, elicited a Th1-biased immune response with two injections on day 0 and day 21 of two different protein doses (5 and 25 μg) [6]. Additionally, both 5 and 25 μg doses of antigen were able to induce high neutralizing antibody titers (IC99 = 3906 and 3309, respectively), which exceeded those seen in human convalescent serum (IC99 = 983) was observed [6]. This immunogenicity profile fulfilled the FDA guideline for an ideal COVID-19 vaccine candidate [98]. In addition, no serious adverse effects were reported. Novavax has initiated a Phase 3 clinical trial in the UK [153] and is continuing Phase 2/3 studies in Australia and the US, as well as a Phase 2b trial in South Africa that will also include adults infected with HIV [154]. To prepare for global distribution, Novavax has made manufacturing agreements with multiple manufacturers including Emergent, Fujifilm, AGC Biologics, and the Serum Institute of India to produce 2 billion doses annually, with production slated to start in 2021 [155].
6.1.2. Clover biopharmaceuticals
Clover Biopharmaceuticals’ vaccine candidate, SCB-2019, was shown to trigger a robust immune response in their non-human primate study. In the study, 30 μg of the S-trimer adjuvanted with either AS03 or CpG1018/alum were used to immunize Rhesus macaques on Day 0 and Day 21. On day 35, neutralizing antibody titers in the AS03-adjuvanted S-Trimer group (IC50 = 20,234) were significantly higher than CpG 1018 plus alum group (IC50 = 11,682), however, the lymphocyte response seems to sustain in the CpG 1018 plus alum group longer [11]. Clover biopharmaceuticals have partnered with GlaxoSmithKline to produce the vaccine for the current Phase 1 study [156] and have formed an advisory board for global vaccine development and access [157].
6.1.3. West China Hospital
West China Hospital in collaboration with Sichuan University is developing an insect cell-based RBD vaccine and has so far published its testing in Rhesus macaques. By formulating 20 or 40 μg RBD with alum, and using two injections on days 0 and 7, the vaccine was triggered neutralizing antibody titers of approximately 100 on the 35th-day post-vaccination. While this neutralizing antibody titer appears to be on the lower range of published SARS-CoV-2 vaccines, we acknowledge that there is still no unified way to determine neutralizing antibody titers across different laboratories. West China Hospital also reports that vaccinated non-human primates were protected against viral challenge, and with this encouraging data, the vaccine is currently in a phase I clinical trial [158].
6.1.4. Biological E/Baylor College of Medicine
The RBD-based vaccine developed by Biological E and Texas Children’s Hospital Center for Vaccine Development at Baylor College of Medicine (TCH-CVD) is the first yeast-expressed COVID-19 vaccine that entered clinical trials. Immunogenicity and functionality are being tested in mouse and non-human primate models. After introducing two modifications into the wild-type RBD, the candidate antigen, RBD219-N1C1, not only showed improved production yield and stability but also maintained functionality and immunogenicity [18,19]. The vaccine induced high levels of antigen-specific IgG antibodies and elicited strong neutralizing antibody titers (IC50= 103-104). The alum-formulated vaccine induced a Th2-bias immune response with higher levels of secreted IFN-γ, IL-6, and IL-10 [18]. In the current clinical trial, CpG1018 is being introduced to this alum formulation to induce a more Th1/Th2 balanced immune response [17,80].
6.1.5. Medigen Vaccine Biologics Corporation/NIAID/Dynavax
Albeit without any published efficacy data yet, Medigen’s vaccine candidate, a CHO-cell expressed spike protein (S-2P), has been shown to elicit high neutralizing titers in mice after formulation with alum and CpG-1018. The sera from mice immunized with S-2P adjuvanted with alum and CpG-1018 demonstrated a higher neutralizing ability against SARS-CoV-2 (ID50= 1,500) than the alum-alone formulation [13].
6.2. Pre-clinical Stage
6.2.1. Indian Institute of Science, Bangalore (IISB)/Mynvax
An India-based startup vaccine developer incubated by IISB has developed an RBD-based vaccine candidate (residues 332-532 of S protein) expressed in a HEK293 mammalian cell line. The vaccine candidate was highly heat-resistant in lyophilized form and maintained functionality at 70°C for 16 hours, 100°C for 90 minutes, and 37°C for four weeks. When guinea pigs were immunized with two doses of AddaVax-formulated RBD, high RBD-specific antibody titers (6,400-102,400), and neutralizing antibody titers (NT100 = 160-1,280) were observed [159].
6.2.2. AdaptVac/ExpreS2ion/Bavarian Nordic/Copenhagen University/AGC Biologics
AdaptVac, the developer of capsid virus-like particles (cVLP), has previously demonstrated the ability to boost long-lasting protection in mice for their flu vaccine with just one dose [160]. By joining venture with ExpreS2ion, Bavarian Nordic, and Copenhagen University and incorporating cVLP with the S protein, their vaccine induced high levels of neutralizing antibodies in mice [161] and non-human primates even after one dose [162]. This vaccine has been manufactured by AGC Biologics and is being analyzed for its quality before a clinical trial, expected for Q1 of 2021 [162].
6.2.3. Heat biologics/University of Miami
Heat biologics use cells that express a cellular heat shock chaperone protein, glycoprotein 96 (gp96), fused with the IgG1 Fc domain (gp69-Ig) as a vaccine platform. Vaccines using this platform technology have been shown to elicit potent, antigen-specific CD8+T-cells against several infectious diseases and tumors in animal models [[163], [164], [165]]. Their COVID-19 vaccine is composed of a HEK293 or human lung adenocarcinoma cell lines (AD100) cell line that was transfected with plasmids encoding gp96-Ig and the SARS-CoV-2 S protein. In mice, the vaccine induced tissue-resident memory T (TRM) cells, capable of rapidly responding to infection in the tissue. It also elicited CD4+ and CD8+T cell responses that are specific to the S-protein epitopes in the lung interstitium and the airways [166]. Unlike other COVID-19 vaccines, this vaccine technology provided an approach to induce the desired cellular response directly in the target tissues.
7. Challenges and perspectives
In an earlier assessment of Operation Warp Speed, Moore and Klasse noted that while recombinant proteins are “by far the most immunogenic vaccine candidates for antibody responses”, they were not included in the first wave of vaccine candidates [167]. DNA and mRNA vaccines inactivated viruses, as well as vector-based strategies, were able to attract more attention (and funding). Early on, these platforms offered a faster time to the clinic and the ability to produce the necessary quantities of vaccine. In the meantime, of course, a recombinant protein antigen-based vaccine has been added to the government-supported OWS portfolio and initial data from human clinical trials has begun to enter the public domain, and the first vaccines will have been administered by mid-December 2020. How these first-generation vaccines will perform in the long-term, and whether some of these new vaccine technologies will be received well by an increasingly vaccine-hesitant public remains to be seen [168].
While arguably requiring more time for development and production, recombinant protein vaccines do offer distinct advantages over the mRNA and viral-vector vaccines [169]. First and foremost, they have a history of triggering a safe and robust immune response. Second, they are much less demanding when it comes to production, storage, and transportation. This is of paramount importance for transferring this proven vaccine technology to low- and middle-income countries. There, facilities to reproduce the new production platforms (e.g. mRNA vaccines) are unavailable [103], and establishing the infrastructure to distribute fastidious nucleic acid vaccines, with storage and transportation required at -94° F [170], is out of reach. Third, while viral-vector vaccines have been clinically successful as evidenced by, for example, the recent rVSV-ZEBOV vaccine against Ebola [171], concerns remain about how exposure to the viral vector backbone could impact the robustness of the immune response [172], or may even impose limitations on the ability to use the same viral vector platform for any possible booster vaccinations [173]. A recombinant protein vaccine does not carry that risk and may even be an ideal complement to elicit a more desired and prolonged immune response in a prime-boost approach.
Data from the first clinical studies with protein vaccine front-runners are highly encouraging. In the absence of a standardized virus neutralization assay and considering the fact that ELISAs are run using varying protocols, the absolute numbers need to be reviewed cautiously, but the NVX-CoV2373 recombinant protein vaccine showed high titers of total as well as neutralizing antibodies (Table 3 ).
Table 3.
Reported neutralizing antibody titers for a selection of COVID-19 vaccines that have been tested in Phase 1 and Phase 1-2 human clinical trials.
| Vaccine candidate | Category | Doses | Neutralizing AB titersa | Ref. |
|---|---|---|---|---|
| ChAdOx1 nCoV-19 | Vectored Vaccine | 2 | Ic50: 451b | [174] |
| Ad26.COV2.S | Vectored Vaccine | 1 | Ic50: 243c | [175] |
| mRNA-1273 | RNA | 2 | Ic50: 374d | [176] |
| BNT162b2 | RNA | 2 | Ic50: 363e | [177] |
| NVX-CoV2373 | Recombinant Protein | 2 | Ic99: 3,906f | [6] |
Highest reported value in the referenced publication.
50% neutralization titer, 5x109 viral particles, 42 days post first vaccination.
50% neutralization titer, 1x1011 viral particles, 29 days post first vaccination.
Ic50, 250 μg, 36 days post first vaccination.
50% neutralization titer, 20 μg RNA vaccine, 28 days post first vaccination.
Wild-type SARS-CoV-2 microneutralization, inhibitory concentration greater than 99% (MN IC>99%) titer response, 5 μg adjuvanted protein, 35 days post first vaccination.
So, as we continue to struggle to contain the pandemic, and as there is news about the first-generation COVID-19 vaccine candidates, concerns and questions remain, be it about the ability to supply the vaccine to everybody who needs it, or about the vaccine’s manufacture and its trial performance [178], recombinant protein vaccines so far check all the marks for offering a relevant and urgently needed contribution to control COVID-19 in the long-term, in the U.S. and elsewhere.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Figures were created using Biorender.com.
References
- 1.Rainsford S. BBC; 2020. Coronavirus: Sputnik V vaccine rushed out to wary Russians. [Google Scholar]
- 2.McGregor G. China drugmaker fact-checks claim that its leading COVID vaccine is 97% effective. Fortune. 2020 https://fortune.com/2020/12/08/china-covid-vaccine-sinovac-efficacy-seroconversion-rate-pfizer-moderna/ [Google Scholar]
- 3.Mueller B. The New York Times; 2020. As U.K. Begins Vaccinations, A Glimpse of Life After Covid. [Google Scholar]
- 4.Dwyer C. NPR; 2020. Canada Authorizes Use Of Pfizer COVID-19 Vaccine. [Google Scholar]
- 5.WHO . 2020. DRAFT Landscape of COVID-19 Candidate Vaccines. [Google Scholar]
- 6.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., Smith G., Patel N., Frieman M.B., Haupt R.E., Logue J., McGrath M., Weston S., Piedra P.A., Desai C., Callahan K., Lewis M., Price-Abbott P., Formica N., Shinde V., Fries L., Lickliter J.D., Griffin P., Wilkinson B., Glenn G.M. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinicaltrials_gov . 2020. A Study Looking at the Efficacy, Immune Response, and Safety of a COVID-19 Vaccine in Adults at Risk for SARS-CoV-2. [Google Scholar]
- 8.Clinicaltrials_gov . 2020. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS Nanoparticle Vaccine With/Without Matrix-M Adjuvant. [Google Scholar]
- 9.Dalton M., Walker J. Sanofi-GSK covid-19 vaccine is set back by lab mistake. Wall Street J. 2020 https://www.wsj.com/articles/broader-vaccine-plan-in-west-dealt-setback-by-sanofi-gsk-delay-11607696740 [Google Scholar]
- 10.Clinicaltrials_gov . 2020. SCB-2019 as COVID-19 Vaccine. [Google Scholar]
- 11.Liang J.G., Su D., Song T.Z., Zeng Y., HUang W., Wu J., Xu R., Luo P., Yang X.L., Zhang X., Luo S., Liang Y., Li X., Huang J., Wang Q., Huang X., Xu Q., Luo M., Huang A., Luo D., Zhao C., Yang F., Han J.B., Zheng Y.T., Liang P. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. bioRxiv. 2020 doi: 10.1101/2020.09.24.311027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinicaltrials_gov . 2020. A Study to Evaluate the Safety and Immunogenicity of MVC-COV1901 Against COVID-19. [Google Scholar]
- 13.Kuo T.Y., Lin M.Y., Coffman R.L., Campbell J.D., Traquina P., Lin Y.J., Liu L.T., Cheng J., Wu Y.C., Wu C.C., Tang W.H., Huang C.G., Tsao K.C., Chen C. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-77077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinicaltrials_gov . 2020. Monovalent Recombinant COVID19 Vaccine (COVAX19) [Google Scholar]
- 15.Genengnews . 2020. Vaxine and Medytox – COVAX-19®. [Google Scholar]
- 16.Clinicaltrials_gov . 2020. A Study to Evaluate the Safety and Immunogenicity of COVID-19 (AdimrSC-2f) Vaccine. [Google Scholar]
- 17.CTRI . 2020. Biological E’s Novel Covid-19 Vaccine of SARS-CoV-2 for Protection Against Covid-19 Disease. [Google Scholar]
- 18.Pollet J., Chen W.H., Versteeg L., Keegan B., Zhan B., Wei J., Liu Z., Lee J., Kundu R., Adhikari R., Poveda C., Mondragon M.V., de Araujo Leao A.C., Rivera J.A., Gillespie P.M., Strych U., Hotez P.J., Bottazzi M.E. SARS-CoV-2 RBD219-N1C1: a yeast-expressed SARS-CoV-2 recombinant receptor-binding domain candidate vaccine stimulates virus neutralizing antibodies and T-cell immunity in mice. bioRxiv. 2020 doi: 10.1101/2020.11.04.367359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W.H., Wei J., Kundu R., Adhikari R., Liu Z., Lee J., Versteeg L., Poveda C., Keegan B., Villar M.J., Araujo-Leao A.C., Rivera J.A., Gillespie P.M., Pollet J., Strych U., Zhan B., Hotez P.J., Bottazzi M.E. Cloning, expression and biophysical characterization of a yeast-expressed recombinant SARS-CoV-2 receptor binding domain COVID-19 vaccine candidate. bioRxiv. 2020 doi: 10.1101/2020.11.09.373449v1. [DOI] [Google Scholar]
- 20.RPCEC . 2020. Soberano 01 - Estudio Fase I/II, aleatorizado, controlado, adaptativo, a doble ciego y multicéntrico para evaluar la seguridad, reactogenicidad e inmunogenicidad del Candidato Vacunal profiláctico FINLAY- FR-1 anti SARS – CoV – 2 en un esquema de dos dosis. (COVID-19) [Google Scholar]
- 21.Morales Y.C., Montero A.R. 2020. Soberana is Cuba’s, The First Candidate Vaccine Against COVID-19 in Latin America and the Caribbean, Granma - The OFFICIAL VOICE OF THE COMMUNIST PARTY OF CUBA CENTRAL COMMITTEE. [Google Scholar]
- 22.Clinicaltrials_gov . 2020. KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. [Google Scholar]
- 23.Clinicaltrials_gov . 2020. Clinical Study of Recombinant Novel Coronavirus Vaccine. [Google Scholar]
- 24.CHICTR . 2020. A Randomized, Double-blind, Placebo-controlled Phase III Clinical Trial of the Effectiveness and Safety of Inoculation of Recombinant New Coronavirus Vaccine (CHO cells) in the Prevention of COVID-19 in People 18 Years and Older. [Google Scholar]
- 25.Yang J., Wang W., Chen Z., Lu S., Yang F., Bi Z., Bao L., Mo F., Li X., Huang Y., Hong W., Yang Y., Zhao Y., Ye F., Lin S., Deng W., Chen H., Lei H., Zhang Z., Luo M., Gao H., Zheng Y., Gong Y., Jiang X., Xu Y., Lv Q., Li D., Wang M., Li F., Wang S., Wang G., Yu P., Qu Y., Yang L., Deng H., Tong A., Li J., Wang Z., Yang J., Shen G., Zhao Z., Li Y., Luo J., Liu H., Yu W., Yang M., Xu J., Wang J., Li H., Wang H., Kuang D., Lin P., Hu Z., Guo W., Cheng W., He Y., Song X., Chen C., Xue Z., Yao S., Chen L., Ma X., Chen S., Gou M., Huang W., Wang Y., Fan C., Tian Z., Shi M., Wang F.S., Dai L., Wu M., Li G., Wang G., Peng Y., Qian Z., Huang C., Lau J.Y., Yang Z., Wei Y., Cen X., Peng X., Qin C., Zhang K., Lu G., Wei X. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586(7830):572–577. doi: 10.1038/s41586-020-2599-8. [DOI] [PubMed] [Google Scholar]
- 26.CHICTR . 2020. Recombinant COVID-19 Vaccine (Sf9 cells) Phase II Clinical Trial. [Google Scholar]
- 27.CHICTR . 2020. Randomized Double Blind, Placebo Controlled Phase I Trial for Anti Novel Coronavirus Pneumonia (COVID-19) Recombinant Vaccine (Sf9) [Google Scholar]
- 28.Genetic_Engineering&Biotechnology_News . 2020. COVAXX – UB-612. [Google Scholar]
- 29.Clinicaltrials_gov . 2020. A Study to Evaluate the Safety, Tolerability, and Immunogenicity of UB-612 COVID-19 Vaccine. [Google Scholar]
- 30.Clinicaltrials_gov . 2020. Study of the Safety, Reactogenicity and Immunogenicity of “EpiVacCorona” Vaccine for the Prevention of COVID-19 (EpiVacCorona) [Google Scholar]
- 31.Clinicaltrials_gov . 2020. Safety and Immunogenicity Trial of Multi-peptide Vaccination to Prevent COVID-19 Infection in Adults (pVAC) [Google Scholar]
- 32.Nelde A., Bilich T., Heitmann J.S., Maringer Y., Salih H.R., Roerden M., Lubke M., Bauer J., Rieth J., Wacker M., Peter A., Horber S., Traenkle B., Kaiser P.D., Rothbauer U., Becker M., Junker D., Krause G., Strengert M., Schneiderhan-Marra N., Templin M.F., Joos T.O., Kowalewski D.J., Stos-Zweifel V., Fehr M., Rabsteyn A., Mirakaj V., Karbach J., Jager E., Graf M., Gruber L.C., Rachfalski D., Preuss B., Hagelstein I., Marklin M., Bakchoul T., Gouttefangeas C., Kohlbacher O., Klein R., Stevanovic S., Rammensee H.G., Walz J.S. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2020;22(1):74–85. doi: 10.1038/s41590-020-00808-x. [DOI] [PubMed] [Google Scholar]
- 33.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y.W., Schmitz J.E., Persing D.H., Stratton C.W. Laboratory diagnosis of COVID-19: current issues and challenges. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D., Yazdanpanah Y., Le Hingrat Q., Descamps D., Houhou-Fidouh N., Reusken C., Bosch B.J., Drosten C., Koopmans M.P.G., Haagmans B.L. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo L., Ren L., Yang S., Xiao M., Chang F. Yang, Dela Cruz C.S., Wang Y., Wu C., Xiao Y., Zhang L., Han L., Dang S., Xu Y., Yang Q.W., Xu S.Y., Zhu H.D., Xu Y.C., Jin Q., Sharma L., Wang L., Wang J. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S., Kase R., Sekiguchi S., Morita K., Hishima T., Suzuki H., Karamatsu K., Yasutomi Y., Shida H., Kidokoro M., Mizuno K., Matsushima K., Kohara M. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J. Immunol. 2008;181:6337–6348. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 38.Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. bioRxiv. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia S., Lan Q., Su S., Wang X., Xu W., Liu Z., Zhu Y., Wang Q., Lu L., Jiang S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Trans. Targ. Therapy. 2020;5:92. doi: 10.1038/s41392-020-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvatori G., Luberto L., Maffei M., Aurisicchio L., Roscilli G., Palombo F., Marra E. SARS-CoV-2 SPIKE PROTEIN: an optimal immunological target for vaccines. J. Transl. Med. 2020;18:222. doi: 10.1186/s12967-020-02392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ter Meulen J., van den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E., Preiser W., Doerr H.W., Chow V.T., de Kruif J., Peiris J.S., Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chappell K.J., Watterson D., Young P.R. WIPO. 2018. Chimeric molecules and uses thereof. [Google Scholar]
- 46.Watterson D., et al. 2020. Molecular Clamp Stabilised Spike Protein For Protection Against SARS- CoV-2. [Google Scholar]
- 47.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., Kong W.P., Andres E.L., Kettenbach A.N., Denison M.R., Chappell J.D., Graham B.S., Ward A.B., McLellan J.S. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo T.Y., Lin M.Y., Coffman R.L., Campbell J.D., Traquina P., Lin Y.J., Liu L.T.C., Cheng J., Wu Y.C., Wu C.C., Tang W.H., Huang C.G., Tsao K.C., Shih S.R., Chen C. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. bioRxiv. 2020 doi: 10.1101/2020.08.11.245704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H., Su D., Zhang J., Ge S., Li Y., Wang F., Gravel M., Roulston A., Song Q., Xu W., Liang J.G., Shore G., Wang X., Liang P. Improvement of pharmacokinetic profile of TRAIL via trimer-tag enhances its antitumor activity in vivo. Sci. Rep. 2017;7:8953. doi: 10.1038/s41598-017-09518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasan S., Schlesinger S.J., Huang Y., Hurley A., Lombardo A., Chen Z., Than S., Adesanya P., Bunce C., Boaz M., Boyle R., Sayeed E., Clark L., Dugin D., Schmidt C., Song Y., Seamons L., Dally L., Ho M., Smith C., Markowitz M., Cox J., Gill D.K., Gilmour J., Keefer M.C., Fast P., Ho D.D. Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B' HIV-1 candidate vaccine. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovsky N., Larena M., Siddharthan V., Prow N.A., Hall R.A., Lobigs M., Morrey J. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J. Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinen B., Petrovsky N., Verma A., Merkel T.J. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin. Vaccine Immunol.: CVI. 2014;21:580–586. doi: 10.1128/CVI.00019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon D., Kelley P., Heinzel S., Cooper P., Petrovsky N. Immunogenicity and safety of Advax, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: a randomized controlled Phase 1 study. Vaccine. 2014;32:6469–6477. doi: 10.1016/j.vaccine.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrovsky N., Cooper P.D. Advax, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–5926. doi: 10.1016/j.vaccine.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi M., Aoshi T., Haseda Y., Kobiyama K., Wijaya E., Nakatsu N., Igarashi Y., Standley D.M., Yamada H., Honda-Okubo Y., Hara H., Saito T., Takai T., Coban C., Petrovsky N., Ishii K.J. Advax, a delta inulin microparticle, potentiates in-built adjuvant property of co-administered vaccines. EBioMedicine. 2017;15:127–136. doi: 10.1016/j.ebiom.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honda-Okubo Y., Rajapaksha H., Sajkov D., Gordon D., Cox M.M.J., Petrovsky N. Panblok-H1+advax H1N1/2009pdm vaccine: insights into rapid development of a delta inulin adjuvanted recombinant pandemic influenza vaccine. Hum. Vaccines Immunother. 2017;13:1–11. doi: 10.1080/21645515.2017.1279765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomar J., Patil H.P., Bracho G., Tonnis W.F., Frijlink H.W., Petrovsky N., Vanbever R., Huckriede A., Hinrichs W.L.J. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J. Control. Rel.: Off. J. Control. Rel. Soc. 2018;288:199–211. doi: 10.1016/j.jconrel.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norville D. Development of unique Australian COVID-19 vaccine halted. Science. 2020 doi: 10.1126/science.abg1208. [DOI] [Google Scholar]
- 60.Dai L., Zheng T., Xu K., Han Y., Xu L., Huang E., An Y., Cheng Y., Li S., Liu M., Yang M., Li Y., Cheng H., Yuan Y., Zhang W., Ke C., Wong G., Qi J., Qin C., Yan J., Gao G.F. A Universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell. 2020;182:722–733. doi: 10.1016/j.cell.2020.06.035. e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.PipelineReviews . 2020. COVAXX Announces First Multitope Peptide-Based Vaccine to Enter Human Trials. [Google Scholar]
- 62.Hotez P.J., Corry D.B., Bottazzi M.E. COVID-19 vaccine design: the Janus face of immune enhancement. Nat. Rev. Immunol. 2020;20:347–348. doi: 10.1038/s41577-020-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merlin M., Gecchele E., Capaldi S., Pezzotti M., Avesani L. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/136419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puetz J., Wurm F.M. Recombinant proteins for industrial versus pharmaceutical purposes: a review of process and pricing. Processes. 2019;7:476. [Google Scholar]
- 65.Rosano G.L., Ceccarelli E.A. Recombinant protein expression in Escherichia coli: advances and challenges. Front. Microbiol. 2014;5:172. doi: 10.3389/fmicb.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang M., Bao J., Nielsen J. Biopharmaceutical protein production by Saccharomyces cerevisiae: current state and future prospects. Pharm. Bioprocess. 2014;2:167–182. [Google Scholar]
- 67.Vetter V., Denizer G., Friedland L.R., Krishnan J., Shapiro M. Understanding modern-day vaccines: what you need to know. Ann. Med. 2018;50:110–120. doi: 10.1080/07853890.2017.1407035. [DOI] [PubMed] [Google Scholar]
- 68.Nigrovic L.E., Thompson K.M. The Lyme vaccine: a cautionary tale. Epidemiol. Infect. 2007;135:1–8. doi: 10.1017/S0950268806007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Comstedt P., Hanner M., Schuler W., Meinke A., Lundberg U. Design and development of a novel vaccine for protection against Lyme borreliosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Comstedt P., Schuler W., Meinke A., Lundberg U. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gandhi A., Balmer P., York L.J. Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba(R)) Postgrad. Med. 2016;128:548–556. doi: 10.1080/00325481.2016.1203238. [DOI] [PubMed] [Google Scholar]
- 72.Shirley M., Dhillon S. Bivalent rLP2086 vaccine (Trumenba((R))): a review in active immunization against invasive meningococcal group b disease in individuals aged 10-25 years. BioDrugs: Clin. Immunother. Biopharm. Gene Therapy. 2015;29:353–361. doi: 10.1007/s40259-015-0139-0. [DOI] [PubMed] [Google Scholar]
- 73.Toneatto D., Pizza M., Masignani V., Rappuoli R. Emerging experience with meningococcal serogroup B protein vaccines. Expert Rev. Vaccines. 2017;16:433–451. doi: 10.1080/14760584.2017.1308828. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen K.H. Protein expression-yeast. Methods Enzymol. 2014;536:133–147. doi: 10.1016/B978-0-12-420070-8.00012-X. [DOI] [PubMed] [Google Scholar]
- 75.Valenzuela P., Medina A., Rutter W.J., Ammerer G., Hall B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 76.Mello C.F. Vaccination against human papillomavirus. Einstein. 2013;11:547–549. doi: 10.1590/S1679-45082013000400027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jacobs P.P., Geysens S., Vervecken W., Contreras R., Callewaert N. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat. Protoc. 2009;4:58–70. doi: 10.1038/nprot.2008.213. [DOI] [PubMed] [Google Scholar]
- 78.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A., Guo X.V., Cerutti G., Bimela J., Gorman J., Zhou T., Chen Z., Yuen K.Y., Kwong P.D., Sodroski J.G., Yin M.T., Sheng Z., Huang Y., Shapiro L., Ho D.D. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 79.PTI . The Hindu; 2020. Coronavirus|U.S.-Based Baylor College of Medicine Ties Up with India’s Biological E for COVID-19 Vaccine. [Google Scholar]
- 80.Dynavax . 2020. Biological E. Limited Starts Phase I/II Clinical Trial of its COVID-19 Vaccine Candidate. [Google Scholar]
- 81.Chen W.H., Du L., Chag S.M., Ma C., Tricoche N., Tao X., Seid C.A., Hudspeth E.M., Lustigman S., Tseng C.T., Bottazzi M.E., Hotez P.J., Zhan B., Jiang S. Yeast-expressed recombinant protein of the receptor-binding domain in SARS-CoV spike protein with deglycosylated forms as a SARS vaccine candidate. Hum. Vaccines Immunother. 2014;10:648–658. doi: 10.4161/hv.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen W., Tao X., Agrawal A., Algaissi A., Peng B., Pollet J., Strych U., Bottazz M., Hotez P., Lustigman S., Du L., Jiang S., Tseng C. Yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1) formulated with alum induces protective immunity and reduces immune enhancement. bioRxiv. 2020 doi: 10.1101/2020.05.15.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tripathi N.K., Shrivastava A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front. Bioeng. Biotechnol. 2019;7:420. doi: 10.3389/fbioe.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Norden R., Nilsson J., Samuelsson E., Risinger C., Sihlbom C., Blixt O., Larson G., Olofsson S., Bergstrom T. Recombinant glycoprotein E of varicella zoster virus contains glycan-peptide motifs that modulate B cell epitopes into discrete immunological signatures. Int. J. Mol. Sci. 2019:20. doi: 10.3390/ijms20040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith G.E., Summers M.D., Fraser M.J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983;3:2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kost T.A., Condreay J.P., Jarvis D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luckow V.A., Summers M.D. Trends in the development of baculovirus expression vectors. Bio/Technology. 1988;6:47–55. [Google Scholar]
- 88.Caron A.W., Archambault J., Massie B. High-level recombinant protein production in bioreactors using the baculovirus-insect cell expression system. Biotechnol. Bioeng. 1990;36:1133–1140. doi: 10.1002/bit.260361108. [DOI] [PubMed] [Google Scholar]
- 89.Contreras-Gomez A., Sanchez-Miron A., Garcia-Camacho F., Molina-Grima E., Chisti Y. Protein production using the baculovirus-insect cell expression system. Biotechnol. Prog. 2013;30:1–18. doi: 10.1002/btpr.1842. [DOI] [PubMed] [Google Scholar]
- 90.Shi X., Jarvis D.L. Protein N-glycosylation in the baculovirus-insect cell system. Curr. Drug Targets. 2007;8:1116–1125. doi: 10.2174/138945007782151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.BAT . 2020. BAT Working on Potential COVID-19 Vaccine through US Bio-tech Subsidiary. [Google Scholar]
- 92.Komarnytsky S., Borisjuk N.V., Borisjuk L.G., Alam M.Z., Raskin I. Production of recombinant proteins in tobacco guttation fluid. Plant Physiol. 2000;124:927–934. doi: 10.1104/pp.124.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pearson M.S., Jariwala A.R., Abbenante G., Plieskatt J., Wilson D., Bottazzi M.E., Hotez P.J., Keegan B., Bethony J.M., Loukas A. New tools for NTD vaccines: A case study of quality control assays for product development of the human hookworm vaccine Na-APR-1M74. Hum. Vaccines Immunother. 2015;11:1251–1257. doi: 10.4161/21645515.2014.980199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seid C.A., Curti E., Jones R.M., Hudspeth E., Rezende W., Pollet J., Center L., Versteeg L., Pritchard S., Musiychuk K., Yusibov V., Hotez P.J., Bottazzi M.E. Expression, purification, and characterization of the Necator americanus aspartic protease-1 (Na-APR-1 (M74)) antigen, a component of the bivalent human hookworm vaccine. Hum. Vaccines Immunother. 2015;11:1474–1488. doi: 10.1080/21645515.2015.1036207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tremblay R., Wang D., Jevnikar A.M., Ma S. Tobacco, a highly efficient green bioreactor for production of therapeutic proteins. Biotechnol. Adv. 2010;28:214–221. doi: 10.1016/j.biotechadv.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Owczarek B., Gerszberg A., Hnatuszko-Konka K. A brief reminder of systems of production and chromatography-based recovery of recombinant protein biopharmaceuticals. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/4216060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soler E., Houdebine L.M. Preparation of recombinant vaccines. Biotechnol. Annu. Rev. 2007;13:65–94. doi: 10.1016/S1387-2656(07)13004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.FDA . 2020. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry. [Google Scholar]
- 99.Shardlow E., Mold M., Exley C. Unraveling the enigma: elucidating the relationship between the physicochemical properties of aluminium-based adjuvants and their immunological mechanisms of action. Allergy Asthma Clin. Immunol.: Off. J. Can. Soc. Allergy Clin. Immunol. 2018;14:80. doi: 10.1186/s13223-018-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He P., Zou Y., Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015;11:477–488. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J. Virol. 2015;89:2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hotez P.J., Corry D.B., Strych U., Bottazzi M.E. COVID-19 vaccines: neutralizing antibodies and the alum advantage. Nat. Rev. Immunol. 2020;20:399–400. doi: 10.1038/s41577-020-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hotez P.J., Bottazzi M.E. 2020. Developing a Low-Cost and Accessible COVID-19 Vaccine for Global Health. Preprints.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ko E.J., Kang S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccines Immunother. 2018;14:3041–3045. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O'Hagan D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- 106.CDC . 2020. Adjuvanted Flu Vaccine. [Google Scholar]
- 107.Dupuis M., Denis-Mize K., LaBarbara A., Peters W., Charo I.F., McDonald D.M., Ott G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur. J. Immunol. 2001;31:2910–2918. doi: 10.1002/1521-4141(2001010)31:10<2910::aid-immu2910>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 108.Valensi J.P., Carlson J.R., Van Nest G.A. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J. Immunol. 1994;153:4029–4039. [PubMed] [Google Scholar]
- 109.O'Hagan D.T., Rappuoli R., De Gregorio E., Tsai T., Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev. Vaccines. 2011;10:447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 110.Oberemok V.V., Laikova K.V., Yurchenko K.A., Marochkin N.A., Fomochkina A.V., II, Kubyshkin SARS-CoV-2 will constantly sweep its tracks: a vaccine containing CpG motifs in 'lasso' for the multi-faced virus. Inflamm. Res.: Off. J. Eur. Histam. Res. Soc. … [et al.] 2020;69:801–812. doi: 10.1007/s00011-020-01377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Campbell J.D. In: Vaccine Adjuvants: Methods and Protocols. Fox C., editor. Humana Press; New York: 2016. Development of the CpG adjuvant 1018: A case study; pp. 15–27. [Google Scholar]
- 112.Cohet C., van der Most R., Bauchau V., Bekkat-Berkani R., Doherty T.M., Schuind A., Tavares Da Silva F., Rappuoli R., Garçon N., Innis B.L. Safety of AS03-adjuvanted influenza vaccines: a review of the evidence. Vaccine. 2019;37:3006–3021. doi: 10.1016/j.vaccine.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 113.Canelle Q., Dewé W., Innis B.L., van der Most R. Evaluation of potential immunogenicity differences between Pandemrix™ and Arepanrix™. Hum. Vaccines Immunother. 2016;12:2289–2298. doi: 10.1080/21645515.2016.1168954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilkins A.L., Kazmin D., Napolitani G., Clutterbuck E.A., Pulendran B., Siegrist C.A., Pollard A.J. AS03- and MF59-adjuvanted influenza vaccines in children. Front. Immunol. 2017;8:1760. doi: 10.3389/fimmu.2017.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Magnusson S.E., Altenburg A.F., Bengtsson K.L., Bosman F., de Vries R.D., Rimmelzwaan G.F., Stertman L. Matrix-M adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol. Res. 2018;66:224–233. doi: 10.1007/s12026-018-8991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Magnusson S.E., Reimer J.M., Karlsson K.H., Lilja L., Bengtsson K.L., Stertman L. Immune enhancing properties of the novel matrix-M adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine. 2013;31:1725–1733. doi: 10.1016/j.vaccine.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 117.Osterhaus A.D., Rimmelzwaan G.F. Induction of virus-specific immunity by iscoms. Dev. Biol. Stand. 1998;92:49–58. [PubMed] [Google Scholar]
- 118.Cox R.J., Pedersen G., Madhun A.S., Svindland S., Saevik M., Breakwell L., Hoschler K., Willemsen M., Campitelli L., Nostbakken J.K., Weverling G.J., Klap J., McCullough K.C., Zambon M., Kompier R., Sjursen H. Evaluation of a virosomal H5N1 vaccine formulated with matrix M adjuvant in a phase I clinical trial. Vaccine. 2011;29:8049–8059. doi: 10.1016/j.vaccine.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 119.Clinicaltrials_gov . 2011. Study of Parenterally Administrated Adjuvanted Seasonal Influenza Vaccine in Healthy Elderly Volunteers. [Google Scholar]
- 120.Clinicaltrials_gov . 2014. A(H7N9) VLP Antigen Dose-Ranging Study With Matrix-M1™ Adjuvant. [Google Scholar]
- 121.Clinicaltrials_gov . 2017. Safety and Immunogenicity Study to Evaluate Single- or Two-Dose Regimens Of RSV F Vaccine With and Without Aluminum Phosphate or Matrix-M1™ Adjuvants In Clinically-Stable Older Adults. [Google Scholar]
- 122.Clinicaltrials_gov . 2019. A Study to Assess the Safety and Immunogenicity of the Malaria Vaccine, R21, Administered With and Without Matrix-M1. [Google Scholar]
- 123.Heddle R., Smith A., Woodman R., Hissaria P., Petrovsky N. Randomized controlled trial demonstrating the benefits of delta inulin adjuvanted immunotherapy in patients with bee venom allergy. J. Allergy Clin. Immunol. 2019;144:504–513. doi: 10.1016/j.jaci.2019.03.035. e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hotez P.J., Bottazzi M.E., Corry D.B. The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect. 2020;22:165–167. doi: 10.1016/j.micinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He Y., Barker S.J., MacDonald A.J., Yu Y., Cao L., Li J., Parhar R., Heck S., Hartmann S., Golenbock D.T., Jiang S., Libri N.A., Semper A.E., Rosenberg W.M., Lustigman S. Recombinant Ov-ASP-1, a Th1-biased protein adjuvant derived from the helminth Onchocerca volvulus, can directly bind and activate antigen-presenting cells. J. Immunol. 2009;182:4005–4016. doi: 10.4049/jimmunol.0800531. [DOI] [PubMed] [Google Scholar]
- 127.MacDonald A.J., Tawe W., Leon O., Cao L., Liu J., Oksov Y., Abraham D., Lustigman S. Ov-ASP-1, the Onchocerca volvulus homologue of the activation associated secreted protein family is immunostimulatory and can induce protective anti-larval immunity. Parasite Immunol. 2004;26:53–62. doi: 10.1111/j.0141-9838.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 128.Xiao W., Du L., Liang C., Guan J., Jiang S., Lustigman S., He Y., Zhou Y. Evaluation of recombinant Onchocerca volvulus activation associated protein-1 (ASP-1) as a potent Th1-biased adjuvant with a panel of protein or peptide-based antigens and commercial inactivated vaccines. Vaccine. 2008;26:5022–5029. doi: 10.1016/j.vaccine.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao G., Lin Y., Du L., Guan J., Sun S., Sui H., Kou Z., Chan C.C., Guo Y., Jiang S., Zheng B.J., Zhou Y. An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol. J. 2010;7:9. doi: 10.1186/1743-422X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clements J.D., Norton E.B. The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere. 2018;3 doi: 10.1128/mSphere.00215-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Norton E.B., Bauer D.L., Weldon W.C., Oberste M.S., Lawson L.B., Clements J.D. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine. 2015;33:1909–1915. doi: 10.1016/j.vaccine.2015.02.069. [DOI] [PubMed] [Google Scholar]
- 132.Chao Y.X., Rotzschke O., Tan E.K. The role of IgA in COVID-19. Brain Behav. Immun. 2020;87:182–183. doi: 10.1016/j.bbi.2020.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L., Wang W., Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines. 2015;14:1509–1523. doi: 10.1586/14760584.2015.1081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bonnotte B., Gough M., Phan V., Ahmed A., Chong H., Martin F., Vile R.G. Intradermal injection, as opposed to subcutaneous injection, enhances immunogenicity and suppresses tumorigenicity of tumor cells. Cancer Res. 2003;63:2145–2149. [PubMed] [Google Scholar]
- 135.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L., Korkmaz E., Falo L.D., Jr., Gambotto A. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang M., Fu T., Hao J., Li L., Tian M., Jin N., Ren L., Li C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020;160:736–740. doi: 10.1016/j.ijbiomac.2020.05.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liebowitz D., Lindbloom J.D., Brandl J.R., Garg S.J., Tucker S.N. High titre neutralising antibodies to influenza after oral tablet immunisation: a phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 2015;15:1041–1048. doi: 10.1016/S1473-3099(15)00266-2. [DOI] [PubMed] [Google Scholar]
- 138.Arthur R. 2020. Vaxart's oral COVID-19 Tablet Vaccine to Enter Clinical Trials. [Google Scholar]
- 139.Eaton E.S. 2020. Merck Looks to Lower Barriers to COVID-19 Vaccination with Oral Approach. [Google Scholar]
- 140.Simin W., Luan I. 2020. Chinese Covid-19 Vaccine Candidate Becomes First Nasal Spray to Start Clinical Trial. [Google Scholar]
- 141.ClinicalTrialsArena . 2020. Nasal Spray Vaccine for Covid-19. [Google Scholar]
- 142.Altimmune . 2020. Single-Dose Intranasal COVID-19 Vaccine. [Google Scholar]
- 143.University_of_Wisconsin, UW–Madison . 2020. FluGen, Bharat Biotech to develop CoroFlu, a coronavirus vaccine. [Google Scholar]
- 144.Siepmann J.R., Siegel R.A., Rathbone M.J., Controlled Release Society . Springer: Controlled Release Society; New York: 2012. Fundamentals and Applications of Controlled Release Drug Delivery. [Google Scholar]
- 145.Criscuolo E., Caputo V., Diotti R.A., Sautto G.A., Kirchenbaum G.A., Clementi N. Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. J Immunol Res. 2019;2019 doi: 10.1155/2019/8303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Taddio A., Ipp M., Thivakaran S., Jamal A., Parikh C., Smart S., Sovran J., Stephens D., Katz J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–4812. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 147.Hutcheon C.J., Becker J.O., Russell B.A., Bariola P.A., Peterson G.J., Stroop S.D. Physiochemical and functional characterization of antigen proteins eluted from aluminum hydroxide adjuvant. Vaccine. 2006;24:7214–7225. doi: 10.1016/j.vaccine.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 148.Jones L.S., Peek L.J., Power J., Markham A., Yazzie B., Middaugh C.R. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J. Biol. Chem. 2005;280:13406–13414. doi: 10.1074/jbc.M500687200. [DOI] [PubMed] [Google Scholar]
- 149.Borges O., Borchard G., Verhoef J.C., de Sousa A., Junginger H.E. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int. J. Pharm. 2005;299:155–166. doi: 10.1016/j.ijpharm.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 150.Amacker M., Smardon C., Mason L., Sorrell J., Jeffery K., Adler M., Bhoelan F., Belova O., Spengler M., Punnamoottil B., Schwaller M., Bonduelle O., Combadiere B., Stegmann T., Naylor A., Johnson R., Wong D., Fleury S. New GMP manufacturing processes to obtain thermostable HIV-1 gp41 virosomes under solid forms for various mucosal vaccination routes. NPJ Vaccines. 2020;5:41. doi: 10.1038/s41541-020-0190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huckriede A., Bungener L., Stegmann T., Daemen T., Medema J., Palache A.M., Wilschut J. The virosome concept for influenza vaccines. Vaccine. 2005;23(Suppl. 1):S26–S38. doi: 10.1016/j.vaccine.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 152.Kempers R. 2020. Mymetics Starts Second Preclinical Study for Virosome-based Covid-19 Vaccine. [Google Scholar]
- 153.Clinicaltrials_gov . 2020. A Study Looking at the Effectiveness, Immune Response, and Safety of a COVID-19 Vaccine in Adults in the United Kingdom. [Google Scholar]
- 154.Novavax . 2020. Novavax Initiates Efficacy Trial of COVID-19 Vaccine in South Africa. [Google Scholar]
- 155.Sagonowsky E., Liu A., Blankenship K., Hale C., Kansteiner F. 2020. COVID-19 Tracker: Warp Speed Chief Eyes Timeline for Pfizer, Moderna, AZ Shot Data; FDA can Reveal Safety info If Vaccine Makers Don't, Official Says, Fierce_Pharma. [Google Scholar]
- 156.Biospace . 2020. Dynavax Announces First Participants Dosed in Phase 1 Clinical Trial Evaluating Clover Biopharmaceuticals’ COVID-19 S-Trimer Vaccine Candidate with CpG 1018 Adjuvant. [Google Scholar]
- 157.Clover_Biopharma . 2020. Clover Announces Formation of a Global Scientific Advisory Board for its COVID-19 Vaccine Program. [Google Scholar]
- 158.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Malladi S.K., Singh R., Pandey S., Gayathri S., Kanjo K., Ahmed S., Khan M.S., Kalita P., Girish N., Upadhyaya A., Reddy P., Pramanick I., Bhasin M., Mani S., Bhattacharyya S., Joseph J., Thankamani K., Raj V.S., Dutta S., Singh R., Nadig G., Varadarajan R. Design of a highly thermotolerant, immunogenic SARS-CoV-2 spike fragment. J. Biol. Chem. 2020 doi: 10.1074/jbc.RA120.016284. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thrane S., Aves K.L., Uddback I.E.M., Janitzek C.M., Han J., Yang Y.R., Ward A.B., Theander T.G., Nielsen M.A., Salanti A., Thomsen A.R., Christensen J.P., Sander A.F. A vaccine displaying a trimeric influenza-a ha stem protein on capsid-like particles elicits potent and long-lasting protection in mice. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.de Jongh W. 2020. COVID-19 cVLP Vaccine Demonstrated as Effective in Mice. [Google Scholar]
- 162.Frandsen B.U. 2020. ExpreS2ion Announces Successful Manufacturing of the cVLP COVID-19 Vaccine and Further Positive Updates. [Google Scholar]
- 163.Strbo N., Garcia-Soto A., Schreiber T.H., Podack E.R. Secreted heat shock protein gp96-Ig: next-generation vaccines for cancer and infectious diseases. Immunol. Res. 2013;57:311–325. doi: 10.1007/s12026-013-8468-x. [DOI] [PubMed] [Google Scholar]
- 164.van den Brand J.M., Haagmans B.L., van Riel D., Osterhaus A.D., Kuiken T. The pathology and pathogenesis of experimental severe acute respiratory syndrome and influenza in animal models. J. Comp. Pathol. 2014;151:83–112. doi: 10.1016/j.jcpa.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Selinger C., Strbo N., Gonzalez L., Aicher L., Weiss J.M., Law G.L., Palermo R.E., Vaccari M., Franchini G., Podack E.R., Katze M.G. Multiple low-dose challenges in a rhesus macaque AIDS vaccine trial result in an evolving host response that affects protective outcome. Clin. Vaccine Immunol.: CVI. 2014;21:1650–1660. doi: 10.1128/CVI.00455-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fisher E., Padula L., Podack K., O'Neill K., Seavey M.M., Jayaraman P., Jasuja R., Strbo N. Induction of SARS-CoV-2 protein S-specific CD8+T cells in the lungs of gp96-Ig-S vaccinated mice. BioRxiv. 2020 doi: 10.1101/2020.08.24.265090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moore J.P., Klasse P.J. COVID-19 vaccines: “Warp Speed” needs mind melds, not warped minds. J. Virol. 2020;94 doi: 10.1128/JVI.01083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Callaghan T., Moghtaderi A., Lueck J.A., Hotez P.J., Strych U., Dor A., Franklin-Fowler E., Motta M. 2020. Correlates and Disparities of COVID-19 Vaccine Hesitancy. SSRN, prepublication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.King A. Protein-based Covid-19 vaccines could overshadow rivals. Chem. World. 2020 https://www.chemistryworld.com/news/protein-based-covid-19-vaccines-could-overshadow-rivals/4012450.article [Google Scholar]
- 170.Blankenship K. 2020. Pfizer, Moderna's Coronavirus Shot Rollouts Could Freeze Up, Experts Say, Citing Cold-storage Needs. [Google Scholar]
- 171.Goldstein N., Bockstal V., Bart S., Luhn K., Robinson C., Gaddah A., Callendret B., Douoguih M. Safety and immunogenicity of heterologous and homologous two dose regimens of Ad26- and MVA-vectored ebola vaccines: a randomized, controlled phase 1 study. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Casimiro D.R., Bett A.J., Fu T.M., Davies M.E., Tang A., Wilson K.A., Chen M., Long R., McKelvey T., Chastain M., Gurunathan S., Tartaglia J., Emini E.A., Shiver J. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J. Virol. 2004;78:11434–11438. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Oxford C.V.T.G. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sadoff J., De Paepe E., Haazen W., Omoruyi E., Bastian A.R., Comeaux C., Heijnen E., Strout C., Schuitemaker H., Callendret B. Safety and immunogenicity of the Ad26.RSV.preF investigational vaccine coadministered with an influenza vaccine in older adults. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa409. [DOI] [PubMed] [Google Scholar]
- 176.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., II, Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H., mRNA-1273 Study Group An Mrna vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Walsh E.E., Frenck R., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Thompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. RNA-based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv: Preprint Server Health Sci. 2020 doi: 10.1101/2020.08.17.20176651. [DOI] [Google Scholar]
- 178.Robbins R., Mueller B. New York Times; 2020. After Admitting Mistake, AstraZeneca Faces Difficult Questions About Its Vaccine. [Google Scholar]