Abstract
This comprehensive review article aims to provide some definitive statements on the factors like clinical syndromes, radiological findings, and decompressive surgery, that may influence the outcomes in cervical spinal cord injury management. Literature search on these factors published in the last decade were analyzed and definite statements prepared and voted for consensus opinion by the WFNS Spine Committee members and experts in this field at a meeting in Moscow in June 2019 using Delphi method. This was re-evaluated in a meeting in Pakistan in November 2019. Finally, the consensus statements were brought out as recommendations by the committee to the world literature. Traumatic Spinal Cord Syndromes have good prognosis except in elderly and when the presenting neurological deficit was very poor. Though conservative management provides satisfactory results, results can be improved with surgery when instability and progressive compression was present. Locked facet with spinal cord injury denotes poor prognosis. Magnetic resonance imaging T2 imaging is the essential prognostic indicator that apart from sagittal grade, length of injury, maximum canal compromise, maximum spinal cord compression, axial grading (BASIC) score. Diffusion tensor imaging is the next promising predictor in the pipeline. Decompressive surgery when done earlier especially within 24 hours of injury provides better result and there is no clear evidence to show medical management is better or equivalent to delayed surgical management. Clinical syndromes, radiological syndromes, and surgical decompression have strong impact on the out comes in the management of cervical spinal cord injury. Our comprehensive review and final recommendations on this subject will be of great importance in understanding the complex treatment methods in use.
Keywords: Spinal cord injury, Outcome of spinal cord injury, Central cord syndrome, Clinical syndromes, Magnetic resonance imaging, Decompressive surgery
INTRODUCTION
Spinal cord injury (SCI) is a devastating disorder that demands attention of highest order and pose great challenge. There has been ever debating medical and surgical management protocols. However, there are many factors like clinical syndromes, radiological findings, and decompressive surgery that have significant impact on the outcomes. In this article, a comprehensive study on these factors is enumerated in separate subheadings after reviewing last 10 years of literature and finally the salient points are recommended from World Federation of Neurosurgical Societies (WFNS) Spine Committee.
METHODS
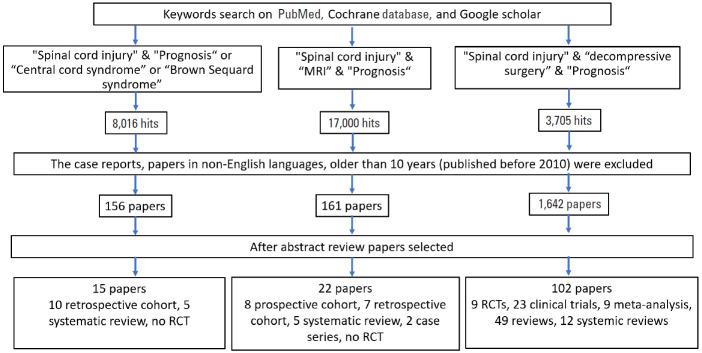
Literature search was done on google scholar, PubMed, and Cochrane data base. There were 3 main subtitles we searched for: (1) “impact of clinical syndromes on spinal cord injury outcomes.” The key words used for this were “spinal cord injury” and “prognosis” (8,016 hits) or “traumatic central cord syndrome” (184 hits) or “traumatic anterior cord syndrome” (76 hits) or “traumatic Brown-Séquard syndrome” (60 hits). The case reports, papers in languages other than English, papers older than 10 years (published before 2010), unrelated papers were removed. Remaining 38 papers were reviewed. (2) “impact of radiology on spinal cord injury outcomes.” The key words used for this were “spinal cord injury” and “prognosis” and “MRI.” On search, Google scholar gave 17,000 hits, on specifying further key words they were reduced to 250, PubMed gave 1,230, on searching for specific key words there were 208 hits, whereas Cochrane gave 189 hits. On excluding animal studies and including only human studies, 161 articles were shortlisted. Of these, 55 articles were identified to be relevant to our topic. Twenty-two of these studies were selected and studied in detail. No randomized controlled trial (RCT) was identified, 8 prospective cohort studies (level 2), 7 retrospective cohort studies (level 3), 5 systematic reviews (level 3), and 2 case series (level 4) were identified. (3) “impact of decompressive surgery on spinal cord injury outcomes”. Using keyword “decompressive surgery in spinal cord injury” in PubMed search there were 3,705 papers. Between 2010 and 2020, we found 1,642 articles with 28 clinical trials, 10 RCTs, 11 meta-analysis, and 111 review articles. Further narrowing search with “outcomes of decompressive surgery in spinal cord” we found 489 articles with 23 clinical trials, 9 RCTs, 9 meta-analysis, 49 reviews, and 12 systemic reviews. Best studies that contributed for our review came from few RCTs, meta-analysis and important articles (Fig. 1) reflects a flowchart depicting how we did literature search.
Fig. 1.
Flowchart: literature search for the effect of radiological findings on prognosis. MRI, magnetic resonance imaging; RCT, randomized controlled trials.
First consensus meeting was conducted on June 1, 2019 in Moscow. A re-evaluation meeting was done on November 13, 2019 in Peshawar, Pakistan. Based on the literature review the authors prepared statements on factors that influence outcomes in cervical SCI, viz. clinical syndromes, radiological findings and decompressive surgery. A presentation based on the literature review and the prepared statements were subjected to discussions, followed by voting process by the members of the WFNS Spine Committee using Delphi method. Answering to the questionnaire each expert voted for all of the statements grading every item on a 5-point scale according to Delphi method. 1=total disagreement, 2=disagreement, 3=agreement, 4=more than agreement, 5=total agreement. Consensus is reached when the sum of items “1”+“2” or “3”+“4”+“5” exceeds 66%. We called a negative consensus if 1-2> 66%, positive consensus=3-4-5> 66%, nonconsensus=1-2 or 3-4-5< 66%. The recommendations were prepared from those statements after consensus meeting.
1. Impact of Clinical Syndromes on SCI Outcomes
SCI clinical syndromes are: central cord syndrome (CCS), Brown-Séquard syndrome, anterior cord syndrome, posterior cord syndrome, cauda equina syndrome, and conus medullaris syndrome. In this paper, we will stress the most common syndrome, the CCS.
1) Incidence
McKinley et al. [1] have searched for the incidence and outcomes of the SCI syndromes. From 839 acute SCI, 175 patients (21%) had diagnosed with SCI clinical syndromes. The most common was CCS (44%), followed by cauda equina syndrome (25%), Brown-Séquard syndrome (17%), and anterior cord syndrome (5%).
(1) Central cord syndrome
CCS occurs most frequently after an extension injury in older patients with pre-existing spinal canal stenosis. It was first described by Schneider et al. [2] in 1954. Clinical presentation consists of more severe paresis in the upper extremities than the lower extremities. The reason for that is said to be due to central white matter tracts are most affected. It accounts for approximately 9% of all traumatic SCIs [1].
(2) Cauda equina syndrome
In fact, it is not a true SCI, but the injury of roots. In addition to lower extremity weakness, perianal anesthesia, and sphincter dysfunction are the clinical characteristics. The main difference from spinal cord syndromes is its nature as lower motor neuron lesion and greater chance of recovery since roots can regenerate [1].
(3) Brown-Séquard syndrome
Brown-Séquard syndrome accounts for 1%–4% of all traumatic SCIs. Only a limited number of patients have the pure form of Brown-Séquard syndrome – much more common is Brown-Séquard plus syndrome [3], which refers to a relative ipsilateral hemiplegia with a relative contralateral hemianalgesia. Etiology in most of the cases is penetrating injuries. It has the best prognosis among syndromes with up to 90% become ambulated by the time. If the upper limb is weaker than the lower limb, patients are more likely to ambulate [3].
(4) Anterior cord syndrome
Anterior cord syndrome is a lesion involving the anterior twothirds of the spinal cord. Since the posterior columns are preserved, touch, position, and vibrators sensation are preserved, but there are complete paralysis and hypoalgesia below the level of the lesion. Its incidence is 2.7% of all traumatic SCIs [1]. It has a poor prognosis, with only 10%–20% chance of motor recovery.
2) Acute traumatic CCS
(1) Diagnosis of CCS
Although the original description for trauma mechanism in CCS is a hyperextension injury in a person with already narrow spinal canal [2], flexion, and vertical compression can also cause this syndrome [4]. The upper extremity weakness should be attributed to the damage at the corticospinal tract and the motor neurons in the anterior horn. Hyperpathia probably resulted from injuries to the posterior horn.
Magnetic resonance imaging (MRI) is the diagnostic imaging of choice, showing typical intramedullary hypersignal on T2-weighted image. There is probably an overlooked instability in some cases of traumatic CCS. Krappinger et al. [5] have correlated the intraoperative disco-ligamentous injury with preoperative MRI findings. During surgery, they revealed 25 cervical spine segments with hyperextension instability in 22 of 23 patients (95.7%). While the radiologist on call correctly assessed segmental hyperextension instability in 15 of 25 segments, the specialized MRI radiologist was correct in 22 segments. The authors concluded that in most of the cases with acute CCS there is a disco-ligamentous injury, the magnetic resonance (MR) images are important for the diagnosis as well as the radiologist’s level of experience [5].
(2) Nonsurgical treatment of CCS
In the past, these patients had been managed more conservatively than other spinal cord injuries because the earliest descriptions showed poor results with surgery and significant neurological improvement with nonsurgical management [2]. However, because surgical techniques have improved and become safer, most central cord injuries are now treated surgically [6-8]. The incidence of surgery in the United States has increased 40% from 2003 to 2010 [6].
Nonsurgical treatment relies on external cervical immobilization, maintenance of a sufficient systolic blood pressure, and early rehabilitation, and should be reserved for patients suffering from mild acute traumatic central cord syndrome (ATCCS). Surgical management of ATCCS consists of posterior, anterior, or combined approaches, in order to achieve spinal cord decompression, with or without stabilization. The benefits of early surgical decompression in the setting of ATCCS remain controversial due to the lack of clinical randomized trials; recent studies suggest that early surgery (less than 72 hours after trauma) appears to be safe and effective, especially for patients with evidence of focal anatomical cord compression.
Early orthotic (collar) and medical management (volume resuscitation and blood pressure augmentation) are essential to maximize the chances of neurological recovery, by preventing the secondary injury cascade. Nonsurgical treatment may be proposed to patients with mild ATCCS [9]. Nevertheless, this treatment may predispose to occurrence of persisting neuropathic pain and spasticity. Contrary to what historically advocated, early surgical decompression seems indicated especially in patients who exhibit progressive neurological deficits. Controversy persists in the literature and no clear consensus can be proposed because of the lack of prospective controlled studies. However, recent studies of class III evidence suggest that early surgery for ATCCS is safe and effective, especially for patients with focal anatomical cord compression [9].
In general, an aggressive medical management in intensive care unit by providing a good oxygenation and maintaining the blood pressure on normal levels are mainstay for all patients with a SCI, including CCS [10].
(3) Surgery for CCS
There is no discussion that patients with cervical spinal fracture or dislocation will need a surgery. For patients with focal cord compression and ATCCS, surgery will be a more viable option. However, the role of surgery for patients with ATCCS with long segment cord compression and injury or with spinal stenosis without bony injury remains a subject of debate in the literature [10].
Yoshihara and Yoneoka [8] have investigated the treatment trend in the United States. The ratio of patients who underwent surgical treatment was 27.1%. The surgical treatment ratio has increased from 15% in 2000 to 30% in 2009. A total of 47.2% of surgical procedures were performed during the first 2 days [8].
Recently, there are increasing number of papers defending a surgery for ATCCS. They report that surgical management of acute traumatic CCS has been shown to improve neurological function, limit neuropathic pain, and prevent further SCI [8,11-14]. Patient age and comorbidities are important factors when considering surgical treatment for patients with ATCCS.
In a retrospective review of 69 patients, Anderson et al. [11] have used a posterior approach in 33 patients (48%), anterior approach in 22 patients (32%), and combined anteroposterior approach in 14 patients (20%). They, however, have not found any correlation between type of surgery and surgical timing with outcome scores.
Time of surgery is another concern. In a meta-analysis in 2010, Lenehan et al. [7] have looked for the outcomes of early and late surgery. They concluded that it is reasonable and safe to consider early surgical decompression in patients with profound neurologic deficit (American Spinal Injury Association [ASIA] C) and persistent spinal cord compression due to developmental cervical spinal canal stenosis without fracture or instability. Those with less severe deficit (ASIA D) can be treated with initial observation with surgery potentially at a later date depending on the extent and temporal profile of the patient’s neurologic recovery [7].
In a meta-analysis in 2015, Anderson et al. [12] have found that patients operated on less than 24 hours have better ASIA scores 1 year after surgery than those operated later. However, in a retrospective study taken from National Trauma Data Bank Research [13], among 1,060 operated patients, delayed surgery was associated with a decreased inpatient mortality.
A guideline for treatment options has been published by Fehlings et al. [14] in 2017. Their recommendation is “We suggest that early surgery (< 24 hours after injury) be considered as a treatment option in adult patients with traumatic CCS.” But, the quality of evidence is low. On the other hand, a late recurrent neurologic deterioration after conservative treatment may happen. Jin et al. [15] have reviewed 17 cases with late deterioration. They report that neurological deficits of all patients on admission were not serious and recovered quickly after conservative treatment. No fractures or dislocation were found. Because of deterioration approximately 6 weeks after trauma, and since new MR images showing more anterior cord compression, they operated all of them with anterior approach. During surgery, they have observed obvious ruptures of disk, anterior and longitudinal ligaments. They concluded that ruptures of anterior longitudinal ligaments, posterior longitudinal ligaments, and disks resulting in cervical instability and secondary compression on spinal cord are the reasons of late deterioration [15].
(4) Outcome of CCS
The most effective predictors of the outcome in acute traumatic CCS were found admission ASIA motor score, midsagittal diameter of the canal, and age of the patient [16]. Some others have found the importance of premorbid diseases.
3) Statements
Statement 1: Traumatic central cord syndrome (TCCS) has a good prognosis, although factors such as older age and more severe neurological damage during development are associated with a lower likelihood of neurological recovery. This statement reached a positive consensus (100% yes).
Statement 2: Conservative treatment (with use of hemodynamic support and maintaining mean arterial pressure [MAP] 85–90 mmHg) remains the most useful treatment for TCCS. This statement reached a positive consensus (91% yes).
Statement 3: To improve the outcomes of TCCS treatment, when there are signs of spinal instability or continuing compression of the spinal cord, an early surgery should be considered. This statement reached a positive consensus (91% yes).
2. Radiological Findings and Outcomes of SCI
Modern multidetector computed tomography (CT) imaging of the spine has essentially replaced plain radiographs due to the widespread availability and efficiency. CT scans can readily identify the bony abnormalities and types of fracture. Prompt and accurate diagnosis and care could be possible once we detect even slight changes in bone marrow, softtissue, and spinal cord. A typical lesion on MRI in a traumatic spinal cord is a spindle-shaped lesion containing hemorrhage in the center surrounded by a halo of edema. The edema has a greater rostralcaudal extent than the central hemorrhage. The identification of parenchymal SCI on MRI tallies well with the degree of neurologic deficit and the chances for the recovery [17]. The ability to predict the functional outcome of radiology can influence rehabilitation strategies. It may provide an understanding of the neurological impairment in SCI cases where a concomitant head injury or another level of SCI exists. This may help in determining the cellular changes in response to neural repair or biological therapies focused towards healing the damaged spinal cord.
1) Role of MRI
MRI should be obtained in the first 48 hours after trauma. 6% of the patients with normal CT scans may have an abnormality on MRI, especially the ligamentous injury. MRI assesses prognosis based on the findings of hemorrhage, the extent of edema, and the severity of the initial compression. On a T2 weighted MRI, a grave prognosis is symbolized by intraspinal hemorrhages more than 1 cm in length and a longitudinal signal more than 3 cm. A complete recovery is associated with normal initial MR image. Parashari et al. [18] looked at the prognostic role of MRI and its association with the clinical outcome. Patients with a sizable focus of hemorrhage (> 1 cm) had larger cord edema and a more severe grade of initial ASIA Impairment Scale (AIS) with poor recovery at follow-up. Scoring is calculated on the basis of a single axial image with the most severe SCI [19,20].
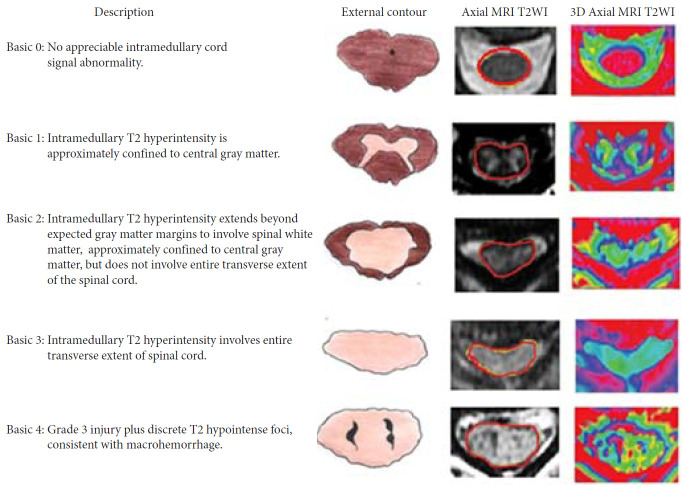
Talbott et al. [20] assessed the MRI findings of SCI patients according to a 5-point score, in 95 patients with MR done within 24 hours of injury. They showed excellent prognosis through all SCI severities. The data showed that the BASIC score helped in identifying AIS grade A patients, who may have potential to improve before discharge (Figs. 2, 3) [21]. The score showed improvement in patients with low scores with no improvement in BASIC score 4. In 2017, Kurpad et al. [22] carried out a systematic review of patients with SCI using MRI. MRI characteristics appear to be predictive of outcomes in acute SCI, including the length of intramedullary hemorrhage (moderate-quality evidence), canal diameter at maximal spinal cord compression, and spinal cord swelling (low-quality evidence) (Table 1).
Fig. 2.
Basic score. The picture depicts MRI findings in cervical spinal cord injury patients with 5 basic scores ranging from 0 to 4. MRI, magnetic resonance imaging; T2WI, T2-weighted image; 3D, 3-dimensional. Reprinted from Sharif and Jazaib. World Neurosurg 2020;140:574-590. with permission [21].
Fig. 3.
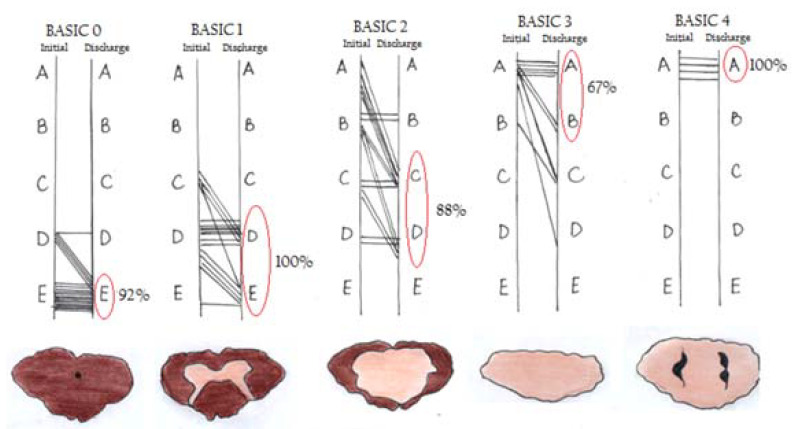
Basic scores in cervical spinal cord injury on admission and discharge. AIS grade improvement is shown in each BASIC score group, with a sketch of the SCI below. The percentages of patients within each group with a discharge AIS grade (circled in red). AIS, American Spinal Injury Association Impairment Scale. Reprinted from Sharif and Jazaib. World Neurosurg 2020;140:574-590. with permission [21].
Table 1.
Magnetic resonance imaging predictors of neurologic recovery
| Study | Data collection | Study type | Type of lesion | Association with outcome | Overall evidence |
|---|---|---|---|---|---|
| Boldin et al. [27], 2006 | Prospective | Cohort | Hemorrhage | No | Low |
| Long cord edema | Worse | ||||
| Cord swelling | Unspecified | ||||
| Miyanji et al. [25], 2007 | Prospective | Cohort | Hemorrhage | Worse | Moderate |
| Cord edema | No | ||||
| Cord swelling | Worse | ||||
| Shepard and Bracken [28] | Prospective | Cohort | Hemorrhage | No | Moderate |
(1) Treatment strategies and SCI outcomes according to pretreatment MRI
Papadopoulos et al. [23] evaluated the effect of pretreatment MRI on the neurological outcome in 91 consecutive patients, suffering from traumatic closed cervical (C1–T1) cord injury. Seventy-two percent of patients were managed according to an MRI-protocol group. Results were used to choose on emergency decompression. There were no adverse events in all 66 patients, and ongoing cord compression was seen in half of these patients, leading to surgical treatment. Disc herniation, epidural hematoma, or bone fragments were identified on MRI, causing the anterior spinal cord compression in 25% of the patients, leading to an anterior surgery. 50% of the MRI-protocol group had an improvement in the outcome, compared to 24% of the reference group. One in 9 patients improved from a complete motor injury in the MRI-protocol group, to independent walking ability [23]. Six studies showed an association between MRI features and neurological functions. AIS grade, ASIA motor score, Frankel grade, motor function score, upper/lower extremity motor function, and minimally useful function, pinprick score, and light touch score were used to assess functional outcome.
(2) Intramedullary spinal cord hemorrhage and neurological outcomes
Three of the 5 studies identified the presence of intramedullary hemorrhage was predictive of worse neurologic recovery [24-26]. Selden et al. [26] demonstrated that intra-axial hematoma in Frankel grade A patients is associated with no improvement in follow-up neurology. In 2 studies, worse neurological outcome was seen in longer rostrocaudal intramedullary hematoma [26,27]. Boldin et al. [27] observed that longer hematoma length (median, 10.5 mm) was associated with complete injuries, and these patients did not improve on follow-up AIS, whereas hemorrhage length of less than 4 mm showed improvement. [27] Neurological outcome and cord edema on MRI (high signal intensity on T2-weighted images) were not associated with neurologic recovery in 3 studies [25,27,28]. The longer length of edema had worse neurologic recovery in 2 papers [24,27]. Increase in spinal cord diameter was a predictor of worse neurological outcomes in a study [25].
(3) Prognosticators of MRI and function
The MRI features predicting functional outcomes were studied by Wilson et al. [29] The measures of functional recovery used were Functional Independence Measure (FIM) Motor score, functional dependence, self-reported manual dexterity, and dysesthesia. Aarabi et al. [10] showed that lower maximal canal compromise was associated with worse FIM scores. Functional recovery was not associated with maximum spinal cord compression (MSCC) and the spinal canal diameter. Similarly, SCI lesion length and FIM scores did not show an association, although, manual dexterity and dysesthesia were related to a longer spinal injury lesion. Unusually, Wilson et al. [29] suggested that MRI consistent with edema or hemorrhage are not important predictors of functional outcomes. Conventional MRI cannot distinguish recoverable from nonrecoverable tissue injury. Contusion and edema have similar signal changes on T2-weighted images. Though, edema is recoverable and contusion is relatively irreversible, with permanent loss of neurons, they have similar change on T2-weighted images. Advanced and innovative MRI techniques are required to have a comprehensive study of the spinal cord parenchyma, including diffusion tensor imaging (DTI), functional MRI, MR spectroscopy, and perfusion imaging [10,24]. Intrinsic measures of spinal cord pathology on acute MR imaging, particularly the BASIC score, accurately predict neurologic impairment in acute SCI (Table 2) [18].
Table 2.
Magnetic resonance imaging sequences and their value in spinal cord injury
| MRI sequences | Various findings in traumatic spinal cord injury | Logical basis |
|---|---|---|
| Recommended | ||
| Sagittal T2 | Cord compression, edema, hemorrhage | For management and prognosis |
| Axial T2 | Disc herniation, cord compression | For management and prognosis |
| Optional | ||
| Sagittal and axial T1 | Ligaments, cysts | Identify the mechanism of injury |
| STIR/GRASS | Ligaments | Identify the mechanism of injury |
| fMRI | Blood oxygen level-dependent contrast | For investigation and mapping out areas of spinal cord |
| DTI | White matter tracts | Investigational and Prognostic? |
STIR, Sequences of fast short TI inversion recovery, GRASS, gradient recalled acquisition in steady state; fMRI, functional magnetic resonance imaging; DTI, diffusion tensor imaging.
(4) Diffusion tensor imaging
DTI evaluates spinal cord structural integrity and microstructural alterations that affect the diffusion of water molecules in the pathology. It is more sensitive for assessing spinal cord damage than standard T2-weighted imaging (Fig. 4) [30].
Fig. 4.
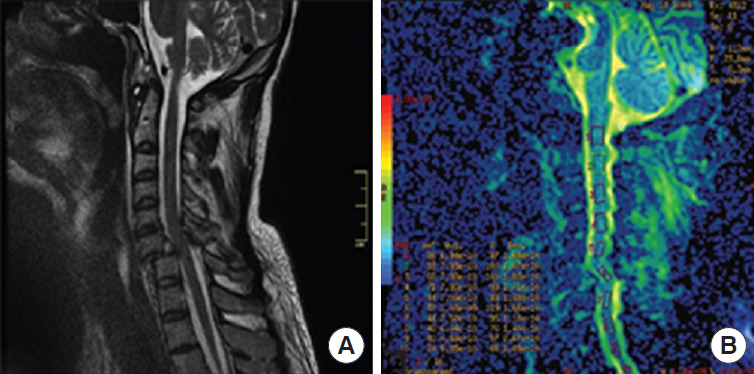
The diffusion tensor imaging of a patient with C6/C7 spinal cord injury. (A) Sagittal image of the T2-weighted image of the cervical spine. (B) Diffusion tensor imaging sagittal section. Reprinted from Czyz et al. J Spinal Study Surg. 2017;1:25-28, under the terms of Open Access [30].
There are only 39 human clinical studies that have been conducted with DTI up to now, with only a couple of studies with a year long-term follow-up. Conventional imaging and DTI were performed on 25 patients with blunt SCI and 11 normal persons. DTI depict the severity of SCI and correspond with ASIA motor scores in patients with nonhemorrhagic cord injury. The strongest association with both motor and SCIM III scores (Spinal Cord Independence Measure) at one year was found with axial diffusivity. Axial diffusivity is a more specific parameter for axonal injury than radial diffusivity. Hence axonal injury in the cord can be the main factor affecting patient recovery [31]. Fractional anisotropy (FA) defined as “the degree of anisotropy” ranges from 0 to 1, where tissues with high anisotropy such as white matter have values closer to 1. The injured portion of the spinal cord shows a decrease in anisotropy secondary to the disruption of longitudinally arranged fibres (Fig. 5) [32].
Fig. 5.
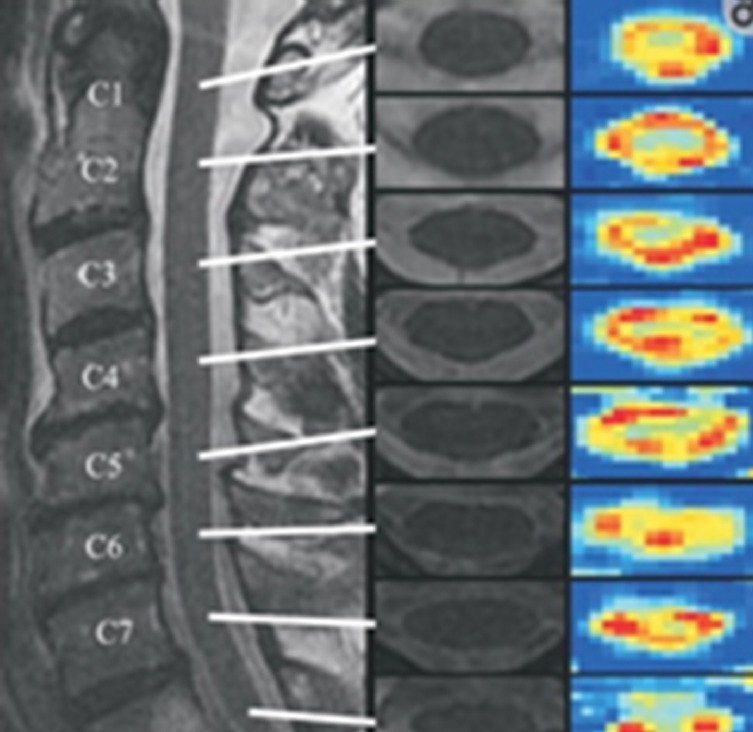
Sagittal T2-weighted (T2W) magnetic resonance image (left) of the cervical spine in a normal individual with axial T2W images at each cervical level (middle). Fractional anisotropy maps (right) at each cervical level show the cross-sectional anatomy with lower anisotropy in the central gray matter and higher anisotropy in the white matter funiculi. Lower cervical segments show poorer spatial resolution as compared to superior levels. Reprinted from Vedantam et al. J Magn Reson Imaging 2013;38:861-7, under the terms of Open Access [32].
DTI in chronic cervical spine injury demyelination and axonal degeneration of spinal tracts lead to reduce DTI values in SCI. The quantitative evaluation of spinal cord damage and the effect of future regeneration-inducing treatments can be monitored by DTI [33].
(5) Functional MRI and neural activity
Functional MRI (fMRI) is a noninvasive imaging tool that relies on the changes in the flow and oxygen levels of the metabolically active neural tissue. The change in signal is related to both the blood oxygen level-dependent contrast and signal enhancement from water protons in extracellular space caused by an increase in water content in the area. The spinal fMRI can map out areas of sensory and motor spinal function, related to utilizing an active and passive lower limb task paradigm. Kornelsen and Stroman [34] showed that the neural activity was present in all patients irrespective of the degree of injury and that both the active and passive motion could be elicited below the level of injury.
(6) Assessment of MRI sequences
In 2015, Martin et al. [35] evaluated different techniques used in MRI sequences. The FA had the robust evidence of utility, with moderate-quality evidence as a biomarker, showing correlation with disability in the various clinical scenario. New MRI techniques are evolving with the excellent potential for improvement in the diagnosis and management of spinal pathologies. This has been shown to have limited clinical use [35].
Efficacy of these advanced spinal imaging techniques will play a vital role in determining the role of novel treatments. Serial DTI may evaluate spinal cord integrity and monitor microstructural changes during therapies. Perfusion MRI may determine whether blood flow has been re-established. An increase in glucose uptake is expected in healing spinal tissue, 18F-fluorodeoxyglucose positron emission tomography imaging may be valuable for monitoring neural repair.
2) Statements
Statement 4: Presence of facet dislocation on CT is suggestive of poor neurological outcome. This statement reached a positive consensus (91% yes).
Statement 5: MRI T2 sequences is an acceptable method to rapidly screen patients with a cervical injury. This statement reached a positive consensus (100% yes).
Statement 6: Predictive findings on T2 sequences, including sagittal grade, length of injury, maximum canal compromise, and MSCC, axial grading (BASIC) score provide the best and easy means to predict the outcome. This statement reached a positive consensus (100% yes).
Statement 7: DTI sequences may be promising to predict outcome in both acute and chronic spinal injury patients. This statement reached a positive consensus (91% yes).
3. Impact of Decompressive Surgery on SCI Outcomes
Surgical decompression in acute SCI was debated in the past while continuous studies were undertaken to search the validity of the same. It was hypothesized that the secondary changes in the cascade of events modified with less tissue damage following decompression and hence clinical improvements were better following surgery done at the earliest. Preclinical studies and clinical trials clearly highlight the value of surgical management in SCI, and if a surgery was done earlier after injury, the outcomes are better at the end.
1) Decompressive surgery
Traumatic spinal cord injuries are increasing due to fast improvement in living style in last decade with an average report of 750 cases per million population all over the world. Therapies are aimed to reduce the extent of injury and prevent the cascades of events in secondary injury by doing decompressive surgeries at the earliest possible time in these victims. Laboratory research has shown significant benefits towards application of decompressive surgery in acute SCI. Furlan et al. [36] summarized the results of various experimental studies emphasizing the impact of early decompression and outcome. Ziu et al. [37] demonstrated in a mouse model that prolonged compression leads to altered micro-RNA expression compared with shortduration compression, suggesting that secondary injury not simply mediated by acute cytotoxicity, but by active protein synthesis in surviving cells. These preclinical studies have shown that early surgical decompression improves outcomes of SCI [38,39]. Initially assessment of early decompression was done with a period of 72 hours as cutoff. It was hypothesized that early decompression will provide less tissue damage and improved outcome compared to those who were treated conservatively or with delayed surgery. However, Molliqaj et al. [9] suggested that the final results were controversial due to lack of randomized clinical trials, in spite, they are safe and effective. Lenehan et al.7 reported early surgery provided better results as mentioned earlier. However, later one systematic review supported early surgery with 24 hours cutoff. Fehlings et al. [40] in his multicenter prospective cohort study examining surgical timing in acute SCI with comparison of early (less than 24 hours of injury) versus late (24 hours or later) surgery with respect to neurological improvement at 6 months post cervical SCI. They concluded early surgery is safe and at least 2 AIS grade clinical improvement were observed at 6 months after surgery. While 19.8% of early surgical group showed 2 grade improvement, only 8.8% improved in the late group. Complications were moderately less in early group (24.2%) compared to late group (30.5%). However, the strength of evidence was very low, that timing of surgery is associated with functional outcomes [41,42]. In the recent clinical reports, favoring surgeries done within a period of 8 hours have been analyzed by Lee et al. [42] in a meta-analysis. They have opened newer thoughts on how earlier will be the best for SCI patients. In their analysis, 6 out of the 7 groups showed neurological improvement and 4 of them had methylprednisolone infused. While one group reported no improvement in spite of methylprednisolone infusion, another group showed improvement without the same. This meta-analysis has really opened a new area of research in the future not only to identify how early is the surgery to be recommended and infusion of methylprednisolone as a routine in all indicated cases.
2) Statements
Statement 8: Decompressive surgery is an effective treatment in SCI and must be performed as early as possible. Data suggest that better outcomes are correlated with surgery performed within 24 hours from trauma. This statement reached a positive consensus (100% yes).
Statement 9: There is no clear evidence that nonoperative treatment is better or equivalent to delayed decompression. This statement reached a positive consensus (100% yes).
CONCLUSION
TCCS has a good prognosis, although factors such as older age and severe neurological injury are associated with limited neurological recovery. Conservative treatment (maintaining MAP 85–90 mmHg) remains the most useful treatment for TCCS. Early surgery should be considered when there are signs of spinal instability or continued spinal cord compression. BASIC scoring system remains a good and easy means to measure the outcome following spinal cord injuries. MRI T2 sequence is an acceptable method to rapidly screen patients with a cervical injury. Predictive findings on T2 sequences, including sagittal grade, length of injury, maximum canal compromise and MSCC, axial grading (BASIC) score provide the best and easy means to predict the outcome. Decompressive surgery is an effective treatment in SCI and must be performed as early as possible. Data suggest that better outcomes are correlated with surgery performed within 24 hours from trauma.
WFNS SPINE COMMITTEE RECOMMENDATIONS
Impact of Clinical Syndromes on Outcomes
• TCCS has a good prognosis, although factors such as older age and severe neurological damage are associated with a lower likelihood of neurological recovery.
• Conservative treatment (with use of hemodynamic support and maintaining MAP 85–90 mmHg) remains the most useful treatment for TCCS.
• To improve the outcomes of TCCS treatment, when there are signs of spinal instability or continuing compression of the spinal cord, early surgery should be considered.
Impact of Radiological Findings on Outcomes
• Presence of facet dislocation on CT is suggestive of poor neurological outcome.
• MRI T2 sequences is an acceptable method to rapidly screen patients with a cervical injury.
• Predictive findings on T2 sequences, including sagittal grade, length of injury, maximum canal compromise, and MSCC, axial grading (BASIC) score provide the best and easy means to predict the outcome.
• DTI sequences may be promising to predict outcome in both acute and chronic SCI patients.
Impact of Decompressive Surgery on Outcomes
• Decompressive surgery is an effective treatment in SCI and must be performed as early as possible. Data suggest that better outcomes are correlated with surgery performed within 24 hours from trauma.
• There is no clear evidence that nonoperative treatment is better or equivalent to delayed decompression.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.McKinley W, Santos K, Meade M, et al. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30:215–24. doi: 10.1080/10790268.2007.11753929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider RC, Cherry G, Pantek H. The syndrome of acute central cervical spinal cord injury; with special reference to the mechanisms involved in hyperextension injuries of cervical spine. J Neurosurg. 1954;11:546–77. doi: 10.3171/jns.1954.11.6.0546. [DOI] [PubMed] [Google Scholar]
- 3.Roth EJ, Park T, Pang T, et al. Traumatic cervical Brown-Sequard and Brown-Sequard-plus syndromes: the spectrum of presentations and outcomes. Paraplegia. 1991;29:582–9. doi: 10.1038/sc.1991.86. [DOI] [PubMed] [Google Scholar]
- 4.Li XF, Dai LY. Acute central cord syndrome: injury mechanisms and stress features. Spine (Phila Pa 1976) 2010;35:E955–64. doi: 10.1097/BRS.0b013e3181c94cb8. [DOI] [PubMed] [Google Scholar]
- 5.Krappinger D, Lindtner RA, Zegg MJ, et al. Spondylotic traumatic central cord syndrome: a hidden discoligamentous injury? Eur Spine J. 2019;28:434–41. doi: 10.1007/s00586-018-5796-5. [DOI] [PubMed] [Google Scholar]
- 6.Brodell DW, Jain A, Elfar JC, et al. A national trends in the management of central cord syndrome: an analysis of 16,134 patients. Spine J. 2015;15:435–42. doi: 10.1016/j.spinee.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenehan B, Fisher CG, Vaccaro A, et al. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976) 2010;35(21 Suppl):S180–6. doi: 10.1097/BRS.0b013e3181f32a44. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara H, Yoneoka D. Trends in the treatment for traumatic central cord syndrome without bone injury in the United States from 2000 to 2009. J Trauma Acute Care Surg. 2013;75:453–8. doi: 10.1097/TA.0b013e31829cfd7f. [DOI] [PubMed] [Google Scholar]
- 9.Molliqaj G, Payer M, Schaller K, et al. Acute traumatic central cord syndrome: a comprehensive review. Neurochirurgie. 2014;60:5–11. doi: 10.1016/j.neuchi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Aarabi B, Alexander M, Mirvis SE, et al. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J Neurosurg Spine. 2011;14:122–30. doi: 10.3171/2010.9.SPINE09922. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DG, Sayadipour A, Limthongkul W, et al. Traumatic central cord syndrome: neurologic recovery after surgical management. Am J Orthop (Belle Mead NJ) 2012;41:E104–8. [PubMed] [Google Scholar]
- 12.Anderson KK, Tetreault L, Shamji MF, et al. Optimal timing of surgical decompression for acute traumatic central cord syndrome: a systematic review of the literature. Neurosurgery. 2015;77 Suppl 4:S15–32. doi: 10.1227/NEU.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 13.Samuel AM, Grant RA, Bohl DD, et al. Delayed surgery after acute traumatic central cord syndrome is associated with reduced mortality. Spine (Phila Pa 1976) 2015;40:349–56. doi: 10.1097/BRS.0000000000000756. [DOI] [PubMed] [Google Scholar]
- 14.Fehlings MG, Tetreault LA, Wilson JR, et al. A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤24 hours versus >24 hours) of decompressive surgery. Global Spine J. 2017;7(3 Suppl):195S202S. doi: 10.1177/2192568217706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin W, Sun X, Shen K, et al. Recurrent neurological deterioration after conservative treatment for acute traumatic central cord syndrome without bony injury: seventeen operative case reports. J Neurotrauma. 2017;34:3051–7. doi: 10.1089/neu.2016.4706. [DOI] [PubMed] [Google Scholar]
- 16.Aarabi B, Hadley MN, Dhall SS, et al. Management of acute traumatic central cord syndrome (ATCCS) Neurosurgery. 2013;72 Suppl 2:195–204. doi: 10.1227/NEU.0b013e318276f64b. [DOI] [PubMed] [Google Scholar]
- 17.Wittenberg RH, Boetel U, Beyer HK. Magnetic resonance imaging and computer tomography of acute spinal cord trauma. Clin Orthop Relat Res. 1990;(260):176–85. [PubMed] [Google Scholar]
- 18.Parashari UC, Khanduri S, Bhadury S, et al. Diagnostic and prognostic role of MRI in spinal trauma, its comparison and correlation with clinical profile and neurological outcome, according to ASIA impairment scale. J Craniovertebr Junction Spine. 2011;2:17–26. doi: 10.4103/0974-8237.85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishna V, Andrews H, Varma A, et al. Spinal cord injury: how can we improve the classification and quantification of its severity and prognosis? J Neurotrauma. 2014;31:215–27. doi: 10.1089/neu.2013.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talbott JF, Whetstone WD, Readdy WJ, et al. The Brain and Spinal Injury Center score: a novel, simple, and reproducible method for assessing the severity of acute cervical spinal cord injury with axial T2-weighted MRI findings. J Neurosurg Spine. 2015;23:495–504. doi: 10.3171/2015.1.SPINE141033. [DOI] [PubMed] [Google Scholar]
- 21.Sharif S, Jazaib Ali MY. Outcome prediction in spinal cord injury: myth or reality. World Neurosurg. 2020;140:574–90. doi: 10.1016/j.wneu.2020.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Kurpad S, Martin AR, Tetreault LA, et al. Impact of baseline magnetic resonance imaging on neurologic, functional, and safety outcomes in patients with acute traumatic spinal cord injury. Global Spine J. 2017;7(3 Suppl):151S–174S. doi: 10.1177/2192568217703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulos SM, Selden NR, Quint DJ, et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52:323–32. doi: 10.1097/00005373-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Flanders AE, Spettell CM, Tartaglino LM, et al. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology. 1996;201:649–55. doi: 10.1148/radiology.201.3.8939210. [DOI] [PubMed] [Google Scholar]
- 25.Miyanji F, Furlan JC, Aarabi B, et al. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome--prospective study with 100 consecutive patients. Radiology. 2007;243:820–7. doi: 10.1148/radiol.2433060583. [DOI] [PubMed] [Google Scholar]
- 26.Selden NR, Quint DJ, Patel N, et al. Emergency magnetic resonance imaging of cervical spinal cord injuries: clinical correlation and prognosis. Neurosurgery. 1999;44:785–92. doi: 10.1097/00006123-199904000-00057. [DOI] [PubMed] [Google Scholar]
- 27.Boldin C, Raith J, Fankhauser F, et al. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine (Phila Pa 1976) 2006;31:554–9. doi: 10.1097/01.brs.0000201274.59427.a4. [DOI] [PubMed] [Google Scholar]
- 28.Shepard MJ, Bracken MB. Magnetic resonance imaging and neurological recovery in acute spinal cord injury: observations from the National Acute Spinal Cord Injury Study 3. Spinal Cord. 1999;37:833–7. doi: 10.1038/sj.sc.3100927. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JR, Grossman RG, Frankowski RF, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma. 2012;29:2263–71. doi: 10.1089/neu.2012.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Czyz M, Tykocki T, Szewczyk P, et al. Application of diffuse tensor imaging in the prognosis of outcome after cervical spinal cord injury. J Spinal Study Surg. 2017;1:25–8. [Google Scholar]
- 31.Cheran S, Shanmuganathan K, Zhuo J, et al. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011;28:1881–92. doi: 10.1089/neu.2010.1741. [DOI] [PubMed] [Google Scholar]
- 32.Vedantam A, Jirjis MB, Schmit BD, et al. Characterization and limitations of diffusion tensor imaging metrics in the cervical spinal cord in neurologically intact subjects. J Magn Reson Imaging. 2013;38:861–7. doi: 10.1002/jmri.24039. [DOI] [PubMed] [Google Scholar]
- 33.Petersen JA, Wilm BJ, von Meyenburg J, et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012;29:1556–66. doi: 10.1089/neu.2011.2027. [DOI] [PubMed] [Google Scholar]
- 34.Kornelsen J, Stroman PW. Detection of the neuronal activity occurring caudal to the site of spinal cord injury that is elicited during lower limb movement tasks. Spinal Cord. 2007;45:485–90. doi: 10.1038/sj.sc.3102019. [DOI] [PubMed] [Google Scholar]
- 35.Martin AR, Aleksanderek I, Cohen-Adad J, et al. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. Neuroimage Clin. 2015;10:192–238. doi: 10.1016/j.nicl.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furlan JC, Noonan V, Cadotte DW, et al. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of preclinical and clinical studies. J Neurotrauma. 2011;28:1371–99. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziu M, Fletcher L, Savage JG, et al. Spatial and temporal expression levels of specific microRNAs in a spinal cord injury mouse model and their relationship to the duration of compression. Spine J. 2014;14:353–60. doi: 10.1016/j.spinee.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Batchelor PE, Wills TE, Skeers P, et al. Meta-analysis of preclinical studies of early decompression in acute spinal cord injury: a battle of time and pressure. PLoS One. 2013;8:e72659. doi: 10.1371/journal.pone.0072659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Walker CL, Zhang YP, et al. Surgical decompression in acute spinal cord injury: a review of clinical evidence, animal model studies, and potential future directions of investigation. Front Biol (Beijing) 2014;9:127–36. doi: 10.1007/s11515-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson JR, Tetreault LA, Kwon BK, et al. Timing of decompression in patients with acute spinal cord injury: a systematic review. Global Spine J. 2017;7(3 Suppl):95S–115S. doi: 10.1177/2192568217701716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DY, Park YJ, Kim HJ, et al. Early surgical decompression within 8 hours for traumatic spinal cord injury: is it beneficial? A meta-analysis. Acta Orthop Traumatol Turc. 2018;52:101–8. doi: 10.1016/j.aott.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]