Abstract
The G protein–coupled estrogen receptor 1 (GPER1) mediates rapid estrogenic signaling. We recently reported that activation of GPER1 in the renal medulla evokes endothelin-1–dependent natriuresis in female, but not male, rats. However, the involvement of the ET receptors, ETA and ETB, underlying GPER1 natriuretic action remain unclear. In this study, we used genetic and pharmacologic methods to identify the contributions of ETA and ETB in mediating this female-specific natriuretic effect of renal medullary GPER1. Infusion of the GPER1-selective agonist G1 (5 pmol/kg per minute) into the renal medulla for 40 minutes increased Na+ excretion and urine flow in anesthetized female ETB-deficient (ETB def) rats and littermate controls but did not affect blood pressure or urinary K+ excretion in either group. Pretreatment with the selective ETA inhibitor ABT-627 (5 mg/kg, intravenous) abolished G1-induced natriuresis in ETB def rats. To further isolate the effects of inhibiting either receptor alone, we conducted the same experiments in anesthetized female Sprague-Dawley (SD) rats pretreated or not with ABT-627 and/or the selective ETB inhibitor A-192621 (10 mg/kg, intravenous). Neither antagonism of ETA nor antagonism of ETB receptor alone affected the G1-induced increase in Na+ excretion and urine flow in SD rats. However, simultaneous antagonism of both receptors completely abolished these effects. These data suggest that ETA and ETB receptors can mediate the natriuretic and diuretic response to renal medullary GPER1 activation in female rats.
SIGNIFICANCE STATEMENT
Activation of G protein–coupled estrogen receptor 1 (GPER1) in the renal medulla of female rats evokes natriuresis via endothelin receptors A and/or B, suggesting that GPER1 and endothelin signaling pathways help efficient sodium excretion in females. Thus, GPER1 activation could be potentially useful to mitigate salt sensitivity in females.
Introduction
Hypertension is the leading cause of cardiovascular morbidity and mortality among women (Benjamin et al., 2018). In the United States, the prevalence of hypertension among adult women from 2017 to 2018 was 39.7% (Ostchega et al., 2020), and one in three deaths of women were attributed to cardiovascular disease (Benjamin et al., 2018). Hypertension control resulted in the largest reduction, 38%, in cardiovascular mortality in women, according to NHANES (National Health and Nutrition Examination Survey) data modeling (Patel et al., 2015). Yet despite the availability of multiple antihypertensive regimens, almost half of hypertensive individuals in the United States do not have their blood pressure under control, highlighting the need for personalized therapeutic treatment options for the management of high blood pressure. Impaired natriuresis is a fundamental mechanism in the initiation of hypertension (Hall et al., 2012; Elijovich et al., 2016). Therefore, it is vital that we expand our knowledge of the varied mechanisms of natriuresis. As studies indicate that women often receive suboptimal care for cardiovascular disease (Bairey Merz et al., 2015; Leifheit-Limson et al., 2015), improved understanding of the female-specific mechanisms underlying hypertension will help narrow the gender-related gap in health care.
We recently provided evidence for the G protein–coupled estrogen receptor 1 (GPER1) as a novel pronatriuretic factor in female rats but not male rats (Gohar et al., 2020). GPER1 is a nonclassic estrogen receptor that elicits rapid activation of signaling pathways (Revankar et al., 2005; Thomas et al., 2005). In particular, this heptahelical membrane-associated receptor has been shown to elicit protective actions in the cardiovascular and renal systems of several animal models (Lindsey et al., 2009, 2011; Jessup et al., 2010; Kurt et al., 2016; Liu et al., 2016; Qiao et al., 2018; Chang et al., 2019; Gohar et al., 2020). Indeed, many studies have shown that GPER1 activation mitigates salt-induced cardiovascular and kidney disease (Jessup et al., 2010; Lindsey et al., 2011; Liu et al., 2016), although the underlying mechanism has not been completely defined. Within the kidney, GPER1 expression has been detected in tubular and epithelial cells (Lindsey et al., 2011; Cheng et al., 2014). We found that activation of GPER1 within the renal medulla of female rats promotes an increase in urinary Na+ excretion via an endothelin-1 (ET-1)–dependent pathway (Gohar et al., 2020), but the contribution of endothelin receptors ETA and/or ETB, involved in GPER1-induced natriuretic effect, have not been identified.
ET-1 is a well established pronatriuretic peptide that inhibits the activity of epithelial Na+ channels and Na+/K+ ATPase (Zeidel et al., 1989; Kohan, 2011; Speed et al., 2015). In fact, ET-1 has a fundamental role in the maintenance of blood pressure and Na+ homeostasis (Kohan, 2011; Speed et al., 2015). ET-1 elicits downstream actions via activation of the G protein–coupled receptors ETA and ETB, which are expressed at high levels within the medulla of the kidney (Kohan et al., 2011). In particular, established evidence indicates that the ETB receptor has a central role in mediating the excretory effects of ET-1 (Ge et al., 2006), and loss of ETB receptor function results in a salt-sensitive phenotype (Hocher and Ehrenreich, 2002). However, natriuretic actions have also been ascribed to ETA receptors specifically in female rats under conditions of ETB receptor dysfunction (Nakano and Pollock, 2009). Additional evidence indicates that ETA and ETB receptors may act synergistically to facilitate Na+ and water excretion (Ge et al., 2008; Nakano and Pollock, 2009; Boesen and Pollock, 2010). Of note, ET receptor antagonists, combined ETA and ETB or selective ETA receptor–selective agents, are currently used in the treatment of pulmonary hypertension (Boesen, 2015). Despite limitations related to higher doses causing fluid retention, ETA receptor–selective agents hold promise in the management of hypertension and renal disease (Meyers and Sethna, 2013; Boesen, 2015; Pollock and Pollock, 2019), whereas concomitant ETB receptor antagonists are not beneficial clinically, as they induce vasoconstriction and salt and water retention (Dhaun and Webb, 2008; Meyers and Sethna, 2013; Moorhouse et al., 2013).
The current study was designed to use genetic and pharmacologic approaches to identify the relative contributions of ETA and ETB receptors to renal medullary GPER1-induced natriuresis. Considering that the pronatriuretic effects of GPER1 are evident in female rats but absent in males (Gohar et al., 2020), the experiments in this study were conducted solely in female animals.
Materials and Methods
Animals.
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and the ARRIVE ((Animal Research: Reporting of In Vivo Experiments) guidelines (Kilkenny et al., 2010) and approved and monitored by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Female ETB-deficient (ETB def) rats and transgenic (TG) control littermates were obtained from our in-house colony. ETB-deficient rats were originally produced from the spotting lethal rat, which has a naturally occurring 301‐bp deletion in the gene encoding ETB that results in a lethal phenotype of congenital intestinal megacolon (Gariepy et al., 1998). This model was rescued by expressing a human ETB transgene only in sympathetic tissue using the human dopamine‐β‐hydroxylase promoter (Gariepy et al., 1998). Thus, the ETB def rats express ETB receptors only in nervous tissue because of the transgene, whereas TG controls express the transgene and the normal ETB receptor. In total, 20 ETB def and TG rats were used. Separate experiments used an additional 38 Sprague-Dawley (SD) rats purchased from Envigo (Indianapolis, IN). All rats were aged 18–22 weeks and weighed 200–300 g at time of the experimental protocol. Rats were housed at the institutional animal facilities at the University of Alabama at Birmingham in temperature- and humidity-controlled rooms with a 12-hour light/dark cycle. Experiments were conducted during the light period. Rats had free access to water and food (7917 Irradiated NIH-31 Mouse/Rat diet, 0.8% NaCl; Envigo).
Surgical Procedure.
Female ETB def rats and TG littermates were surgically prepared for acute intramedullary infusion experiments as detailed in our previous studies (Gohar et al., 2016a, 2017). Briefly, rats were anesthetized by intraperitoneal injections of thiobutabarbitone (Inactin hydrate, 100 mg/kg per milliliter, catalog number T133; Sigma-Aldrich Co., St. Louis, MO). The trachea was cannulated with polyethylene tubing (PE-205) to facilitate free breathing. The left femoral vein was cannulated with a PE-50 catheter for intravenous supplementation of 3% bovine serum albumin (catalog number A7906; Sigma-Aldrich Co.) in phosphate-buffered saline (catalog number 2810305; MP Biomedicals, Irvine, CA) at a rate of 1.2 ml/h to compensate for fluid loss and maintain euvolemia. Then, the left femoral artery was cannulated with a PE-50 catheter for blood pressure recording, after which a midline incision was made and the left ureter was cannulated by a PE-10 catheter to collect urine. Finally, 5 mm of stretched PE-10 catheter was inserted into the left kidney and positioned to deliver fluids at the outer-inner medullary junction at a rate of 0.5 ml/h. After surgery, the animals were allowed to equilibrate for 60 minutes before baseline urine collection. At the end of each experiment, the kidney was dissected to confirm the proper positioning of the catheter within the renal medullary interstitium. Surgeries were performed on a heated surgical table to avoid hypothermia. In separate experiments, the female SD rats underwent surgical procedures identical to those described above. Importantly, the intramedullary infusion technique we used has been shown to induce localized effects in the renal medulla, not the renal cortex (Stec et al., 1997; Speed and Hyndman, 2016).
Blood Pressure Measurement.
Blood pressure was recorded in anesthetized animals via femoral artery catheterization. The catheter was connected to a blood pressure transducer (DPT-200, Deltran II; Utah Medical Products Ltd., Midvale, UT) with an output to PowerLab analog-to-digital converter (4/35; ADInstruments, Colorado Springs, CO) via a Quad Bridge Amplifier (FE224; ADInstruments). LabChart software version 7 was used to record and analyze blood pressure. Blood pressure data are presented as mean arterial pressure (MAP).
Experimental Protocol.
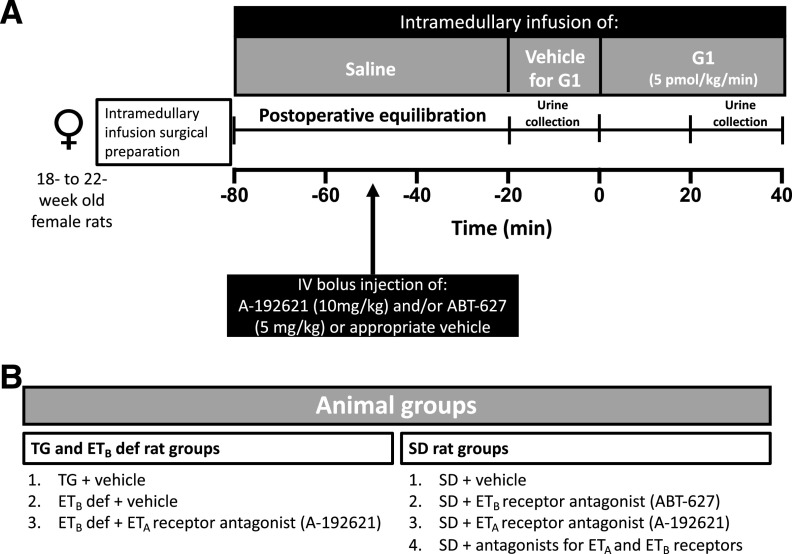
Our experimental protocol is depicted in Fig. 1A. After the 60-minute postsurgical stabilization period, urine was collected over a 20-minute baseline period, and vehicle (0.02% DMSO in saline) for the selective GPER1 agonist G1 was infused into the renal medulla (DMSO, catalog number D8418; Sigma-Aldrich Co.). Then, G1 (5 pmol/kg per minute, catalog number 41004001; Sandia Biotec Inc. Albuquerque, NM) was infused into the renal medulla for 40 minutes (Fig. 1A). This intramedullary dose of G1 promotes natriuresis in female SD rats (Gohar et al., 2020). After 20 minutes of G1 infusion, a 20-minute urine sample was collected while G1 was still being infused to the renal medulla (Fig. 1A). Urine volume was determined gravimetrically. To pharmacologically inhibit the ETA receptor, ETB def rats were treated with an intravenous bolus injection of the ETA receptor antagonist ABT-627 (5 mg/kg; AbbVie Inc., Abbott Park, IL; n = 5) or vehicle (n = 6) 30 minutes before the end of the postsurgical stabilization period (Fig. 1A). Animal groups used are depicted in Fig. 1B. To pharmacologically inhibit ET receptors, SD rats were treated with an intravenous bolus injection of ABT-627 (5 mg/kg; n = 8) and/or the ETB receptor antagonist A-192621 (10 mg/kg; PepTech Corp., Bedford, MA; n = 10 and 9, respectively) 30 minutes before the end of the postsurgical stabilization period (Fig. 1A). These doses of ABT-627 and A-192621 have been shown to elicit efficient pharmacologic blockade of ETA and ETB, respectively, for this experimental duration (Gohar et al., 2016a), as ABT-627 and A-192621 have half-lives of 6 and 5 hours, respectively, in rats (Wessale et al., 2002). A separate experimental group of SD (n = 11) and TG rats (n = 9) received an intravenous bolus injection of vehicle (0.5 ml/kg) and served as controls (Fig. 1B). The vehicle used to solubilize ABT-627 and A-192621 was composed of 60% polyethylene glycol 400 (catalog number 91893; Sigma-Aldrich Co.) and 25% ethanol (molecular-grade, catalog number E7023; Sigma-Aldrich Co.) in saline. Urine samples were stored at −80°C until further analysis.
Fig. 1.
Schematic presentation of the experimental timeline (A) and animal groups (B) used in intramedullary infusion experiments.
Of note, G1 is a highly selective agonist for GPER1 and displays no activity against classic estrogen receptors (Bologa et al., 2006; Albanito et al., 2007) or a panel of 25 other G protein–coupled receptors (Blasko et al., 2009). Moreover, the physiologic actions of G1 are not evident in GPER1-knockout mice (Haas et al., 2009; Liu et al., 2009; Wang et al., 2009).
Measurement of Urinary Electrolytes.
Urine Na+ and K+ concentrations were determined using an atomic absorption spectrometer (iCE 3000 series paired with a CETAC ASX-520 AutoSampler; ThermoFisher Scientific, Waltham, MA) in the flame photometry mode.
Statistics.
Comparisons between groups were analyzed by a repeated-measures two-way ANOVA followed by Dunnett’s post hoc tests. Parameters measured during the first 20 minutes of G1 infusion were included in the statistical analysis. For clarity in data presentation, only responses during the second 20 minutes and not the first 20 minutes of G1 infusion are presented in the figures. Values are presented as means ± S.E.M. P values less than 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism version 8.
Results
ETB-Deficient Rats.
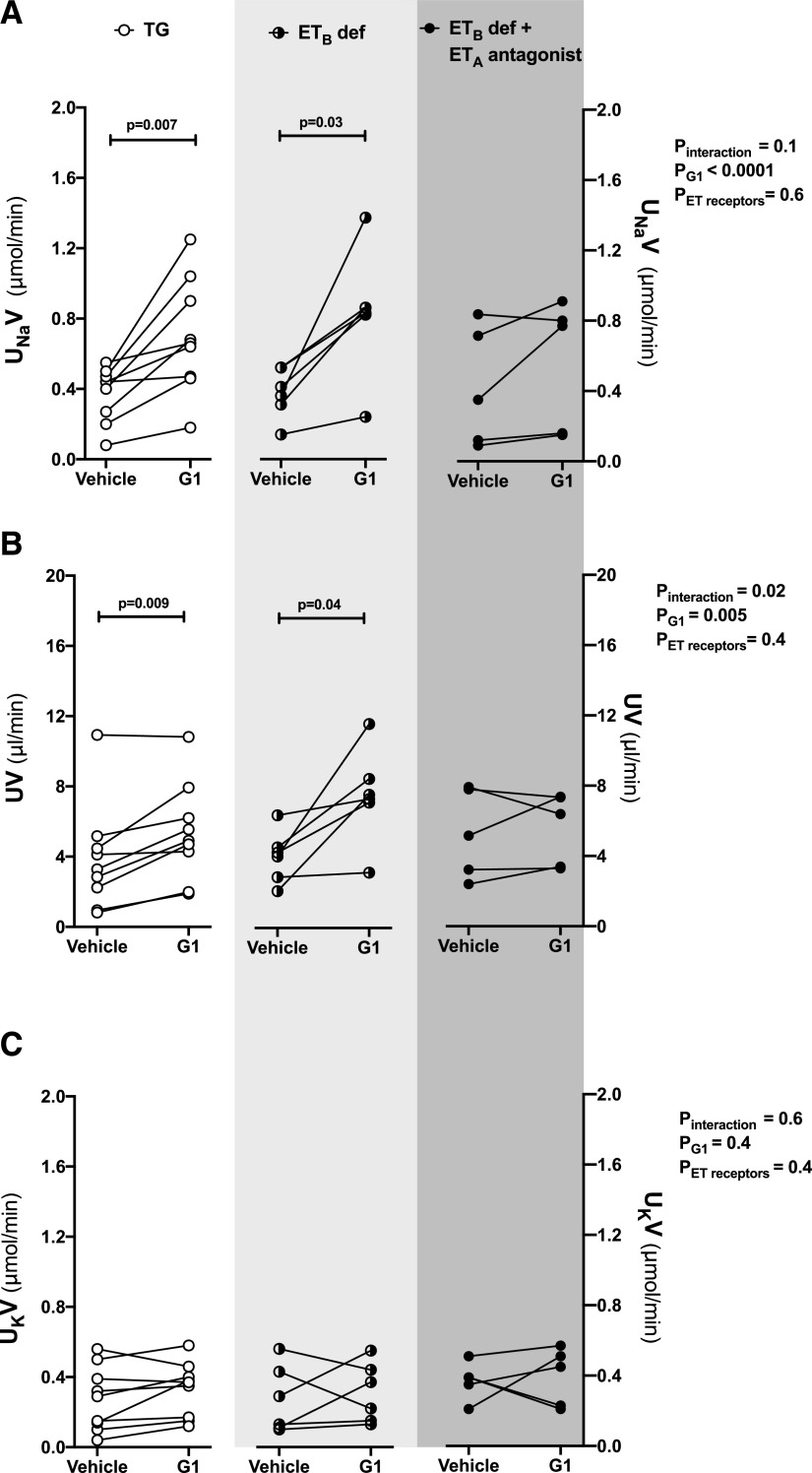
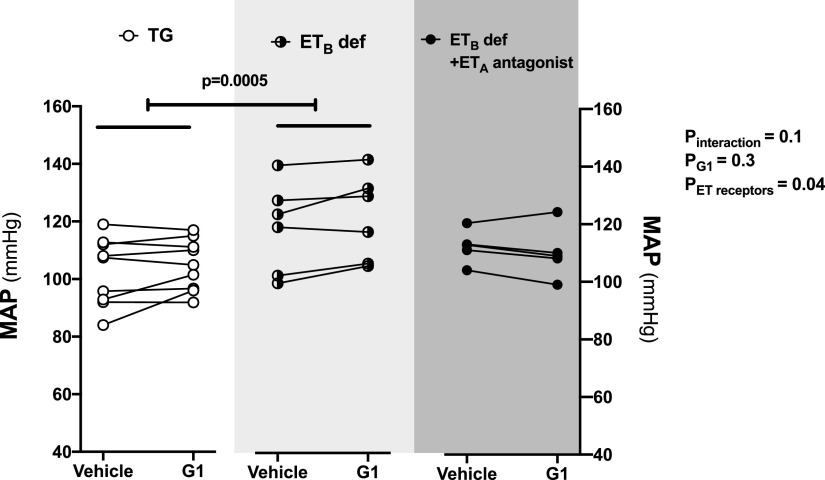
Intramedullary infusion of G1, the selective GPER1 agonist, increased the urinary Na+ excretion by 97% ± 19% and 126% ± 36% relative to baseline in TG control rats and ETB def rats, respectively (Fig. 2A). Urine flow also increased in response to G1 infusion in female TG control rats and ETB def rats (Fig. 2B. In both groups, urinary K+ excretion remained relatively stable during the infusion (Fig. 2C). As previously reported, MAP was higher in ETB def rats than in TG littermates at baseline and did not change after G1 infusion in either group (Fig. 3).
Fig. 2.
Blockade of ETA receptor abolished the natriuretic response to renal medullary GPER1 activation in female ETB def rats. Urinary Na+ excretion (UNaV) (A), urine flow (UV) (B), and urinary K+ excretion (UKV) (C) were measured during two 20-minute urine collection periods in anesthetized female ETB def rats or TG control rats: at baseline during the intramedullary infusion of vehicle and 20 minutes after the infusion of the GPER1 agonist G1 (5 pmol/kg per minute) was initiated. ETA receptor blockade was achieved by intravenous bolus injections of ABT-627 (5 mg/kg) 30 minutes before the beginning of the baseline urine collection period; TG and ETB def controls received intravenous bolus injections of vehicle. Each line shows data for an individual animal. Statistical comparisons were performed by repeated-measures two-way ANOVA followed by Dunnett’s post hoc tests.
Fig. 3.
Effect of ETA receptor blockade on blood pressure during renal medullary GPER1 activation in female ETB def rats. MAP was measured during two 20-minute urine collection periods in anesthetized female ETB def rats or TG control rats: at baseline during the intramedullary infusion of vehicle and 20 minutes after the infusion of the GPER1 agonist G1 (5 pmol/kg per minute) was initiated. ETA receptor blockade was achieved by intravenous bolus injection of ABT-627 (5 mg/kg) 30 minutes before the beginning of the baseline urine collection period in female ETB def rats; TG and ETB def controls received intravenous bolus injections of vehicle. Each line shows data for an individual animal. Statistical comparisons were performed by repeated-measures two-way ANOVA followed by Dunnett’s post hoc tests.
Pretreatment of ETB def rats with the ETA receptor antagonist ABT-627 prevented the G1-induced increase in urinary Na+ excretion and urine flow (Fig. 2, A and B) but did not affect urinary K+ excretion (Fig. 2C). Pretreatment of ETB def rats with ABT-627 also caused a slight decrease in MAP that did not reach statistical significance (Fig. 3) but did eliminate the difference in MAP between ETB def and TG controls.
Sprague-Dawley Rats.
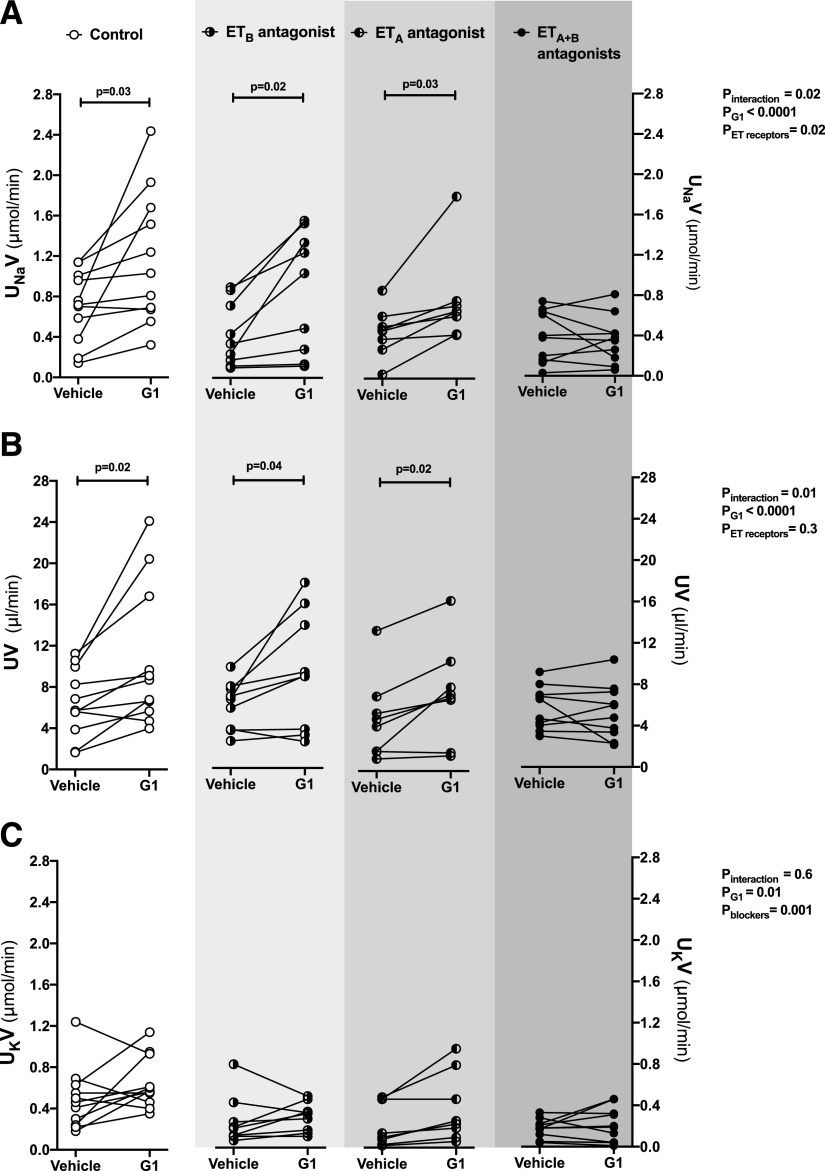
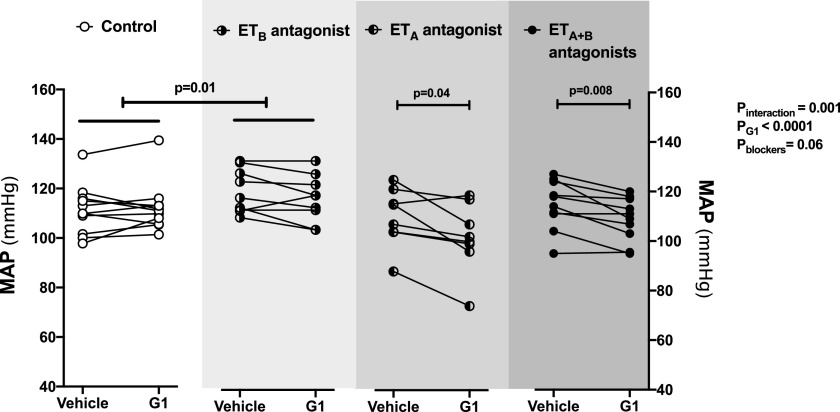
To examine the effects of blocking either ETA or ETB receptor alone, we conducted similar experiments in female SD rats. Intramedullary infusion of G1 in SD rats increased urinary Na+ excretion by 70% ± 24% relative to baseline (Fig. 4A), which was similar to our recent study (Gohar et al., 2020). Urine flow was also increased in response to G1 infusion in SD rats (Fig. 4B). No significant differences were observed in urinary K+ excretion or MAP during G1 infusion in these rats when compared with corresponding baseline values (Fig. 4C; Fig. 5). Pretreatment of the SD rats with either ABT-627 or A-192621 did not alter the G1-induced increase in urinary Na+ excretion or urine flow (Fig. 4, A and B). However, pretreatment with ABT-627 and A-192621 simultaneously completely abolished the natriuretic and diuretic response to G1 infusion (Fig. 4, A and B). G1 elicited a significant overall effect on urinary K+ excretion in SD rats (Fig. 4C). However, there was no significant increase in comparison with corresponding baseline values in SD rats pretreated with either ET-1 receptor antagonist alone or in combination (Fig. 4C). Similar to the increase in MAP observed in ETB def rats, treatment with A-192621 alone resulted in an increase in MAP that was stable across the experimental period (Fig. 5). However, this increase in MAP did not occur after pretreatment with ABT-627 and A-192621 simultaneously (Fig. 5). In fact, pretreatment with ABT-627 alone or in combination with A-192621 resulted in a small but statistically significant decline in MAP during G1 infusion (Fig. 5).
Fig. 4.
Combined blockade of ETA and ETB receptors abolished the natriuretic response to renal medullary GPER1 activation in female SD rats. Urinary Na+ excretion (UNaV) (A), urine flow (UV) (B), and urinary K+ excretion (UKV) (C) were measured during two 20-minute urine collection periods in anesthetized female SD rats: at baseline during the intramedullary infusion of vehicle and 20 minutes after intermedullary infusion of the GPER1 agonist G1 (5 pmol/kg per minute) was initiated. ETA and ETB receptor blockade was achieved by intravenous bolus injection of ABT-627 (5 mg/kg) and A-192621 (10 mg/kg), respectively, 30 minutes before the beginning of the baseline urine collection period; controls received intravenous bolus injections of vehicle. Each line shows data for an individual animal. Statistical comparisons were performed by repeated-measures two-way ANOVA followed by Dunnett’s post hoc tests.
Fig. 5.
Effect of ETA and/or ETB receptor blockade on blood pressure during renal medullary GPER1 activation in female SD rats. MAP was measured during two 20-minute urine collection periods in anesthetized female SD rats: at baseline during the intramedullary infusion of vehicle and 20 minutes after intermedullary infusion of the GPER1 agonist G1 (5 pmol/kg per minute) was initiated. ETA and ETB receptor blockade was achieved by intravenous bolus injection of ABT-627 (5 mg/kg) and A-192621 (10 mg/kg), respectively, 30 minutes before the beginning of the baseline urine collection period; controls received intravenous bolus injections of vehicle. Each line shows data for an individual animal. Statistical comparisons were performed by repeated-measures two-way ANOVA followed by Dunnett’s post hoc tests.
Discussion
In this study, we sought to elucidate the contribution of ETA and ETB receptors as downstream mediators of renal medullary GPER1-induced natriuresis. Overall, we provide genetic and pharmacologic evidence for a functional role of these receptors within the renal medulla in mediating GPER1-induced natriuresis. These results are compatible with previous evidence showing that ETA and ETB receptors promote water and Na+ excretion (Ge et al., 2008; Boesen and Pollock, 2010).
In the present study, we demonstrated that activation of GPER1 within the medulla of the kidney of female SD rats evokes a natriuretic and a diuretic effect, consistent with our recent findings (Gohar et al., 2020). Notably, several studies have demonstrated that GPER1 activation ameliorates salt-induced cardiovascular and kidney damage (Jessup et al., 2010; Lindsey et al., 2011; Liu et al., 2016). Specifically, GPER1 activation was shown to attenuate salt-induced cardiac remodeling (Jessup et al., 2010), vascular injury (Liu et al., 2016), and proteinuria (Lindsey et al., 2011) in mRen2.Lewis rats in a blood pressure–independent manner. The renoprotective actions of GPER1 activation were linked to decreased tubular oxidative stress and increased megalin-mediated protein reabsorption (Lindsey et al., 2011). However, the relationship between GPER1 activation and Na+ homeostasis was not defined. Our recently published study showed that renal medullary GPER1 functions as a novel female-specific pronatriuretic factor via an ET-1–dependent signaling pathway. We also revealed that the mRNA expression of ET-1 and the ETA and ETB receptors is diminished in female GPER1-knockout mice compared with wild-type controls (Gohar et al., 2020). This effect was not evident in male mice (Gohar et al., 2020).
In the current study, we found that genetic deficiency or pharmacologic blockade of ETB receptors does not attenuate the G1-induced natriuresis observed in female SD rats, suggesting that the pronatriuretic actions of GPER1 can be mediated via ETA receptors. Hence, the inhibition of GPER1-induced natriuresis requires concomitant antagonism of ETA and ETB receptors in female rats. Nakano and Pollock (2009) demonstrated that ETA receptor contributes to ET-1–dependent natriuresis in female rats, and it has been shown that ETA and ETB receptors work synergistically to promote water and Na+ excretion (Ge et al., 2008; Boesen and Pollock, 2010). These latter findings are consistent with our present findings, suggesting that ETA and ETB receptors help efficient excretion of salt and water.
Our observation that ETA or ETB receptor can mediate the G1 natriuretic effect alternatively under conditions of loss of the other receptor subtype is interesting, suggesting the capability of one ET receptor to compensate for the loss of function of the other receptor. However, this observation does not negate the possibility that one receptor may be solely or predominantly mediating GPER1 natriuretic action under normal physiologic conditions when both ET receptors are functional.
This interaction between ETA and ETB receptors has also been documented within the vasculature (White et al., 1993; Seo et al., 1994; Inscho et al., 2005). Inscho et al. (2005) showed that both ETA and ETB receptors contribute to ET-1–induced vasoconstriction of afferent arterioles, highlighting a possible interaction between ETA and ETB receptors in the control of afferent arteriolar vascular tone. Similarly, ET-1 actions via ETB and possibly ETA receptors contribute to the blunted renal autoregulation in salt-loaded rats (Fellner et al., 2015). It has also been demonstrated that ETA receptor expression in the lung is diminished in ETB knockout mice (Kuc et al., 2006). Notably, fluorescence resonance energy transfer experiments demonstrated that ETA and ETB receptors exist as constitutive homodimers and heterodimers (Gregan et al., 2004a,b). In addition, Kapsokalyvas et al. (2014) provided imaging evidence for ETA and ETB receptor heterodimerization in rat mesenteric resistance arteries. Further experiments are required to identify whether heterodimers exist in epithelial cells and whether such dimers have relevance to the functional interaction between ETA and ETB receptors.
We demonstrated that ETA receptor blockade alone does not mitigate G1-induced natriuresis in female SD rats. However, whether the GPER1/ETA receptor–mediated natriuretic effect is a compensatory mechanism that occurs only under conditions of ETB receptor dysfunction remains unclear. The observation that both ETA and ETB receptors contribute to mediating the natriuretic response to GPER1, which is endogenously activated by estradiol in females, may have important implications for therapeutic use of dual ETA and ETB antagonists in female patients.
Changes in blood pressure and consequently pressure natriuresis do not appear to account for G1 natriuretic actions. We found that pharmacologic blockade of ETA in the presence or absence of ETB receptor blockade in SD rats decreases blood pressure before and after GPER1 activation. Importantly, GPER1-induced natriuresis was still evident despite the lower blood pressure produced by the ETA antagonist. We also found that genetic deficiency or pharmacologic blockade of ETB receptors elevates blood pressure, similar to previous observations (Gariepy et al., 2000; Pollock and Pollock, 2001; Ge et al., 2006). Furthermore, G1 evoked a natriuretic action in animals with elevated blood pressure due to loss of ETB receptor function. Whether GPER1-induced natriuresis is evident in other experimental models of hypertension remains to be determined.
The localization of GPER1 and ETA and ETB receptors is also appropriate to support the crosstalk we observed between these receptors in the kidney. Multiple studies have shown that GPER1 is expressed in kidney cells (Hazell et al., 2009; Lindsey et al., 2011; Cheng et al., 2014; Li et al., 2014; Cheema et al., 2015). Earlier studies in which Davidoff et al. (1980) tested the binding capacity and affinity for [3H]estradiol in the cytosolic fraction of rat renal homogenates revealed radioactivity within the distal part of the tubule, specifically in the inner medullary collecting duct cells (Davidoff et al., 1980). In line with this finding, immunohistology revealed that GPER1 is expressed in the rat renal inner medulla (Hazell et al., 2009; Gohar et al., 2020). Similarly, studies with radioactively labeled ET-1 showed ET-1 binding primarily in the renal medulla (Davenport et al., 1989), and a high density of ET receptors was detected in inner medullary collecting duct cells (Kohan et al., 1992). Overall, our study identifies a novel role for rapid estrogenic signaling as an upstream regulator of an ET-1/ETA/ETB pronatriuretic signaling system, supporting the importance of the intrarenal ET-1 system in renal Na+ handling, particularly in females.
The regulation of natriuresis is highly integrated and involves many neural, vascular, and tubular signaling events within the kidney. We previously demonstrated that G1 downregulates Na+/K+ ATPase activity in the renal outer medulla (Gohar et al., 2020). However, we do not know the contribution of the vascular and neuronal components to GPER1-induced natriuresis, if any. Evidence points to a vasodilatory response to GPER1 activation in multiple extrarenal vascular beds (Haynes et al., 2002; Broughton et al., 2010; Lindsey et al., 2014; Tropea et al., 2015; Peixoto et al., 2017; Fredette et al., 2018). Similarly, the GPER1 agonist, G1, induces a vasodilation in isolated perfused rat kidneys preconstricted with phenylephrine (Kurt and Buyukafsar, 2013). In contrast, G1 evokes a vasoconstrictor response in the isolated perfused rat kidney under basal renal perfusion pressure (Kurt and Buyukafsar, 2013). Importantly, renal medullary GPER1 activation did not change blood flow in the medulla of the kidney (Gohar et al., 2020). Despite reports that GPER1 can modulate neuronal function, its role in regulating renal nerves is not clear. Li et al. (2016) provided evidence that GPER1 inhibits colonic motility by enhancing nitric oxide release from nitrergic nerves. GPER1 also appears to exert an inhibitory effect on neuronal apoptosis in the hippocampus (Han et al., 2019). Furthermore, GPER1 activation induces bradycardic effects via activation of cardiac parasympathetic neurons (Brailoiu et al., 2013). Additional studies are required to determine the contribution of renal nerves, if any, to GPER1-dependent natriuresis and how that relates to hypertension.
Previous studies have shown that systemic GPER1 activation lowers blood pressure in ovariectomized SD rats (Gohar et al., 2020) and mRen2.Lewis rats (Lindsey et al., 2009). Further, natriuresis evoked by renal medullary GPER1 activation is evident after ovariectomy (Gohar et al., 2020). Indeed, ovariectomy did not impact GPER1 protein expression in the renal medulla (Gohar et al., 2020). Of note, ovariectomy increased the mRNA expression of ETA and ETB in renal inner medulla (Gohar et al., 2016b). However, the contribution of ET receptors to GPER1-induced natriuresis under conditions of sex hormonal deficiency remains to be determined.
Collectively, we have demonstrated that G1 activates the GPER1/ETA/ETB axis, which in turn triggers natriuresis, suggesting that targeting GPER1 may be a useful approach to maintaining cardiovascular health in women. The question of whether GPER1 activation triggers an increase in ET-1 release and/or production remains to be answered. The effects of GPER1 activity on transport activity related to Ca2+ influx and specific renal Na+ transporters need to be investigated. Further research is also required to determine the functional significance of the interaction between GPER1 and ET receptors with regard to Na+ balance and hypertension and to expand our understanding of GPER1 actions on renal electrolyte handling and salt sensitivity.
Acknowledgments
The authors would like to thank Rawan N. Almutlaq for technical assistance with atomic absorption measurement.
Abbreviations
- ET-1
endothelin-1
- ETA
endothelin receptor subtype A
- ETB
endothelin receptor subtype B
- ETB def
ETB-deficient
- GPER1
G protein–coupled estrogen receptor 1
- MAP
mean arterial pressure
- SD
Sprague-Dawley
- TG
transgenic
Authorship Contributions
Participated in research design: Gohar, Pollock
Conducted experiments: Gohar.
Performed data analysis: Gohar.
Wrote or contributed to the writing of the manuscript: Gohar, Pollock.
Footnotes
This work was supported by the American Heart Association [Grant 18CDA34110010] (to E.Y.G.) and National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant K99-DK119413] (to E.Y.G.) and National Heart, Lung, and Blood Institute [Grant P01-HL136267] (to D.M.P.).
References
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, et al. (2007) G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866. [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Andersen HS, Shufelt CL. (2015) Gender, cardiovascular disease, and the sexism of obesity. J Am Coll Cardiol 66:1958–1960. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2018) Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation 137:e67–e492. [DOI] [PubMed] [Google Scholar]
- Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, Horuk R. (2009) Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J Neuroimmunol 214:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesen EI. (2015) Endothelin receptors, renal effects and blood pressure. Curr Opin Pharmacol 21:25–34. [DOI] [PubMed] [Google Scholar]
- Boesen EI, Pollock DM. (2010) Cooperative role of ETA and ETB receptors in mediating the diuretic response to intramedullary hyperosmotic NaCl infusion. Am J Physiol Renal Physiol 299:F1424–F1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, et al. (2006) Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Arterburn JB, Oprea TI, Chitravanshi VC, Brailoiu E. (2013) Bradycardic effects mediated by activation of G protein-coupled estrogen receptor in rat nucleus ambiguus. Exp Physiol 98:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Miller AA, Sobey CG. (2010) Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol 298:H1055–H1061. [DOI] [PubMed] [Google Scholar]
- Chang Y, Han Z, Zhang Y, Zhou Y, Feng Z, Chen L, Li X, Li L, Si JQ. (2019) G protein-coupled estrogen receptor activation improves contractile and diastolic functions in rat renal interlobular artery to protect against renal ischemia reperfusion injury. Biomed Pharmacother 112:108666. [DOI] [PubMed] [Google Scholar]
- Cheema MU, Irsik DL, Wang Y, Miller-Little W, Hyndman KA, Marks ES, Frøkiær J, Boesen EI, Norregaard R. (2015) Estradiol regulates AQP2 expression in the collecting duct: a novel inhibitory role for estrogen receptor α. Am J Physiol Renal Physiol 309:F305–F317. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, Thomas P, Filardo EJ. (2014) Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol Cell Endocrinol 382:950–959. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Nunez DJ, Brown MJ. (1989) Binding sites for 125I-labelled endothelin-1 in the kidneys: differential distribution in rat, pig and man demonstrated by using quantitative autoradiography. Clin Sci (Lond) 77:129–131. [DOI] [PubMed] [Google Scholar]
- Davidoff M, Caffier H, Schiebler TH. (1980) Steroid hormone binding receptors in the rat kidney. Histochemistry 69:39–48. [DOI] [PubMed] [Google Scholar]
- Dhaun N, Webb DJ. (2008) Endothelin-receptor antagonism: the future is bright. Lancet 371:2061–2062. [DOI] [PubMed] [Google Scholar]
- Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL, American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council (2016) Salt sensitivity of blood pressure: a scientific statement from the American heart association. Hypertension 68:e7–e46. [DOI] [PubMed] [Google Scholar]
- Fellner RC, Guan Z, Cook AK, Pollock DM, Inscho EW. (2015) Endothelin contributes to blunted renal autoregulation observed with a high-salt diet. Am J Physiol Renal Physiol 309:F687–F696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredette NC, Meyer MR, Prossnitz ER. (2018) Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J Steroid Biochem Mol Biol 176:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. (2000) Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest 105:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. (1998) Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest 102:1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. (2006) Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291:F1274–F1280. [DOI] [PubMed] [Google Scholar]
- Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. (2008) Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol 295:F1635–F1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar EY, Daugherty EM, Aceves JO, Sedaka R, Obi IE, Allan JM, Soliman RH, Jin C, De Miguel C, Lindsey SH, et al. (2020) Evidence for G-protein-coupled estrogen receptor as a pronatriuretic factor. J Am Heart Assoc 9:e015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar EY, Kasztan M, Becker BK, Speed JS, Pollock DM. (2017) Ovariectomy uncovers purinergic receptor activation of endothelin-dependent natriuresis. Am J Physiol Renal Physiol 313:F361–F369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar EY, Speed JS, Kasztan M, Jin C, Pollock DM. (2016a) Activation of purinergic receptors (P2) in the renal medulla promotes endothelin-dependent natriuresis in male rats. Am J Physiol Renal Physiol 311:F260–F267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar EY, Yusuf C, Pollock DM. (2016b) Ovarian hormones modulate endothelin A and B receptor expression. Life Sci 159:148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan B, Jürgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. (2004a) Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem 279:27679–27687. [DOI] [PubMed] [Google Scholar]
- Gregan B, Schaefer M, Rosenthal W, Oksche A. (2004b) Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol 44 (Suppl 1):S30–S33. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanović M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, et al. (2009) Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104:288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. (2012) Hypertension: physiology and pathophysiology. Compr Physiol 2:2393–2442. [DOI] [PubMed] [Google Scholar]
- Han ZW, Chang YC, Zhou Y, Zhang H, Chen L, Zhang Y, Si JQ, Li L. (2019) GPER agonist G1 suppresses neuronal apoptosis mediated by endoplasmic reticulum stress after cerebral ischemia/reperfusion injury. Neural Regen Res 14:1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes MP, Li L, Russell KS, Bender JR. (2002) Rapid vascular cell responses to estrogen and membrane receptors. Vascul Pharmacol 38:99–108. [DOI] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. (2009) Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol 202:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocher B, Ehrenreich H. (2002) ETB receptor deficiency causes salt-sensitive hypertension. J Mol Med (Berl) 80:747–749; author reply 750–752. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Imig JD, Cook AK, Pollock DM. (2005) ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol 146:1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. (2010) Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLoS One 5:e15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsokalyvas D, Schiffers PM, Maij N, Suylen DP, Hackeng TM, van Zandvoort MA, De Mey JG. (2014) Imaging evidence for endothelin ETA/ETB receptor heterodimers in isolated rat mesenteric resistance arteries. Life Sci 111:36–41. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE. (2011) Endothelin and collecting duct sodium and water transport. Contrib Nephrol 172:94–106. [DOI] [PubMed] [Google Scholar]
- Kohan DE, Hughes AK, Perkins SL. (1992) Characterization of endothelin receptors in the inner medullary collecting duct of the rat. J Biol Chem 267:12336–12340. [PubMed] [Google Scholar]
- Kohan DE, Inscho EW, Wesson D, Pollock DM. (2011) Physiology of endothelin and the kidney. Compr Physiol 1:883–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuc RE, Maguire JJ, Davenport AP. (2006) Quantification of endothelin receptor subtypes in peripheral tissues reveals downregulation of ET(A) receptors in ET(B)-deficient mice. Exp Biol Med (Maywood) 231:741–745. [PubMed] [Google Scholar]
- Kurt AH, Bozkus F, Uremis N, Uremis MM. (2016) The protective role of G protein-coupled estrogen receptor 1 (GPER-1) on methotrexate-induced nephrotoxicity in human renal epithelium cells. Ren Fail 38:686–692. [DOI] [PubMed] [Google Scholar]
- Kurt AH, Buyukafsar K. (2013) Vasoconstriction induced by G1, a G-protein-coupled oestrogen receptor1 (GPER-1) agonist, in the isolated perfused rat kidney. Eur J Pharmacol 702:71–78. [DOI] [PubMed] [Google Scholar]
- Leifheit-Limson EC, D’Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, Krumholz HM, Lichtman JH. (2015) Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO study. J Am Coll Cardiol 66:1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu J, Jiang F, Jiang Z, Liu C, Li L, Luo Y, Lu R, Mu Y, Liu Y, et al. (2016) G protein-coupled estrogen receptor is involved in modulating colonic motor function via nitric oxide release in C57BL/6 female mice. Neurogastroenterol Motil 28:432–442. [DOI] [PubMed] [Google Scholar]
- Li YC, Ding XS, Li HM, Zhang Y, Bao J. (2014) Role of G protein-coupled estrogen receptor 1 in modulating transforming growth factor-β stimulated mesangial cell extracellular matrix synthesis and migration. Mol Cell Endocrinol 391:50–59. [DOI] [PubMed] [Google Scholar]
- Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. (2009) Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology 150:3753–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Liu L, Chappell MC. (2014) Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. (2011) Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Kashyap S, Murphy B, Hutson DD, Budish RA, Trimmer EH, Zimmerman MA, Trask AJ, Miller KS, Chappell MC, et al. (2016) GPER activation ameliorates aortic remodeling induced by salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 310:H953–H961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. (2009) Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 58:2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers KE, Sethna C. (2013) Endothelin antagonists in hypertension and kidney disease. Pediatr Nephrol 28:711–720. [DOI] [PubMed] [Google Scholar]
- Moorhouse RC, Webb DJ, Kluth DC, Dhaun N. (2013) Endothelin antagonism and its role in the treatment of hypertension. Curr Hypertens Rep 15:489–496. [DOI] [PubMed] [Google Scholar]
- Nakano D, Pollock DM. (2009) Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. (2020) Hypertension prevalence among adults aged 18 and over: United States, 2017-2018. NCHS Data Brief:1–8. [PubMed] [Google Scholar]
- Patel SA, Winkel M, Ali MK, Narayan KM, Mehta NK. (2015) Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med 163:245–253. [DOI] [PubMed] [Google Scholar]
- Peixoto P, Aires RD, Lemos VS, Bissoli NS, Santos RLD. (2017) GPER agonist dilates mesenteric arteries via PI3K-Akt-eNOS and potassium channels in both sexes. Life Sci 183:21–27. [DOI] [PubMed] [Google Scholar]
- Pollock DM, Pollock JS. (2001) Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281:F144–F150. [DOI] [PubMed] [Google Scholar]
- Pollock JS, Pollock DM. (2019) SONAR propels endothelin A receptor antagonists to success. Nat Rev Nephrol 15:461–462. [DOI] [PubMed] [Google Scholar]
- Qiao C, Ye W, Li S, Wang H, Ding X. (2018) Icariin modulates mitochondrial function and apoptosis in high glucose-induced glomerular podocytes through G protein-coupled estrogen receptors. Mol Cell Endocrinol 473:146–155. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630. [DOI] [PubMed] [Google Scholar]
- Seo B, Oemar BS, Siebenmann R, von Segesser L, Lüscher TF. (1994) Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation 89:1203–1208. [DOI] [PubMed] [Google Scholar]
- Speed JS, Fox BM, Johnston JG, Pollock DM. (2015) Endothelin and renal ion and water transport. Semin Nephrol 35:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed JS, Hyndman KA. (2016) In vivo organ specific drug delivery with implantable peristaltic pumps. Sci Rep 6:26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec DE, Mattson DL, Roman RJ. (1997) Inhibition of renal outer medullary 20-HETE production produces hypertension in Lewis rats. Hypertension 29:315–319. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632. [DOI] [PubMed] [Google Scholar]
- Tropea T, De Francesco EM, Rigiracciolo D, Maggiolini M, Wareing M, Osol G, Mandalà M. (2015) Pregnancy augments G protein estrogen receptor (GPER) induced vasodilation in rat uterine arteries via the nitric oxide - cGMP signaling pathway. PLoS One 10:e0141997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, Offner H. (2009) Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol 182:3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessale JL, Adler AL, Novosad EI, Calzadilla SV, Dayton BD, Marsh KC, Winn M, Jae HS, von Geldern TW, Opgenorth TJ. (2002) Pharmacology of endothelin receptor antagonists ABT-627, ABT-546, A-182086 and A-192621: ex vivo and in vivo studies. Clin Sci (Lond) 103 (Suppl 48):112S–117S. [DOI] [PubMed] [Google Scholar]
- White DG, Cannon TR, Garratt H, Mundin JW, Sumner MJ, Watts IS. (1993) Endothelin ETA and ETB receptors mediate vascular smooth-muscle contraction. J Cardiovasc Pharmacol 22 (Suppl 8):S144–S148. [DOI] [PubMed] [Google Scholar]
- Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. (1989) Endothelin, a peptide inhibitor of Na(+)-K(+)-ATPase in intact renaltubular epithelial cells. Am J Physiol 257:C1101–C1107. [DOI] [PubMed] [Google Scholar]