Abstract
Drug interactions between warfarin and sulfonylureas are suggested by pharmacokinetic information and prior studies. However, clinical evidence on the association of such interactions and the risk of bleeding is lacking. Using health care claims data from five US Medicaid programs from 1999–2011 and a self-controlled case series design with warfarin as an object drug, we examined confounder-adjusted rate ratios (RRs) for three outcomes separately: 1) serious bleeding as a composite outcome of gastrointestinal bleeding (GIB) and non-traumatic intracranial hemorrhage (ICH); 2) GIB; and 3) ICH. In 6,463 warfarin users experiencing serious bleeding, an increased rate of serious bleeding was not associated with concomitant use of glimepiride (RR: 0.93; 95% confidence interval: 0.75 to 1.15), glipizide (RR: 0.97; 0.84 to 1.13), glyburide (RR: 0.89; 0.76 to 1.06), or metformin (RR: 0.85; 0.76 to 0.96), nor was the occurrence of the component outcomes of GIB or ICH. These results suggest that use of sulfonylureas or metformin was not associated with an increased rate of serious bleeding in warfarin users.
INTRODUCTION
Warfarin, a vitamin K antagonist, remains the most widely prescribed oral anticoagulant in the United States, and is used for the prevention and treatment of thromboembolic events and complications.1–3 Approximately 15 million prescriptions for warfarin are written in the US annually.4 Although warfarin is an effective anticoagulant, warfarin-associated bleeding is among the most serious and common adverse events associated with any drug.5–10 Indeed, it is one of three common, serious, and preventable adverse drug events targeted by the US National Action Plan for Adverse Drug Event Prevention (ADE Action Plan).5 That Plan calls specifically for research examining the effects of potential drug-drug interactions that may increase these adverse events, including warfarin-associated bleeding.
Diabetes is a risk factor for the development of atrial fibrillation11—a primary therapeutic indication for warfarin—and also a common comorbidity of atrial fibrillation. One study estimated that about 17% of adult atrial fibrillation patients in the UK have diabetes.12 In the US, the prevalence of atrial fibrillation in adults is projected to increase substantially from about 5 million in 2010 to about 12 million in 203013 with the aging of the population. Based on pharmacokinetic considerations, drug-drug interactions between warfarin and sulfonylureas are predictable given that glyburide14,15 and glimepiride15 exhibit in vitro inhibition of cytochrome P450 (CYP) 2C9, a principal enzyme for warfarin metabolism, which could lead to over-anticoagulation. Widely-used drug references warn that concurrent use of warfarin and glyburide16 or sulfonylureas17 may result in an increased risk of bleeding. In contrast, several studies have reported that initiation of metformin18,19 or sulfonylureas19 in persons receiving vitamin K antagonist anticoagulants was associated with a 11–22% reduction in the mean international normalized ratio (INR), suggesting an increase in warfarin dose requirements and possibly a reduced risk of bleeding. Clinical evidence, however, on whether sulfonylureas and metformin are associated with an increase or reduction in bleeding risk of warfarin is lacking. We therefore investigated whether the risk of serious bleeding in patients taking warfarin (the object drug in a drug-drug interaction; i.e., the drug whose pharmacokinetics or pharmacodynamics is affected20) is influenced by the concomitant use of the most commonly used sulfonylureas—glimepiride, glipizide, and glyburide—or of metformin (precipitant drugs; i.e., the drugs that affect the pharmacokinetics or pharmacodynamics of the object drug20), using real-world health care claims data.
MATERIALS AND METHODS
Study design, population, and data
We used the self-controlled case series design21–23 to calculate outcome occurrence rate ratios (RRs) for individual sulfonylureas and metformin, separately, as precipitant drugs when used concomitantly with warfarin as the object drug. The self-controlled case series design is a case-only study design that includes only persons who experienced the outcome, with each person serving as their own control. Therefore, this design inherently controls for confounding by factors that do not change within individual over the observation period.24 The study population was adult warfarin users between the ages of 18 and 100 years in the US Medicaid population who also received glimepiride, glipizide, glyburide, or metformin, although not necessarily concomitantly with warfarin. We further required a 6-month baseline period free of enrollment gaps immediately before the first warfarin episode. In case an individual dis-enrolled from Medicaid after contributing to observation time and re-enrolled later, we re-applied the baseline period criterion immediately before the first warfarin episode appearing after that re-enrollment. A flow chart that describes the identification of our study individuals, application of inclusion/exclusion criteria, and sample sizes is presented in Figure S1. The data used were administrative health care claims data from five states’ Medicaid programs25—California, Florida, New York, Ohio, and Pennsylvania—from 1999 to 2011, supplemented with Medicare claims for Medicaid-Medicare dual-enrollees, including Medicare Part D, the outpatient prescription drug coverage program for Medicare that began in 2006. These five states account for approximately 40% of the nationwide Medicaid population.26 We linked these data to the Social Security Administration Death Master File to ascertain dates of death.
Outcome of interest
The outcome of interest was hospital presentation for serious bleeding. Specifically, we examined the following three outcomes of interest separately: 1) serious bleeding as a composite outcome of gastrointestinal bleeding (GIB) and non-traumatic intracranial hemorrhage (ICH); 2) GIB; and 3) ICH. GIB and ICH are the most common among serious bleeding events associated with drugs.27 We ascertained outcomes using validated algorithms based on discharge diagnoses (Table 1) in any position appearing in hospital inpatient claims (for GIB and ICH) or emergency department claims (for ICH). These algorithms have a positive predictive value of 81%28 for GIB and 97–98%29 for ICH. Outcomes that occurred during a warfarin episode that met the baseline criterion were included, regardless of whether the outcome occurred during a period of concomitancy or non-concomitancy with a sulfonylurea or metformin.
Table 1.
Operational definitions of outcomes of interest and performance measures of the outcome ascertainment algorithms
| Outcome | ICD-9-CM code | Description | Diagnosis position and claim type | Performance of algorithm |
|---|---|---|---|---|
| Gastrointestinal bleeding | 530.21 | Esophageal ulcer, with hemorrhage | Any position of discharge diagnosis in inpatient claims | PPV ~81%28 |
| 531.0X, 531.2X, 531.4X, 531.6X | Gastric ulcer, with hemorrhage | |||
| 532.0X, 532.2X, 532.4X, 532.6X | Duodenal ulcer, with hemorrhage | |||
| 533.0X, 533.2X, 533.4X, 533.6X | Peptic ulcer, with hemorrhage | |||
| 534.0X, 534.2X, 534.4X, 534.6X | Gastrojejunal ulcer, with hemorrhage | |||
| 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 535.71 | Gastritis and duodenitis, with hemorrhage | |||
| 537.83, 537.84 | Other specified disorder of stomach and duodenum, with hemorrhage | |||
| 562.02, 562.03, 562.12, 562.13 | Diverticula of intestine, with hemorrhage | |||
| 569.85, 569.86 | Other disorders of intestine, with hemorrhage | |||
| 578.X | Gastrointestinal hemorrhage | |||
| Non-traumatic intracranial hemorrhage | 430 | Subarachnoid hemorrhage | Any position of discharge diagnosis in ED or inpatient claims, excluding cases with traumatic brain injury diagnosis (ICD-9-CM codes 800–804, 850–854) in any position on the same admission date | PPV ~98% (subarachnoid hemorrhage), ~97% (intracerebral hemorrhage)29 |
| 431 | Intracerebral hemorrhage |
ED: emergency department. ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification. PPV: positive predictive value.
Exposure of interest and covariates
The exposure of interest was current use of either one of the sulfonylureas—glimepiride, glipizide, and glyburide, which together accounted for more than 99% of the sulfonylurea prescriptions in the Medicaid and Medicare claims data that we used—or metformin, concomitantly with warfarin. We ascertained the exposure of interest using National Drug Codes (NDCs), dispensing dates, and days supplied in prescription drug claims data. Because prescription claims data from Ohio lack information on days supplied, we imputed 30 days, given that days supplied in more than 80% of prescriptions for these precipitant drugs from each of the other four states was 30 days.
Given that the self-controlled case series design inherently controls for time-invariant confounders, we adjusted only for time-varying potential confounders (Table 2), including: a) drugs that can increase the risk of bleeding; b) drugs that can increase the INR; c) drugs that can reduce INR; and d) major acute condition that may affect bleeding (Table S1). Average daily dose (defined as the product of the quantity and strength divided by the days supplied) of the most recent prescription of the object drug (i.e., warfarin) and an indicator of therapeutic drug monitoring for warfarin (“warfarin monitoring”) that day or within the past seven days were additionally controlled for in separate sensitivity analyses. To control for warfarin average daily dose, we excluded person-days with potentially implausible warfarin average daily doses—those greater than 2 times maximum daily dose of warfarin (i.e., >20 mg/day)—in dose-adjusted analyses. Warfarin monitoring was assessed using Current Procedural Terminology (CPT) codes and Healthcare Common Procedure Coding System (HCPCS) codes (CPT: 99363, 99364, 3555F, 85610, 85611, HCPCS: G0249, G0250) (Table 2).
Table 2.
Prespecified time-varying covariates included in conditional Poisson regression models
| Category | Subcategory | Component | Identification method |
|---|---|---|---|
| Drugs that can increase the risk of bleeding | Antiplatelet agentsa | abciximab, aspirin, cangrelor, cilostazol, clopidogrel, dipyridamole, eptifibatide, prasugrel, ticagrelor, ticlopidine, tirofiban, vorapaxar | NDC, dispensing date, days supplied |
| NSAIDsa | celecoxib, diclofenac, diflunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, meclofenamate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, salsalate, sulindac, tolmetin | NDC, dispensing date, days supplied | |
| SNRIsa | desvenlafaxine, duloxetine, levomilnacipran, milnacipran, venlafaxine | NDC, dispensing date, days supplied | |
| SSRIsa | citalopram, escitalopram, fluoxetine, fluvoxamine, milnacipran, paroxetine, sertraline | NDC, dispensing date, days supplied | |
| Drugs that can increase INR | CYP1A2 inhibitors | enoxacinb, gatifloxacinb, propranolola | NDC, dispensing date, days supplied |
| CYP2C9 inhibitors | amiodaronea, amoxicillinb, co-trimoxazoleb, etravirinea, fluconazoleb, fluvoxaminea, metronidazoleb, miconazoleb, oxandrolonea, voriconazoleb | NDC, dispensing date, days supplied | |
| CYP3A4 inhibitors | atorvastatina, azithromycinb, erythromycinb, gemfibrozila, prednisonea, propafenonea, rosuvastatina, simvastatina | NDC, dispensing date, days supplied | |
| Drugs increasing vitamin K catabolisma | thyroid hormones (levothyroxine, liothyronine, liotrix) | NDC, dispensing date, days supplied | |
| Drugs that can cause protein displacementa | phenytoin, sulfinpyrazone | NDC, dispensing date, days supplied | |
| Drugs with unknown mechanism | acetaminophena, cefamandoleb, cefazolinb, quinidinea | NDC, dispensing date, days supplied | |
| Drugs that can reduce INR | Inducers of drug metabolism | amobarbitala, carbamazepinea, cholestyraminea, griseofulvinb, phenobarbitala, propylthiouracila, rifampinb, secobarbitala | NDC, dispensing date, days supplied |
| Major non-chronic condition that may affect bleeding risk | Acute infectionb | Acute infection identified at any position of discharge diagnosis on inpatient or outpatient claims | ICD-9-CM diagnosis codes, admission or service date |
| Warfarin monitoring* | Therapeutic drug monitoring for warfarinc | INR testing/monitoring, prothrombin time testing/monitoring, identified at any-position of any claim-type procedure codes | CPT, HCPCSd |
| Average daily dose of object drug* | Average daily dose of warfarine | Defined by [(quantity × strength) / (days supplied)] | NDC, dispensing date, days supplied, quantity, strength |
NDC: National Drug Code. NSAID: nonsteroidal anti-inflammatory drug. SNRI: serotonin and norepinephrine reuptake inhibitor. SSRI: selective serotonin reuptake inhibitor. INR: international normalized ratio. CYP: cytochrome P450 enzyme. ICD-9-CM: International Classification of Diseases 9th Revision Clinical Modification. CPT: Current Procedural Terminology. HCPCS: Healthcare Common Procedure Coding System.
Measured as a day-level binary variable indicating being dispensed on the current day (refers to each day during the observation time as current) or any time during the 31 days prior to the current day.
Measured as a day-level binary variable indicating being diagnosed on the current day or any time during the 15 days prior to the current day.
Measured as a day-level binary variable indicating warfarin monitoring conducted on the current day or any time during the 7 days prior to the current day.
CPT codes 99363, 99364, 3555F, 85610, 85611 and HCPCS codes G0249, G0250.
Measured as a day-level continuous variable on the current day, based on the prescription active on the current day. In the analysis controlling for warfarin average daily dose, while Medicare claims from Ohio were included, Medicaid claims from Ohio were excluded due to lack of information on days supplied.
Covariates additionally adjusted in the sensitivity analyses.
Observation time
Observation time consisted of one or more warfarin episodes during which at least one serious bleeding event occurred. We constructed observation time for each outcome category (i.e., serious bleeding as a composite outcome, GIB, and ICH) separately. Individuals were allowed to contribute more than one episode if inclusion/exclusion criteria were met for each episode. An episode was defined as one or continuous prescriptions that allowed for a 14-day grace period at the end of each prescription to account for potential incomplete adherence. Each episode began at the dispensing date of the first warfarin prescription and ended at the first occurrence of the following: a) end of the days supplied (including the grace period); b) Medicaid enrollment discontinuation; c) death; and d) end of dataset (i.e., December 31, 2011). The occurrence of bleeding was not a reason to end an episode. Episodes for each sulfonylurea and metformin were defined by the same method except for censoring by death occurrence. As death permanently ends an episode, we additionally performed a sensitivity analysis that only excluded episodes that were terminated by death.24 Within a warfarin episode, days of concomitant use with a sulfonylurea or metformin constituted precipitant-exposed time, and the other days constituted precipitant-unexposed time.
Statistical analysis
We performed conditional Poisson regression analyses21,22 for each endpoint of interest and for each warfarin-precipitant drug pair to estimate confounder-adjusted outcome occurrence RRs—i.e., outcome occurrence rate during precipitant-exposed time versus outcome occurrence rate during precipitant-unexposed time—and 95% confidence intervals (CIs). The unit of analysis was a person-day of observation time. The dependent variable was an indicator of outcome occurrence. The independent variables included were indicators of exposure to precipitant drugs and prespecified time-varying covariates (potential confounders) listed in Table 2. Further, to examine the robustness of the results, we performed sensitivity analyses as follows: 1) controlling for average daily dose of warfarin; 2) controlling for warfarin monitoring; 3) excluding warfarin episodes terminated by death (because death permanently ends observation time, thereby making subsequent exposures that might have otherwise occurred during observation time impossible24); and 4) excluding warfarin episodes during which individuals had potentially incomplete data (defined operationally as data from individuals enrolled in a managed care plan or a private health insurance, individuals with restricted benefits, or Medicaid-Medicare dual-enrollees who were enrolled in a group health organization or a Medicare Advantage plan for which the Centers for Medicare and Medicaid Services does not process provider claims). All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina, US).
This study was approved by the institutional review board of the University of Pennsylvania, which waived the requirement for informed consent.
RESULTS
We identified 6,463 warfarin users who met inclusion/exclusion criteria and experienced a serious bleeding event while on warfarin. These persons contributed a total of 1,433,447 person-days of observation time. Table 3 shows the characteristics of study participants. There were 7,388 occurrences of the composite outcome of serious bleeding (including 27 concurrences of GIB with ICH); 6,419 GIB occurrences; and 996 ICH occurrences. For the composite outcome, median age at start of the observation time was 73.1 (Q1: 64.9, Q3: 80.3) years, 66.5% of the individuals were women, and the proportion of person-days exposed to glimepiride, glipizide, glyburide, and metformin during observation time was 7.1%, 18.1%, 12.8%, and 26.9%, respectively.
Table 3.
Characteristics of study participants by outcome of interest
| Outcome | |||
|---|---|---|---|
| Serious bleedinga | Gastrointestinal bleeding | Non-traumatic intracranial hemorrhage | |
| Persons, person-days, and outcome occurrence | |||
| Number of persons | 6,463 | 5,832 | 684 |
| Person-days of observation time | 1,433,447 | 1,303,047 | 141,179 |
| During precipitant-exposed timeb | 740,318 | 666,694 | 80,453 |
| During precipitant-unexposed timec | 693,129 | 636,353 | 60,726 |
| Observation time per person in days, median (Q1; Q3) | 114 (48; 268) | 115 (49; 268) | 106 (45; 266) |
| Number of outcome occurrences during observation time | 7,388 | 6,419 | 996 |
| During precipitant-exposed time | 3,585 | 3,065 | 534 |
| During precipitant-unexposed time | 3,803 | 3,354 | 462 |
| Rate of outcome occurrence per 1,000 person-days during observation time | 5.2 | 4.9 | 7.1 |
| Demographic characteristics, number of persons (%), unless otherwise noted | |||
| Age in years at start of observation time, median (Q1; Q3) | 73.1 (64.9; 80.3) | 73.3 (65.1; 80.4) | 72.3 (63.5; 79.4) |
| Sex, female | 4,301 (66.5%) | 3,893 (66.8%) | 446 (65.2%) |
| Race/ethnicity | |||
| White | 3,307 (51.2%) | 3,063 (52.5%) | 262 (38.3%) |
| Black | 1,145 (17.7%) | 1,043 (17.9%) | 112 (16.4%) |
| Hispanic/Latino | 908 (14.0%) | 795 (13.6%) | 126 (18.4%) |
| Other/unknown | 1,103 (17.1%) | 931 (16.0%) | 184 (26.9%) |
| State of residence | |||
| California | 2,201 (34.1%) | 1,909 (32.7%) | 314 (45.9%) |
| Florida | 944 (14.6%) | 861 (14.8%) | 92 (13.5%) |
| New York | 1,669 (25.8%) | 1,525 (26.1%) | 156 (22.8%) |
| Ohio | 865 (13.4%) | 808 (13.9%) | 65 (9.5%) |
| Pennsylvania | 784 (12.1%) | 729 (12.5%) | 57 (8.3%) |
| Calendar year at start of observation time | |||
| 1999 | 210 (3.2%) | 190 (3.3%) | 21 (3.1%) |
| 2000 | 413 (6.4%) | 372 (6.4%) | 48 (7.0%) |
| 2001 | 456 (7.1%) | 419 (7.2%) | 44 (6.4%) |
| 2002 | 451 (7.0%) | 411 (7.0%) | 44 (6.4%) |
| 2003 | 458 (7.1%) | 408 (7.0%) | 54 (7.9%) |
| 2004 | 475 (7.3%) | 430 (7.4%) | 50 (7.3%) |
| 2005 | 521 (8.1%) | 474 (8.1%) | 49 (7.2%) |
| 2006 | 718 (11.1%) | 645 (11.1%) | 76 (11.1%) |
| 2007 | 541 (8.4%) | 478 (8.2%) | 70 (10.2%) |
| 2008 | 578 (8.9%) | 527 (9.0%) | 54 (7.9%) |
| 2009 | 672 (10.4%) | 601 (10.3%) | 75 (11.0%) |
| 2010 | 574 (8.9%) | 520 (8.9%) | 57 (8.3%) |
| 2011 | 396 (6.1%) | 357 (6.1%) | 42 (6.1%) |
| Medicare dual-enrollment, anytime during baseline period | 5,462 (84.5%) | 4,956 (85.0%) | 550 (80.4%) |
| Precipitant drugsd, number of person-days during precipitant-exposed time (%)e | |||
| glimepiride | 101,700 (7.1%) | 92,882 (7.1%) | 9,457 (6.7%) |
| glipizide | 259,066 (18.1%) | 236,553 (18.2%) | 25,667 (18.2%) |
| glyburide | 183,101 (12.8%) | 162,723 (12.5%) | 22,488 (15.9%) |
| metformin | 385,586 (26.9%) | 343,808 (26.4%) | 43,526 (30.8%) |
| Time-varying covariatesf, number of person-days (%), unless otherwise noted | |||
| Acute infection | 214,885 (15.0%) | 198,882 (15.3%) | 17,434 (12.3%) |
| Antiplatelet agents | 224,404 (15.7%) | 203,391 (15.6%) | 22,379 (15.9%) |
| CYP2C9 inhibitors | 114,769 (8.0%) | 108,115 (8.3%) | 8,406 (6.0%) |
| CYP1A2 inhibitors | 7,274 (0.5%) | 6,823 (0.5%) | 451 (0.3%) |
| CYP3A4 inhibitors | 577,352 (40.3%) | 527,600 (40.5%) | 54,396 (38.5%) |
| Drugs that can cause protein displacement | 30,917 (2.2%) | 27,580 (2.1%) | 3,443 (2.4%) |
| Drugs with unknown mechanism | 322,795 (22.5%) | 299,682 (23.0%) | 24,452 (17.3%) |
| Inducers of drug metabolism | 14,746 (1.0%) | 12,631 (1.0%) | 2,198 (1.6%) |
| NSAIDs | 120,204 (8.4%) | 110,194 (8.5%) | 10,165 (7.2%) |
| SNRIs | 46,348 (3.2%) | 42,773 (3.3%) | 3,677 (2.6%) |
| SSRIs | 280,375 (19.6%) | 256,594 (19.7%) | 25,815 (18.3%) |
| Thyroid hormones | 203,894 (14.2%) | 189,372 (14.5%) | 15,375 (10.9%) |
| Average daily dose of warfarin, in milligrams (Q1; Q3)g | 4.0 (2.5; 5.0) | 4.0 (2.5; 5.0) | 4.0 (2.5; 5.0) |
| Therapeutic drug monitoring for warfaring | 419,475 (29.3%) | 385,002 (29.5%) | 38,048 (27.0%) |
CYP: cytochrome P450. NSAID: nonsteroidal anti-inflammatory drug. Q1: First quartile. Q3: Third quartile. SNRI: serotonin and norepinephrine reuptake inhibitor, SSRI: selective serotonin reuptake inhibitors.
Composite outcome: occurrence of gastrointestinal bleeding or non-traumatic intracranial hemorrhage or both.
Precipitant-exposed time: time exposed to a precipitant drug.
Precipitant-unexposed time: time unexposed to a precipitant drug.
Exposure to precipitant drugs: indicator of the exposure status.
% of person-days: % of person-days for “yes” of the variable.
Time-varying covariates: indicator of the exposure status, measured in prior 31 days on the person-day level except for acute infection which were measured in prior 15 days. Detailed information on diagnosis codes used to identify acute infections is presented in Table S1.
Average daily dose of warfarin and therapeutic drug monitoring for warfarin were adjusted for in sensitivity analyses.
Table 4 and Figure 1 present confounder-adjusted RRs and 95% CIs for each outcome. RRs across precipitant drugs did not substantially differ across outcomes, although ICH seemed to have greater variations in RRs and CIs, which is probably attributable to the smaller number of ICH events. All RRs were numerically lower than 1.0, except for glipizide for ICH (RR: 1.04, 95% CI: 0.70 to 1.55). Metformin had RRs that were statistically significantly lower than the null, suggesting a potentially lower risk of bleeding by concomitant use compared with use of warfarin alone, for the composite outcome (RR: 0.85, 95% CI: 0.76 to 0.96) and ICH (RR: 0.51, 95% CI: 0.36 to 0.71). All other CIs included the null value.
Table 4.
Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin
| Outcome | Person-days during observation time | Number of outcomes during observation time | Precipitant drug | Person-days during precipitant-exposed timea | Number of outcomes during precipitant-exposed time | Rate ratiob | 95% CI |
|---|---|---|---|---|---|---|---|
| Serious bleedingc | 1,433,447 | 7,388 | glimepiride | 101,700 | 496 | 0.93 | 0.75, 1.15 |
| glipizide | 259,066 | 1,276 | 0.97 | 0.84, 1.13 | |||
| glyburide | 183,101 | 913 | 0.89 | 0.76, 1.06 | |||
| metformin | 385,586 | 1,757 | 0.85 | 0.76, 0.96 | |||
| Gastrointestinal bleeding | 1,303,047 | 6,419 | glimepiride | 92,882 | 441 | 0.98 | 0.78, 1.24 |
| glipizide | 236,553 | 1,090 | 0.96 | 0.82, 1.13 | |||
| glyburide | 162,723 | 756 | 0.89 | 0.74, 1.06 | |||
| metformin | 343,808 | 1,487 | 0.92 | 0.81, 1.05 | |||
| Non-traumatic intracranial hemorrhage | 141,179 | 996 | glimepiride | 9,457 | 58 | 0.68 | 0.37, 1.25 |
| glipizide | 25,667 | 191 | 1.04 | 0.70, 1.55 | |||
| glyburide | 22,488 | 161 | 0.88 | 0.57, 1.36 | |||
| metformin | 43,526 | 276 | 0.51 | 0.36, 0.71 |
CI: confidence interval (based on a two-tailed test).
Precipitant-exposed time: days of warfarin-precipitant drug concomitant use during observation time since the initiation of the concomitant use.
Rate ratio: [(outcome occurrence rate during precipitant-exposed time) / (outcome occurrence rate during precipitant-unexposed time)], for each warfarin-precipitant drug pair.
Serious bleeding as a composite outcome; occurrence of gastrointestinal bleeding or non-traumatic intracranial hemorrhage or both.
Figure 1.
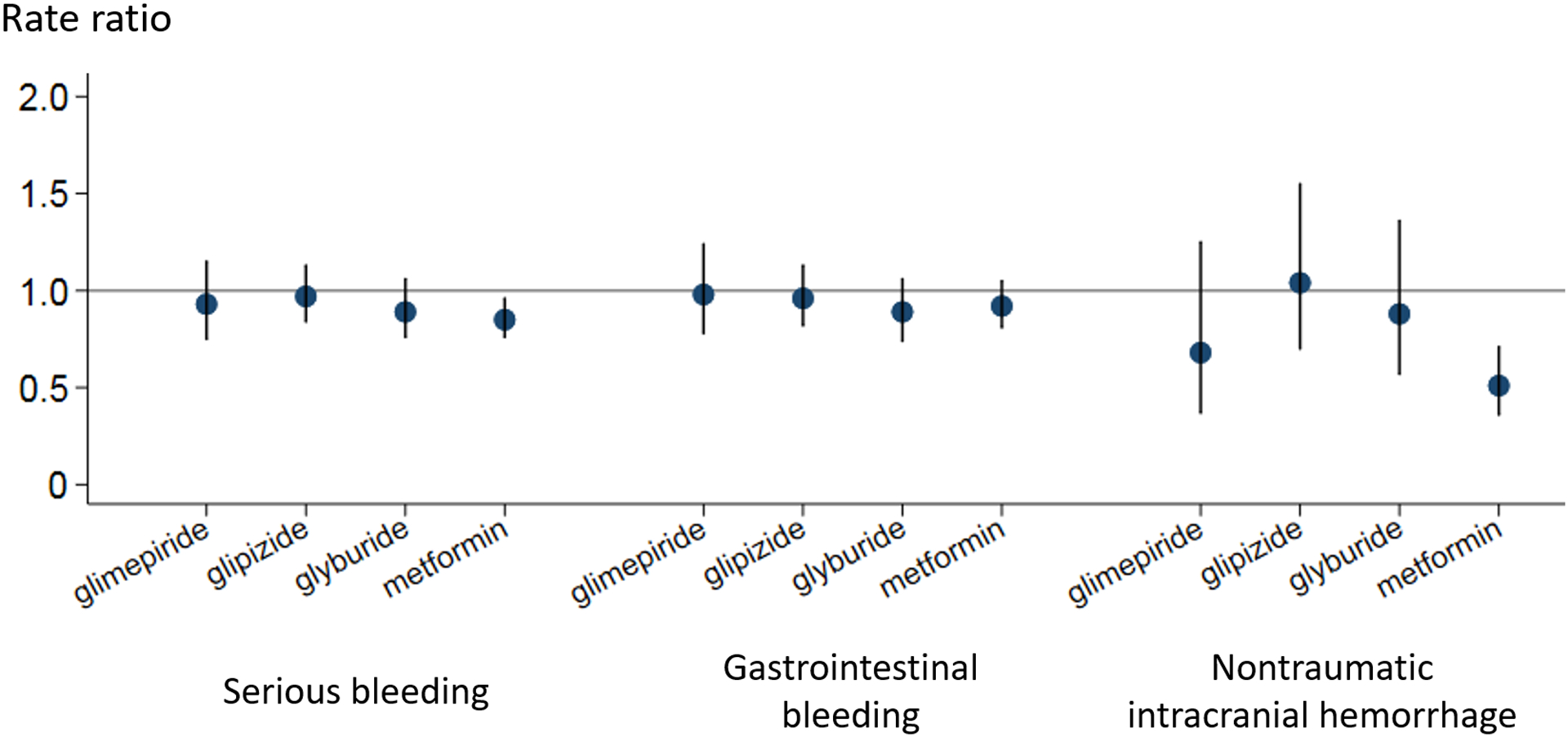
Outcome occurrence rate ratios and 95% confidence intervals for the association between the risk of serious bleeding and concomitant use of sulfonylureas or metformin in warfarin users
Serious bleeding: a composite outcome of gastrointestinal bleeding and non-traumatic intracranial hemorrhage
The results of sensitivity analyses—i.e., adjusting for average daily dose of warfarin (Table S2), adjusting for warfarin monitoring (Table S3), excluding warfarin episodes terminated by death (Table S4), and excluding warfarin episodes from individuals with potentially incomplete data (Table S5)—were similar to the primary analysis results. Notably, when warfarin average daily dose was additionally controlled for, concomitancy with glyburide showed a statistically reduced rate for ICH (RR: 0.56, 95% CI: 0.34 to 0.92) and the composite outcome (RR: 0.82, 95% CI: 0.68 to 0.99).
DISCUSSION
We found that commonly used sulfonylureas and metformin were not associated with an increased rate of serious bleeding in warfarin users. Prior to this study, there was reason to predict that some of these precipitants might either increase or reduce the risk of bleeding in warfarin users. For example, both glyburide14,15 and glimepiride15 exhibit in vitro inhibition of CYP2C9, and widely used drug references warn that concurrent use of warfarin plus glyburide16 or sulfonylureas17 may increase the risk of bleeding. Our results suggest that an increase in average bleeding risk is not observed in real-world practice, and are consistent with previous clinical studies that found that sulfonylureas and metformin are associated with reduced INR18,19 and increased dose requirements of vitamin K antagonists.18,19,30 Potential mechanisms of a reduced INR include: increased metabolism of vitamin K antagonists by increased liver blood flow by metformin,30 increased clearance of vitamin K antagonists through increased bile salt excretion by metformin18,31, reduced hepatic inflammation as a result of antidiabetes drug treatment, which leads to higher expression of CYP enzymes (especially CYP2C9), and increased metabolism of vitamin K antagonists.19 While such mechanisms might be expected to increase the risk of thromboembolism if the warfarin dose is not increased in response to a reduction in INR, whether this results in an observable increase in the risk has not yet been studied, but is an important research question.
This study has several important strengths. The self-controlled case series design inherently controls for confounding by static patient factors. We further controlled for potential time-varying confounders, and examined the robustness of the results through a series of sensitivity analyses. Further, the algorithms to identify the study outcomes have good performance characteristics. In addition, the large number of events (e.g., n=7,388 in the primary analysis for serious bleeding) allowed for relatively narrow CIs. Finally, the study was conducted in Medicaid beneficiaries, a vulnerable population in whom harmful DDIs may be more readily identified. This study also has limitations. Information on actual ingestion of prescribed drugs, non-medical or non-prescription drug therapy, diet, and health behaviors is lacking in the administrative claims data. Notwithstanding our adjustment for a large number of potential time-varying confounders, in addition to the inherent control for time-invariant confounders, residual confounding may have remained.
In summary, we found that use of sulfonylureas and metformin were not associated with an increased rate of serious bleeding in warfarin users. These results might provide epidemiologic evidence against the need to generally avoid these combinations to reduce bleeding risk. Further studies are warranted to investigate whether their concurrent use is associated with an increased rate of thromboembolism and to elucidate potential underlying mechanisms.
Supplementary Material
Figure S1. Identification of study individuals and application of inclusion and exclusion criteria
Table S1. ICD-9-CM diagnosis codes used to identify acute infections
Table S2. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, controlling for warfarin average daily dose
Table S3. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, controlling for warfarin monitoring
Table S4. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, with observation time only from individuals who were alive during that observation time
Table S5. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, excluding individuals with potentially incomplete data
STUDY HIGHLIGHTS.
• What is the current knowledge on the topic?
Warfarin-associated bleeding is among the most serious and common adverse drug events. Concomitant use of sulfonylureas or metformin is common in warfarin users, yet whether these drugs increase or reduce the risk of bleeding when used with warfarin has remained unclear.
• What question did this study address?
This study investigated whether concomitant use of sulfonylureas or metformin affects the risk of serious bleeding in warfarin users.
• What does this study add to our knowledge?
After controlling for potential confounders, using real-world administrative health care data from the US Medicaid population and a self-controlled case series design, this study found that use of sulfonylureas—glimepiride, glipizide, and glyburide—or metformin was not associated with an increased rate of serious bleeding in warfarin users.
• How might this change clinical pharmacology or translational science?
The results provide epidemiologic evidence against the need to generally avoid the concomitant use of sulfonylureas or metformin in warfarin users to reduce bleeding risk.
ACKNOWLEDGMENTS
The authors thank Ms. Qing Liu and Ms. Min Du of the Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania for their assistance with biostatistics computer programming.
Sources of funding: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK102694), the National Institute on Aging (R01AG025152 and R01AG060975), and the National Institute on Drug Abuse (R01DA048001) of the US National Institutes of Health. These organizations had no role in the design and conduct of the study, data collection and analysis, interpretation of the results, writing and review of the manuscript, or the decision to submit the manuscript for publication. This study was conducted by the authors independently from the funders.
Footnotes
Conflict of interest: S.H. consulted for Merck Research Laboratories and the Medullary Thyroid Cancer Consortium (Novo Nordisk Inc, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline LLC, and Eli Lilly and Company) on topics unrelated to this submitted work, and leads a training program that receives support from Pfizer Inc., unrelated to this submitted work. C.E.L. serves on the executive committee of this training program that receives support from Pfizer Inc. All the other authors declare no conflict of interests.
Data availability statement: Medicaid and Medicare claims data are available via a data use agreement with the Centers for Medicare and Medicaid Services (CMS) (https://www.cms.gov/). The authors did not have any special access privileges that others would not have. The procedures to obtain access to these data are described in the CMS website (https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/Data-Disclosures-Data-Agreements/Researchers) and the Research Data Assistance Center (ResDAC) website (https://www.resdac.org/research-identifiable-files-rif-requests).
REFERENCES
- 1.Barnes GD, Lucas E, Alexander GC & Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med 128, 1300–1305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bielecki S, Lee D & Hamad B. The market for oral anticoagulants. Nat Rev Drug Discov 17, 617–618 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Alawan AA, Voils SA, Hartzema AG. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am J Healt Syst Pharm 74, 1237–1244 (2017). [DOI] [PubMed] [Google Scholar]
- 4.The Top 200 Drugs of 2020. ClinCalc.com. Available at https://clincalc.com/DrugStats/Top200Drugs.aspx. Accessed 7 January 2020.
- 5.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention (2014). Available at https://health.gov/hcq/pdfs/ade-action-plan-508c.pdf. Accessed 6 December 2017.
- 6.Holstein A, Holstein JD, Patzer OM, Stumvoll M, Machalke K & Kovacs P. Substantial increase in incidence of severe hypoglycemia between 1997–2000 and 2007–2010. Diabetes Care 35, 972–975 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budnitz DS, Lovegrove MC, Shehab N & Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 365, 2002–2012 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Gurwitz JH. et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 289, 1107–1116 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ & Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 296, 1858–1866 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ & Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 316, 2115–2125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huxley RR, Filion KB, Konety S & Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 108, 56–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colilla S, Crow A, Petkun W, Singer DE, Simon T & Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 112, 1142–1147 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Van Staa TP, Setakis E, Di Tanna GL, Lane DA & Lip GY. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost 9, 39–48 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Kim KA & Park JY. Inhibitory effect of glyburide on human cytochrome p450 isoforms in human liver microsomes. Drug Metab Dispos 31, 1090–1092 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Iwakawa S, Miyashita K, Hashimoto Y & Kuroda T. Effect of glimepiride and glibenclamide on S-warfarin 7-hydroxylation by human liver microsomes, recombinant human CYP2C9.1 and CYP2C9.3. Biol Pharm Bull 29, 1983–1985 (2006). [DOI] [PubMed] [Google Scholar]
- 16.IBM Watson Micromedex. Drug drug interactions. Available at: https://www.ibm.com/watson-health/learn/micromedex. Accessed 28 January 2020.
- 17.Facts & Comparisons eAnswers. Glipizide oral (Drug Facts and Comparions). Available at https://fco.factsandcomparisons.com/lco/action/doc/retrieve/docid/fc_dfc/5548579. Accessed 28 January 2020.
- 18.Wijnen JCF, van de Riet IR, Lijfering WM & van der Meer FJM. Metformin use decreases the anticoagulant effect of phenprocoumon. J Thromb Haemost 12, 887–890 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Stage TB. et al. Initiation of glucose-lowering treatment decreases international normalized ratio levels among users of vitamin K antagonists: a self-controlled register study. J Thromb Haemost 14, 129–133 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Hennessy S. et al. Pharmacoepidemiologic methods for studying the health effects of drug-drug interactions. Clin Pharmacol Ther 99, 92–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitaker HJ, Farrington CP, Spiessens B & Musonda P. Tutorial in biostatistics: The self-controlled case series method. Stat Med 25, 1768–1797 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Whitaker HJ, Hocine MN & Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res 18, 7–26 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Weinberg CR. Invited commentary: Self-control is a virtue. Am J Epidemiol 185, 1184–1186 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Petersen I, Douglas I & Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 354, i4515 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Hennessy S, Freeman CP & Cunningham F. US Government claims databases In Pharmacoepidemiology 5th edn (ed. Strom BL.) 209–223 (John Wiley & Sons, Sussex, UK, 2012). [Google Scholar]
- 26.Kaiser Family Foundation. Medicaid enrollment: June 2010 data snapshot. Report #8050–03 Available at https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8050-03.pdf. Accessed 12 August 2016.
- 27.Burgos KD, Sienko SE, Hoffman JL, Koerber JM & Smythe MA. Characteristics, management, and outcomes of patients with atrial fibrillation experiencing a major bleeding event while on rivaroxaban. Clin Appl Thromb Hemost 24, 372–378 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schelleman H, Bilker WB, Brensinger CM, Wan F, Yang YX & Hennessy S. Fibrate/statin initiation in warfarin users and gastrointestinal bleeding risk. Am J Med 123, 151–157 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokotailo RA & Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke 36, 1776–1781 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Ohnhaus EE, Berger W, Duckert F & Oesch F. The influence of dimethylbiguanide on phenprocoumon elimination and its mode of action. A drug interaction study. Klin Wochenschr 61, 851–858 (1983). [DOI] [PubMed] [Google Scholar]
- 31.Scarpello JH, Hodgson E & Howlett HC. Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet Med 15, 651–656 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Identification of study individuals and application of inclusion and exclusion criteria
Table S1. ICD-9-CM diagnosis codes used to identify acute infections
Table S2. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, controlling for warfarin average daily dose
Table S3. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, controlling for warfarin monitoring
Table S4. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, with observation time only from individuals who were alive during that observation time
Table S5. Sensitivity analysis: Confounder-adjusted outcome occurrence rate ratios for the association between serious bleeding and warfarin when used concomitantly with a sulfonylurea or metformin, excluding individuals with potentially incomplete data


