Abstract
Background
England's National Institute for Health and Care Excellence (NICE) and the US’ Institute for Clinical and Economic Review (ICER) both conduct cost-effectiveness evaluations for new cancer drugs to help payers make drug coverage decisions. However, NICE and ICER assessments have been noted to reach different conclusions. We aim to better understand the degree to which their recommendations diverge and what drives these apparent differences.
Methods
We compared the methods and results of publicly available cost-effectiveness evaluations performed by ICER and NICE of similarly assessed cancer drugs. Assessments were compared based on incremental cost-effectiveness ratio, comparator treatment, price, recommendation, and the design of the economic evaluation.
Findings
Among 11 commonly assessed cancer drugs, ICER and NICE were in concordance for 7 evaluations and in discordance on the cost-effectiveness and coverage decisions for 4 drugs. Most new cancer drugs were not cost-effective in either the US (7/11) or England (7/11). Furthermore, NICE's capacity to negotiate price discounts and access schemes result in much lower cost per QALY valuations and more favourable recommendations than those of ICER for similarly assessed cancer drugs.
Interpretations
NICE and ICER employ similar health technology assessment (HTA) methodologies and were aligned with most recommendations, finding that many new and expensive cancer drugs are cost ineffective. Growing use of ICER assessments will continue to send stronger price signals to manufacturers that cancer drugs with low value for money will be viewed less favourably by private insurers. NICE provides an important reminder of how much lower other countries pay for drugs when comparative effectiveness and value-based pricing are integrated into public drug coverage decisions.
Keywords: Cancer drugs, Cost-effectiveness, United States, United Kingdom, Institute for Clinical and Economic Review, National Institute for Health and Care Excellence
Research in Context.
Evidence before this study
The US does not have a formal HTA agency for evaluating the clinical and cost-effectiveness of new drugs, therefore private insurers have increasingly begun to rely on the Institute for Clinical and Economic review (ICER) to make drug coverage decisions in medicinal formularies. Whereas in England, the National Institute for Health and Care Excellence (NICE) makes national coverage decisions for the publicly funded National Health Service. To our knowledge, no study has evaluated the difference in cost-effectiveness valuations of cancer drugs between these two agencies.
Added value of this study
We searched ICER and NICE appraisals for all similarly assessed cancer drugs until November 2019. As they use similar data and methods for determining cost-effectiveness, they come out with remarkably similar results. But there are important ways in which they diverge, largely due to contextual differences between the US and English health systems, and because of NICE's capacity to engage in negotiating price discounts and access schemes to drugs with uncertain therapeutic benefit or economic value.
Implications of all the available evidence
Most new cancer drugs evaluated by HTA agencies in the US and England are not cost-effective. Furthermore, discordance between the two agencies regarding the cost-effectiveness of newer cancer therapeutics is driven by the higher cost of healthcare and pharmaceuticals in the US inputted into economic modeling.
Alt-text: Unlabelled box
1. Introduction
New therapeutics for cancer such as immune checkpoint inhibitors and chimeric antigen receptor t-cell therapy (CAR-T), have become available for treatment of advanced or metastatic tumors. Many of these drugs have been priced very highly, raising important debates about the degree to which they provide value [1], [2], [3]. When comparing the value gained from new cancer drugs, the United States is an outlier behind several high-income countries including Australia, Canada and the United Kingdom on health gains per dollar spent [4]. In several countries, cancer drugs are regularly appraised by national health technology assessment (HTA) agencies to determine whether they represent a cost-effective use of public resources.
In England, the National Institute for Health and Care Excellence (NICE) appraises the clinical and cost-effectiveness of newly approved therapies and issues recommendations for public coverage in the National Health Service (NHS). In contrast, the US does not have a national HTA agency largely for political and ideological reasons that the government should not be involved in drug coverage decisions. The Institute for Clinical and Economic Review (ICER) is an independent, non-governmental research organization that is working to fill the HTA void in the US. ICER does not have a formal role for evaluation or reimbursement. Rather, they conduct an analysis for value-based pricing and budget impact which are being increasingly used by private payers to inform price negotiations with pharmaceutical companies. ICER's assessments appear to be gaining traction with a 2016 survey of US private payers finding that 59% of respondents had used ICER reports in formulary decisions [5].
While NICE and ICER represent two of the largest and most consequential HTA agencies, we know very little about how their methodologies compare or the degree to which they reach similar conclusions. Studies by Chabot and Rocchi in 2014 [6], and Pinto and colleagues in 2020 compared English and Canadian HTA for oncology drugs [7] and both studies found variation in recommendations due to differences in available policy tools to address issues of clinical uncertainty and unfavorable cost-effectiveness.
We compared the methods and results of cost-effectiveness evaluations performed by ICER and NICE of similarly assessed cancer drugs. We focused on cancer drugs because a host of them have recently come on the market, many are purported to be quite clinically useful, and most of them have very high price tags. We further examined where these two agencies have concordance or discordance in their recommendations to payers and the reasons underlying any differences.
2. Methods
2.1. Sample
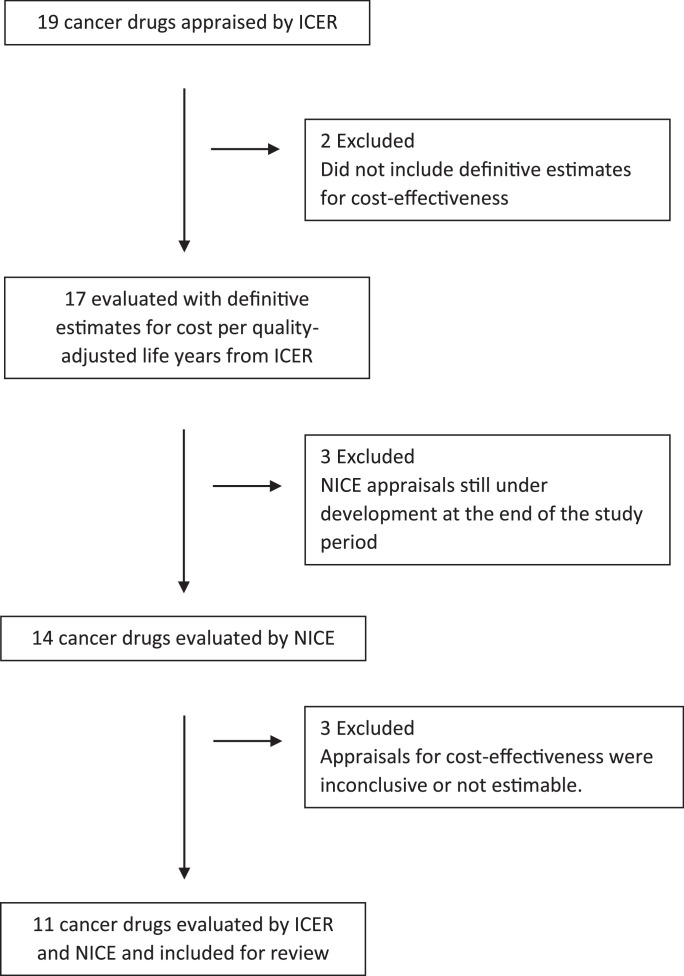
Both ICER and NICE publish publicly available assessment reports on the clinical and cost-effectiveness of new drugs. Every assessment of a cancer drug evaluated by ICER was included in the study. [8] As of November 2019, ICER has evaluated six cancer indications (categorized under five ‘topics’) including; b-cell acute lymphoblastic leukemia and b-cell lymphoma (included under the topic chimeric antigen receptor T-cell therapies), multiple myeloma, non-small cell lung cancer, ovarian cancer and prostate cancer. In total, 19 cancer drugs were appraised across the five topics, 17 were evaluated with definitive estimates for cost-effectiveness measured in cost per quality adjusted life year (QALY) from January 2016 through October 2018. The assessments of abiraterone acetate for prostate cancer and daratumumab for multiple myeloma did not include definitive estimates for cost-effectiveness and were excluded from the analysis.
The 17 drugs with available estimates for cost-effectiveness were similarly compared with publicly available technology appraisals from NICE. Six drugs were excluded; three technology appraisals for drugs were in development and three had estimates for cost-effectiveness which were inconclusive or not estimable. Reports from NICE often included estimates with greater than or less than figures relative to established cost-effectiveness thresholds. These were presented in the table, but the boundary value was used to calculate the statistical mean and median cost-effectiveness values for the sampled drugs.
Eleven cancer drugs were ultimately included in our study (Fig. 1). Each was a biologic or immunotherapy, indicated for treatment of recurrent, relapsed, advanced or metastatic tumor. Three drugs were indicated for treatment of non-small cell lung cancer and ovarian cancer, two were CAR-T therapies (one for acute leukemia and the other for lymphoma), and two were for multiple myeloma and one for prostate cancer (eTable 1 and eTable 2).
Fig. 1.
Flowchart of cancer drugs evaluated for cost-effectiveness included in our study. Abbreviations: The Institute for Clinical and Economic Review; ICER, the National Institute for Health and Care Excellence, NICE.
2.2. Statistical analysis
The primary outcome for selection was cost-effectiveness measured using the incremental cost-effectiveness ratio. This was available in US dollars for ICER and Great British pounds per QALY for NICE. The incremental cost-effectiveness ratio is used to evaluate the cost-effectiveness of health interventions measuring the difference in cost divided by the difference in clinical effect.
Assessment reports were analyzed and data was extracted for the following characteristics: cost-effectiveness using the incremental cost-effectiveness ratio, clinical benefit measured in QALYs gained, date of assessment, comparator treatment used in determining cost-effectiveness, price the calculation was based upon (often list price or wholesale acquisition cost), final recommendation and the criteria and design of the economic evaluation. Other variables collected from the assessment reports for the methodology and criteria included; the perspective of the economic evaluation, type of model, time horizon, cycle length, discount rate, outcomes, clinical trials and the assumptions used in the estimate (eTable 1).
A detailed analysis of the modeling of clinical efficacy and health equities used to determine incremental QALYs gained by a drug was not completed in our assessment because the majority of clinical data from NICE appraisals was redacted. Therefore, it was not possible to compare how ICER and NICE evaluated the clinical benefit of cancer drugs included in our study. The incremental cost-effectiveness ratio calculated by NICE was converted to US dollars using US gross domestic product purchasing power parity from the year the final recommendation was made or the nearest year with available data [9].
2.3. Role of funding
None.
3. Results
3.1. Methodology
We found that eleven cancer drugs were commonly assessed by ICER and NICE from 2016 to 2019 and evaluated for cost-effectiveness using the measure of incremental cost per QALY (Table 1)[10]. ICER uses a cost-effectiveness threshold of $100,000 to $150,000 per QALY or below. In comparison, NICE utilizes a much lower threshold of £20,000 to £30,000 per QALY ($28,471 - 42,857) [11].
Table 1.
Cost-effectiveness evaluations and coverage recommendations from ICER and NICE.
| Indication | Drug |
Incremental cost-effectiveness ratio |
Recommendation |
Concordance of Recommendations | Reason for Discordance | ||
|---|---|---|---|---|---|---|---|
| ICER | NICE | ICER | NICE | ||||
| Non-small Cell Lung Cancer | Atezolizumab (Tecentriq) | $219,179 | < $71,429 | High certainty for benefit despite uncertain evidence, exceeds cost-effectiveness (factor of uncertainty) | Recommended with financial agreement | Yes | N/A; not cost-effective in either US or England |
| Nivolumab (Opdivo) | $415,950 | $72,379 | High certainty for benefit despite uncertain evidence, exceeds cost-effectiveness (factor of uncertainty) | Recommended with a financial and post-market efficacy agreement | Yes | N/A; not cost-effective in either US or England | |
| Pembrolizumab (Keytruda) | $236,492 | < $71,429 | High certainty for benefit despite uncertain evidence, exceeds cost-effectiveness (factor of uncertainty) | Recommended with financial agreement | Yes | N/A; not cost-effective in either US or England | |
| Ovarian, Fallopian, & Peritoneal Cancer | Rucaparib (Rubraca) | $369,175 | > $42,857 | Quality adjusted and OS benefit but not priced in alignment with benefit | Recommended with a financial and post-market efficacy agreement | Yes | N/A; not cost-effective in either US or England |
| Niraparib (Zejula) | $291,454 |
$53,804 | Quality adjusted and OS benefit, but the price is not aligned with the benefit | Recommended with a financial and post-market efficacy agreement | Yes | N/A; not cost-effective in either US or England | |
| Olaparib (Lynparza) | $324,100 | > $42,857 | Quality adjusted and OS benefit but not priced in alignment with benefit for platinum sensitive disease | Recommended with a financial and post-market efficacy agreement | Yes | N/A; not cost-effective in either US or England | |
| Multiple Myeloma | Panobinostat (Farydak) | $10,230 | < $35,765 |
Promising but concerns over toxicity, long-term cost-effectiveness is uncertain | Recommended with financial agreement | Yes | N/A; cost-effective in both US and England |
| Ixazomib (Ninlaro) | $433,794 | < $42,857 | Moderate certainty for health benefit, not representative of long-term value at list price | Recommended with a financial and post-market efficacy agreement | No | Higher price in the US | |
| Acute Lymphoblastic Leukemia | Tisagenlecleucel (Kymriah) | $45,871 | > $42,857 – $64,286 | Net health benefit, potentially cost-effective but more evidence for PFS and OS is needed to reduce uncertainty of clinical and cost-effectiveness | Recommended with a financial and post-market efficacy agreement | No | Higher cost-effectiveness threshold in the US |
| Lymphoma | Axicabtagene ciloleucel (Yescarta) | $136,078 | > $71,429 | Net health benefit, cost-effective | Recommended with a financial and post-market efficacy agreement | No | Higher cost-effectiveness threshold in the US |
| Prostate Cancer | Enzalutamide (Xtandi) | $84,000 | $80,240 | High certainty of substantial net health benefit (based on MFS and immature OS data), cost-effective | Not recommended; immature OS evidence not significant, not cost-effective with financial agreement | No | Higher cost-effectiveness threshold in the US, discordance regarding clinical effectiveness |
Abbreviations: ICER; Institute for Clinical and Economic Review, NICE; the National Institute for Health and Care Excellence, PFS; progression-free survival, MFS; metastasis-free survival, OS; overall survival.
Notes: Drug evaluations from ICER and NICE differ because of their function within the two healthcare systems. In the United Kingdom, NICE makes recommendations for funding decisions in the NHS whereas in the United States, ICER does not have a funding mandate and does not make formal decisions for reimbursement. Therefore, the recommendations from the two agencies are distinct and presented differently.
1. For NICE's assessment of atezolizumab the ICER was confidential due to the patient access scheme. NICE explained the ICER was similar to pembrolizumab and likely cost-effective. Less than $71,429 per QALY was used as an educated assumption based on the information given.
2. For the assessment of rucaparib, ICER used comparators of Pegylated liposomal doxorubicin + carboplatin while NICE used comparators of routine surveillance or olaparib.
3. For NICE's assessment of olaparib the base-case ICER was $42,857 per QALY but this was stated to over value treatment. NICE stated treatment was not a cost-effective use of resources compared with routine surveillance therefore an educated assumption (greater than £30 K per QALY) was used.
4. ICER compared a combination therapy of panobinostat with bortezomib and dexamethasone versus bortezomib and dexamethasone. NICE compared panobinostat with bortezomib and dexamethasone versus lenalidomide and dexamethasone. ICER also made this comparison but found that lenalidomide and dexamethasone was cheaper and more cost-effective than the therapy with panobinostat.
5. Ixazomib is indicated with lenalidomide and dexamethasone.
6. For tisagenlecleucel, NICE and ICER used different comparators. ICER compared tisagenlecleucel to clofarabine while NICE compared it with a composite of salvage chemotherapy as well as blinatumomab. NICE determined that tisagenlecleucel had an incremental cost effectiveness ratio > $42,857 when compared with salvage chemotherapy and > $64,286when compared with blinatumomab.
Methodologically, both ICER and NICE utilize similar comparators and approaches for estimating cost-effectiveness. They use comparable economic tools and assumptions: lifetime time horizon for measuring effect, economic modeling using partitioned and Markov models, an economic perspective from the payer's point of view, discount rates of approximately 3%, length of treatment cycle matched to the drug, and key outcomes measured including cost, life years gained, QALYs, and the incremental cost-effectiveness ratio. However, some appraisals had discordant comparators for evaluating cost-effectiveness, such as tisagenlecleucel (ICER used clofarabine as a comparator and NICE used blinatumomab or salvage chemotherapy) (Table 1). Both ICER and NICE encounter similar limitations with modeling clinical effectiveness. Many surrogate measures used for cancer therapies are not strongly correlated with clinically meaningful outcomes, such as overall survival [12]. Consequently, calculating QALYs gained using surrogate measures or immature clinical data lead to less than definitive results by relying on uncertain assumptions and extrapolation of multiple variables. This is because the final estimate for cost-effectiveness requires statistical modeling, which may be based upon information from surrogate measures, immature data for clinical outcomes, or small or unrepresentative patient cohorts.
3.2. Cost-effectiveness: the Institute for Clinical and Economic Review
We find that four drugs were deemed cost-effective by ICER and valued below the $150,000 per QALY threshold, recommended for formulary inclusion at the stated list price (Table 1). For the remaining seven drugs, ICER does not recommend including them at the stated list price without further rebates or discounts. For drugs which are not cost-effective, ICER performs a budget impact analysis based on the available list or net price to calculate discount rates that would make the drug fall below their cost-effective threshold. The rationale is that individual payers will use ICER reports as evidence in negotiations with manufacturers to achieve lower prices.
The mean and median incremental cost per QALY appraised by ICER was $233,302 and $236,492, respectively. Panobinostat had the lowest cost per QALY valuation at $10,230 while ixazomib had the highest, $433,794. On average, a price discount of 51% is required to improve the cost-effectiveness of these seven drugs to $150,000 per QALY and a discount of 68% is needed to achieve a cost-effectiveness of $100,000 per QALY.
3.3. Cost-effectiveness: England's National IInstitute for Health and Care Excellence
NICE rarely identifies a single cost per QALY value for cost-effectiveness. Instead, it deliberates with the manufacturer to propose a range of possible cost-effectiveness values and then broadly decides whether the drug's value likely falls above or below the established threshold. Two drugs were within the cost-effectiveness threshold accepted by NICE at less than $28,471 - $42,857 per QALY, however, with a degree of uncertainty (panobinostat and ixazomib). The majority of cancer drugs (7/11) were valued above $42,857 but less than $71,429 (£50,000), a second higher threshold for end-of-life care and rare conditions that applies to most of these cancer therapies [11]. Only one drug, enzalutamide, was not recommended for use in England as it was definitively above the end-of-life threshold at $80,240 and had questionable certainty for overall survival. In contrast, axicabtagene ciloleucel was not cost-effective but recommended for coverage via the NHS subject to a financial agreement (since the manufacturer was able to reach a price agreement where the drug was cost-effective) and additional data for efficacy.
In total, ten drugs were recommended by NICE for coverage via the NHS and all ten drugs included a financial agreement to improve cost-effectiveness. Further, seven of the ten recommended drugs were subject to additional evidence for efficacy and recommended for use via England's Cancer Drugs Fund (CDF). The CDF provides access to cancer drugs that have significant uncertainty for clinical and cost-effectiveness but have potential for routine use. Through the CDF, real world evidence for clinical effectiveness is collected to reduce uncertainty and inform clinical decision making in the NHS[13].
3.4. Discordance
Despite large discrepancies in their cost per QALY valuations, ICER and NICE agreed with the majority (7/11) of recommendations to payers. Cancer drugs with positive recommendations tended to be more reasonably priced in the US or received discounts with financial agreements in England. Alternatively, drugs with negative recommendations often had high prices, were cost-ineffective, or had a significant budget impact. ICER and NICE were in discordance for 4/11 drugs. For three drugs, discordance was due to the higher cost-effectiveness threshold set by ICER, and higher US drug prices. For the remaining drug (enzalutamide), clinical effectiveness data from a surrogate endpoint resulted in opposing recommendations. ICER's appraisal for enzalutamide was based upon evidence from metastasis-free survival (a surrogate endpoint) and immature overall survival data to validate clinical benefit. In contrast, NICE did not believe enzalutamide was clinically effective as the data for overall survival was both immature and not significant.
4. Discussion
ICER and NICE evaluate cancer drugs to support cost-effective healthcare decisions and value-based pricing (according to the value of the drug rather than market dynamics, cost, or historical prices). As they use similar data and methods for determining cost-effectiveness, they come out with remarkably similar results. But there are important ways in which they diverge, largely due to contextual differences between the US and English health systems and because ICER and NICE have different remits.
Our analysis shows that NICE's capacity to negotiate price discounts and access schemes result in much lower cost per QALY valuations and more favourable recommendations than those of ICER for similarly assessed cancer drugs – largely due to its sanctioned role in healthcare decisions for England in contrast to ICER's voluntary non-profit role which has no enforcement over pricing or coverage decisions in the US. Differences in NICE's role within healthcare decisions for coverage and funding are part of the reason why it helps achieve lower drug prices in England. Almost all cancer drugs assessed by ICER were cost-ineffective at the list or net price and required substantial discounts to reach their cost-effectiveness threshold (a threshold that is much higher than the one used by NICE). ICER's higher cost-effectiveness valuations and chosen cost-effectiveness thresholds likely reflect the higher US drug prices and cost of healthcare inputted into their economic models. In 2018, US gross domestic product per capita spent on healthcare ($10,586) was almost three times greater than the UK ($4070) [14]. While challenges with estimating clinical effectiveness in new cancer drugs, such as relying on poorly validated surrogate measures, may contribute to cost-effectiveness variation, it does not appear to be a major factor in the consistently higher cost-effectiveness ratios presented by ICER.
With growing adoption of ICER assessments, US private insurers now have a standard for measuring the comparative value gained from new cancer treatments. This will continue to send price signals to the US market that high cost cancer drugs with marginal or uncertain benefit are likely to receive unfavorable coverage recommendations. Private insurers are then in a better, more informed position to negotiate price discounts off the list price. In contrast to private insurers which have some discretion in limiting formulary inclusion, Medicare and Medicaid, two of the largest payers in the US, are required to include nearly every FDA-approved cancer drug within the public formulary [15]. Consequently, public financing for cancer drugs in the US particularly suffers from inefficiencies, whereby Medicare and Medicaid are required to pay for me-too cancer drugs and drugs without validated improvements in clinical or cost-effectiveness over the standard of care [16]. Since public payors such as Medicare are required to cover most new cancer drugs regardless of price, private insurers have less flexibility to negotiate price discounts because Medicare is willing to cover the full cost of the drug.
Due to the unique structure of the US health system and its sociopolitical context, the US market is unlikely to accept a lower cost-effectiveness threshold or integrate a public HTA agency. In fact, there may be consequences to the pharmaceutical market if the US, the largest payer of new innovative pharmaceutical products, substantially lowers drug prices. However, NICE provides a stark reminder of how much lower other countries pay for cancer drugs and other pharmaceuticals. NICE employs several policy tools, such as value-based pricing, direct price negotiations and patient access schemes (which lower the acquisition cost of the drug to improve cost-effectiveness or limit prescribing to patient subgroups), that could be modified for the US market to help insurers, particularly Medicare and Medicaid, achieve better value.
This study has limitations. From the eleven drugs assessed by NICE, all eleven included a financial agreement used to improve the cost-effectiveness of drugs under review for coverage from the English National Health Service (NHS). This requires a negotiated agreement between the manufacturer and the regulator for public healthcare in England (NHS England), to submit the drug to the NHS at a discounted, confidential price. As such, the list price without the patient access scheme was available in public assessment reports produced by NICE [17]. The discounted price was used by NICE in the assessment of cost-effectiveness; however, this price remains confidential under the agreement. Therefore, the cost-effectiveness results reflect the list price after accounting for rebates and discounts from the manufacturer.
As noted above, NICE makes recommendations based on a range of possible cost-effectiveness values. The evidence review committee will broadly decide whether the balance of evidence suggests that the drug can be considered cost-effective relative to established cost-effectiveness thresholds (£20,000 to £30,000 per QALY). Consequently, a single cost per QALY often cannot be used to compare with that determined by ICER. In these cases, we provided the ‘greater than’ or ‘less than’ value identified by NICE. When calculating the mean and median cost per QALY we use the threshold value that NICE provides, which will trend these values toward the threshold itself. However, due to the nature of discount negotiations the range of cost per QALYs is not far from the threshold value. In contrast, ICER uses variable prices in their assessment of cost-effectiveness. These included the Medicare price, the wholesale acquisition cost (list price) or the net price depending on the drug. The list price did not reflect future discounts, voucher schemes, and rebates. Payers will then conduct their own private negotiations, similar to NICE, to achieve more cost-effective prices. Therefore, this study will overstate the true difference in cost-effectiveness of these cancer drugs listed in the US and England. However, even if payers were able to achieve the theoretical discount levels recommended by ICER, the cost-effectiveness will still be higher than that obtained by NICE because ICER utilizes a higher cost-effectiveness threshold. Lastly, cost-ineffective was used to describe both drugs with known clinical efficacy per unit of cost (e.g. pembrolizumab), and drugs with uncertain efficacy and more reasonable cost (e.g. panobinostat).
Spending on costly new cancer therapeutics is increasing for payers and health systems in the US and England. Among 11 new cancer drugs commonly evaluated by ICER and NICE, both drug assessment agencies used similar HTA methods and were in concordance for the majority of their cost-effectiveness recommendations. They concluded that most new cancer drugs represent low value for money at current prices. Both higher US prices and a higher cost-effectiveness threshold used by ICER were the primary reasons for divergence in the remaining assessments. A growing number of private US insurers look to ICER for cost-effectiveness valuations which will help send price signals to manufacturers that expensive cancer drugs with marginal or uncertain benefit are less likely to be covered by these insurers. While differences in health system and sociopolitical context may prevent US acceptance of a lower cost-effectiveness threshold or public integration of an HTA body, NICE provides an important perspective for how much lower other countries pay for pharmaceuticals and provides examples of policy tools that may help US health insurers, particularly Medicare and Medicaid, achieve better value.
Data sharing
All data is provided in the supplementary content.
Author contributions
AC and EM designed the analysis. AC and MR collected the data and wrote the initial draft. All authors provided critical insight and revised the manuscript.
Funding
None.
Declaration of Competing Interests
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100625.
Appendix. Supplementary materials
References
- 1.Salas-Vega S., Shearer E., Mossialos E. Relationship between costs and clinical benefits of new cancer medicines in Australia, France, the UK, and the US. Soc Sci Med. 2020;258 doi: 10.1016/j.socscimed.2020.113042. [DOI] [PubMed] [Google Scholar]
- 2.Vokinger K.N., Hwang T.J., Grischott T., Reichert S., Tibau A., Rosemann T. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol [Internet] 2020;21(May 1(5)):664–670. doi: 10.1016/S1470-2045(20)30139-X. http://www.thelancet.com/article/S147020452030139X/fulltext [cited 2020 Sep 7]Available from. [DOI] [PubMed] [Google Scholar]
- 3.Salas-Vega S., Iliopoulos O., Mossialos E. Assessment of overall survival, quality of life, and safety benefits associated with new cancer medicines. JAMA Oncol [Internet] 2017;3(Mar 1(3)):382–390. doi: 10.1001/jamaoncol.2016.4166. http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2016.4166 [cited 2019 Aug 8]Available from. [DOI] [PubMed] [Google Scholar]
- 4.Salas-Vega S., Mossialos E. Cancer drugs provide positive value in nine countries, but the united states lags in health gains per dollar spent. Health Aff [Internet] 2016;35(May(5)):813–823. doi: 10.1377/hlthaff.2015.1453. http://www.healthaffairs.org/doi/10.1377/hlthaff.2015.1453 [cited 2019 Dec 16]Available from. [DOI] [PubMed] [Google Scholar]
- 5.Lising A., Drummond M., Barry M., Augustovski F. Payers’ use of independent reports in decision making – will there be an ICER effect? Value Outcomes Spotlight. 2017 [Google Scholar]
- 6.Chabot I., Rocchi A. Vol. 6. Dove Medical Press Ltd; 2014. Oncology drug health technology assessment recommendations: Canadian versus UK experiences; pp. 357–367. (Clinico economics and outcomes research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto A., Naci H., Neez E., Mossialos E. Association between the use of surrogate measures in pivotal trials and health technology assessment decisions: a retrospective analysis of NICE and CADTH reviews of cancer drugs. Value Heal. 2020 doi: 10.1016/j.jval.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Institute for Clinical and Economic Review. Assessments [Internet]. Available from: https://icer-review.org/. Accessed 6 August 2020.
- 9.OECD. Purchasing power parities (PPP) (indicator). doi: 10.1787/1290ee5a-en. 2019. Accessed 6 August 2020.
- 10.Institute for Clinical and Economic Review. 2020 Value Assessment Framework: Final Framework [Internet]. [cited 2020 Jun 23]. Available from: https://icer-review.org/material/2020-value-assessment-framework-final-framework/
- 11.The National Institute for Health and Care Excellence. Consultation paper - value based assessment of health technologies [Internet]. 2014 [cited 2020 Aug 6]. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisals/VBA-TA-Methods-Guide-for-Consultation.pdf
- 12.Prasad V., Kim C., Burotto M., Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med [Internet] 2015;175(Aug 1(8)):1389–1398. doi: 10.1001/jamainternmed.2015.2829. http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2015.2829 [cited 2020 Jul 20]Available from. [DOI] [PubMed] [Google Scholar]
- 13.NHS England. Cancer Drugs Fund [Internet]. [cited 2020 Aug 5]. Available from: https://www.england.nhs.uk/cancer/cdf/
- 14.OECD. Health spending (indicator). 2020 [cited 2020 Jun 24]; Available from: https://data.oecd.org/healthres/health-spending.htm
- 15.Chambers J.D., Kim D.D., Pope E.F., Graff J.S., Wilkinson C.L., Neumann P.J. Specialty drug coverage varies across commercial health plans in the US. Health Aff. 2018 Jul 1;37(7):1041–1047. doi: 10.1377/hlthaff.2017.1553. [DOI] [PubMed] [Google Scholar]
- 16.Gagne J.J., Choudhry N.K. How many “me-too” drugs is too many? JAMA - J Am Med Ass Am Med Ass. 2011:711–712. doi: 10.1001/jama.2011.152. https://jamanetwork.com/journals/jama/fullarticle/645581 [Internet]. Vol. 305. [cited 2020 Sep 7]Available from. [DOI] [PubMed] [Google Scholar]
- 17.The National Institute for Health and Care Excellence [Internet]. NICE; Available from: https://www.nice.org.uk/. Accessed 6 August 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.