Abstract
Objective:
Many insurance payers are hesitating to cover interventional treatments in patients with isolated symptomatic varicose veins. In this study, we sought to determine the outcomes of patients with varicose veins who were treated with venous ablation alone or ablation plus phlebectomy using the Vascular Quality Initiative Varicose Vein Registry.
Methods:
Using data from the Varicose Vein Registry between January 2015 and March 2019, we investigated immediate postoperative as well as long-term clinical and patient-reported outcomes among patients with documented symptomatic C2 disease undergoing truncal endovenous ablations alone and combined ablation and phlebectomy. Preprocedural and postprocedural comparisons were performed using t-test, χ2 test, or nonparametric tests when appropriate. Multivariable ordinal logistic regression was performed on ordinal outcome variables.
Results:
Among 3375 patients with symptomatic C2 disease, 40.1% of patients (1376) underwent isolated truncal ablation and 59.9% (1999) underwent ablation and phlebectomy. Complications overall were low (8.6%) and varied between 8.4% and 8.7% in patients undergoing ablation alone and ablation plus phlebectomy, respectively (P = .820). The most common complication noted was paresthesia, 3.4% overall, which occurred more commonly after ablation and phlebectomy (4.5%) than after ablation alone (1.3%; P < .001). An improvement in Venous Clinical Severity Score (VCSS) was experienced by 87.4% of patients; median change in VCSS was 4 points (interquartile range [IQR], 2–5 points), with an improvement of 3 points among patients undergoing ablation alone (IQR, 1–5 points) and 5 points among patients undergoing ablation and phlebectomy (IQR, 3–5 points; P < .001). An improvement in overall symptoms was experienced by 94.4% of patients (median improvement, 11 points; (maximum, 30 points), with more significant decreases among patients undergoing ablation and phlebectomy (median, 12 points; IQR, 8–17 points) compared with ablation alone (median, 9 points; IQR, 5–13 points; P < .001).
Conclusions:
Among patients with isolated symptomatic varicose veins (C2 disease), ablation and ablation with phlebectomy are safe and effective in improving both patient-reported outcomes and clinical severity (VCSS). Given these data, payers should continue to cover these treatments.
Keywords: Venous insufficiency, Varicose veins, Chronic venous disease, Endovenous ablation, C2 disease, CEAP
Varicose veins affect a significant portion of the population, with long-term effects including pain and development of more severe consequences of chronic venous insufficiency, such as swelling and ulcers from venous stasis.1–3 This is often accompanied by decreased quality of life, even for mild disease, and contributes to a significant cost to the health care system in excess of $1 billion annually.4–6
In recent years, payers have been hesitant to pay for procedures for the treatment of varicose veins, with the Centers for Medicare and Medicaid Services recommending a low confidence of evidence supporting current venous practice during the Medicare Evidence Development & Coverage Advisory Committee meeting in 2016 because of insufficient evidence for the benefit of lower extremity venous disease treatments.7 Multiple previous studies have revealed a significant benefit for those undergoing endovenous ablation, and several medical societies and organizations, including the American Venous Forum, the National Institute for Health and Care Excellence, and the European Society for Vascular Surgery, have recommended surgical treatment of symptomatic C2 disease.8–10 The addition of concomitant phlebectomy at the time of ablation has been shown to improve outcomes in patients with venous insufficiency.11,12 Furthermore, medical treatments for isolated varicose veins are limited, with previous studies suggesting that there may be no improvement in discomfort with compression therapy for patients with mild disease severity.13 Evidence regarding outcomes of mild lower extremity venous insufficiency are currently lacking with respect to both traditional clinical outcomes, such as morbidity and mortality, and patient-reported outcomes (PROs).
In this study, we sought to determine the effect of truncal endovenous ablation on patients with isolated symptomatic varicose veins (C2 disease) in terms of both the procedure-specific morbidity rates and PROs. In addition, we sought to compare the addition of phlebectomy to ablation for these outcomes. We hypothesized that patients with symptomatic C2 disease would have substantial benefit in terms of PROs after endovenous ablation, with low rates of complications, and that the addition of phlebectomy would result in moderately higher rates of complications.
METHODS
Vascular Quality Initiative (VQI) Varicose Vein Registry (VVR).
The VQI VVR is a prospectively maintained surgical registry and was retrospectively reviewed for this study. Inclusion criteria included patients with symptomatic C2 disease who underwent procedures to ablate truncal veins (including the great saphenous vein, anterior accessory great saphenous vein, superficial accessory great saphenous vein, small saphenous vein, and other truncal vein) using radiofrequency or laser in the lower extremity between 2015 and 2019. Patients who underwent procedures from 2014 were excluded as these were entered into the registry retrospectively. Patients who underwent concomitant sclerotherapy were excluded from this analysis to minimize confounding. Cases are entered, and follow-up includes one early follow-up (0–3 months) and one late follow-up (>3 months after the procedure). Data regarding complications are collected at initial (early, 0–3 months) follow-up; data regarding PROs are collected at later (late, >3 months) follow-up, including Venous Clinical Severity Score (VCSS) and the quality of life questionnaire detailed later. At initial reporting, Clinical, Etiology, Anatomy, and Pathophysiology (CEAP) class and VCSS are recorded for both legs. Follow-up reporting includes the treated leg’s clinical classification and VCSS along with outcomes. All analyses of outcome data used the most recent VCSS measurement, which is obtained at late follow-up.
Patients’ demographic, diagnostic, preoperative, intraoperative, and postoperative data were collected prospectively by trained support staff at each center.14–16 Participation by venous disease treatment centers is not required. There are webinars to train data managers, online support staff to field questions about all the variables, and strong data definitions. Entry of consecutive procedures by each participating center is ensured by annual audit against hospital claims data submitted by each center. VQI data forms contain error tracking software to prevent erroneous entry. Data that are entered are periodically checked for statistical aberration. Data that appear to be in error are then audited within centers to ensure accuracy and completeness. The University of Michigan Institutional Review Board waived the need for review, and informed consent of the patient was waived for this exempt analysis.
Patients also complete a quality of life survey before the procedure and then at late follow-up. This survey defines seven different parameters, including leg heaviness, achiness, swelling, throbbing, itching, appearance, and work impact, and asks the participant to grade each parameter on an ordinal scale (0, none of the time; and 5, all of the time). Each PRO was rated on a scale of 0 to 4 or 5, depending on the specific outcome:
For heaviness, achiness, swelling, throbbing, and itching, the scale was as follows: 0, none of the time; 1, a little of the time; 2, some of the time; 3, a good bit of the time; 4, most of the time; and 5, all of the time.
For appearance, the scale was as follows: 0, not at all noticeable; 1, slightly noticeable; 2, moderately noticeable; 3, very noticeable; and 4, extremely noticeable.
For impact on work/activity, the scale was as follows: 0, none; 1, symptoms but full work/activity; 2, mildly reduced work/activity; 3, moderately reduced work/activity; 4, severely reduced work/activity; and 5, unable to do work/activity.
The total possible scores range from 0 to 34.
This PRO assessment instrument resembles the HASTI instrument, which has been validated previously, with the addition of the impact on work/activity questions.14–18
Statistical analysis.
Patients’ demographics as well as procedural and clinical characteristics are described using summary statistics. Categorical variables are presented as frequency counts with percentages, and continuous variables are presented as mean ± standard deviation or median with interquartile range (IQR). Student t-tests and χ2 tests and Mann-Whitney for nonparametric measurements when the underlying distribution was non-normal were used. For the multivariable analyses, covariates significant at the P < .30 level in the univariate analysis were included in a multivariable logistic regression model. The α level was set at .05 unless otherwise noted. Stata version 16 (StataCorp LLC, College Station, Tex) was used for all analyses.
RESULTS
Characteristics of the patients.
A total of 12,005 patients underwent truncal endovenous ablation during the study period, of whom 3375 had C2 disease and were included in the analysis (Fig 1). Mean age was 52.4 ± 13.8 years, with the majority of patients (n = 2552 [75.6%]) being female. Most of the patients included were white (n = 2730 [94.2%]), and median length of follow-up was 431 days (IQR, 91–889 days). Mean body mass index was 27.7 ± 6.1 kg/m2, with a minority of patients having undergone previous treatment for varicose veins (n = 773 [23.0%]). History of deep venous thrombosis (DVT) was uncommon (n = 129 [3.8%]), as was preprocedural anticoagulation (n = 208 [6.2%]). Median preprocedural VCSS was 6 (IQR, 5–7). For the women included in the study, the median number of pregnancies was two (IQR, one to three). Patients undergoing ablation alone were less likely to be white (92.1% vs 95.4%; P < .001) and had shorter median follow-up compared with patients who underwent ablation with phlebectomy (median, 136 days [IQR, 42.5–532.5 days] vs 652 days [IQR, 140–988 days]; P < .001). Patients who underwent ablation alone also had lower mean BMI (27.4 ± 5.8 kg/m2 vs 28.0 ± 6.3 kg/m2; P =.004), higher rates of prior varicose vein treatment (26.2% vs 20.7%; P < .001), and lower rates of therapeutic anticoagulation at the time of the procedure (4.1% vs 7.6%; P < .001) compared with those undergoing ablation with phlebectomy (Table I).
Fig 1.
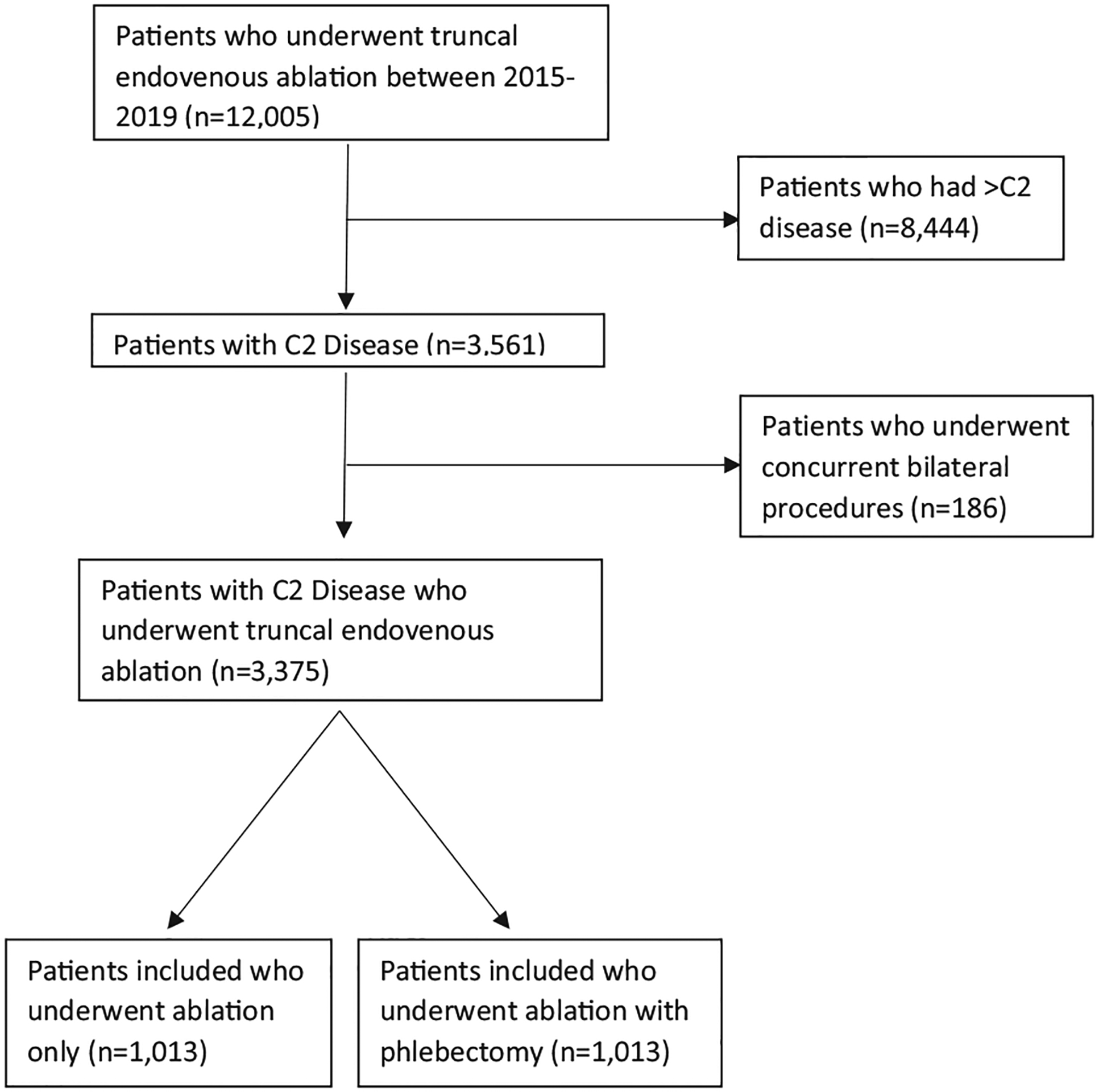
Enrollment schema for patients who underwent endovenous ablation and were included.
Table I.
Patient and procedure characteristics
| Characteristic | Ablation only (n = 1376) | Ablation and phlebectomy (n = 1999) | P value |
|---|---|---|---|
| Age, years | 52.7 (14.0) | 52.2 (13.7) | .331 |
| Sex, female | 1042 (75.7) | 1510 (75.5) | .900 |
| Race, white | 954 (92.1) | 1776 (95.4) | <.001 |
| Length of follow-up, days | 136 (42.5–532.5) | 652 (140–988) | <.001 |
| BMI, kg/m2 | 27.4 (5.8) | 28.0 (6.3) | .004 |
| Prior W treatment | 360 (26.2) | 413 (20.7) | <.001 |
| History of DVT | 44 (3.2) | 85 (4.3) | .117 |
| Receiving anticoagulation | 56 (4.1) | 152 (7.6) | <.001 |
| No. of pregnancies | 2 (1–3) | 2 (1–3) | .015 |
BMI, Body mass index; DVT, deep venous thrombosis; VV, varicose vein.
Categorical variables are presented as number (%). Continuous variables are presented as mean (standard deviation) or median (interquartile range).
Procedure-specific complications.
A variety of procedure-specific complications were evaluated and are summarized in Table II. Overall, complications were low, and no difference was seen between patients undergoing ablation only and patients undergoing ablation with phlebectomy (8.4% vs 8.7%; P = .820). Furthermore, no difference was seen between the two groups for DVT, bleeding requiring intervention, skin blistering, hematoma, superficial phlebitis, induced ulcer requiring intervention, wound infection, or proximal thrombus extension (P > .05 for all comparisons). Patients who underwent ablation alone had lower rates of paresthesias (1.3% vs 4.5%; P < .001) but were found to have higher rates of skin pigmentation (2.0% vs 0.6%; P = .004) compared with patients who underwent concomitant phlebectomy.
Table II.
Procedure-specific complications for patients undergoing ablation and phlebectomy compared with ablation alone
| Ablation only (n = 1376), No. (%) | Ablation and phlebectomy (n = 1999), No. (%) | P value | |
|---|---|---|---|
| Any complication | 65 (8.4) | 132 (8.7) | .820 |
| DVT | 13 (1.7) | 14 (0.9) | .150 |
| Bleeding requiring intervention | 0 | 0 | |
| Skin blistering | 1 (0.1) | 4 (0.3) | .669 |
| Hematoma | 1 (0.1) | 11 (0.7) | .071 |
| Paresthesia | 10 (1.3) | 68 (4.5) | <.001 |
| Pigmentation | 15 (2.0) | 9 (0.6) | .004 |
| Superficial phlebitis | 11 (1.4) | 11 (0.7) | .116 |
| Induced ulcer requiring intervention | 1 (0.1) | 3 (0.2) | 1.000 |
| Wound infection | 6 (0.8) | 4 (0.3) | .096 |
| Proximal thrombus extension | 9 (1.2) | 10 (0.7) | .227 |
DVT, Deep venous thrombosis.
VCSS.
No differences were seen between the two groups in VCSS in the preprocedural period (median, 6 [IQR, 4–7] for ablation only vs 6 [IQR, 5–6.5] for ablation and phlebectomy; P = .372). After ablation and phlebectomy, median VCSS was lower at 1 (IQR, 0–2) compared with those who underwent ablation only (median, 2; IQR, 1–4; P < .001 for the comparison). This translated to a higher median improvement in VCSS for those undergoing ablation and phlebectomy (median improvement, 5 [IQR, 3–5]) compared with those undergoing ablation only (median improvement, 3 [IQR 1–5]; P < .001 for the comparison). VCSS scores are shown graphically in Fig 2.
Fig 2.
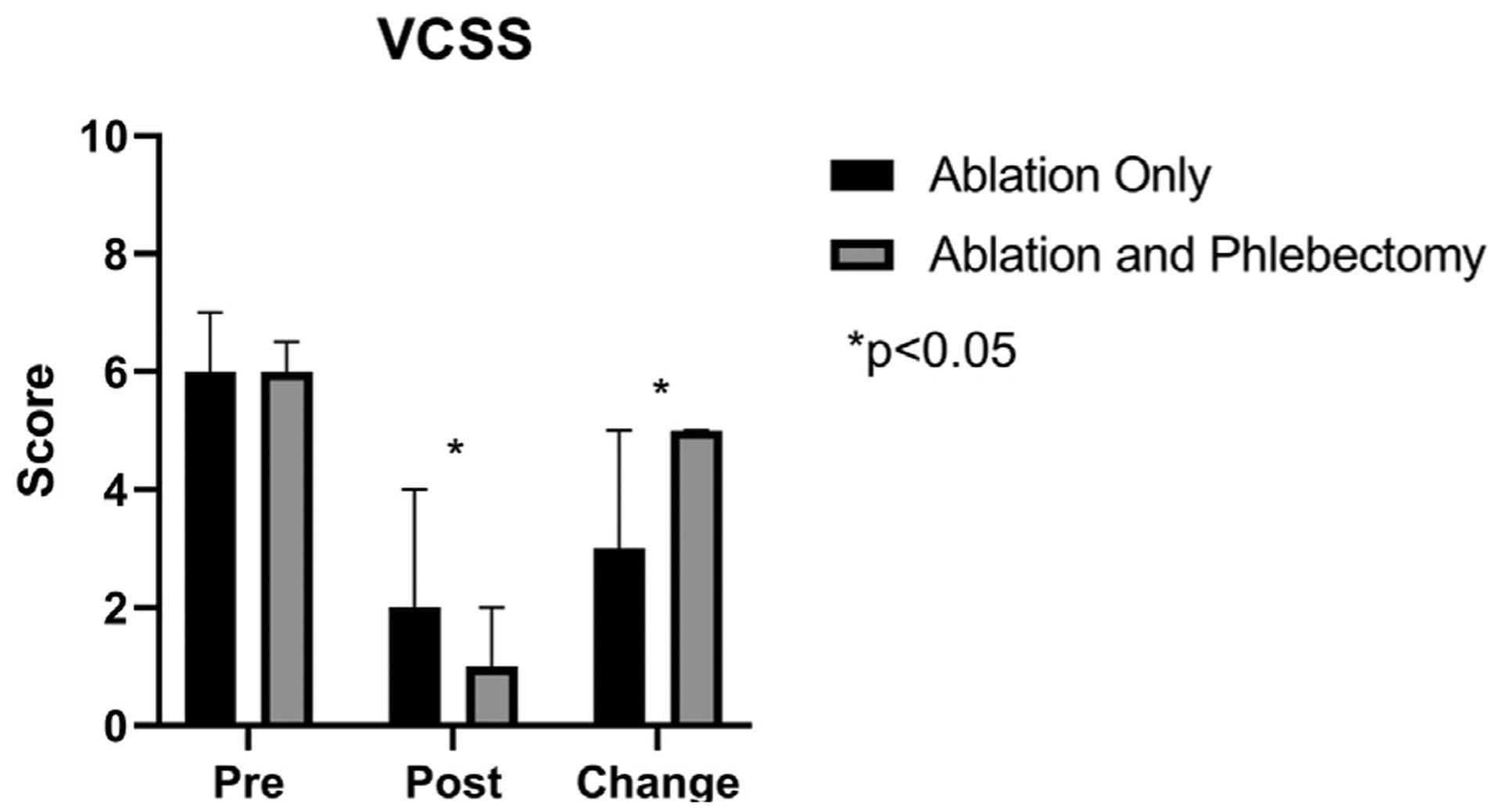
Comparison of Venous Clinical Severity Score (VCSS) among patients undergoing ablation alone or ablation with phlebectomy. *P values < .05.
PROs.
Preprocedural symptoms were overall less in the group that underwent ablation alone (median total symptom score, 13 [IQR, 8–17] vs 14 [IQR, 9–19]; P < .001) compared with those who underwent ablation and phlebectomy (Fig 3, A). After evaluation of individual symptom scores, preprocedural scores were better for heaviness (P = .017), achiness (P < .001), swelling (P < .001), throbbing (P < .001), appearance (P < .001), and impact on work (P < .001), whereas no difference was found for itching (P = .929), although this was a rare complaint in the preintervention or postintervention period (Fig 3, B).
Fig 3.
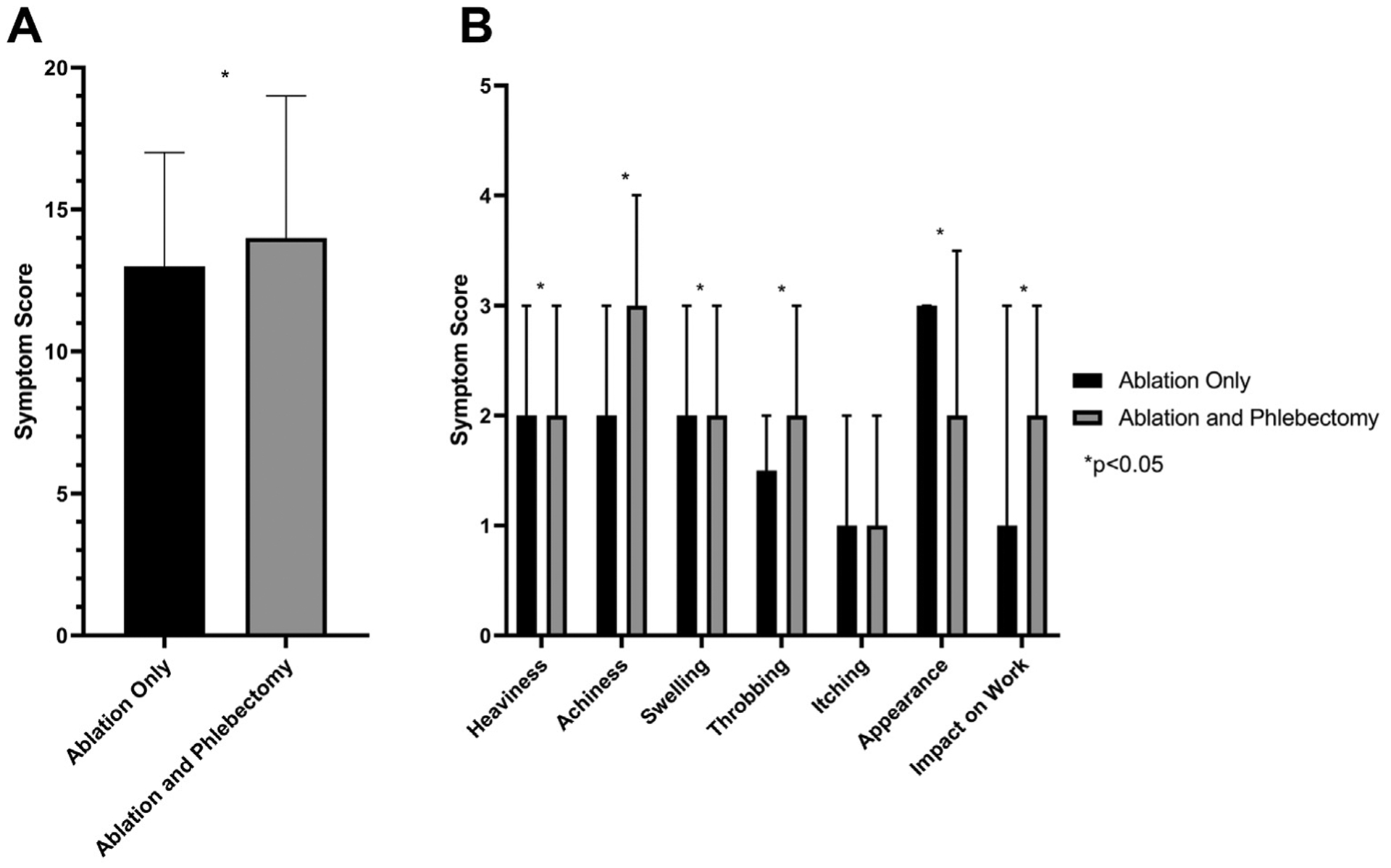
Comparison of preprocedural patient-reported outcomes (PROs) among patients undergoing ablation alone or ablation with phlebectomy. A, Total symptom score. B, Individual symptom score comparisons are shown. *P values < .05.
Improvement was seen in all symptoms for both groups. Median total symptom change was lower for those undergoing ablation alone (9; IQR, 5–13) compared with those undergoing ablation and phlebectomy (12; IQR, 8–17; P < .001 for the comparison; Fig 4, A). As with postprocedural symptoms, the change in symptom scores for heaviness (P < .001), achiness (P < .001), swelling (P < .001), throbbing (P < .001), itching (P = .005), appearance (P < .001), pain (P < .001), and impact on work (P < .001) was lower for ablation alone compared with ablation with phlebectomy (Fig 4, B).
Fig 4.
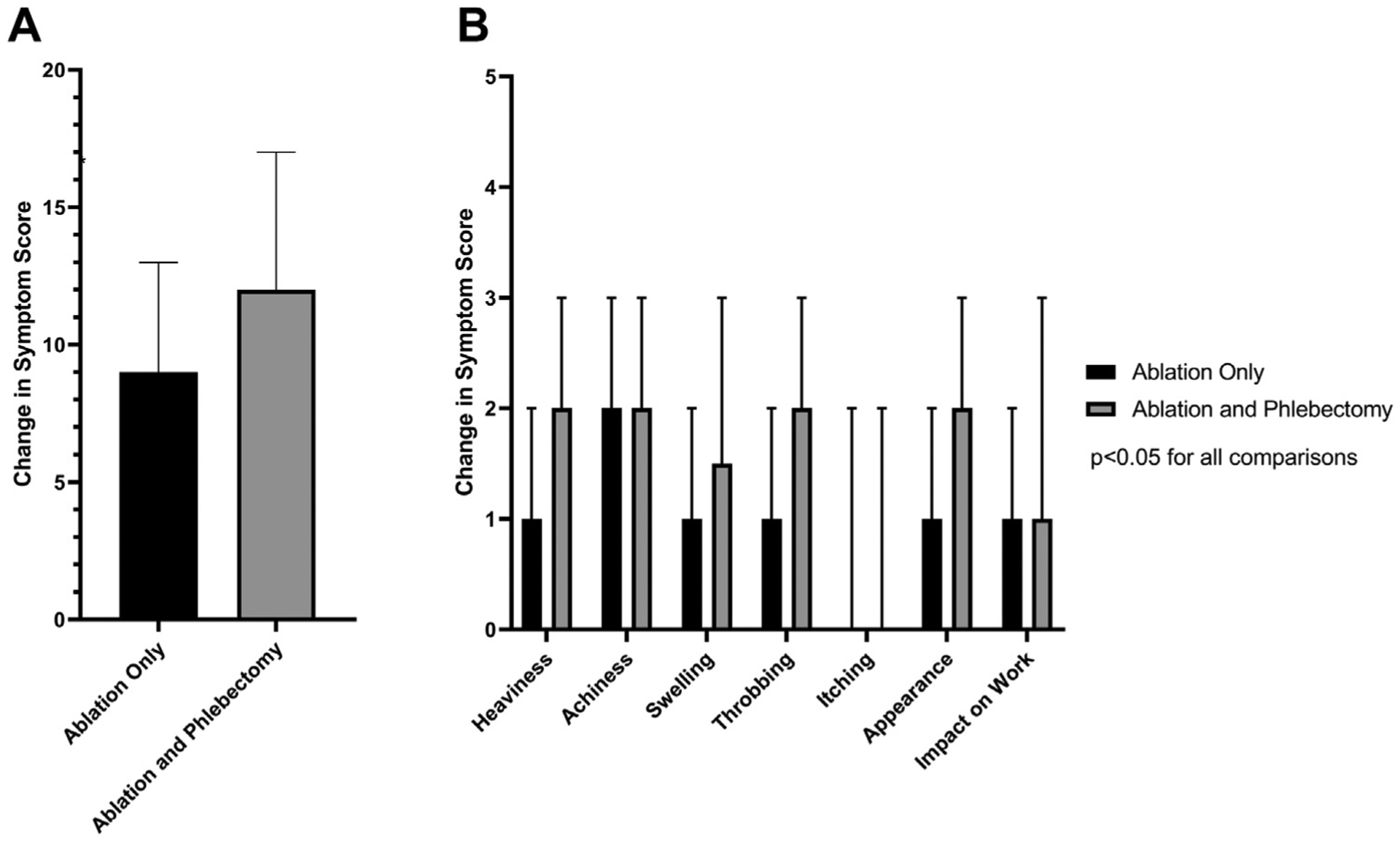
Comparison of change in patient-reported outcomes (PROs) from before to after the procedure among patients undergoing ablation alone or ablation with phlebectomy. A, Change in total symptom score. B, Change in individual symptom score comparisons are shown. P values were < .05 for all direct comparisons between ablation only and ablation with phlebectomy.
Interestingly, postprocedural symptoms were overall less improved in the ablation only group (median total symptom score, 2 [IQR, 1–6]) compared with the ablation and phlebectomy group (median total symptom score, 1 [IQR, 0–3]; P < .001 for the comparison; Fig 5, A). All individual postprocedural symptom scores including heaviness, achiness, swelling, throbbing, itching, appearance, pain, and impact on work were higher in the ablation only group compared with the ablation and phlebectomy group (P < .001 for all comparisons; Fig 5, B).
Fig 5.
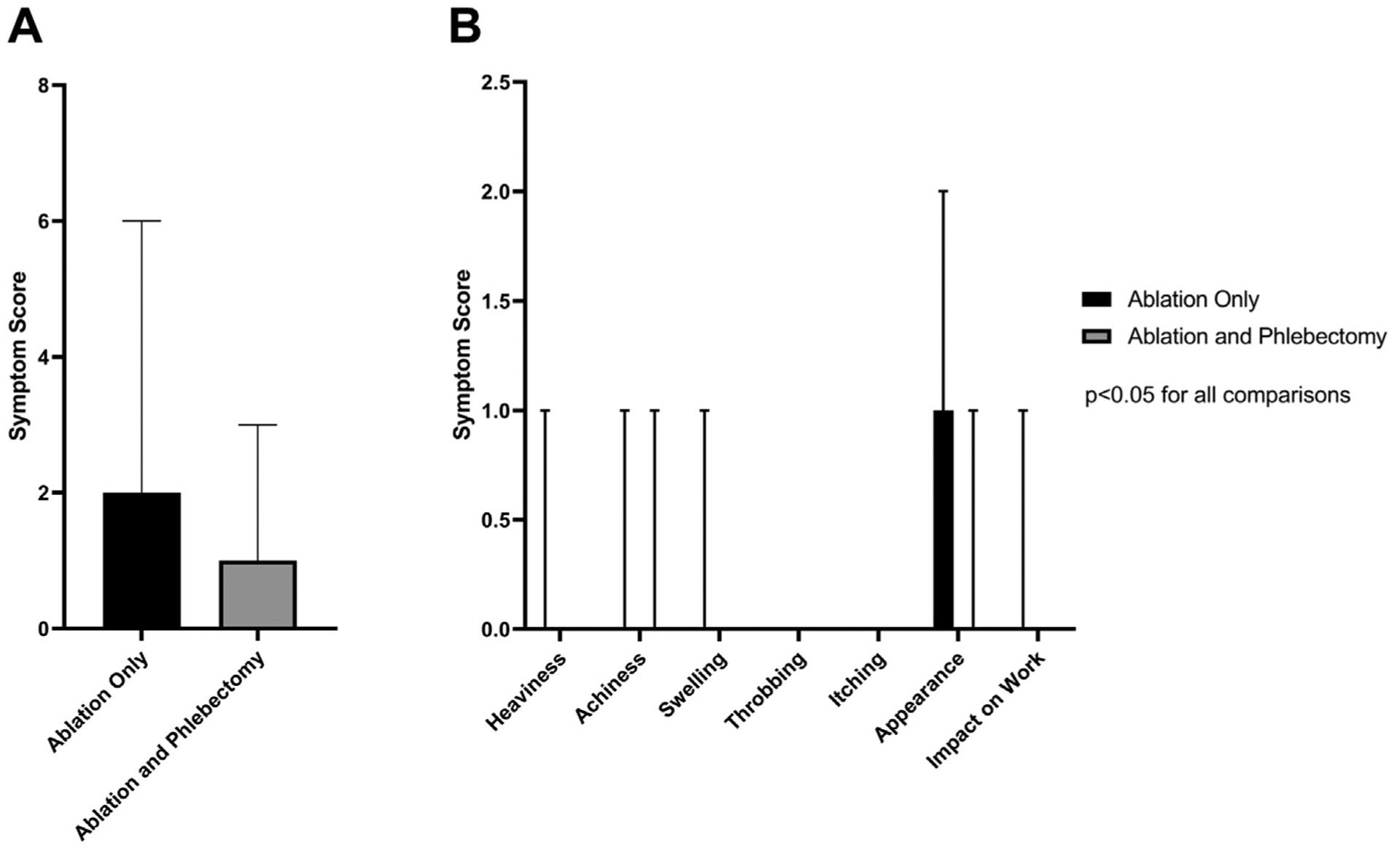
Comparison of postprocedural patient-reported outcomes (PROs) among patients undergoing ablation alone or ablation with phlebectomy. A, Total symptom score. B, Individual symptom score comparisons are shown. P values were < .05 for all direct comparisons between ablation only and ablation with phlebectomy.
Multivariable logistic regression.
As there were significant differences between the group of patients undergoing ablation alone and the group of patients undergoing ablation with phlebectomy, a multivariable logistic regression model was used to compare the likelihood of improvement in VCSS between ablation alone and ablation with phlebectomy with the following covariates: year of surgery, age, sex, BMI, history of prior pregnancy, race, history of varicose vein procedure, history of DVT, preoperative anticoagulation, and preoperative VCSS. After adjustment for these covariates, ablation with phlebectomy was associated with a significantly higher odds of improvement in VCSS compared with ablation alone (odds ratio [OR], 4.12; 95% confidence interval [CI], 2.96–5.75). In addition, significant predictors were treatment in 2018 (OR, 0.53; 95% CI; 0.32–0.89), history of prior varicose vein procedure (OR, 0.57; 95% CI, 0.40–0.81), and preoperative VCSS (OR, 2.01; 95% CI, 1.80–2.25, per point). These results are summarized in Table III.
Table III.
Multivariable analysis for predictors of improvement in Venous Clinical Severity Score (VCSS) among patients undergoing ablation of truncal veins
| OR | 95% Cl | P value | |
|---|---|---|---|
| Characteristics | |||
| Ablation and phlebectomy | 4.12 | 2.96–5.75 | <.001 |
| Year of surgery | |||
| 2016 | 0.86 | 0.51–1.43 | .556 |
| 2017 | 0.96 | 0.57–1.62 | .885 |
| 2018 | 0.53 | 0.32–0.89 | .017 |
| 2019 | - | - | - |
| Age (per year) | 1.00 | 0.99–1.01 | .715 |
| Female | 1.06 | 0.61–1.83 | .831 |
| BMI (per point) | 0.99 | 0.96–1.02 | .482 |
| Prior pregnancy | 1.00 | 0.61–1.65 | .993 |
| Nonwhite race | 0.56 | 0.3–1.06 | .074 |
| Prior varicose vein procedure | 0.57 | 0.4–0.81 | .002 |
| Preoperative anticoagulation | 1.08 | 0.55–2.15 | .818 |
| History of DVT | 0.80 | 0.36–1.79 | .592 |
| Preoperative VCSS (per point) | 2.01 | 1.8–2.25 | <.001 |
BMI, Body mass index; CI, confidence interval; DVT, deep venous thrombosis; OR, odds ratio.
DISCUSSION
The evidence regarding outcomes after truncal endovenous ablation for patients with symptomatic C2 disease has been insufficient, with a resultant hesitation by many payers to cover ablation procedures for patients with this mild level of venous insufficiency. Our results suggest that patients with isolated symptomatic C2 disease have low rates of complications after truncal ablation and moreover have substantial benefits from truncal ablation, with or without combined phlebectomy. These improvements include all measured symptoms, including traditional symptoms associated with venous insufficiency, such as pain and swelling, as well as the others included in the VQI PRO assessment, such as heaviness, achiness, throbbing, itching, appearance, and impact that C2 disease has on patients’ ability to work. The addition of phlebectomy was associated with a substantially higher rate of paresthesia, but paresthesia has previously been shown to rarely be life or lifestyle limiting.19 Whereas treatment decisions should continue to be made on a per-case basis, these results will help inform a thorough conversation with our patients about expected improvements in symptoms and help guide the decision around therapy. These data support the use of either ablation or ablation with phlebectomy in patients with symptomatic C2 disease and should serve as further evidence to support the coverage of truncal endovenous ablation in patients with isolated symptomatic C2 disease.
Previous work has investigated more severe disease, with many surgeons suggesting that isolated symptomatic C2 disease is associated with mild enough symptoms that the risks of truncal endovenous ablation outweigh the minor benefits.20 Here we show that even those with symptomatic C2 disease have significant symptoms that affect their quality of life and furthermore that the complications associated with ablation are relatively minor and appear to be outweighed by significant improvements in symptoms across the spectrum of those assessed.
The results of this study must be interpreted within the context of several limitations. First and foremost, the VVR is a prospectively collected clinical registry, but there may exist additional clinically relevant unmeasured factors that may bias the results found in this analysis. Because there is no randomization, there may be operator bias. Surgeons may systematically choose which procedure to perform (ablation alone vs ablation with phlebectomy) on the basis of a set of unmeasured clinical criteria that may bias the effect estimates. The strength of this database, though, is that it represents a sample of “real-world” patients, that is, these data represent care that is actually being delivered and has the same biases that surgeons do in actual practice. We did not include patients who underwent sclerotherapy, which is still commonly used in practice, and this may limit the generalizability of this work to patients who may undergo a combination of ablation with or without phlebectomy and sclerotherapy. Given the inability to completely control for confounding between the two groups of patients presented, we caution against interpretation of these data as evidence of superiority for one procedure type over the other. Follow-up was significantly longer for those undergoing concomitant phlebectomy than for those undergoing ablation alone. The trajectory of recovery and its relationship to quality of life assessment is not well understood for these patients. It remains possible that differences seen in PROs are due to this difference in follow-up or other systematic differences between the two groups rather than the effect of concomitant phlebectomy. Furthermore, missing data may represent another avenue for selection bias as the missingness may not be at random. In this way, patients with missing data may systematically differ from patients who do not have missing data.
The VVR is a procedural registry, and as such, there may be significant selection bias against patients who were not candidates for or offered surgery or even against patients whose health insurance plans would not cover the cost of treatment and therefore deferred surgery. Little previous work has focused on these patients, particularly those with less severe disease. The question of how these patients progress or not remains to be conclusively answered, but prior results from the Bonn Vein Study among others suggest that patients, on average, have progression in severity of their venous insufficiency and furthermore that the prevalence of severe venous disease has diminished substantially since the advent of surgical therapy for lower extremity chronic venous insufficiency.21 Further research, including registry development, will need to include not only patients treated procedurally but also patients treated with medical therapy and compression. Future studies using registries that include nonoperatively managed patients, such as the American Vein and Lymphatic Society PRO Venous Registry, represent opportunities to answer these questions, and future studies should use these powerful resources.22
Despite these limitations, this study represents the most comprehensive assessment of morbidity and PROs for patients with symptomatic C2 disease and leverages the high-quality data available within the VQI VVR.
CONCLUSIONS
Our results underscore the low risks and relatively high benefits that patients with isolated symptomatic C2 disease experience after endovenous ablation with and without phlebectomy and should guide decision-making in treatment of these patients as well as policy surrounding the issue of payment for symptomatic C2 disease. Additional work is required to understand the costs associated with ablation in this group of patients to allow an assessment of treatment value, but previous studies have suggested cost-effectiveness in patients with more severe disease, and these results suggest that even patients with mild disease experience substantial benefit that should be covered by payers moving forward.23,24
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective analysis of prospectively collected registry data (Vascular Quality Initiative)
Key Findings: Truncal endovenous ablation with or without phlebectomy in 3375 patients with symptomatic varicose veins (C2 class) was completed with l8.6% complication rate and improvement of symptoms in 94.4% of the patients. Ablation with phlebectomy resulted in higher odds of improvement in Venous Clinical Severity Score compared with ablation alone.
Take Home Message: Patients with symptomatic C2 venous disease can be treated with truncal endovenous ablation with and without phlebectomy with excellent outcomes and low complication rates, and they can experience significant improvements in symptoms.
Acknowledgments
The National Institutes of Health funded this work through NIH 5T32HS000053-28, which provides salary support for C.S.B.
Footnotes
Author conflict of interest: A.T.O. reports serving as the principal investigator for a device contract with Medtronic related to deep venous thrombosis.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Callam MJ. Epidemiology of varicose veins. Br J Surg 1994;81: 167–73. [DOI] [PubMed] [Google Scholar]
- 2.Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health 1999;53:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fowkes FG, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology 2001;52(Suppl 1): S5–15. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg 2003;37:1047–53. [DOI] [PubMed] [Google Scholar]
- 5.Smith JJ, Garratt AM, Guest M, Greenhalgh RM, Davies AH. Evaluating and improving health-related quality of life in patients with varicose veins. J Vasc Surg 1999;30:710–9. [DOI] [PubMed] [Google Scholar]
- 6.Korn P, Patel ST, Heller JA, Deitch JS, Krishnasastry KV, Bush HL, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg 2002;35:950–7. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services. MEDCAC meeting 7/20/2016—lower extremity chronic venous disease. Available at: https://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=72. Accessed March 10, 2020.
- 8.National Clinical Guideline Centre. Varicose veins in the legs. London: National Institute for Health and Care Excellence; 2013. [Google Scholar]
- 9.Masuda E, Ozsvath K, Vossler J, Woo K, Kistner R, Lurie F, et al. The 2020 appropriate use criteria for chronic lower extremity venous disease of the American Venous Forum, the Society for Vascular Surgery, the American Vein and Lymphatic Society, and the Society of Interventional Radiology. J Vasc Surg Venous Lymphat Disord 2020. March 2.. [DOI] [PubMed] [Google Scholar]
- 10.Wittens C, Davies AH, Baekgaard N, Broholm R, Cavezzi A, Chastanet S, et al. Editor’s choice—management of chronic venous disease clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2015;49:678–737. [DOI] [PubMed] [Google Scholar]
- 11.Carradice D, Mekako AI, Hatfield J, Chetter IC. Randomized clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. Br J Surg 2009;96:369–75. [DOI] [PubMed] [Google Scholar]
- 12.Lane TR, Kelleher D, Shepherd AC, Franklin IJ, Davies AH. Ambulatory varicosity avulsion later or synchronized (AVULS). Ann Surg 2015;261:654–61. [DOI] [PubMed] [Google Scholar]
- 13.Amsler F, Blättler W. Compression therapy for occupational leg symptoms and chronic venous disorders—a meta-analysis of randomised controlled trials. Available at: www.amslerconsulting.ch. Accessed February 8, 2020. [DOI] [PubMed]
- 14.Sutzko DC, Andraska EA, Obi AT, Sadek M, Kabnick LS, Wakefield TW, et al. Age is not a barrier to good outcomes after varicose vein procedures. J Vasc Surg Venous Lymphat Disord 2017;5:647–57.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutzko DC, Obi AT, Kimball AS, Smith ME, Wakefield TW, Osborne NH. Clinical outcomes after varicose vein procedures in octogenarians within the Vascular Quality Initiative Varicose Vein Registry. J Vasc Surg Venous Lymphat Disord 2018;6:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obi AT, Sutzko DC, Almeida JI, Kabnick L, Cronenwett JL, Osborne NH, et al. First 10-month results of the Vascular Quality Initiative Varicose Vein Registry. J Vasc Surg Venous Lymphat Disord 2017;5:312–20.e2. [DOI] [PubMed] [Google Scholar]
- 17.Paty J, Turner-Bowker DM, Elash CA, Wright D. The VVSymQ instrument: use of a new patient-reported outcome measure for assessment of varicose vein symptoms. Phlebology 2016;31:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd KL, Wright D, Gibson K, Goldman M, Hertzman P, Hirsch S, et al. The VANISH-2 study: a randomized, blinded, multicenter study to evaluate the efficacy and safety of polidocanol endovenous microfoam 0.5% and 1.0% compared with placebo for the treatment of saphenofemoral junction incompetence. Phlebology 2014;29:608–18. [DOI] [PubMed] [Google Scholar]
- 19.Obi AT, Reames BN, Rook TJ, Mouch SO, Zarinsefat A, Stabler C, et al. Outcomes associated with ablation compared to combined ablation and transilluminated powered phlebectomy in the treatment of venous varicosities. Phlebology 2016;31:618–24. [DOI] [PubMed] [Google Scholar]
- 20.Hassanin A, Aherne TM, Greene G, Boyle E, Egan B, Tierney S, et al. A systematic review and meta-analysis of comparative studies comparing nonthermal versus thermal endovenous ablation in superficial venous incompetence. J Vasc Surg Venous Lymphat Disord 2019;7:902–13.e3. [DOI] [PubMed] [Google Scholar]
- 21.Rabe E, Pannier-Fischer F, Bromen K, Schuldt K, Stang A, Poncar C, et al. Bonner Venenstudie der Deutschen Gesellschaft für Phlebologie. Phlebologie 2003;32:1–14. [Google Scholar]
- 22.Lurie F, Obi A, Schul M, Hofmann LV, Kasper G, Wakefield T. Venous disease patient registries available in the United States. J Vasc Surg Venous Lymphat Disord 2018;6:118–25. [DOI] [PubMed] [Google Scholar]
- 23.Brittenden J, Cotton SC, Elders A, Tassie E, Scotland G, Ramsay CR, et al. Clinical effectiveness and cost-effectiveness of foam sclerotherapy, endovenous laser ablation and surgery for varicose veins: results from the Comparison of LAser, Surgery and foam Sclerotherapy (CLASS) randomised controlled trial. Health Technol Assess 2015;19:1–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsden G, Perry M, Bradbury A, Hickey N, Kelley K, Trender H, et al. A cost-effectiveness analysis of surgery, endothermal ablation, ultrasound-guided foam sclerotherapy and compression stockings for symptomatic varicose veins. Eur J Vasc Endovasc Surg 2015;50:794–801. [DOI] [PubMed] [Google Scholar]


