Abstract
The role of α1-adrenergic receptors (α1-ARs) and their subtypes in metabolism is not well known. Most previous studies were performed before the advent of transgenic mouse models and utilized transformed cell lines and poorly selective antagonists. We have now studied the metabolic regulation of the α1A- and α1B-AR subtypes in vivo using knock-out (KO) and transgenic mice that express a constitutively active mutant (CAM) form of the receptor, assessing subtype-selective functions. CAM mice increased glucose tolerance while KO mice display impaired glucose tolerance. CAM mice increased while KO decreased glucose uptake into white fat tissue and skeletal muscle with the CAM α1A-AR showing selective glucose uptake into the heart. Using indirect calorimetry, both CAM mice demonstrated increased whole body fatty acid oxidation, while KO mice preferentially oxidized carbohydrate. CAM α1A-AR mice displayed significantly decreased fasting plasma triglycerides and glucose levels while α1A-AR KO displayed increased levels of triglycerides and glucose. Both CAM mice displayed increased plasma levels of leptin while KO mice decreased leptin levels. Most metabolic effects were more efficacious with the α1A-AR subtype. Our results suggest that stimulation of α1-ARs results in a favorable metabolic profile of increased glucose tolerance, cardiac glucose uptake, leptin secretion, and increased whole body lipid metabolism that may contribute to its previously recognized cardioprotective and neuroprotective benefits.
Keywords: Adrenergic Receptor, metabolism, fatty acid oxidation, leptin
Introduction
α1-adrenergic receptors (ARs) are G-protein coupled receptors that mediate the sympathetic nervous system. They are well documented for their role in regulating neurotransmission, heart function, cardiac hypertrophy and blood pressure (reviewed in 1). There are nine AR subtypes that all bind epinephrine and norepinephrine (β1, β2, β3, α2A, α2B, α2C, α1A, α1B, α1D), but each with both common, yet distinctive, and sometimes opposite, functional roles. Previous studies on AR regulation of metabolism concentrated on β-ARs or α2-ARs, not α1-ARs. α1-AR-mediated metabolism was explored mostly in the 1980s and used poorly selective antagonists and utilized mostly transformed cell lines to assess functions before transgenic approaches were made available.
The autonomic nervous system is well known to regulate both glucose and fatty acid metabolism through ARs via both neurotransmission as well as hormonal effects. In general, catecholamines direct metabolic effects towards substrate mobilization and utilization in order to meet the increased energy requirements of stress or the “fight or flight” response.
It is well known that norepinephrine mediates multi-faceted aspects of metabolism. Early studies indicated that α1-ARs stimulate gluconeogenesis and ketogenesis in the liver (2) and suppress triglyceride secretion (3). α1-ARs have also been shown to regulate glucose uptake into various cell lines (4–6), but never shown to regulate glycolysis. α1B-AR KO mice were previously reported to be insulin resistant and impaired glucose homeostasis (7). The β-ARs are well known mediators of adipocyte metabolism through their regulation of cAMP levels in both white and brown fat (8) and are lipolytic (9). However, the β1/β2/β3-AR triple KO mice still display norepinephrine-induced lipolysis, suggesting involvement of another AR subtype (10). α2-AR and in particular α2A-AR stimulation inhibits insulin secretion (11) and are anti-lipolytic (12). Therefore, there was significant previous data to suggest that α1-ARs can regulate whole body and tissue-specific metabolism that was different from either β-or α2-ARs.
Early studies also indicted that α1-ARs stimulate fatty acid oxidation in hepatocytes but there are no reports outside of the liver or in whole body metabolism (3, 13–16). In the present study, we made use of novel transgenic mice with systemic overexpression of the α1A- and α1B-ARs using large fragments of the isogenic promoters. These receptors also contain mutations that render them constitutively active and are chronically activated even when an agonist is not present. These mice provide tools for assessing α1-AR subtype-selective in vivo signaling. Using these and the corresponding KO mice, we now describe the in vivo metabolic functions regulated by this receptor family.
Materials and Methods
Animal use.
Transgenic mouse models (mixed B6CBA background) that systemically over express the α1A-AR or α1B-AR subtypes were previously described and characterized in our laboratory (17–18). These mice express constitutively active mutants (CAM) of the receptors under the control of their native mouse promoter to increase subtype-selective signaling in tissues that naturally express that subtype. The α1-AR KO mice were obtained from Paul Simpson, MD (19–20), maintained on a C57BL6 background, and backcrossed every 10 generations. B6CBA WT mice were obtained from Jackson Laboratory, then bred internally and used as controls for the CAM mice. C57BL/6 WT were also obtained from Jackson Laboratory, bred internally, the used as controls for the KO mice. Equal numbers of both male and female mice (unless otherwise indicated) from 2–4 mo of age were used in a randomized fashion in each experiment. Mice were given a normal standard laboratory chow diet (Harlan, #2918), housed on a 12-hour light-dark cycle, temperature-controlled facility at 70°F with free access to food and water unless otherwise indicated. Mice were provided veterinary care in an AAALAC-accredited animal care facility. The experimental protocols employed in this study conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the Animal Care and Use Committee at our institutions (Protocol 0844).
Blood Profiles.
Mice were transferred to wood chip bedding and fasted for 6 hours. Mice were then anesthetized with Nembutal (ip, 50mg/ml) and blood collected from the vena cava into 1.5ml polypropylene microfuge tubes. The blood samples were allowed to sit at room temperature for 30 min to coagulate, then centrifuged at 2000 x g for 10 min at 4°C. The sera was transferred to fresh tubes and re-centrifuged to remove blood cells. Samples are stored at −80°C. Measurement of cholesterol, triglycerides, urea and electrolytes were analyzed by the Veterinary Diagnostic Services of Marshfield Laboratories (Cleveland, Ohio) using standard clinical procedures.
Glucose Tolerance.
Mice were fasted for 6 hours on hardwood bedding and weighed. Sterile 20% D-Glucose (Sigma) solution was injected intraperitoneal at a concentration of 2g/kg body weight. Whole blood samples were taken from a tail cut immediately before the injection of glucose (designated as time 0 min) and at 30, 60, 30, 90 and 120 min after the injection of glucose. Glucose levels were measured using a Nova Max Plus glucometer according to manufacture’s instructions (Nova Biomedical Corporation).
Indirect Calorimetry.
Case Western Reserve University Mouse Metabolic Phenotyping Center (MMPC) performed the indirect calorimetry using established procedures (21–22). Metabolic rates were measured by indirect calorimetry in mice using an 8-chamber open-circuit Oxymax system (CLAMS, Columbus Instruments, Columbus, OH). Briefly, mice were acclimated to the experimental room for 1 week prior to the experiment. The mice were individually housed in acrylic calorimeter chambers through which air with a known O2 concentration is passed at a constant flow rate. The system automatically withdraws gas samples from each chamber hourly for 24 h. The system then calculated the volumes of O2 consumed (VO2) and CO2 generated (VCO2) by each mouse in 1 h. The respiratory quotient (RQ), which is the ratio of VCO2 to VO2, is calculated. Heat or energy expenditures were measured throughout the study and measurements are carried out in both light and dark cycles and both fed and fasting conditions. Mice were maintained at 25°C and had free access to water in all conditions.
Tissue 2-Deoxyglucose Uptake.
Equal numbers of male and female mice were fasted for 6 hours then injected with [3H]-2-deoxyglucose (2DG)(20uCi/mouse; ip). Blood samples were taken from the tail vein at 30, 60, 90 mins post-injection to determine blood glucose levels using a Nova Max Plus glucometer according to manufacturer’s instructions (Nova Biomedical Corporation, Waltham, MA). Plasma samples were also prepared at the same time to determine [3H]-2DG specific activity. After the final collection of blood, mice were euthanized with pentobarbitol (i.p. 60mg/kg body weight) and various tissues removed, rinsed in PBS, diced and frozen in dry ice. For skeletal muscles, hindlimb muscle was obtained from the gatrocnemius while forelimb muscle was from the tricep. Tissues were processed with perchloric acid, barium hydroxide/zinc sulfate and glucose uptake rate was calculated according to the method of Ferre’, et al (23), using the following equation, Rate= [2-deoxyglucose 6-phosphate]τ/ LC 0 ∫ τ (CB*/CB)dt where τ is the sampling time, CB* is the blood 2-deoxyglucose expressed in terms of radioactivity and CB is the blood glucose concentration. The lump constant (LC) is a correction factor for the discrimination against 2-deoxyglucose in glucose transport and phosphorylation pathways determined in vitro by comparing glucose and 2-deoxyglucose fractional extraction by the different tissues.
Leptin.
Mice were put into cages with wood-chip bedding and were fasted for 6 hours prior to drawing blood. Blood was drawn from mice by making a small cut in the tail after anesthesia with ketamine (87.5mg/kg) /xylazine (12.5mg/kg) cocktail. The blood was centrifuged for 10 minutes at 2000x g, the sera collected, and stored at −80° until processed. The sera were analyzed for leptin using the mouse/rat leptin enzyme immunoassay kit from SPI BIO (Montigny le Bretonneux, France) (cat#A05176) according to manufacturer’s procedures. Briefly, samples and standards were pipetted onto the 96-well plate pre-coated with a polyclonal anti-mouse leptin antibody. The plate was covered and incubated for 1 hour with shaking. The wells were rinsed and biotin-labelled anti-leptin antibody was dispensed in each well and incubated as before for another hour. The wells were washed followed by incubation with Streptavidin-HRP conjugate for 30 minutes. The wells were washed and substrate solution was added to each well and the plate was incubated in the dark for 10 minutes at room temperature. The color development was stopped and the absorbance at 450 nm was read within 5 minutes. The concentration of the samples was read comparing the absorbance to the standards plotted on a log-log graph.
Statistical Analysis.
One Way Analysis of Variance and Newman-Keuls post-test were used to compare functional and signaling parameters in the different mouse models and experimental conditions. A repeated-measures One Way Analysis of Variance was assessed on the glucose tolerance and respiration quotient data. A probability value p< 0.05 was considered statistically significant. Prism software (GraphPad, San Diego, CA) was used for all data analyses.
Results
α1-AR activation increased glucose clearance from the blood.
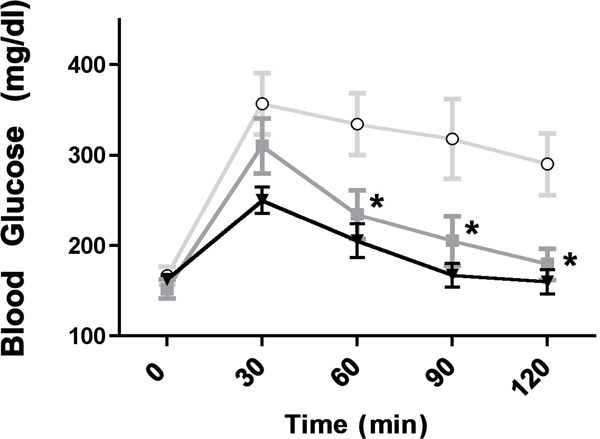
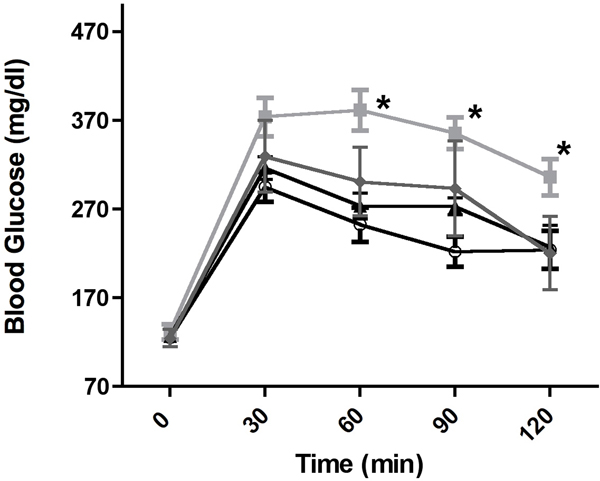
We recently found that α1-ARs protect against low glucose damage during ischemia by increasing the uptake of glucose into myocytes through activating GLUT transporters GLUT1 and GLUT4 (24). Therefore, we hypothesized that α1-AR activation may also affect blood glucose levels. Utilizing a glucose tolerance test, we found that both CAM α1-AR mice (p<0.01) selectively enhanced glucose clearance from the blood using the Repeated Measures ANOVA followed by the Newman-Keuls Multiple Comparison Test. (F(2,4)=8.78, p<0.01, N=8 or 10 mice) (Fig 1A). While both α1B-AR KO or α1A/B double KO mice displayed elevated blood glucose levels, only the α1A-AR KO (p<0.01) mice displayed significantly reduced glucose uptake compared to the control (F(3,4)=44.39, p<0.001).
Figure 1. α1-AR regulation of glucose tolerance.
Mice are fasted for 6 hours and weighed. 20% glucose is injected intra-peritoneal at a concentration of 2g/kg body weight. Whole blood samples are taken from a tail cut immediately before the injection of glucose (designated as time 0 min) and at 30, 60, 30, 90 and 120 min after the injection of glucose. Glucose levels were measured using a Nova Max Plus glucometer according to manufacture’s instructions. A. Glucose tolerance levels in CAM α1A-AR (squares), CAM α1B-AR (triangles) mice and WT control (circles). B. Glucose tolerance levels in α1A-AR KO (squares), α1B-AR KO (triangle), α1A/B-AR double KO (diamonds) and WT controls (circles). * p≤ 0.01, statistically significant from WT control using the Repeated Measures ANOVA followed by the Newman-Keuls Multiple Comparison Test. N=8 or 10 individual mice.
α1-AR activation increased 2-deoxyglucose uptake into heart, white fat, and faster twitch skeletal muscle.
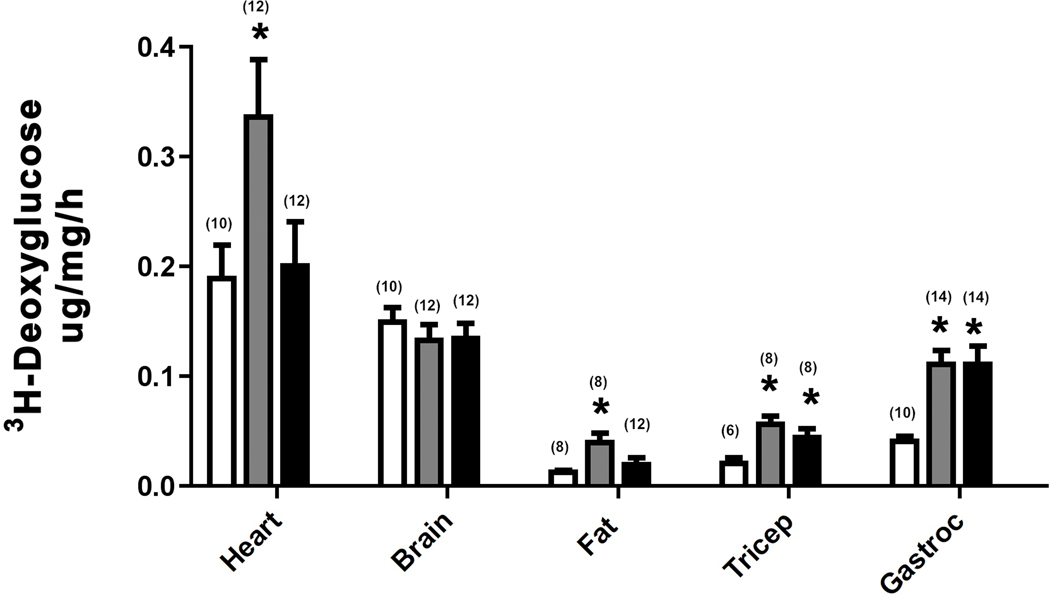
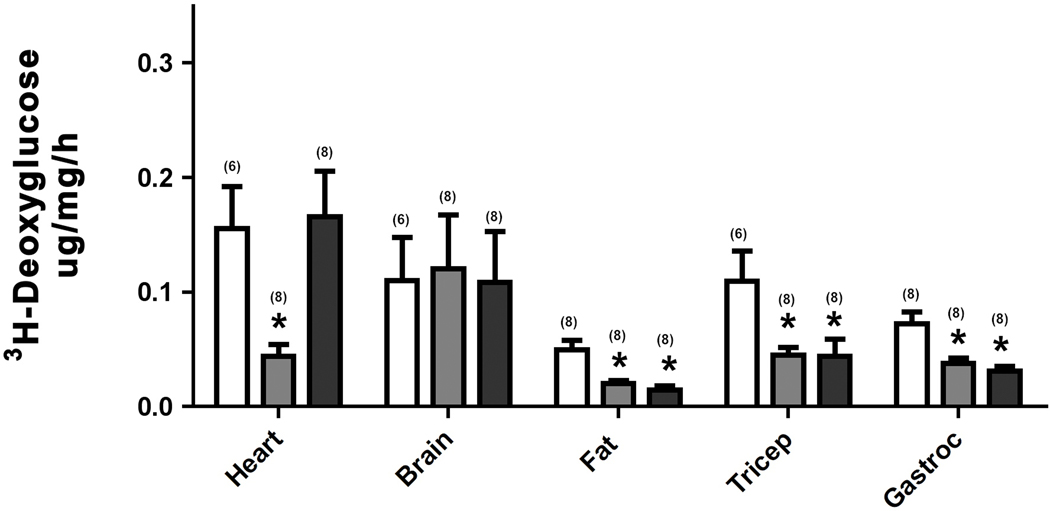
We next used deoxyglucose to determine which tissues were utilizing glucose through α1-AR regulation. Deoxyglucose is used for tissue uptake studies because it is not metabolized and becomes trapped inside the cell after phosphorylation by hexokinase. We could not analyze liver due to the presence of an enzyme that interferes with the assay. We found that CAM α1A-AR mice selectively enhanced glucose uptake into the heart while both α1-AR subtypes increased glucose uptake in white fat and tricep or gastrocnemius skeletal muscle (p<0.05) (Fig 2A). Utilizing the KO mice, we found that only α1A-AR KO selectively reduced glucose uptake in the heart, but either KO was sufficient in reducing glucose uptake in either fat tissue or skeletal muscle (p<0.05)(Fig 2B).
Figure 2. α1-AR regulation of 3H-deoxyglucose uptake.
Mice were fasted for 6 hours then injected with [3H]-2DG (20uCi/mouse; ip). Blood samples were taken from the tail vein at 30, 60, 90 mins post-injection using a Nova Max Plus glucometer. After the final collection of blood, mice were euthanized and tissues removed and frozen in dry ice. Tissues were processed and the glucose uptake rate was calculated according to the method of Ferre’, et al (1985). A. 3H-deoxyglucose uptake in tissues isolated from CAM α1A-AR (grey bars), CAM α1B-AR (black bars) mice and WT control (white bars). B. 3H-deoxyglucose uptake in tissues isolated from α1A-AR KO (grey bars), α1B-AR KO (black bars), and WT controls (white bars). * p≤ 0.05, statistically significant from WT control. N=6–14 mice (in parentheses). Gastroc (gastrocnemius muscle).
α1-AR activation increased whole body fatty acid oxidation.
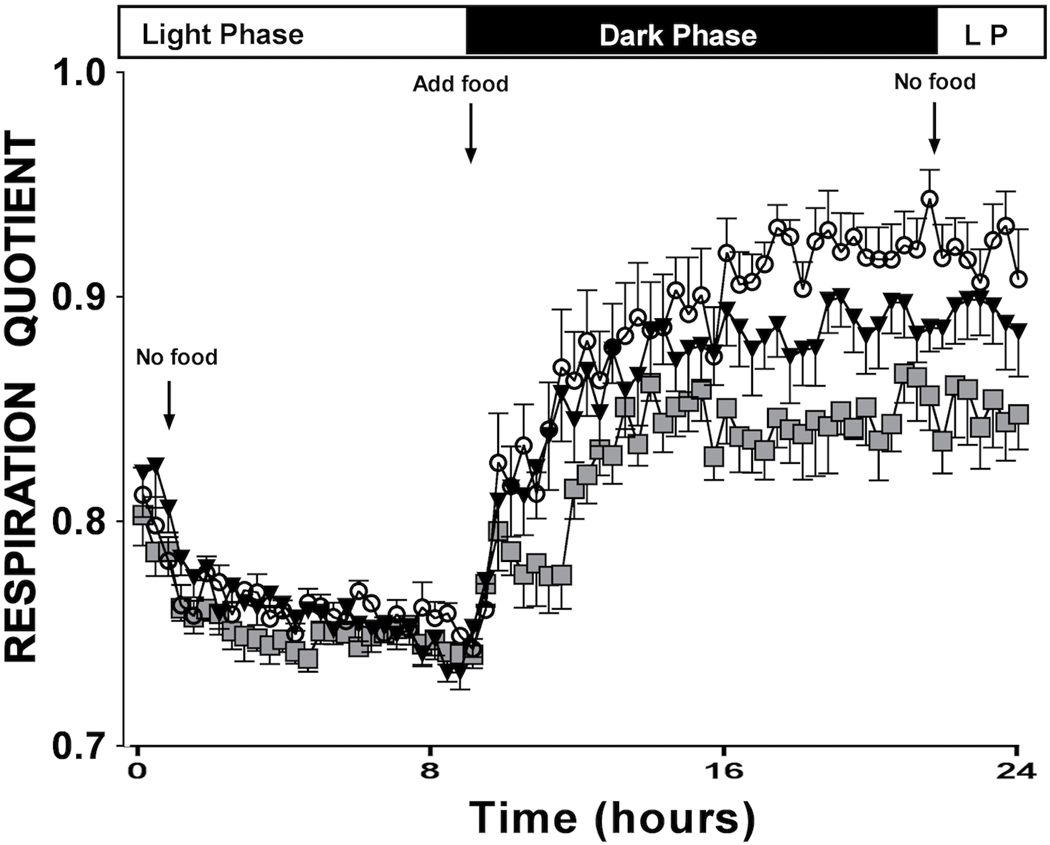
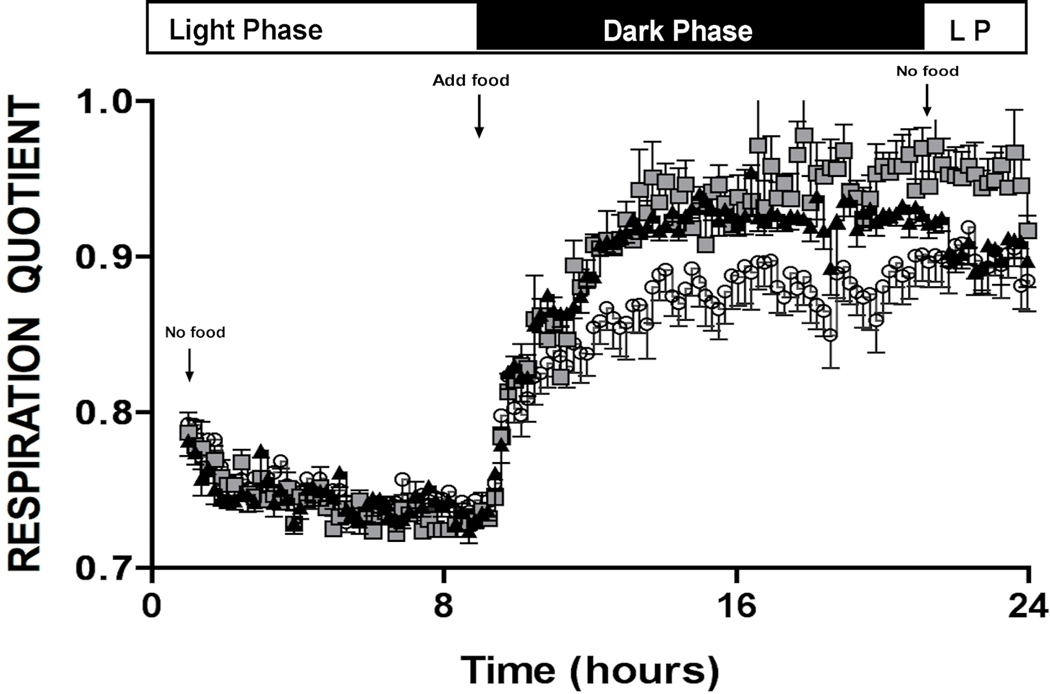
To investigate α1-AR effects on whole body energy metabolism, we performed indirect calorimetry on conscious mice through collaboration with the NIH-sponsored Mouse Metabolic Phenotyping Core (MMPC) at nearby Case Western Reserve University. The Respiratory Quotient (RQ) is the ratio of CO2 eliminated/O2 consumed and can indicate which type of fuel is being preferentially metabolized. RQ range from 1.0–0.7 where 1.0 is expected for pure glucose oxidation and 0.7 for pure fatty acid oxidation (25). Proteins are oxidized in the range of 0.8–0.9 RQ. We found that both CAM α1A-AR and α1B-AR mice showed a lower RQ than WT control mice (Fig 3A) when feeding suggesting increased lipid metabolism (F(2,75)=36.76, p≤ 0.001) using the Repeated Measures ANOVA. Both α1A-AR KO and α1B-AR KO mice had higher RQs than WT control (F(2,130)=48.65, p<.001)(Fig 3B) suggesting that they burn more carbohydrate than WT control mice. CAM α1A-AR displayed lower RQ than CAM α1B-AR mice while α1A-AR KO mice displayed higher RQ than α1B-AR KO mice, suggesting there were subtype-specific differences in the rate of metabolism.
Figure 3. α1-AR regulation of whole body metabolism.
Metabolic rates are measured by indirect calorimetry in mice using an 8-chamber open-circuit Oxymax system. Briefly, mice are acclimated to the experimental room for 1 week prior and individually housed in acrylic calorimeter chambers through which air with a known O2 concentration is passed at a constant flow rate. The system automatically withdraws gas samples from each chamber hourly for 24 h and calculates the volumes of O2 consumed (VO2) and CO2 generated (VCO2). The respiratory quotient (RQ), which is the ratio of VCO2 to VO2, is calculated. A. RQ profile for CAM α1A-AR (squares), CAM α1B-AR (triangles) mice and WT control (circles). F(2,75)=36.76, p≤ 0.001. Both CAM statistically significant (P<0.001) from WT control using Repeated Measures ANOVA. B. RQ profile for α1A-AR KO (squares), α1B-AR KO (triangles), and WT controls (circles). N=6 or 8 mice. (F(2,130)=48.65, p<.001), Both KO statistically significant (p<0.001) from WT control using Repeated measures ANOVA.
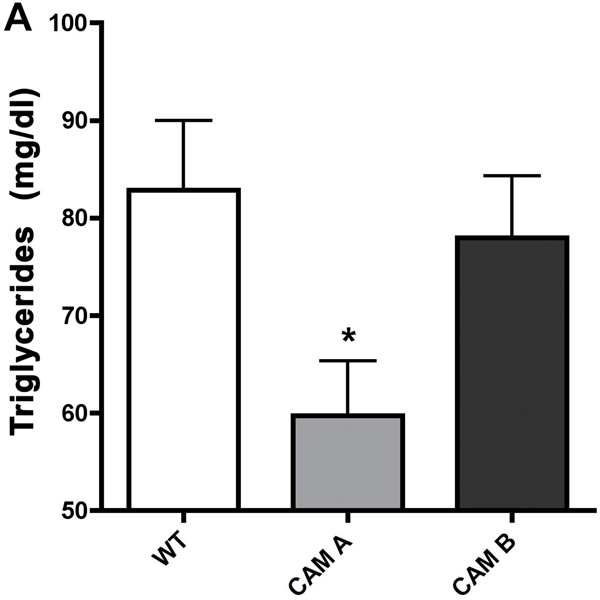
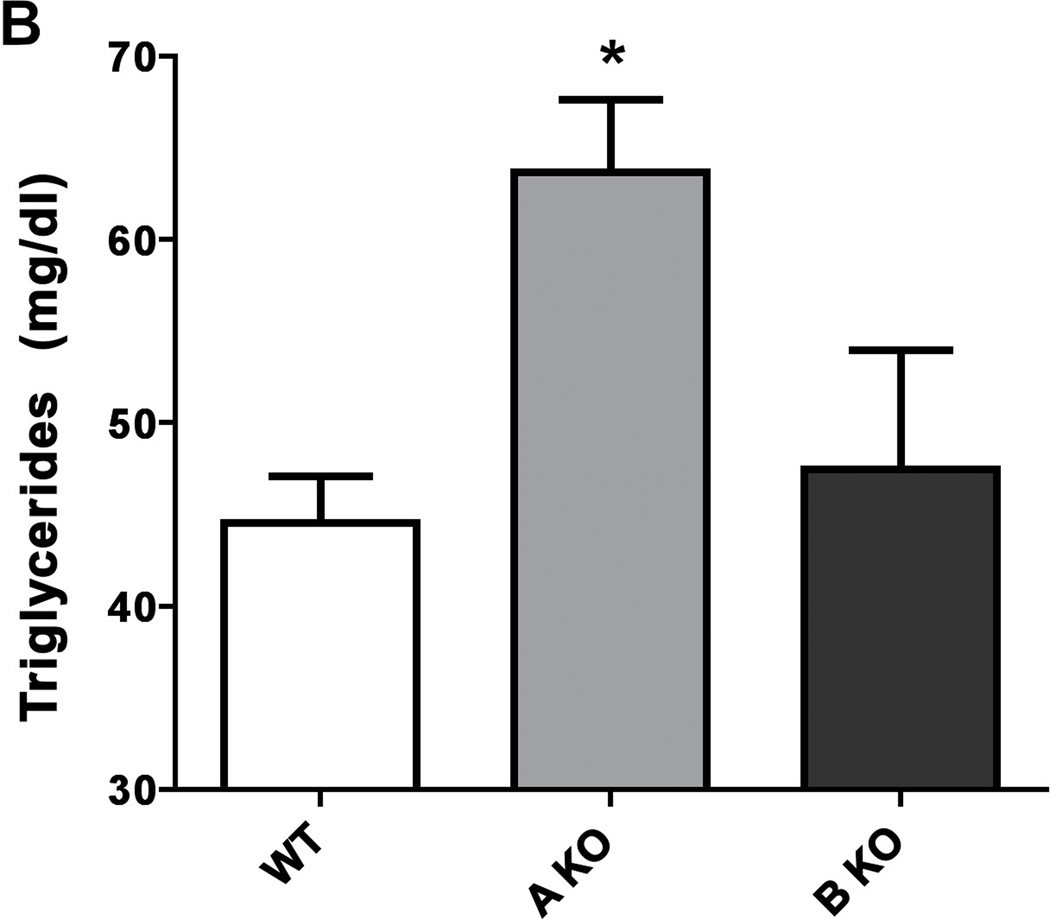
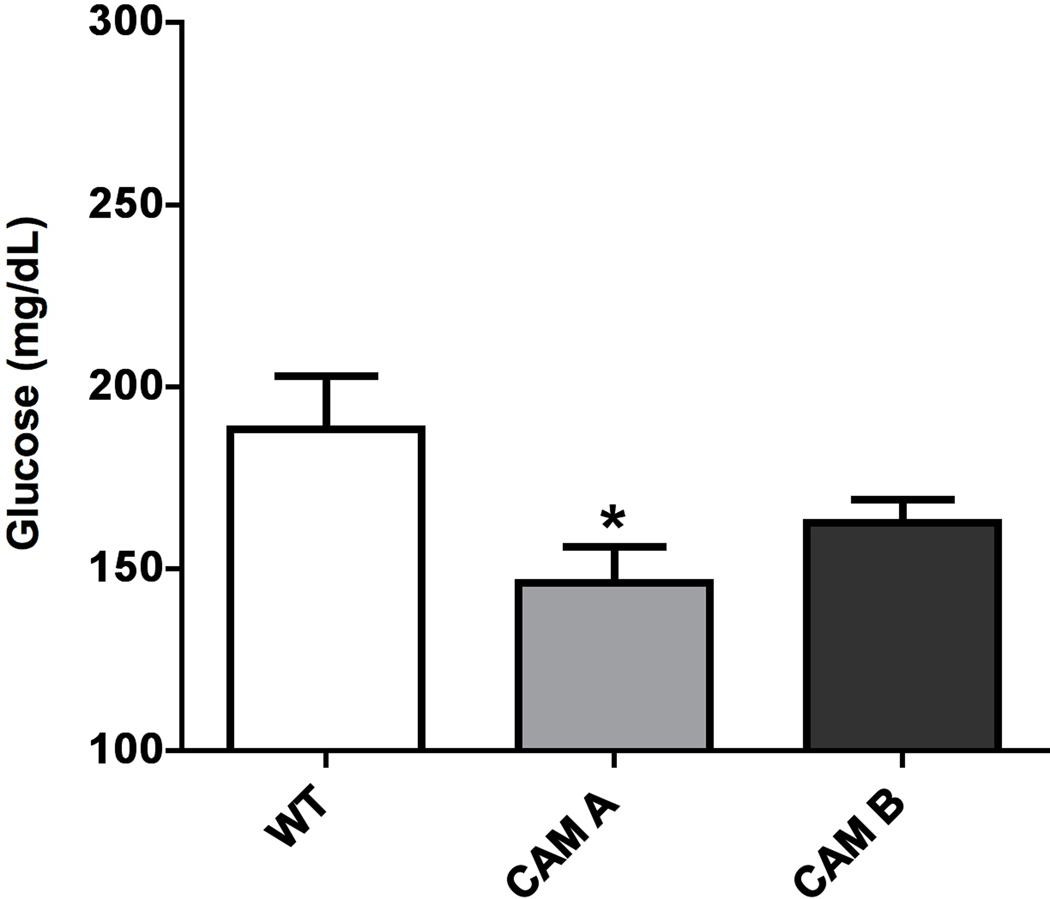
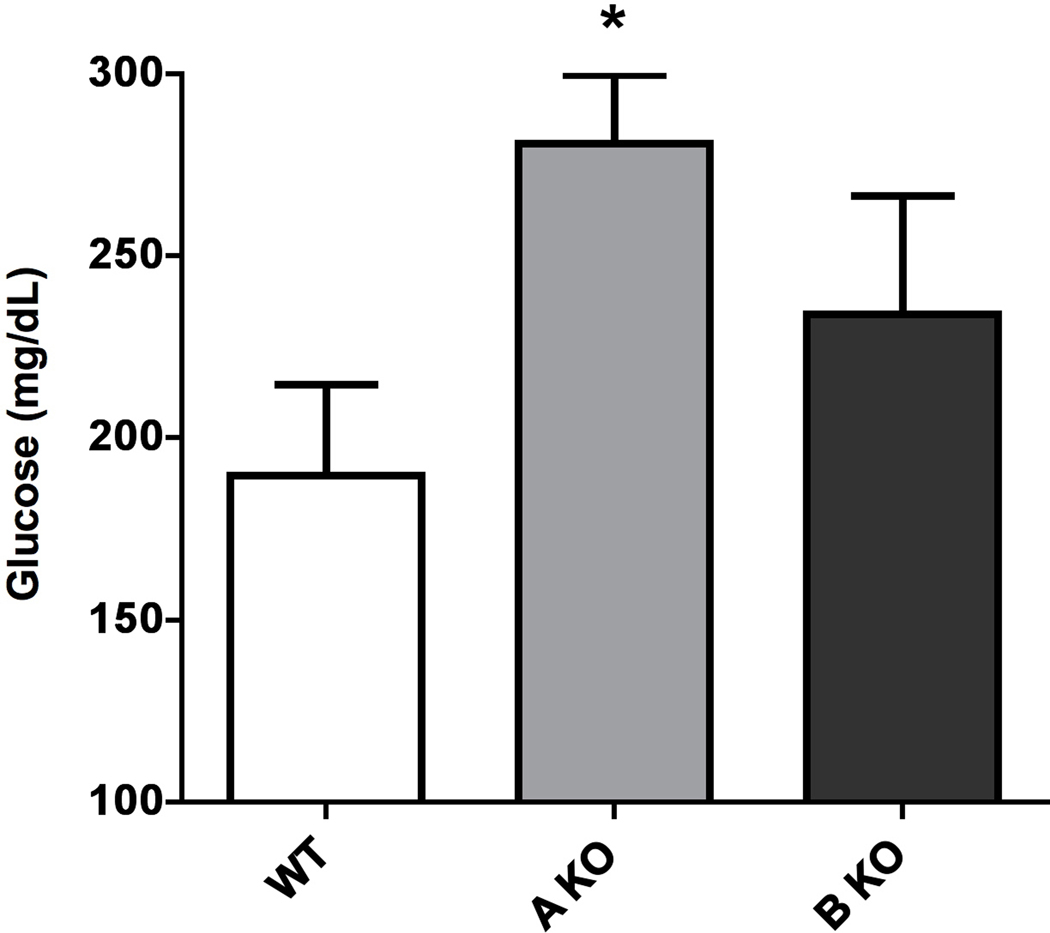
α1-AR activation lowers fasted triglyceride and glucose levels.
We next determined if there were differences in the blood lipid and glucose profiles that might reflect and confirm the results from indirect calorimetry. Indeed, we found that CAM α1A-AR mice significantly lowered triglyceride levels (p<0.05)(Fig 4A) than WT control while the α1A-AR KO mice displayed elevated triglycerides (p<0.05)(Fig 4B). Likewise, we found that CAM α1A-AR mice significantly lowered fasting glucose levels (p<0.05)(Fig 5A) than WT control while the α1A-AR KO mice displayed elevated glucose levels (p<0.05)(Fig 5B). Similarly, but not significantly, both CAM α1B-AR and α1B-AR KO mice displayed intermediate values from WT controls.
Figure 4. α1-AR regulation of plasma fasting triglyceride levels in CAM (A) or KO (B) mice.
Mice were first fasted for 6 hours, anesthetized, then blood collected from the vena cava into 1.5ml polypropylene microfuge tubes. The blood samples were allowed to sit at room temperature for 30 min to coagulate, then centrifuged at 2000x g for 10 min at 4°C. The sera was transferred to fresh tubes and re-centrifuged to remove blood cells. Blood samples were analyzed by the Veterinary Diagnostic Services of Marshfield Laboratories (Cleveland, Ohio) using standard clinical procedures. * p≤ 0.05, statistically significant from WT control. N=6–8 mice.
Figure 5. α1-AR regulation of fasting glucose levels in CAM (A) or KO (B) mice.
Mice were first fasted for 6 hours, anesthetized, then blood collected from the vena cava into 1.5ml polypropylene microfuge tubes. The blood samples were allowed to sit at room temperature for 30 min to coagulate, then centrifuged at 2000x g for 10 min at 4°C. The sera was transferred to fresh tubes and re-centrifuged to remove blood cells. Blood samples were analyzed by the Veterinary Diagnostic Services of Marshfield Laboratories (Cleveland, Ohio) using standard clinical procedures. * p≤ 0.05, statistically significant from WT control. N=6–10 mice.
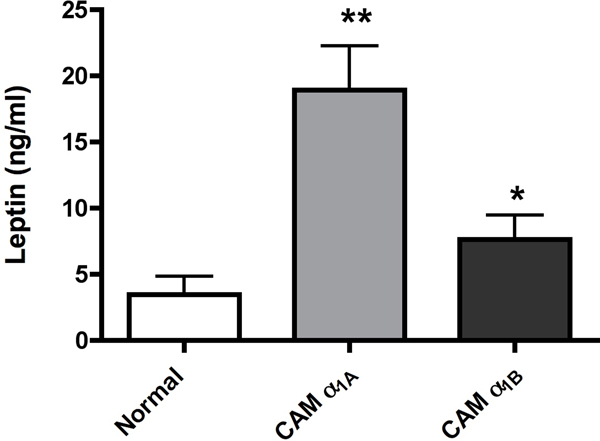
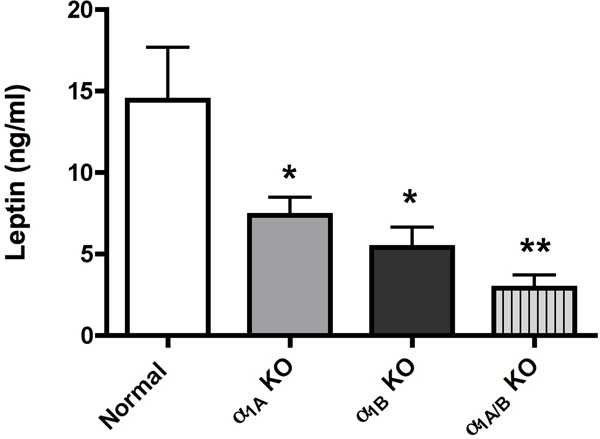
α1-AR stimulation results in higher plasma leptin levels.
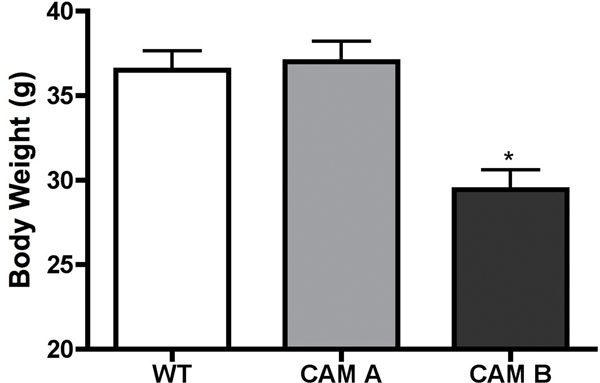
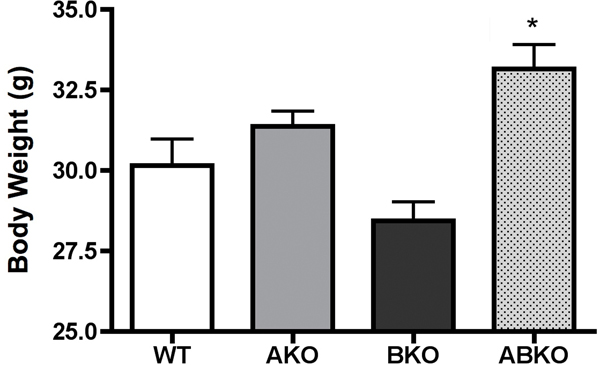
As leptin is a major regulator of metabolism and energy balance, we next determine plasma levels of leptin in both CAM and KO mice. Both CAM mice had significantly higher levels of plasma leptin with the CAM α1A-AR being more efficacious (p<0.01 vs p<0.05) (Fig 6A). Either KO mice had significantly lower levels of leptin (p<0.05)(Fig 6B) with the α1A/B-AR double KO mice (p<0.01) displaying an additive effect. However, we did not find any correlation in body weight. While CAM α1A-AR mice had higher levels of leptin, it did not have any change in body weight from normal controls (Fig 7A). While CAM α1B-AR mice did have significantly lower body weight, they also have significant neurodegeneration (17), and autonomic dysfunction (26), which leads to weight loss (27). KO mice only displayed significantly weight gain in the α1A/B-AR double KO mice (Fig 7B), which are significantly heavier than single KO or WT controls (P<0.05).
Figure 6. α1-AR regulation of leptin release in CAM (A) or KO (B) mice.
Mice were fasted for 6 hours. Blood was drawn from mice by making a small cut in the tail after anesthesia and centrifuged for 10 minutes at 2000x g. The sera was collected and stored at −80° until processed. The sera were analyzed for leptin using the mouse/rat leptin enzyme immunoassay kit from SPI BIO (Montigny le Bretonneux, France) according to manufacturer’s procedures. * p ≤ 0.05, **p ≤ 0.01, statistically significant from WT control. N=8–10 mice.
Figure 7. Body weight of male CAM (A) or KO (B) mice.
Mice were weighed at 5 months of age. * p ≤ 0.05, statistically significant from WT control. N= 10–12 mice.
Discussion
This is the first comprehensive in vivo report of the metabolic functions regulated through α1-ARs. Stimulation of α1-ARs regulates glucose tolerance, whole body metabolism, leptin release, and is selective in regulating glucose uptake into particular tissues. While both the α1A- and α1B-AR subtypes appear to regulate metabolism in a similar fashion, the α1A-AR is clearly more efficacious and confirmed in the α1A-AR KO mice. Stimulation of α1A-ARs results in a favorable metabolic profile of increased glucose tolerance, cardiac glucose uptake, leptin release, and enhanced whole body lipid metabolism, resulting in a favorable lipid and glucose blood profile that may contribute to CAM α1A-AR’s increased longevity and cardioprotection (1, 18, 28–29). These results suggest that α1-ARs, and in particular the α1A-AR, is a significant, but previously unrecognized, regulator of whole body and organ-specific metabolism.
It is well known that norepinephrine mediates multi-faceted aspects of metabolism. Early studies indicated that α1-ARs stimulate gluconeogenesis and ketogenesis in the liver (2) and suppress triglyceride secretion (3). α1-ARs have also been shown to regulate glucose uptake into various cell lines (4–6), but never shown to regulate glycolysis. α1B-AR KO mice were previously reported to be insulin resistant and impaired glucose homeostasis (7). The β-ARs are well known mediators of adipocyte metabolism through their regulation of cAMP levels in both white and brown fat (8) and are lipolytic (9). However, the β1/β2/β3-AR triple KO mice still display norepinephrine-induced lipolysis, suggesting involvement of another AR subtype (10). α2-AR and in particular α2A-AR stimulation inhibits insulin secretion (11) and are anti-lipolytic (12). Therefore, there was significant previous data to suggest that α1-ARs can regulate whole body and tissue-specific metabolism that was different from either β-or α2-ARs.
Early studies also indicted that α1-ARs stimulate fatty acid oxidation in hepatocytes but there are no reports outside of the liver or in whole body metabolism (3, 13–16). Whole body metabolism is usually regulated through skeletal muscle or adipose tissue because of their mass volume in the body. Both α1-AR subtypes appear to stimulate whole body fatty acid oxidation (Fig 3A). If α1-AR stimulation increases whole body fatty acid oxidation, we might expect that triglyceride levels to be decreased as they are being preferentially oxidized. We found that CAM α1A-AR had decreased plasma triglycerides (Fig 4A) while the α1A-AR KO had increased levels (Fig 4B). Very early multi-centered clinical studies using non-selective prazosin showed little (30) or no changes in triglyceride levels (31). However, selective blockage of the α1A-AR has not been tried. However, a more recent cross-over study (i.e. each subject is compared to themselves) using doxazosin showed an 40% increased in plasma non-esterified fatty acid concentrations (32), similar to increased triglyceride levels in α1A-AR KO mice. Our results suggest that chronic stimulation of the α1A-AR may be an important regulator of lipid metabolism than previously recognized.
The stimulatory effect of sympathetic activity on glucose uptake in human adipocytes (33) and into rat white adipose in vitro (34) can be mediated by the α1-AR and confirmed in mouse fat tissue in vivo in our studies (Fig 2). Norepinephrine enhances glucose entry into human adipocytes independently of insulin action that can be blocked with urapidil, an α1-AR blocker (33). Glucose uptake into adipose tissue plays an important role in glucose homeostasis. In obese subjects with insulin resistance, α1-AR stimulation may provide an important alternative pathway for glucose uptake into adipose tissue.
Glucose uptake in adipocytes is a key regulator of leptin release (35). Leptin (ob) is a mainly adipocyte-secreted hormone that signals through receptors in the hypothalamus that control hunger, energy balance and body fat (36–37). Corroborating with α1-ARs increasing glucose uptake into adipose tissue, we found that CAM α1-AR mice increased leptin levels (Fig 6A), while KO mice decreased leptin release (Fig 6B). There are only a few prior studies indicating association of leptin with α1-ARs. The study of (38) demonstrated that leptin activates hepatic AMPK through α1-ARs. Blocking α1-ARs also reduced leptin levels in obese humans (39) without affecting body weight, similar to our results in KO mice. We also could not see correlation of leptin levels with body weight in our mice (Fig 7), although the α1A/B-AR double KO mice are significantly overweight. α1-AR agonists also enhance leptin transport across the blood brain barrier (40). Leptin improves insulin sensitivity by reducing fat content in the liver and skeletal muscle (41–42). Leptin can stimulate fatty acid oxidation in skeletal muscle through AMPK signaling (43–46). There is significant data to indicate that leptin is involved in the regulation of peripheral lipid metabolism (47–50). An in vitro study of leptin showed an increase of lipolytic activity in adipocytes (51). An in vivo study demonstrated that leptin accelerated lipolysis in adipose tissues (43). In the liver, peripheral treatment with leptin prevented accumulation of lipids by acceleration of fatty acid oxidation (50). Besides regulating the release of leptin, α1-ARs and particularly the α1A-AR are highly expressed in the hypothalamus (52). Thus, we hypothesize that α1-ARs mediate glucose uptake into adipocytes, which causes the release of leptin that regulates peripheral whole body lipid metabolism through the hypothalamus. Interestingly, leptin is also both cardioprotective (53–55) and neuroprotective with increasing cognition and anti-aging effects (56), all similar phenotypes in our CAM α1A-AR mice (1, 18, 28).
We also found that the α1A-AR in vivo appears selective in mediating glucose uptake into the adult heart (Fig 2). Previous studies in the isolated rat heart (57) and neonatal rat myocytes (58) also indicate α1-AR mediated glucose uptake. In the normal adult heart, fatty acids are the preferred substrate for metabolism and generate 67% of the ATP. This is contrasted with the fetal heart, which relies mostly on glucose (57). However, glucose uptake, the rate-limiting step in glycolysis (59) may play an important alternative energy source in specific pathological conditions, such as ischemia in the adult (60–61) suggesting that an increase of glucose uptake in the heart may result in a favorable metabolic guard against ischemic injury. The selectivity of the α1A-AR in mediating glucose uptake into the heart may contribute to its cardioprotective phenotype against ischemia as seen in the CAM α1A-AR mouse (18).
Skeletal muscle as the largest organ in the body with high metabolic rates, utilize a large amount of glucose (62). The increased glucose tolerance (Fig 1) is likely due to the increased glucose uptake into skeletal muscle by α1-ARs (Fig 2). CAM mice also had decreased fasting glucose levels (Fig 5A) while KO had elevated fasting levels (Fig 5B), suggesting either a diabetic or pre-diabetic state. Both the gastrocnemius and triceps in the mouse are considered fast twitch, high glycolytic muscle (63). Increasing capacity of glucose uptake in the skeletal muscle may be a beneficial therapeutic approach for the prevention and treatment of type 2 diabetes (64).
β3-ARs agonists, once pursued for metabolic treatment, was cut short in clinical trial due to poor oral availability, pharmacokinetics and poor efficacy in humans compared with initial studies in rodents (65). α1A-AR partial agonists may overcome pharmacodynamic barriers as novel oral agonists were developed and considered safe for use in treating urinary incontinence (66) with better clinical efficacy in humans. Clinical studies demonstrating effects of α1-AR blockers on lipid and leptin profiles (32, 39) may also suggest better clinical efficacy in humans than β3-AR agonists. While α1-AR agonists have the negative side effect of increasing blood pressure, imidazoline-type partial α1A-AR agonists allow for the baroreflex response, have high oral availability, biased agonism, and CNS penetration that may temper affects on blood pressure (67–70).
CONCLUSIONS
Our results suggest that stimulation of α1-ARs regulates glucose tolerance, whole body metabolism, leptin release, and is selective in regulating glucose uptake into particular tissues. While both the α1A- and α1B-AR subtypes appear to regulate metabolism in a similar fashion, the α1A-AR is clearly more efficacious. α1A-AR regulation of metabolism needs to be furthered explored to determine its potential to treat metabolic syndrome.
Acknowledgements:
The authors wish to thank Satish Kalhan, MD of the Cleveland Clinic for his consulting and insight into these metabolic studies.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. This work was supported by a grant from The National Institutes of Health [1R03AG049394], an American Heart Association Grant in Aid (Great Rivers Affiliate) to DMP, and the Case Western Reserve University Mouse Metabolic Phenotyping Center (MMPC) grant [U24 DK76174].
References
- 1.Perez DM and Doze VA. Cardiac and Neuroprotection Regulated by α1-Adrenergic Receptor Subtypes. J Recept Signal Transduct Res (2011), 31, 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark B and Keller U α1-adrenergic stimulation of ketogenesis and fatty acid oxidation is associate with inhibition of lipogenesis in rat hepatocytes. Experientia (1987), 43, 1104–1106. [DOI] [PubMed] [Google Scholar]
- 3.Brindle NPJ and Ontko JA. α-adrenergic suppression of very-low-density-lipoprotein triacylglycerol secretion by isolated rat hepatocytes. Biochem J (1988), 250(2), 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson DS and Bengtsson T. α1A-adrenoceptors activate glucose uptake in L6 muscle cells through a phospholipase C-, phosphatidylinositol-3 kinase-, and atypical protein kinase C-dependent pathway. Endocrinology (2005), 146(2), 901–912. [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson DS and Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: mediation by α1-adrenoceptors causing glucose uptake. Diabetes (2006), 55(3), 682–690. [DOI] [PubMed] [Google Scholar]
- 6.Liu IM, Tsai CC, Lai TY, and Cheng JT. Stimulatory effect of isoferulic acid on α1A-adrenoceptor to increase glucose uptake into cultured myoblast C2C12 cell of mice. Auton Neurosci (2001), 88(3), 175–180. [DOI] [PubMed] [Google Scholar]
- 7.Burcelin R, Uldry M, Foretz M, Perrin C, Dacosta A, Nenniger-Tosato M, Seydoux J, Cotecchia S, and Thorens B. Impaired glucose homeostasis in mice lacking the α1b-adrenergic receptor subtype. J Biol Chem (2004), 279(2), 1108–1115. [DOI] [PubMed] [Google Scholar]
- 8.Lafontan M, Barbe P, Galitzky J, Tavernier G, Langin D, Carpéné C, Bousquet-Melou A, and Berlan M. Adrenergic regulation of adipocyte metabolism. Hum Reprod (1997), 12 Suppl 1,6–20. [DOI] [PubMed] [Google Scholar]
- 9.Mauriège P, Pergola GD, Berlan M, and Lafontan M Human fat cell β-adrenergic receptors: β agonist-dependent lipolytic responses and characterization of β -adrenergic binding sites on human fat cell membranes with highly selective β1-antagonists. J Lipid Res (1988), 29, 587–601. [PubMed] [Google Scholar]
- 10.Tavernier G, Jimenez M, Giacobino JP, Hulo N, Lafontan M, Muzzin P, and Langin D. Norepinephrine induces lipolysis in β1/β2/β3-adrenoceptor knockout mice. Mol Pharmacol (2005), 68(3), 793–799. [DOI] [PubMed] [Google Scholar]
- 11.Fagerholm V, Haaparanta M, and Scheinin M. α2-Adrenoceptor Regulation of Blood Glucose Homeostasis. Basic & Clinical Pharmacology & Toxicology (2011), 108, 365–370. [DOI] [PubMed] [Google Scholar]
- 12.Lafontan M and Berlan M. Fat cell α2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocrine Rev (1995), 16, 716–738 [DOI] [PubMed] [Google Scholar]
- 13.Sugden MC, Tordoff AF, Ilic V, and Williamson DH. α-adrenergic stimulation of [1–14C]oleate oxidation to 14CO2 in isolated rat hepatocytes. FEBS Lett (1980), 120, 80–84. [DOI] [PubMed] [Google Scholar]
- 14.Kosugi K, Harano Y, Nakano T, Suzuki M, Kashiwagi A, and Shigeta Y. Mechanism of adrenergic stimulation of hepatic ketogenesis. Metabolism (1983), 32, 1081–1087. [DOI] [PubMed] [Google Scholar]
- 15.Oberhaensli RD, Schwendimann R, and Keller U. Effect of norepinephrine on ketogenesis, fatty acid oxidation, and esterification in isolated rat hepatocytes. Diabetes (1985), 34(8), 774–779. [DOI] [PubMed] [Google Scholar]
- 16.Nomura T, Nomura Y, Tachibana M, Nomura H, Ukai K, Yokoyama R, and Hagino Y. α1-adrenergic regulation of ketogenesis in isolated rat hepatocytes. Biochim Biophys Acta (1991), 1092(1), 94–100. [DOI] [PubMed] [Google Scholar]
- 17.Zuscik MJ, Sand S, Ross SA, Waugh DJJ, Gaivin RJ, Morilak D, and Perez DM. Overexpression of the α1b-Adrenergic receptor causes apoptotic neurodegeneration: A multiple system atrophy. Nature Medicine (2000), 6, 1388–1394. [DOI] [PubMed] [Google Scholar]
- 18.Rorabaugh BR, Ross SA, Gaivin RJ, Papay RS, McCune DF, Simpson PC, and Perez DM. α1A- but not α1B-adrenergic receptors precondition the ischemic heart by a staurosporine-sensitive, chelerythrine-insensitive mechanism. Cardiovasc Res (2005), 65, 436–445. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell TD, Ishizaka S, Nakamura A, Swigart PM, Rodrigo MC, Simpson GL, Cotecchia S, Rokosh DG, Grossman W, Foster E, and Simpson PC. The α1A/C- and α1B-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. J Clin Invest (2003), 111,1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, and Simpson PC. α1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest (2006), 116, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millward CA, Desantis D, Hsieh CW, Heaney JD, Pisano S, Olswang Y, Reshef L, Beidelschies M, Puchowicz M, and Croniger CM. Phosphoenolpyruvate carboxykinase (Pck1) in hepatic and adipose tissue contributes to the regulation of the triglyceride fatty acid cycle and development of insulin resistance in mice. J Lipid Res (2010), 51, 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millward CA, Heaney JD, Sinasac DS, Chu EC, Bederman IR, Gilge DA, Previs SF, and Croniger CM. Mice with a deletion in the gene for CCAAT/enhancer-binding protein beta are protected against diet-induced obesity. Diabetes (2007), 56(1), 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferré P, Leturque A, Burnol AF, Penicaud L, and Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J (1985), 228, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi T, Papay RS, and Perez DM. α1A-Adrenergic receptor prevents cardiac ischemic damage through PKCδ/ GLUT1/4-mediated glucose uptake. J Receptor Signal Transduction (2016), 36(3), 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonson DC, and DeFronzo RA. Indirect calorimetry: methodological and interpretative problems. Am J Physiol (1990), 258, E399–E412. [DOI] [PubMed] [Google Scholar]
- 26.Zuscik MJ, Chalothorn D, Hellard D, Deighan C, McGee A, Daly CJ, Waugh DJ, Ross SA, Gaivin RJ, Morehead AJ, Thomas JD, Plow EF, McGrath JC, Piascik MT, and Perez DM. Hypotension, autonomic failure, and cardiac hypertrophy in transgenic mice overexpressing the α1B-adrenergic receptor. J Biol Chem (2001), 276, 13738–13743. [DOI] [PubMed] [Google Scholar]
- 27.Papay R, Zuscik MT, Ross SA, Yun J, McCune DF, Gonzalez-Cabrera P, Gaivin R, Macklin WB, Drazba J and Perez DM. Mice expressing the α1b-Adrenergic receptor induces a synucleinopathy with excessive tyrosine nitration but decreased phosphorylation. Neurochem (2002), 83, 623–634. [DOI] [PubMed] [Google Scholar]
- 28.Doze VA, Papay RS, Collette KM, Gupta MK, Lyons MJ, Davis BA, Luger EJ, Wood SG, Goldenstein BL, Haselton JR, Simpson PC, and Perez DM. Long term α1A-adrenergic receptor stimulation improves synaptic plasticity, cognitive function, mood and longevity. Mol Pharmacology (2011), 80(4), 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collette KM, Zhou XD, Amoth HM, Lyons MJ, Papay RS, Sens DA, Perez DM, and Doze VA. Long-term α1B-adrenergic receptor activation shortens lifespan while α1A-adrenergic receptor stimulation prolongs lifespan in association with decreased cancer incidence. Age (2014), 36, 9675–9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto Y Effects of α- and β-blocker antihypertensive therapy on blood lipids: a multicenter trial. Am J Med (1984), 76(2A),72–78. [DOI] [PubMed] [Google Scholar]
- 31.Ferrara LA, Marotta T, Rubba P, De Simone B, Leccia G, Soro S, and Mancini M. Effects of α-adrenergic and β-adrenergic receptor blockade on lipid metabolism. Am J Med (1986), 80(2A), 104–108. [DOI] [PubMed] [Google Scholar]
- 32.Ulahannan TJ1, Karpe F, Humphreys SM, Matthews DR, and Frayn KN Effects of acute administration of doxazosin on fasting and postprandial haemodynamics and lipid metabolism in healthy subjects. Horm Metab Res (2002), 34(9), 499–503. [DOI] [PubMed] [Google Scholar]
- 33.Flechtner-Mors M1, Jenkinson CP, Alt A, Biesalski HK, Adler G, and Ditschuneit HH Sympathetic regulation of glucose uptake by the α1-adrenoceptor in human obesity. Obes Res (2004), 12(4), 612–620. [DOI] [PubMed] [Google Scholar]
- 34.Cheng JT, Liu IM, Yen ST, and Chen PC. Role of α1A-adrenoceptor in the regulation of glucose uptake into white adipocyte of rats in vitro. Auton Neurosci (2000), 84(3),140–146. [DOI] [PubMed] [Google Scholar]
- 35.Havel PJ. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology (1998), 139(2), 551–558. [DOI] [PubMed] [Google Scholar]
- 36.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, and Friedman JM. Physiological response to long term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA (1997), 94, 8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campfield LA, Smith FJ, Guisez Y, Devos R, and Burn P. Recombinant mouse OB protein. Evidence for a peripheral signal linking adiposity and central neural networks. Science (1995), 269, 546–549. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto L, Ebihara K, Kusakabe T, Aotani D, Yamamoto-Kataoka S, Sakai T, Aizawa-Abe M, Yamamoto Y, Fujikura J, Hayashi T, Hosoda K, and Nakao K. Leptin Activates Hepatic 5’-AMP-activated Protein Kinase through Sympathetic Nervous System and α1-Adrenergic Receptor: A Potential Mechanism For Improvement of Fatty Liver in Lipodystrophy by Leptin. J Biol Chem (2012), 287(48), 40441–40447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ihara S, Shimamoto K, Watanabe H, Sakai R, and Kawana M. An α1-receptor blocker reduces plasma leptin levels in hypertensive patients with obesity and hyperleptinemia. Hypertens Res (2006), 29(10), 805–81. [DOI] [PubMed] [Google Scholar]
- 40.Banks WA. Enhanced leptin transport across the blood-brain barrier by α1-adrenergic agents. Brain Res (2001), 899(1–2), 209–217. [DOI] [PubMed] [Google Scholar]
- 41.Ebihara K, Ogawa Y, Masuzaki H, Shintani M, Miyanaga F, Aizawa- Abe M, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Gavrilova O, Reitman ML, and Naka K Transgenic overexpression of leptin rescues insulin resistance and diabetes in a mouse model of lipoatrophic diabetes. Diabetes (2001), 50,1440–1448. [DOI] [PubMed] [Google Scholar]
- 42.Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, and Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest (2002), 109, 1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marti A, Novo FJ, Martinez-Anso E, Zaratiegui M, Aguado M, and Martinez JA. Leptin gene transfer into muscle increases lipolysis and oxygen consumption in white fat tissue in ob/ob mice. Biochem Biophys Res Commun (1998), 246, 859–862. [DOI] [PubMed] [Google Scholar]
- 44.Minokoshi Y and Kahn BB. Role of AMP-activated protein kinase in leptin-induced fatty acid oxidation in muscle. Biochem Soc Trans (2003), 31(Pt 1), 196–201. [DOI] [PubMed] [Google Scholar]
- 45.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, and Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP activated protein kinase. Nature (2002), 415, 339–343. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, and Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the α2 form of AMP-activated protein kinase. Mol Cell Biol (2007), 27(12), 4317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fruhbeck G, Aguado M, and Martinez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun (1997), 240, 590–594. [DOI] [PubMed] [Google Scholar]
- 48.Kamohara S, Burcelin R, Halaas JL, Friedman JM, and Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature (1997), 389, 374–377. [DOI] [PubMed] [Google Scholar]
- 49.Muller G, Ertl J, Gerl M, and Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem (1997), 272, 10585–10593. [DOI] [PubMed] [Google Scholar]
- 50.Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, and Unger RH. Beta-cell function in normal rats made chronically hyperleptinemic by adenovirus-leptin gene therapy. Diabetes (1997), 46, 1276–1280. [DOI] [PubMed] [Google Scholar]
- 51.Fruhbeck G, Aguado M, Gomez-Ambrosi J, and Martinez JA. Lipolytic effect of in vivo leptin administration on adipocytes of lean and ob/ob mice, but not db/db mice. Biochem Biophys Res Commun (1998), 250, 99–102. [DOI] [PubMed] [Google Scholar]
- 52.Papay R, Gaivin R, Archana J, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA, and Perez DM. Localization of the Mouse α1A-Adrenergic Receptor in the Brain: α1A-AR is expressed in Neurons, GABAergic Interneurons and NG2 Oligodendrocyte Progenitors. J Comparative Neurology (2006), 497, 209–222. [DOI] [PubMed] [Google Scholar]
- 53.McGaffin KR, Witham WG, Yester KA, Romano LC, O’Doherty RM, McTiernan CF, and O’Donnell CP. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc Res (2011), 89(1), 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, and Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol (2008), 295(4), H1580-H1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith CC, Mocanu MM, Davidson SM, Wynne AM, Simpkin JC, and Yellon DM. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br J Pharmacol (2006), 149(1), 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Folch J, Pedrós I, Patraca I, Sureda F, Junyent F, Beas-Zarate C, Verdaguer E, Pallàs M, Auladell C, and Camins A. Neuroprotective and anti-aging role of leptin. J Mol Endocrinol (2012), 49(3), R149–R156. [DOI] [PubMed] [Google Scholar]
- 57.Doenst T and Taegtmeyer H. α-Adrenergic stimulation mediates glucose uptake through phosphatidylinositol 3-kinase in rat heart. Circ Res (1999), 84, 467–474. [DOI] [PubMed] [Google Scholar]
- 58.Angeloni C, Maraldi T, Ghelli A, Rugolo M, Leoncini E, Hakim G, and Hrelia S. Green tea modulates α1-adrenergic stimulated glucose transport in cultured rat cardiomyocytes. J Agric Food Chem (2007), 55(18), 7553–7558. [DOI] [PubMed] [Google Scholar]
- 59.Manchester J, Kong X, Nerbonne J, Lowry OH, and Lawrence JC Jr. Glucose transport and phosphorylation in single cardiac myocytes: rate limiting steps in glucose metabolism. Am J Physiol Endocrinol Metab (1994), 266, E326–E333. [DOI] [PubMed] [Google Scholar]
- 60.Depre C, Vanoverschelde JL, and Taegtmeyer H. Glucose for the heart. Circulation (1999), 99(4), 578–588. [DOI] [PubMed] [Google Scholar]
- 61.Tian R and Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation (2001), 103(24), 2961–2966. [DOI] [PubMed] [Google Scholar]
- 62.Zurlo F, Larson K, Bogardus C, and Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest (1990), 86, 1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ariano MA, Armstrong RB, and Edgerton VR. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem (1973), 21, 51–55. [DOI] [PubMed] [Google Scholar]
- 64.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, and Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA (2007), 104, 12587–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arch JR. The discovery of drugs for obesity, the metabolic effects of leptin & variable receptor pharmacology: perspectives from β3-AR agonists. Naun Schmied Arch Pharm (2008), 378, 225. [DOI] [PubMed] [Google Scholar]
- 66.Blue DR, Daniels DV, Gever JR, Jett MF, O’Yang C, Tang HM, Williams TJ, and Ford APDW. Pharmacological characteristics of Ro 115–1240, a selective α1A-AR partial agonist: a potential therapy for stress urinary incontinence. BJU Int (2004), 93, 162–170. [DOI] [PubMed] [Google Scholar]
- 67.Modiri AR, Fredrickson MG, Gillberg PG, and Alberts P. Selectivity of oxymetazoline for urethral pressure vs blood pressure in the anaesthetized female rabbit. Scand J Urol Nephrol (2000), 34(3), 151–156. [DOI] [PubMed] [Google Scholar]
- 68.Musselman DM, Ford AP, Gennevois DJ, Harbison ML, Laurent AL, Mokatrin AS, Stoltz RR, and Blue DR. A randomized crossover study to evaluate Ro 115–1240, a selective α1A adrenoceptor partial agonist in women with stress urinary incontinence. BJU Int (2004), 93(1),78–83. [DOI] [PubMed] [Google Scholar]
- 69.DeWire SM and Violin JD. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ Res (2011), 109(2), 205–216. [DOI] [PubMed] [Google Scholar]
- 70.Evans BA, Broxton N, Merlin J, Sato M, Hutchinson DS, Christopoulos A, and Summers RJ. Quantification of functional selectivity at the human α1A-adrenoceptor. Mol Pharmacol (2011), 79(2), 298–307. [DOI] [PubMed] [Google Scholar]