Key Points
Question
What are the pathophysiologic mechanisms behind the clinical outcomes of the sodium-glucose cotransporter-2 inhibitor empagliflozin in patients with heart failure and reduced ejection fraction (HFrEF)?
Findings
This exploratory post hoc substudy of the Empagliflozin in Heart Failure Patients with Reduced Ejection Fraction (Empire HF) randomized clinical trial of 190 patients with HFrEF found that when empagliflozin was compared with placebo, treatment caused a modest but statistically significant reduction in left ventricular and atrial volumes, but not ejection fraction, after 12 weeks of treatment. These findings were consistent across subgroups, including patients with type 2 diabetes.
Meaning
In this analysis, empagliflozin caused a decrease in cardiac volume after 12 weeks compared with placebo.
This exploratory post hoc analysis of a randomized clinical trial examined the outcomes of empagliflozin vs placebo on cardiac remodeling in patients with heart failure with reduced ejection fraction.
Abstract
Importance
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) improve outcomes in patients with heart failure and a reduced ejection fraction (HFrEF). The association with cardiac remodeling has not been investigated.
Objective
To investigate the outcome of the SGLT2i empagliflozin, compared with placebo, on cardiac remodeling in patients with HFrEF.
Design, Setting, and Participants
This exploratory post hoc analysis included participants with stable HFrEF and ejection fractions of 40% or less, who were randomly enrolled in an investigator-initiated, multicenter, double-blind, placebo-controlled randomized clinical trial in Denmark. Enrollment commenced on June 29, 2017, and continued through September 10, 2019, with the last participant follow-up on December 20, 2019.
Interventions
Randomization (1:1) to empagliflozin (10 mg once daily) or matching placebo in addition to recommended heart failure therapy for 12 weeks.
Main Outcomes and Measures
Efficacy measures were changes from baseline to week 12 in left ventricular end-systolic and end-diastolic volume indexes, left atrial volume index, and left ventricular ejection fraction adjusted for age, sex, type 2 diabetes, and atrial fibrillation. Secondary efficacy measures included changes in left ventricular mass index, global longitudinal strain, and relative wall thickness.
Results
A total of 190 patients were randomized (95 each receiving empagliflozin and placebo), with a mean (SD) age of 64 (11) years; 162 were men (85.3%), 97 (51.1%) had ischemic HFrEF, 24 (12.6%) had type 2 diabetes, and the mean (SD) latest recorded left ventricular ejection fraction was 29% (8%). Of the 190, 186 completed the study. Empagliflozin significantly reduced left ventricular end-systolic volume index (−4.3 [95% CI, −8.5 to −0.1] mL/m2; P = .04), left ventricular end-diastolic volume index (−5.5 [95% CI, −10.6 to −0.4] mL/m2; P = .03), and left atrial volume index (−2.5 [95% CI, −4.8 to −0.1] mL/m2; P = .04) compared with placebo at 12 weeks’ follow-up, with no change in left ventricular ejection fraction (1.2% [95% CI, −1.2% to 3.6%]; P = .32). These findings were consistent across subgroups. Of secondary efficacy measures, left ventricular mass index was significantly reduced by empagliflozin (−9.0 [95% CI, −17.2 to −0.8] g/m2; P = .03).
Conclusions and Relevance
In this small, randomized, short-term study, empagliflozin was associated with modest reductions in left ventricular and left atrial volumes with no association with ejection fraction. Effects beyond 12 weeks of SGLT2i use require further study.
Trial Registration
ClinicalTrials.gov Identifier: NCT03198585
Introduction
The Empagliflozin in Heart Failure Patients With Reduced Ejection Fraction (Empire HF) trial1,2 was an investigator-initiated, multicenter, double-blind, placebo-controlled randomized clinical trial in which 190 patients with heart failure (HF) with reduced ejection fraction (HFrEF) were randomly assigned to receive the sodium-glucose cotransporter 2 inhibitor (SGLT2i) empagliflozin or a matching placebo for 12 weeks. The study failed to detect a decrease in its primary end point of N-terminal pro–brain natriuretic peptide (NT-proBNP) levels. The present Empire HF echocardiographic substudy evaluated the association of empagliflozin with left ventricular (LV) and left atrial (LA) volumes and ejection fraction (LVEF).
Methods
Study Participants
Patients with HFrEF who were receiving guideline-directed HF therapy, aged 18 years or older, considered to be in New York Heart Association functional classes I through III, and having LVEF of 40% or less were eligible. Patients with type 2 diabetes were required to have a glycated hemoglobin level of 48 to 83 mmol/mol (6.5%-10.0%; to convert to proportion of total hemoglobin, multiply by 0.01) and be receiving stable doses of antiglycemic treatment. Exclusion criteria included a symptomatic systolic blood pressure level less than 95 mm Hg, estimated glomerular filtration rate of 30 mL/min/1.73 m2 or less, or a hospital admission for HF within 30 days.2 The trial was approved by the Danish National Committee on Health Research Ethics and complied with the Declaration of Helsinki. The study was designed, conducted, and reported in accordance with a protocol in compliance with Good Clinical Practice standards. Written informed consent was obtained from each patient before inclusion.
Randomization
Eligible patients were randomly assigned in a 1:1 ratio to treatment with either empagliflozin (10 mg once daily) or matching placebo. Randomization was performed in a double-blind fashion.
Echocardiography
Transthoracic echocardiography was performed on a Vivid e9 ultrasonography system (General Electric). Images were analyzed under blinding for treatment allocation and in a random order. In addition, to maximize blinding, images obtained at Herlev and Gentofte University Hospital in Copenhagen, Denmark, were analyzed in Odense University Hospital in Odense, Denmark, and vice versa. Interobserver and intraobserver reproducibility are presented in the eAppendix, the eTable, and eFigure 1 in the Supplement.
Left ventricular and LA volumes and LVEF were assessed using the biplane method of disks. All measurements were standardized to body surface area. Endocardial borders were traced, and speckles were tracked throughout the cardiac cycle from 3 standard apical views. Peak global longitudinal strain was calculated as the mean systolic strain in 17 segments.
Efficacy Measures
Primary efficacy measures were changes in LV end-systolic volume index (LVESVI), end-diastolic volume index (LVEDVI), LA volume index (LAVI), and LVEF from baseline to week 12. All measures consisted of between-group differences.
Statistical Analysis
Echocardiographic measurements were not prespecified in the statistical analysis plan and were decided after termination but before unblinding of the study; therefore, no specific sample-size estimation was performed. Thus, sample size was a consequence of the sample size in the main Empire HF trial.2
The primary statistical analysis was based on an intention-to-treat analysis that included all randomized patients with available data, with no imputation for missing data. The association of treatment with the end points were assessed by examining 2-way interactions derived from a linear mixed model, with a random intercept to account for repeated measurements from the same individual, adjusted for age, sex, type 2 diabetes, and atrial fibrillation.
All statistical tests were carried out at a 2-sided .05 level of significance. Statistical analysis were conducted using Stata statistical software version 16 (StataCorp).
Results
A total of 95 patients were randomly assigned to empagliflozin, and 95 were assigned to placebo (190 patients total). They had a mean (SD) age of 64 (11) years; 162 were men (85.3%), 97 (51.1%) had ischemic HFrEF, 24 (12.6%) had type 2 diabetes, and the mean (SD) latest recorded LVEF was 29% (8%). Both baseline and 12-week follow-up echocardiographic results were available for 89 patients in the empagliflozin group and 90 in the placebo group and included in the intention-to-treat analysis (eFigure 2 in the Supplement). The groups were well balanced with respect to baseline characteristics (Table 1).
Table 1. Baseline Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Empagliflozin (n = 95) | Placebo (n = 95) | |
| Age, mean (SD), y | 65 (10) | 63 (12) |
| Male | 79 (83) | 83 (87) |
| White race | 92 (97) | 94 (99) |
| BMI, median (IQR) | 29 (27-33) | 29 (26-33) |
| Smoking | 22 (23) | 18 (19) |
| Systolic blood pressure, mean (SD), mm Hg | 119 (18) | 121 (16) |
| Heart rate, mean (SD), bpm | 69 (11) | 72 (13) |
| Heart failure characteristics | ||
| Duration of heart failure, median (IQR), mo | 35 (12-69) | 27 (13-62) |
| Heart failure type | ||
| Ischemic | 48 (51) | 49 (52) |
| Nonischemic | 47 (49) | 46 (48) |
| Latest recorded ejection fraction, mean (SD), % | 29 (8) | 30 (8) |
| New York Heart Association class | ||
| I | 5 (5) | 7 (7) |
| II | 72 (76) | 77 (81) |
| III | 18 (19) | 11 (12) |
| Comorbidities | ||
| Type 2 diabetes | 11 (12) | 13 (14) |
| Hypertension | 35 (37) | 41 (43) |
| Atrial fibrillation | 33 (35) | 33 (35) |
| Ischemic heart disease | 50 (53) | 53 (56) |
| Chronic kidney diseasea | 11 (12) | 12 (13) |
| Chronic obstructive pulmonary disease | 14 (7) | 20 (11) |
| Laboratory variables, median (IQR) | ||
| N-terminal pro–B-type natriuretic peptide, ng/L | 582 (303-1020) | 605 (309-1080) |
| In sinus rhythm | 414 (276-689) | 473 (244-793) |
| In atrial fibrillation | 1050 (596-1820) | 1020 (581-1490) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 73 (57-89) | 74 (60-90) |
| Hemoglobin A1c, mmol/mol | 40 (36-43) | 39 (36-42) |
| Hematocrit, % | 41 (39-45) | 41 (38-44) |
| Heart failure medication | ||
| Angiotensin-converting enzyme inhibitors/angiotensin-II receptor blockers | 59 (62) | 65 (68) |
| Sacubitril-valsartan | 31 (33) | 27 (28) |
| β-Blockers | 91 (96) | 89 (94) |
| Mineralocorticoid-receptor antagonist | 62 (65) | 63 (66) |
| Diureticsb | 63 (66) | 62 (65) |
| Device type | ||
| Cardiac resynchronization therapy | ||
| Without ICD | 7 (7) | 4 (4) |
| With ICD | 11 (12) | 14 (15) |
| ICD only | 34 (36) | 32 (34) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); bpm, beats per minute; ICD, implantable cardioverter defibrillator; IQR, interquartile range.
Chronic kidney disease was defined with an estimated glomerular filtration rate less than 60 mL/min/1.73 m2.
Diuretics includes loop diuretics or thiazide.
Primary Efficacy Measures
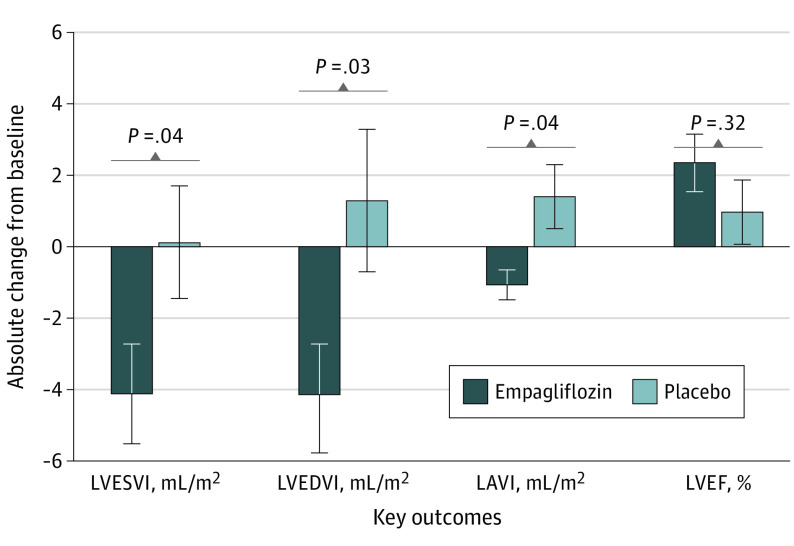
Patients treated with empagliflozin experienced a significant reduction in LVESVI (−4.3 [95% CI, −8.5 to −0.1] mL/m2; P = .04; Table 2; Figure) compared with placebo (adjusted for age, sex, type 2 diabetes, and atrial fibrillation). With empagliflozin compared with placebo, LVEDVI was reduced (−5.5 [95% CI, −10.6 to −0.4] mL/m2; P = .03; Table 2; Figure).
Table 2. Changes in Efficacy Measures in the Intention-to-Treat Population.
| Variable | Empagliflozin, 10 mg/d | Placebo | Adjusted treatment effect (95% CI)a | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Baseline value | No. | 12-wk Value | Change | No. | Baseline value | No. | 12-wk Value | Change | |||
| Primary efficacy measures, mean (SD) | ||||||||||||
| LVESVI, mL/m2b | 89 | 54 (30) | 89 | 49 (23) | −4.1 (13.1) | 90 | 49 (22) | 87 | 48 (21) | 0.1 (14.7) | −4.3 (−8.5 to −0.1) | .04 |
| LVESV, mL | 89 | 112 (66) | 89 | 100 (50) | −8.5 (26.4) | 90 | 104 (48) | 87 | 102 (44) | 0.2 (31.2) | −8.8 (−17.5 to −0.2) | .046 |
| LVEDVI, mL/m2 | 89 | 81 (36) | 89 | 75 (26) | −4.2 (15.2) | 90 | 75 (27) | 87 | 76 (26) | 1.3 (18.6) | −5.5 (−10.6 to −0.4) | .03 |
| LVEDV, mL | 89 | 167 (79) | 89 | 154 (57) | −8.9 (30.4) | 90 | 159 (60) | 87 | 160 (57) | 2.8 (39.5) | −11.8 (−22.4 to −1.2) | .03 |
| LAVI, ml/m2 | 90 | 41 (20) | 92 | 40 (17) | −1.1 (7.9) | 92 | 36 (13) | 86 | 37 (13) | 1.4 (8.3) | −2.5 (−4.8 to −0.1) | .04 |
| In nonatrial fibrillation | 60 | 36 (17) | 59 | 34 (14) | −1.2 (6.9) | 61 | 32 (8) | 59 | 33 (8) | 0.9 (6.6) | NA | NA |
| In atrial fibrillation | 32 | 51 (21) | 33 | 51 (18) | −0.8 (9.6) | 29 | 45 (17) | 27 | 46 (17) | 2.6 (11.3) | NA | NA |
| Ejection fraction, % | 89 | 35 (9) | 89 | 37 (11) | 2.4 (7.5) | 90 | 36 (9) | 86 | 38 (9) | 1.0 (8.3) | 1.2 (−1.2 to 3.6) | .32 |
| Secondary efficacy measures, mean (SD) | ||||||||||||
| LVMI, g/m2a,b | 95 | 130 (64) | 94 | 122 (50) | −3.7 (28.0) | 95 | 128 (46) | 90 | 131 (40) | 5.1 (28.2) | −9.0 (−17.2 to −0.8) | .03 |
| Global longitudinal strain, % | 89 | −11 (4) | 88 | −11 (4) | −0.1 (2.3) | 88 | −11 (3) | 87 | −12 (3) | −0.4 (2.3) | 0.4 (−0.3 to 1.0) | .31 |
| RWT, % | 95 | 0.32 (0.12) | 94 | 0.33 (0.13) | 0.01 (0.09) | 95 | 0.32 (0.13) | 90 | 0.32 (0.13) | 0.01 (0.10) | 0.001 (−0.03 to 0.03) | .97 |
| Blood pressure, mm Hg | ||||||||||||
| Systolic | 95 | 119 (18) | 94 | 115 (14) | −4.4 (14.8) | 95 | 121 (16) | 92 | 121 (14) | 0.2 (12.8) | −4.6 (−8.5 to −0.6) | .02 |
| Diastolic | 95 | 72 (11) | 94 | 71 (10) | −1.0 (10.1) | 95 | 74 (12) | 92 | 27 (11) | −1.3 (9.2) | 0.3 (−2.5 to 3.0) | .84 |
| Hematocrit, % | 95 | 42 (4) | 94 | 44 (4) | 2.1 (2.4) | 95 | 41 (4) | 94 | 41 (4) | −0.1 (2.3) | 2.1 (1.5 to 2.8) | <.001 |
| Weight, kg | 95 | 91 (17) | 94 | 89 (16) | −1.2 (1.8) | 95 | 94 (18) | 92 | 94 (18) | 0.2 (2.6) | −1.3 (−2.0 to −0.7) | <.001 |
Abbreviation: LAVI, left atrial volume index; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVMI, left ventricular mass index; NA, not applicable; RWT, relative wall thickness.
Intention-to-treat population, adjusted for age, sex, type 2 diabetes, and atrial fibrillation.
Indexed to body surface area.
Figure. Changes From Baseline to 12 Weeks in Primary Efficacy Measures.
Error bars indicate 95% CIs. LAVI indicates left atrial volume index; LVEDVI, left ventricular end-diastolic volume indexes; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume indexes.
Empagliflozin was associated with a reduction in LAVI (−2.5 [95% CI, −4.8 to −0.1] mL/m2; P = .04; Figure; Table 2). The reduction in LV volumes was not associated with a significant treatment outcome on LVEF with empagliflozin (1.2% [95% CI −1.2% to 3.6%]; P = .32; Table 2).
No significant interaction was observed for the 4 outcomes across the subgroups except in patients who were naive to diuretics, who exhibited improvement in LVEF by empagliflozin (5.4% [95% CI, 1.52%-9.23%]; interaction P = .01) (eFigure 3 in the Supplement). The associations of empagliflozin with LVEDVI and LVESVI did not differ between patients with ischemic and nonischemic HF, those with or without type 2 diabetes, or those with NT-proBNP levels less than 600 ng/L vs 600 ng/L or greater.
Secondary Efficacy Measures
Empagliflozin significantly reduced LVMI (−9.0 [95% CI, −17.2 to −0.9] g/m2; P = .03; Table 2) compared with placebo (eFigures 4 and 5 in the Supplement). Left ventricular mass was also reduced with empagliflozin compared with placebo (−20.7 [95% CI, −38.0 to −3.4] g; P = .02). Empagliflozin had no association with global longitudinal strain or relative wall thickness compared with placebo (0.001% [95% CI, −0.03% to 0.03%]; P = .97).
Empagliflozin reduced systolic blood pressure (−4.6 [95% CI, −8.6 to −0.6] mm Hg; P = .02) but not diastolic blood pressure (0.29 [95% CI, −2.5 to 3.0] mm Hg; P = .84) at 12 weeks of follow-up. After adjustment for changes in systolic blood pressure, the association of empagliflozin with LVEDVI remained unchanged (−5.5 [95% CI, −10.6 to −0.3] mL/m2; P = .04), but changes in LVESVI (−4.0 [95% CI, −8.2 to 0.2] mL/m2; P = .06) and LAVI (−2.2 [95% CI, −4.6 to 0.2] mL/m2; P = .07) were not significant.
Treatment with empagliflozin increased hematocrit by 5% from baseline, with a significant adjusted treatment effect compared with placebo (2.1% [95% CI, 1.5% to 2.8%]; P < .001; Table 2). After adjustment for the change in hematocrit, the treatment effect of empagliflozin on LVESVI (−5.2 [95% CI, −9.6 to −0.9] mL/m2; P = .02) and LVEDVI (−6.3 [95% CI, −11.6 to −1.0] mL/m2; P = .02) was unchanged, but the treatment effect on LAVI (−2.0 [95% CI, −4.5 to 0.5] mL/m2; P = .12) was nonsignificant.
Discussion
Two recent, large-scale randomized clinical trials unequivocally demonstrated a lower risk of worsening HF or death from cardiovascular causes among patients with HFrEF receiving dapagliflozin or empagliflozin compared with placebo,3,4 but the mechanism is elusive. Earlier studies have shown that a 5% reduction in LV volumes was associated with 14% to 20% reduction in the combined end point of death or hospitalization for HF.5 The present study shows that empagliflozin was associated with a decrease in LV volumes by 5% to 8%, which was apparent after 12 weeks of follow-up. This is in agreement with translational data showing that empagliflozin attenuates adverse cardiac remodeling in pigs without diabetes and with ischemic HF.6 In contrast, a smaller study of 56 patients with type 2 diabetes and symptomatic HF demonstrated no association of 12 months of treatment with dapagliflozin with LVESV or other parameters of LV remodeling. In that study, the mean (SD) LVEF was 46% (12%), suggesting it likely included patients with preserved or mildly reduced LVEF; all patients had diabetes, and the mean body mass index was greater than that in current study. Thus, there are several important differences between that study and the current investigation, and a direct comparison is difficult.
It has been proposed that one of the main mechanisms by which an SGLT2i agent exerts its cardioprotective effects is a reduction in preload, primarily because of the drug’s diuretic and natriuretic effects.7 We observed an increase in hematocrit with empagliflozin, which likely resulted from natriuresis and osmotic diuresis. Although the observed changes in LV volumes with empagliflozin in this study were not associated with the changes in hematocrit levels, the modest but significant decrease in LV volumes could be a consequence of the empagliflozin-induced natriuresis. In the Empire HF trial, no overall association with NT-proBNP1 was observed, and in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF)3,8 and Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR Reduced)4 trials, the SGLT2i association with NT-proBNP levels was minor, suggesting a mode of action beyond this. In the current study, empagliflozin was associated with reduction in LV volumes, despite no overall association with NT-proBNP levels. The potential association between the effects of empagliflozin reductions on chamber size and geometry and improvement of preload requires further investigation with a longer follow-up.
Limitations
The current study should be interpreted in the context of the main Empire HF study, which failed to demonstrate an effect on its primary end point. A specific sample-size estimation was not calculated for the current study, rendering it a post hoc exploratory analysis. This short-term study included a younger population, with better functional capacity as well as lower plasma concentrations of NT-proBNP, compared with other SGLT2i HF trials.3,4,9 Whether these results also apply to patients with HFrEF who have more advanced disease is speculative.
Conclusions
This echocardiographic substudy demonstrated that empagliflozin was associated with a modest reduction in cardiac volumes in patients with HFrEF after 12 weeks of treatment. Outcomes beyond 12 weeks of SGLT2i use in patients with HFrEF require further study.
eAppendix. Intra- and inter-observer variability on primary efficacy measures
eFigure 1. Bland-Altman plots comparing intra- and inter-observer agreement of LVESV, LVEDV, LAVi, and LVEF.
eTable. Primary and derived echocardiographic measurements
eFigure 2. Trial profile
eFigure 3. Forest plot of subgroup analysis
eFigure 4. Empagliflozin exposure for 12 weeks is associated with a decrease in LVMI assessed by echocardiographic imaging
eFigure 5. LVMi outcome, according to pre-specified subgroup
References
- 1.Jensen J, Omar M, Kistorp C, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020;228:47-56. doi: 10.1016/j.ahj.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 2.Jensen J, Omar M, Kistorp C, et al. Empagliflozin in heart failure patients with reduced ejection fraction: a randomized clinical trial (Empire HF). Trials. 2019;20(1):374. doi: 10.1186/s13063-019-3474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Anker SD, Butler J, et al. ; EMPEROR-Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Foster E, Bourgoun M, et al. ; MADIT-CRT Investigators . Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122(10):985-992. doi: 10.1161/CIRCULATIONAHA.110.955039 [DOI] [PubMed] [Google Scholar]
- 6.Lehrke M. SGLT2 inhibition: changing what fuels the heart. J Am Coll Cardiol. 2019;73(15):1945-1947. doi: 10.1016/j.jacc.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 7.Verma S, McMurray JJV, Cherney DZI. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiol. 2017;2(9):939-940. doi: 10.1001/jamacardio.2017.1891 [DOI] [PubMed] [Google Scholar]
- 8.Køber L, Docherty K, Inzucchi SE, et al. Dapaglifozin improves outcomes irrespective of NT-proBNP concentration in patients with HFrEF: an analysis of the DAPA-HF Trial. J Am Coll Cardiol. 2020;75(11, suppl 1):675. doi: 10.1016/S0735-1097(20)31302-4 [DOI] [Google Scholar]
- 9.Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF Trial. Circulation. 2019;140(18):1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Intra- and inter-observer variability on primary efficacy measures
eFigure 1. Bland-Altman plots comparing intra- and inter-observer agreement of LVESV, LVEDV, LAVi, and LVEF.
eTable. Primary and derived echocardiographic measurements
eFigure 2. Trial profile
eFigure 3. Forest plot of subgroup analysis
eFigure 4. Empagliflozin exposure for 12 weeks is associated with a decrease in LVMI assessed by echocardiographic imaging
eFigure 5. LVMi outcome, according to pre-specified subgroup