Abstract
Background
Recent data suggests clinically significant weight gain among non-pregnant HIV-positive adults after starting dolutegravir-based ART (DTG). Excess or insufficient weight gain in pregnancy could adversely impact pregnancy outcomes, but data for pregnant women receiving DTG are limited.
Methods
The Tsepamo Study captured data at delivery sites in Botswana from 2014 to 2019. HIV testing, HIV treatment information, and weight measurements during antenatal care were abstracted from the maternity obstetric record at delivery. HIV-positive women initiating DTG or efavirenz-based ART (EFV) between conception and 17 weeks gestation and HIV-uninfected women first presenting for antenatal care before 17 weeks gestation were included. We evaluated weekly weight gain, total 18-week weight gain, excess weight gain (>0.59 kg/week), insufficient weight gain (<0.18 kg/week), and weight loss between 18±2 and 36±2 weeks gestation, adjusting for demographic and clinical variables.
Findings
Baseline characteristics were similar by exposure group, including pre-pregnancy and early pregnancy weight. Compared with EFV, mean weekly weight gain between 18 and 36 weeks gestation was 0.05 (95% CI 0.03, 0.07) kg/week higher for women initiating DTG and 0.12 (0.10, 0.14) kg/week higher for HIV-uninfected women. Mean 18-week weight gain was 1.05 (95% CI 0.61, 1.49) kg higher for women initiating DTG and 2.31 (1.85, 2.77) kg higher for HIV-uninfected women, compared with EFV. Women initiating DTG were more likely to gain excess weight but less likely to gain insufficient weight or lose weight than women initiating EFV.
Interpretation
Women initiating DTG compared with EFV during pregnancy gained more weight between 18 and 36 weeks gestation. Neither group gained as much weight as HIV-uninfected women. Initiating DTG compared with EFV during pregnancy could increase the risk of excess weight gain but decrease the risk of insufficient weight gain and weight loss, which could have positive and negative consequences in pregnancy. Our findings are consistent with prior studies in non-pregnant adults.
Keywords: Dolutegravir, Pregnancy, Weight gain, Efavirenz, Botswana
Research in Context.
Evidence before this study
Two recent randomized trials found clinically significant weight gain among non-pregnant people living with HIV (PLWH) after initiating antiretroviral therapy (ART) with dolutegraivr (DTG). The ADVANCE trial in South Africa found individuals initiating DTG gained 3kg-6 kg after 48 weeks, compared with 1 kg after initiating efavirenz-based ART (EFV), whereas the NAMSAL trial in Cameroon found individuals initiating DTG gained 5 kg after 48 weeks, compared with 3 kg after initiating EFV. Weight gain was largest among female participants. Neither study included an HIV-uninfected comparator group, leading to debate about whether weight gain with DTG is excessive or simply represents a ‘return to health’.
Added value of this study
This is the first study to evaluate weight gain after initiating DTG in pregnant women, which is important because both excess (more than the maximum recommended) and insufficient (less than the minimum recommended) gestational weight gain can adversely impact pregnancy outcomes. The impact of DTG on gestational weight gain may differ from its impact on weight gain in non-pregnant women. Our study compared gestational weight gain among 1464 women who initiated DTG in pregnancy, 1683 women who initiated EFV in pregnancy and 21,917 HIV-uninfected women within an ongoing birth outcomes surveillance study in Botswana. We found that women initiating DTG during pregnancy gained more weight than women initiating EFV during pregnancy, corresponding to more excess weight gain but also less insufficient weight gain and less weight loss. Neither group gained as much weight as HIV-uninfected pregnant women. Our findings additionally highlight a high overall risk of insufficient weight gain and weight loss in HIV-positive women initiating ART during pregnancy.
Implications of all the available evidence
Initiation of DTG in pregnant women may lead to excess gestational weight gain but may also decrease insufficient weight gain and weight loss in pregnancy compared to initiation of EFV. Future work is needed to understand the impact of weight gain differences by ART regimen on maternal and infant outcomes in HIV-positive women and whether interventions to improve nutrition in pregnancy could mitigate the increased risk of adverse birth outcomes among HIV-infected women on ART.
Alt-text: Unlabelled box
1. Introduction
Antiretroviral therapy (ART) with dolutegravir (DTG) is increasingly used as first-line treatment for people living with HIV (PLWH) because of its efficacy, tolerability, limited side effects, high virus barrier to DTG resistance, and low cost [[1], [2], [3], [4]]. Enthusiasm for global rollout of DTG has been dampened slightly by recent reports of a possible increased risk of neural tube defects with periconceptual exposure to DTG [[5], [6], [7]] and clinically significant weight gain among HIV-infected adults on DTG [8,9]. The ADVANCE trial in South Africa found individuals initiating DTG gained an average of 3 kg (when DTG was combined with tenofovir disoproxil fumarate (TDF)) to 6 kg (when DTG was combined with tenofovir alefanamide (TAF))) after 48 weeks, compared with 1 kg after initiating efavirenz-based ART (EFV) [9]. Weight increase was greatest among female participants and those with an ART regimen that included TAF compared with TDF [9]. The NAMSAL trial in Cameroon found individuals initiating DTG gained 5 kg after 48 weeks (when DTG was combined with TDF), compared with 3 kg after initiating EFV [8]. Weight gain of 10% or more was greater among women than men [8]. A significant proportion of patients on DTG in both trials developed clinical obesity (Body Mass Index (BMI) >30), [8,9] though some argue this weight gain is appropriate as patients who achieve control of their chronic viral infection return to health [10,11].
There is concern that with longer follow up, excess weight gain with DTG will lead to increases in obesity-associated morbidity such as hypertension, diabetes and cardiovascular disease [12]. Given that almost half the global population of PLWH are women of reproductive age, there is also the potential for DTG initiation to increase weight gain in pregnancy which could adversely impact both maternal and fetal outcomes [13]. Excess weight gain in pregnancy is associated with gestational diabetes, [14] hypertension, [15] increased fetal growth and birth weight, [16,17] macrosomia, [17] more difficult labor, [15] and cesarean delivery complications [17]. Pre-pregnancy obesity also increases risk of neural tube defects [18]. In contrast, insufficient weight gain in pregnancy is associated with preterm birth and decreased fetal growth and birth weight [13,16,17] Many low- and middle-income countries are now confronted with a “double burden” of disease, whereby the high prevalence and consequences of infectious diseases and malnutrition are being compounded by an increase in obesity, overweight, and other non-communicable disease risk factors [19]. For example, in Botswana, it is estimated that 41% of individuals are either overweight or obese (BMI at or above 25 kg/m2), [20] and that the prevalence is greater among women than men [20,21].
In May 2016, Botswana became the first country in the world to recommend use of DTG as first-line ART for all adults (including pregnant women), replacing EFV as standard of care [22]. The Tsepamo Study has been conducting birth outcomes surveillance at delivery hospitals throughout Botswana since August 2014, providing the first comparison of outcomes among women initiating DTG with women initiating EFV in pregnancy [5,6,23]. In this analysis, we use Tsepamo data to investigate differences in gestational weight gain among women initiating DTG compared with EFV in pregnancy. To put our results in context and since little is known about differences in excess and insufficient gestational weight gain comparing HIV-infected women initiating ART and HIV-uninfected women, we also assessed gestational weight gain among HIV-uninfected pregnant women.
2. Methods
2.1. The Tsepamo study
Tsepamo is a birth outcomes surveillance study in Botswana [24]. Data are abstracted from the maternity obstetric record (a record of antenatal care) at the time of delivery from all women delivering at selected hospitals throughout the country. Tsepamo included 8 sites (~45% of all births in Botswana) from August 2014-July 2018 and expanded to include 18 sites (increasing coverage to ~72% of all births) from July 2018-March 2019. The maternity sites that were originally included were 2 tertiary referral hospitals, 5 district hospitals, and 1 primary-level hospital; 4 district and 6 primary-level hospitals were added in 2018. The surveillance study captures data on >99% of all births that take place at the included sites as almost all women bring their antenatal medical records (‘maternity card’) to delivery [6,24]. In Botswana, approximately 95% of women deliver at a hospital [25].
Information collected from the maternity obstetric record includes demographics, past medical history, diagnoses, hospitalizations and complications during pregnancy, medications prescribed during pregnancy, HIV history (including timing of diagnoses, ART regimens, CD4 count and viral loads), and clinical information including lab results, blood pressure, and weight measurements. All weight measurements ascertained and recorded by nurses or midwives from the time of the first antenatal care (ANC) visit to delivery are captured in the maternity obstetric record with associated dates. Self-reported pre-pregnancy weight is recorded when available. Height is measured but rarely recorded (approximately 1%) and upper arm circumference is not measured. Gestational age is documented by midwives at the time of delivery based on the estimated date of delivery (EDD). EDD is calculated at the first ANC visit using the reported last menstrual period and confirmed by ultrasound when available. If the last menstrual period date is unknown or suspected to be incorrect, fundal height measurements are used by the midwives to estimate gestational age.
Before May 2016, Botswana recommended initiation of TDF/emtricitabine(FTC)/EFV for all ART naïve adults with CD4 <350 cells/mm3 and for all pregnant women, regardless of CD4 cell count. In May 2016, TDF/FTC/DTG replaced TDF/FTC/EFV as the first-line regimen for all adults and all pregnant women and CD4 restrictions were removed. In September 2018, Botswana began to transition from TDF/FTC/DTG to TDF/lamivudine (3TC)/DTG to decrease the pill burden from 2 pills per day (TDF/FTC plus DTG) to 1 pill per day (TDF/3TC/DTG combined formulation). Women with kidney dysfunction or intolerance/resistance to TDF/FTC could access abacavir/3TC or zidovudine/3TC. TAF is not yet available in Botswana's national HIV program.
2.2. Eligibility criteria, exposure groups, and definition of baseline
HIV-positive women who initiated ART for the first time between the estimated last menstrual period and 17 weeks gestation were included in our analysis. We excluded women who initiated an ART regimen other than a DTG-based or EFV-based regimen. We also included HIV-uninfected women within one standard deviation of the mean age of the HIV-positive women who attended an antenatal clinic between the estimated last menstrual period and 17 weeks gestation. Multiple pregnancies were included. Baseline was defined as the date of ART initiation for HIV-positive women and as the date of the first ANC visit for HIV-uninfected women. Our study population was restricted to women who gave birth from August 2014 to March 2019.
2.3. Outcomes
We evaluated two primary outcomes. First, we calculated weekly weight gain from 18±2 to 36±2 weeks gestation as the difference in weight measured at 36±2 weeks and 18±2 weeks divided by the number of weeks between the measurements. Second, we calculated total 18-week weight gain from 18±2 to 36±2 weeks gestation as the difference in weight measured at 36±2 weeks and 18±2 weeks among women who had two weight measurements recorded 18 weeks apart. We required the 18±2 measurement to occur at or after baseline. We chose 18±2 and 36±2 weeks as the timeframes for the weight measurements to capture a weight measurement soon after baseline (ART initiation if HIV-positive) and to avoid the majority of the variation in gestational duration.
Secondary outcomes included weekly weight gain greater than 0.59 kg/week from 18±2 to 36±2 weeks (‘excess weight gain’), weekly weight gain less than 0.18 kg/week from 18±2 to 36±2 weeks (‘insufficient weight gain’), and any weight loss from 18±2 to 36±2 weeks. These cut-points are based on the Institute of Medicine (IOM) 2009 guidelines on gestational weight gain (converted from pounds to kilograms), which recommend gaining no more than 0.59 kg/week and no less than 0.18 kg/week in the second and third trimesters, regardless of pre-pregnancy BMI category (IOM guidelines recommend women with BMI<18.5 gain the most weight of all BMI categories, up to 0.59 kg/week, and women with BMI≥30 gain the least week of all BMI categories, at least 0.18 kg/week; therefore, these values can be used to define excess and insufficient weight gain regardless of pre-pregnancy BMI) [13]. Follow-up ended at 36±2 weeks for the purpose of all of our analyses.
2.4. Analysis
We examined demographic information by exposure group using sample means and proportions. For weekly weight gain and total 18-week weight gain, we fit linear regression models to estimate mean differences and 95% confidence intervals comparing women initiating DTG to women initiating EFV, and comparing HIV-uninfected women to women initiating EFV. Our models included a 3-level exposure variable (with EFV as the referent) and were adjusted for several baseline covariates: age (<25, 25–30, ≥30 years), pre-ART CD4 in pregnancy (>200 cells/μl or HIV-uninfected, ≤200 cells/μl or missing), employment (salaried, other or unknown), education (secondary education or higher, other or unknown), parity (≥1, 0 or unknown), gravidity (≥2, 1 or unknown), marital status (yes, no or unknown), site (tertiary referral hospital, other), smoking during pregnancy (yes, no or unknown), alcohol use during pregnancy (yes, no or unknown), pre-pregnancy weight (<50 kg, 50–80 kg, ≥80 kg, unknown), baseline weight in pregnancy (<50 kg, 50–80 kg, ≥80 kg, unknown), gestational age at baseline (<12 weeks, ≥12 weeks), and any medical diagnosis prior to pregnancy other than HIV (yes, no or unknown). Examples of common diagnoses prior to pregnancy include sexually transmitted infections (STI), anemia, hypertension, and asthma.
For weekly weight gain greater than 0.59 kg/week, weekly weight gain less than 0.18 kg/week, and weight loss from 18±2 to 36±2 weeks, we fit log-binomial regression models [26] to estimate risk ratios (an appropriate measure of association for non-rare outcomes) and 95% confidence intervals comparing women initiating DTG to women initiating EFV, and comparing HIV-uninfected women to women initiating EFV. Our models were adjusted for the same baseline covariates listed above.
We conducted subgroup analyses to evaluate effect modification by baseline weight in pregnancy (<50 kg and ≥80 kg) and by gravidity (primigravid and non-primigravid).
Women who did not have a weight measurement at 18±2 weeks and/or at 36±2 weeks had missing outcome data for weekly weight gain from 18±2 to 36±2 weeks gestation and women who did not have two weight measurements recorded 18 weeks apart had missing outcome data for total 18-week weight gain. If the factors associated with having a missing weight were also related to the weekly or total weight gain, restricting our analysis to only women who had the weight measurements of interest could induce selection bias. We attempted to adjust for this potential selection bias by estimating inverse probability of censoring weights in a sensitivity analysis [27]. To do so, we fit a logistic regression model for not having missing data on weekly weight gain conditional on the exposure group, the baseline covariates listed above, the number of ANC visits (<10, 10–14, >14), and any maternal diagnosis during pregnancy (yes, no or unknown). Our weights were stabilized [27] and used in the models evaluating weekly weight gain. A similar analysis was conducted for total 18-week weight gain. To further explore the sensitivity of our findings to missing outcome data, we performed additional sensitivity analyses where we restricted our analysis to individuals with baseline weight and where we evaluated weight at 36±2 weeks gestation as an outcome.
To evaluate the potential for residual confounding by missing data for baseline weight in pregnancy, we also conducted a sensitivity analysis restricted to women with a known baseline weight. CD4 cell count was infrequently measured in Botswana after CD4 restrictions were removed from treatment initiation guidelines in 2016. [6,28] To account for unmeasured or residual confounding by CD4 cell count, we varied how we categorized CD4 cell count in several sensitivity analyses (e.g., including a missing indicator for CD4 cell count, dichotomizing CD4 cell count as <200 cells/μl versus ≥200 cells/μl or missing, and using cut-points of 350 cells/μl and 500 cells/μl). In a final sensitivity analysis, we restricted our analysis to singleton pregnancies. All analyses were conducted using SAS. The reporting of this study conforms to the STROBE statement.
2.5. Role of the funding source
The funders had no role in study design, data collection and analysis, data interpretation, or preparation of the manuscript. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
2.6. Ethics approval
Ethics approval for this study was granted by the Health Research and Development Committee in Botswana and by the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health. Maternal consent was waived as data were collected anonymously and via chart abstraction.
3. Results
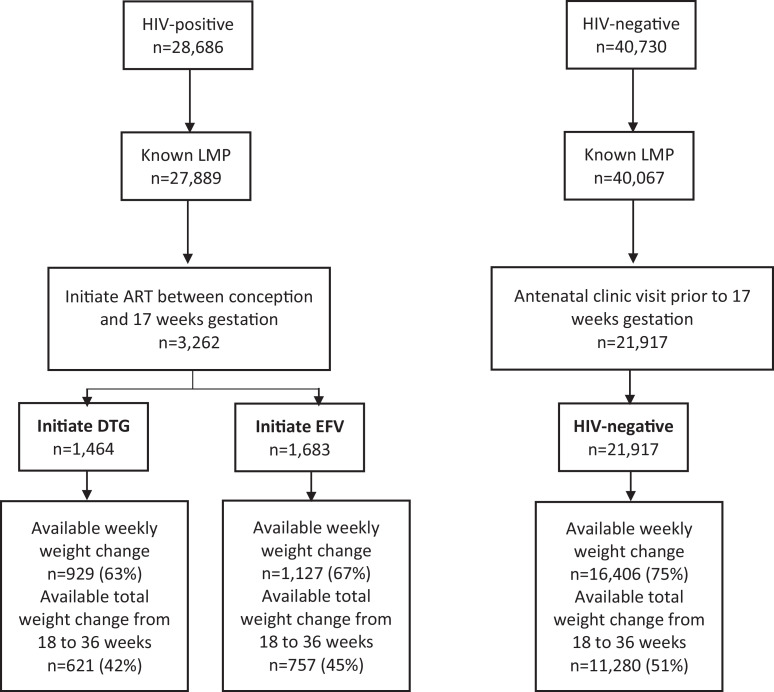
Of 28,686 HIV-positive women included in Tsepamo surveillance, 3262 (11%) initiated ART between conception and 17 weeks gestation. Of these, 1464 initiated DTG (45%), 1683 (52%) initiated EFV, and 115 (4%) initiated other ART or an unknown regimen. TDF/FTC was the most common backbone for both DTG- (98.8%) and EFV- (99.8%) based regimens. Of 40,730 HIV-uninfected women, 21,917 (54%) attended an ANC visit prior to 17 weeks gestation. Fig. 1 shows the study inclusion and available outcome data by exposure group. Women included who initiated DTG, EFV, or were HIV-uninfected had similar baseline characteristics, including age, employment status, educational attainment, pre-pregnancy weight and weight at baseline (Table 1). The proportion of singleton pregnancies was the same in all exposure groups (~98%). There were modest increases over the study period with respect to median baseline weight (63 kg in 2014 to 64.4 kg in 2018) and the proportion of women with baseline weight ≥80 kg (17.3% in 2014 to 19.3% in 2018) (Appendix Fig. 1). Compared with HIV-positive women, HIV-uninfected women were more likely to be nulliparous and primigravid. Compared with women initiating EFV-based ART, women initiating DTG-based ART were less likely to have a CD4 cell count measured at or before baseline (8.9% versus 35.8%), had lower median pre-ART CD4 cell counts (351 versus 399 cells/μl) and initiated ART on average 1 week earlier.
Fig. 1.
Study inclusion by exposure group.
Table 1.
Baseline characteristics by exposure group.
| Baseline characteristics Mean (SD) or Number (%) | EFV-based ART (n = 1683) | DTG-based ART (n = 1464) | HIV-uninfected (n = 21,917) |
|---|---|---|---|
| Age, mean (SD) | 28.78 (5.73) | 28.92 (5.95) | 28.26 (3.07) |
| <25 years | 454 (27.0) | 395 (27.0) | 2723 (12.4) |
| 25 to <30 years | 483 (28.7) | 411 (28.1) | 11,456 (52.3) |
| ≥30 years | 746 (44.3) | 658 (45.0) | 7738 (35.3) |
| First CD4 in pregnancy | |||
| >200 cells/μl | 523 (31.1) | 110 (7.5) | n/a |
| ≤200 cells/μl | 79 (4.7) | 20 (1.4) | n/a |
| missing | 1081 (64.2) | 1334 (91.1) | n/a |
| Median cells/μl | 399 | 351 | n/a |
| Salaried employment | 755 (44.9) | 613 (41.9) | 11,447 (52.2) |
| Secondary education or higher | 1547 (91.9) | 1365 (93.2) | 21,127 (96.4) |
| Parity ≥1 | 1194 (70.9) | 1014 (69.2) | 13,754 (62.8) |
| Gravidity ≥2 | 1262 (75.0) | 1075 (73.4) | 14,747 (67.3) |
| Married | 159 (9.5) | 116 (7.9) | 3562 (16.3) |
| Smoking during pregnancy | 31 (1.8) | 32 (2.2) | 197 (0.9) |
| Alcohol during pregnancy | 148 (8.8) | 177 (12.1) | 2088 (9.5) |
| Tertiary site | 863 (51.3) | 782 (53.4) | 10,557 (48.2) |
| Gestational age at baseline*, mean (SD) weeks | 12.88 (3.45) | 11.85 (3.82) | 12.24 (3.02) |
| <12 weeks gestational age at baseline | 484 (28.8) | 625 (42.7) | 9438 (43.1) |
| Baseline⁎⁎ weight in pregnancy, mean (SD) | 65.62 (15.13) kg | 65.66 (16.14) kg | 66.51 (15.68) kg |
| <50kg | 133 (7.9) | 116 (7.9) | 2195 (10.0) |
| 50–80kg | 780 (46.4) | 519 (35.5) | 12,682 (57.9) |
| ≥80kg | 184 (10.9) | 138 (9.4) | 3326 (15.2) |
| Missing | 586 (34.8) | 691 (47.2) | 3714 (17.0) |
| Pre-pregnancy weight, mean (SD) | 62.60 (14.85) kg | 62.80 (15.36) kg | 63.01 (14.63) kg |
| <50kg | 103 (6.1) | 98 (6.7) | 1418 (6.5) |
| 50–80kg | 463 (27.5) | 379 (25.9) | 6548 (29.9) |
| ≥80kg | 93 (5.5) | 90 (6.2) | 1341 (6.1) |
| Missing | 1024 (60.8) | 897 (61.3) | 12,610 (57.5) |
| Medical diagnosis prior to pregnancy other than HIV | 294 (17.5) | 239 (16.3) | 3189 (14.5) |
Baseline is ART initiation for HIV-positive women, first ANC visit for HIV-uninfected women.
The baseline weight is the first weight in pregnancy at or prior to ART initiation for HIV-positive women and at or prior to the first ANC visit for HIV-uninfected women.
Average weekly weight gain and total 18-week weight gain from 18±2 to 36±2 weeks was lower for women initiating EFV than women initiating DTG and both groups had less weight gain than HIV-uninfected women (Table 2). The adjusted mean difference (95% CI) in weekly weight gain was 0.05 (0.03, 0.07) kg/week for women initiating DTG and 0.12 (0.10, 0.14) kg/week for HIV-uninfected women, compared with women initiating EFV. The adjusted mean difference (95% CI) in total 18-week weight gain was 1.05 (0.61, 1.49) kg for women initiating DTG and 2.31 (1.85, 2.77) kg for HIV-uninfected women, compared with women initiating EFV (Table 2).
Table 2.
Average weekly weight gain and weight gain from 18±2 to 36±2 weeks by exposure group.
| EFV-based ART | DTG-based ART | HIV-uninfected | |
|---|---|---|---|
| Average weekly weight gain from 18±2 weeks to 36±2 weeks | |||
| Mean (SD) | 0.31 (0.23) kg/week | 0.35 (0.22) kg/week | 0.44 (0.23) kg/week |
| Unadjusted mean difference (95% CI) | 0.00 (Reference) | 0.05 (0.03, 0.07) kg/week | 0.13 (0.12, 0.15) kg/week |
| Adjusted* mean difference (95% CI) | 0.00 (Reference) | 0.05 (0.03, 0.07) kg/week | 0.12 (0.10, 0.14) kg/week |
| Total weight gain from 18±2 weeks to 36±2 weeks | |||
| Mean (SD) | 5.30 (4.35) kg | 6.27 (3.96) kg | 7.95 (4.11) kg |
| Unadjusted mean difference (95% CI) | 0.00 (Reference) | 0.97 (0.53, 1.40) kg | 2.64 (2.34, 2.95) kg |
| Adjusted* mean difference (95% CI) | 0.00 (Reference) | 1.05 (0.61, 1.49) kg | 2.31 (1.85, 2.77) kg |
Adjusted for age, first CD4 in pregnancy (if prior to ART initiation), employment, education, parity, gravidity, marital status, site, smoking and alcohol use during pregnancy, pre pregnancy weight, first weight in pregnancy (if prior to ART initiation or first ANC), gestational age at ART initiation, and medical history prior to pregnancy.
Almost one quarter of HIV-uninfected women (23.1%) gained more weight than the IOM recommended amount (0.59 kg/week), compared with 12.9% of women initiating DTG and 9.1% of women initiating EFV. The adjusted risk ratio for excess weight gain was 1.44 (1.11, 1.87) for women initiating DTG and 2.41 (1.81, 3.21) for HIV-uninfected women, compared with women initiating EFV. In contrast, 27.7% of women initiating EFV gained less weight than the IOM recommended amount (0.18 kg/week), compared with 20.2% of women initiating DTG and 11.1% of HIV-uninfected women. The adjusted risk ratio for insufficient weight gain was 0.73 (0.63, 0.86) for women initiating DTG and 0.48 (0.41, 0.57) for HIV-uninfected women, compared with women initiating EFV. More women initiating EFV lost weight (9.4%) than women initiating DTG (4.4%) and HIV-uninfected women (2.2%). The adjusted risk ratio for losing weight was 0.43 (0.28, 0.67) for DTG and 0.30 (0.19, 0.47) for HIV-uninfected women, compared with women initiating EFV (Table 3).
Table 3.
Excess weight gain, insufficient weight gain, and weight loss from 18±2 to 36±2 weeks by exposure group.
| EFV-based ART | DTG-based ART | HIV-uninfected | |
|---|---|---|---|
| Average weekly weight gain greater than 0.59 kg/week (excess weight gain) | (n = 1127) | (n = 929) | (n = 16,406) |
| Total (%) | 102 (9.1) | 120 (12.9) | 3797 (23.1) |
| Unadjusted risk ratio (95% CI) | 1.00 (Reference) | 1.43 (1.11, 1.83) | 2.56 (2.12, 3.08) |
| Adjusted* risk ratio (95% CI) | 1.00 (Reference) | 1.44 (1.11, 1.87) | 2.41 (1.81, 3.21) |
| Average weekly weight gain less than 0.18 kg/week (insufficient weight gain) | (n = 1127) | (n = 929) | (n = 16,406) |
| Total (%) | 312 (27.7) | 188 (20.2) | 1826 (11.1) |
| Unadjusted risk ratio (95% CI) | 1.00 (Reference) | 0.73 (0.62, 0.86) | 0.40 (0.36, 0.45) |
| Adjusted⁎⁎ risk ratio (95% CI) | 1.00 (Reference) | 0.73 (0.63, 0.86) | 0.48 (0.41, 0.57) |
| Weight loss | (n = 757) | (n = 621) | (n = 11,280) |
| Total (%) | 71 (9.4) | 27 (4.4) | 246 (2.2) |
| Unadjusted risk ratio (95% CI) | 1.00 (Reference) | 0.46 (0.30, 0.71) | 0.23 (0.18, 0.30) |
| Adjusted⁎⁎ risk ratio (95% CI) | 1.00 (Reference) | 0.43 (0.28, 0.67) | 0.30 (0.19, 0.47) |
Adjusted for age, first CD4 in pregnancy (if prior to ART initiation), employment, education, parity, gravidity, marital status, site, smoking and alcohol use during pregnancy, pre pregnancy weight, first weight in pregnancy (if prior to ART initiation or first ANC), gestational age at ART initiation, and medical history prior to pregnancy.
Adjusted for all variables listed above except smoking, alcohol, pre pregnancy weight and first weight in pregnancy (these variables were excluded from the model due to model convergence issues).
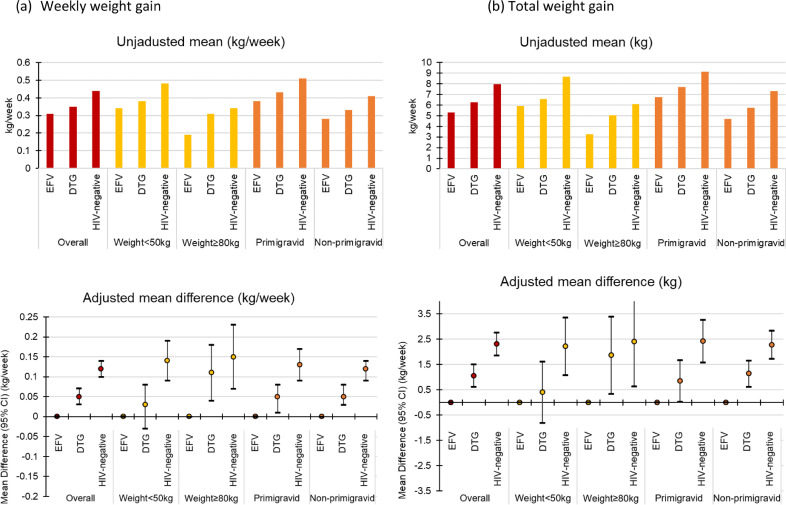
Women weighing <50 kg in early pregnancy gained more weight than women weighing ≥80 kg in early pregnancy. The adjusted mean differences in weight gain comparing women initiating DTG with women initiating EFV were larger among women weighing ≥80 kg in early pregnancy and attenuated among women weighting <50 kg in early pregnancy, but were largely unchanged when comparing HIV-uninfected women to women initiating EFV. Primigravid women gained more weight than non-primigravid women, but the adjusted mean differences were similar (Fig. 2 and Appendix Table 1).
Fig. 2.
Unadjusted means and adjusted mean differences for weekly weight gain (a) and total weight gain (b) from 18±2 to 36±2 weeks by exposure group and in subgroups defined by baseline weight in pregnancy and gravidity.
Approximately 25% of women included in our analysis were missing data on weekly weight gain from 18±2 to 36±2 weeks gestation because they did not have a weight measurement at 18±2 weeks (37%), they did not have a weight measurement at 36±2 weeks (47%), or both (16%). Approximately 50% of women included in our analysis were missing data on total 18-week weight gain from 18±2 to 36±2 weeks gestation because they did not have two weight measurements recorded 18 weeks apart. The proportion of women missing data for the weight gain outcomes was similar according to age, employment, parity, gravidity, marital status, alcohol use, and site; however, missing data for the weight gain outcomes was more likely for women who initiated DTG, had a CD4 cell count ≤200 cells/μl or missing CD4 cell count, had less than secondary education, smoked during pregnancy, had missing baseline weight in pregnancy, did not have a medical diagnosis prior to pregnancy, and had fewer than 10 ANC visits during pregnancy (Appendix Table 2). Including inverse-probability weights for missing data on the outcomes attenuated estimates by approximately 20% (though confidence intervals were overlapping) for average weekly weight gain (mean difference and 95% CI, 0.04 (0.02, 0.06) kg/week) and total 18-week weight gain (mean difference and 95% CI, 0.80 (0.30, 1.31) kg) comparing DTG to EFV. The inverse-probability weighted estimates comparing HIV-uninfected women to women initiating EFV were largely unchanged (Appendix Table 3). Women with baseline weight measures were less likely to have missing outcome data (21% missing for weekly weight gain), and the proportion missing was more balanced by exposure group. Restricting the analysis to individuals with baseline weight measures did not materially affect our estimates (Appendix Table 3). 16% of pregnant women had data on weight at 36±2 weeks gestation. Mean weight at 36±2 weeks gestation was highest among HIV-uninfected women and lowest among women initiating EFV (Appendix Table 4).
Varying assumptions about categorizing missing CD4 cell count also did not materially affect our estimates (Appendix Table 5). Restricting our analysis to singleton pregnancies did not affect our estimates.
4. Discussion
We used a large birth outcomes surveillance study in Botswana to provide the first data on gestational weight gain after initiating DTG-based ART in pregnancy. We found that compared with women initiating EFV during pregnancy, women initiating DTG gained more weight, and were less likely to lose weight, between 18±2 and 36±2 weeks gestation. However, women initiating DTG and EFV both gained less weight, were more likely to gain insufficient weight, and were more likely to lose weight than HIV-uninfected women.
Our findings of increased weight gain with DTG-based ART are consistent with two randomized clinical trials in non-pregnant adults. The ADVANCE trial showed adults initiating DTG gained 2kg-5 kg more than adults initiating EFV after 48 weeks [9] and the NAMSAL trial showed adults initiating DTG gained 2 kg more than adults initiating EFV after 48 weeks, [8] with greater weight gain in women compared to men. Unlike these two trials, our study also compared weight gain to HIV-uninfected women, who had more gestational weight gain than both women on DTG and EFV and less weight loss.
The clinical implications of our study findings may be mixed in terms of pregnancy outcomes. Insufficient weight gain and weight loss in pregnancy are associated with preterm birth and decreased birth weight and fetal growth [13,16,17]. Women on DTG had less insufficient weight gain (20.2%) than women on EFV (27.7%) and were less likely to lose weight (4.4%) than women on EFV (9.4%). However, excess weight gain in pregnancy is associated with pregnancy and delivery complications and increased fetal growth and birth weight, [[14], [15], [16], [17]] and those on DTG were more likely to gain excess weight (12.9%) compared with EFV (9.1%). Still, neither women initiating DTG nor women initiating EFV gained as much weight as HIV-uninfected pregnant women, suggesting that HIV or ART may impact the ability to gain weight in pregnancy. This may be explained by differences in the risk of malnutrition, infection or metabolism in these groups or by a specific effect of ART. A mechanism for excess weight gain with DTG has not yet been identified and further research is needed to understand this finding [29,30].
Our study has several limitations. IOM guidelines on appropriate gestational weight gain vary by pre-pregnancy BMI, which was not available in our study as height is rarely recorded and upper arm circumference is not measured. This made it more difficult to estimate the clinical impact of gestational weight gain. However, the IOM guideline recommended rates of weight gain in the second and third trimester range from 0.18–0.59 kg/week regardless of pre-pregnancy BMI category [13] and so we evaluated weight gain greater than 0.59 kg/week and weight gain less than 0.18 kg/week to approximate excess and insufficient weight gain. This may have underestimated differences at the extremes of BMI. Given that pre-pregnancy weight did not vary substantially by exposure group and median height was unlikely to differ by exposure group, we do not believe this underestimation of excess and insufficient weight gain was differential with respect to exposure group. IOM guidelines also have some limitations [31], including that they are predominantly based on studies conducted in the US population.
In addition to missing data on pre-pregnancy BMI, several other limitations of our study should be noted that could prevent a causal interpretation of our findings. First, data on weekly weight gain was not available for approximately 25% of women eligible for our analysis and data on total 18-week weight gain was not available for approximately 50% of women eligible for our analysis. Using inverse probability weighting to adjust for potential selection bias did not materially affect our estimates, though the weight gain estimates comparing DTG to EFV were slightly attenuated. However, it is possible that there were unmeasured factors impacting both measuring and recording of weight measurements and actual weight gain. Given the magnitude of the missing data for weight gain, our findings should be interpreted with caution. Second, data on important confounding variables such as early pregnancy weight and early pregnancy CD4 cell count were missing for many people. However, our results were largely unchanged in a sensitivity analysis restricted to individuals with available data on early pregnancy weight and in sensitivity analyses where we varied how CD4 cell count was categorized. Third, while our analyses adjusted for several key confounding variables like age, gravidity, smoking, and medical history, unmeasured and residual confounding cannot be ruled out. Specifically, the surveillance study does not collect data on food insecurity, which could differ by HIV status and impact weight gain. Last, our findings may not be generalizable to HIV-infected pregnant women in other regions with different distributions of potential effect modifiers such as race, [[32], [33], [34]] obesity, and malnutrition. An important strength of our study was its large sample size, and ability to compare women with similar demographics and antenatal care in each exposure group.
In summary, women initiating DTG during pregnancy gained more weight than women initiating EFV during pregnancy, corresponding to more excess weight gain but less insufficient weight gain and less weight loss. Neither group gained as much weight as HIV-uninfected pregnant women. HIV-uninfected women have the highest risk of excess weight gain and the lowest risks of insufficient weight gain and weight loss. Our findings highlight a high overall risk of insufficient weight gain (>24%) and weight loss (>7%) in HIV-positive women initiating ART during pregnancy. Further analyses are planned to evaluate weight gain among women who start ART prior to pregnancy and to understand the impact of weight gain differences by ART regimen on maternal and infant outcomes in HIV-positive women. Future studies are needed to evaluate whether interventions to improve nutrition in pregnancy could mitigate the increased risk of adverse birth outcomes among HIV-infected women on ART.
Contributors
ECC and RZ designed the study and wrote the manuscript with significant input from RS, BW, CZ and SL. ECC did the statistical analyses. All authors contributed to interpretation of data and revised and approved the manuscript.
Funding
This research was supported by National Institutes of Health NIH/NICHD R01 HD080471, NIH/NICHD K23 HD088230‐01A1, and NIH/NIAID K24 AI131924.
Data sharing statement
Once the Tsepamo Study has concluded, requests for data can be made, and will be reviewed by the PI and subject to IRB approval in Botswana and the United States.
Declaration of Competing Interest
No conflicts of interest exist.
Acknowledgments
We would like to thank the Tsepamo Study team, including our research assistants, the maternity staff and administrators at the 18 participating hospitals, and the members of the Botswana Ministry of Health and Wellness - in particular, the department of HIV/AIDS Prevention and Care, and the department of Maternal and Child Health. This study would not have been possible without the support of the leadership of the Botswana-Harvard AIDS Institute Partnership. We also acknowledge the funding and support from the National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Diseases.
Footnotes
Funding: National Institutes of Health NIH/NICHD R01 HD080471, NIH/NICHD K23 HD088230‐01A1, NIH/NIAID K24AI131924
Funding: NICHD R01 HD080471, NICHD K23 HD088230‐01A1, NIAID K24AI131924
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100615.
Appendix. Supplementary materials
References
- 1.Gunthard H.F., Saag M.S., Benson C.A. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venter W.F., Kaiser B., Pillay Y. Cutting the cost of South African antiretroviral therapy using newer, safer drugs. S Afr Med J. 2016;107(1):28–30. doi: 10.7196/SAMJ.2016.v107.i1.12058. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart M., Shelton J.D. ARVs: the next generation. Going boldly together to new frontiers of HIV treatment. Glob Health Sci Pract. 2015;3(1):1–11. doi: 10.9745/GHSP-D-14-00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department for Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (accessed Sep 12 2019).
- 5.Zash R., Makhema J., Shapiro R.L. Neural-tube defects with Dolutegravir treatment from the time of conception. N Engl J Med. 2018;379(10):979–981. doi: 10.1056/NEJMc1807653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zash R., Holmes L., Diseko M. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019 doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raesima M.M., Ogbuabo C.M., Thomas V. Dolutegravir use at conception - additional surveillance data from Botswana. N Engl J Med. 2019;381(9):885–887. doi: 10.1056/NEJMc1908155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolutegravir-based or low-dose efavirenz–based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–826. doi: 10.1056/NEJMoa1904340. [DOI] [PubMed] [Google Scholar]
- 9.Venter W.D.F., Moorhouse M., Sokhela S. Dolutegravir plus two different prodrugs of Tenofovir to Treat HIV. N Engl J Med. 2019;381(9):803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 10.Hill A., Waters L., Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity. J Virus Erad. 2019;5(1):41–43. doi: 10.1016/S2055-6640(20)30277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuh B., Tate J., Butt A.A. Weight change after antiretroviral therapy and mortality. Clin. Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(12):1852–1859. doi: 10.1093/cid/civ192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havlir D.V., Doherty M.C. Global HIV treatment - turning headwinds to tailwinds. N Engl J Med. 2019;381(9):873–874. doi: 10.1056/NEJMe1909363. [DOI] [PubMed] [Google Scholar]
- 13.ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013;121(1):210–212. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald S.C., Bodnar L.M., Himes K.P., Hutcheon J.A. Patterns of gestational weight gain in early pregnancy and risk of gestational diabetes mellitus. Epidemiology (Cambridge, Mass) 2017;28(3):419–427. doi: 10.1097/EDE.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchi J., Berg M., Dencker A., Olander E.K., Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev: Off J Int Assoc Study Obes. 2015;16(8):621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]
- 16.Siega-Riz A.M., Viswanathan M., Moos M.K. A systematic review of outcomes of maternal weight gain according to the institute of medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201(4):339. doi: 10.1016/j.ajog.2009.07.002. e1-14. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein R.F., Abell S.K., Ranasinha S. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H.Y., Chen H.L., Feng L.P. Maternal obesity and the risk of neural tube defects in offspring: a meta-analysis. Obes Res Clin Pract. 2017;11(2):188–197. doi: 10.1016/j.orcp.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Regional Office for Africa. Obesity; 2020. https://www.afro.who.int/health-topics/obesity (accessed January 15 2020,).
- 20.Keetile M., Navaneetham K., Letamo G. Socioeconomic and behavioural determinants of overweight/obesity among adults in Botswana: a cross-sectional study. BMJ Open. 2019;9(12) doi: 10.1136/bmjopen-2019-029570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letamo G. The prevalence of, and factors associated with, overweight and obesity in Botswana. J Biosoc Sci. 2011;43(1):75–84. doi: 10.1017/S0021932010000519. [DOI] [PubMed] [Google Scholar]
- 22.Botswana Ministry of Health. Handbook of the Botswana 2016 integrated HIV clinical care guidelines. 2016. http://apps.who.int/medicinedocs/documents/s22413en/s22413en.pdf (accessed Sep 13 2019).
- 23.Zash R., Jacobson D.L., Diseko M. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob health. 2018;6(7):e804–ee10. doi: 10.1016/S2214-109X(18)30218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zash R., Jacobson D.L., Diseko M. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr. 2017;171(10) doi: 10.1001/jamapediatrics.2017.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO statistical profile; Botswana: 2015. World Health Organization.http://www.who.int/gho/countries/bwa.pdf?ua=1&ua=1 (accessed October 21 2019,) [Google Scholar]
- 26.Spiegelman D., Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 27.Hernan M.A., Robins J.M. Chapman & Hall/CRC; Boca Raton: 2019. Causal inference. forthcoming. [Google Scholar]
- 28.Botswana Ministry of Health . 2016. Handbook of the Botswana 2016 integrated hiv clinical care guidelines.https://urldefense.proofpoint.com/v2/url?u=https-3A__aidsfree.usaid.gov_sites_default_files_botswana-5Fart-5F2016.pdf&d=DwIFaQ&c=j5oPpO0eBH1iio48DtsedeElZfc04rx3ExJHeIIZuCs&r=pQ1e-sX7evIuye5vOdRWCn8sKT606H43bihb8WU268w&m=j28ivui2wZVN4vCMBTCZEwLXNlflu00QUyGK0GbB34o&s=cywO_QUNaqJ1ktI3DJBnAhY1_wIeXCBdw0LUp86vCrQ&e= (accessed 1 October 2020, [Google Scholar]
- 29.Eckard A.R., McComsey G.A. Weight gain and integrase inhibitors. Curr Opin Infect Dis. 2020;33(1):10–19. doi: 10.1097/QCO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood B.R. Do Integrase Inhibitors Cause Weight Gain? Clin Infect Dis: Off Publ Infect Dis Soc Am. 2019 [Google Scholar]
- 31.Siega-Riz A., Bodnar L., Stotland N. The current understanding of gestational weight gain among women with obesity and the need for future research. A Natl Acad Med Discuss Pap. 2020 doi: 10.31478/202001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake J.U., Wu K., Erlandson K.M. Risk factors for excess weight gain following switch to integrase inhibitor-based ART. CROI. Seattle Washington. 2019 doi: 10.1093/cid/ciaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatell J.M., Assoumou L., Moyle G. Immediate versus deferred switching from a boosted protease inhibitor-based regimen to a Dolutegravir-based regimen in virologically suppressed patients with high cardiovascular risk or age >/=50 years: final 96-week results of the NEAT022 study. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2019;68(4):597–606. doi: 10.1093/cid/ciy505. [DOI] [PubMed] [Google Scholar]
- 34.Burns J.E., Stirrup O.T., Dunn D. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS. 2020;34(1):109–114. doi: 10.1097/QAD.0000000000002379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.