Abstract
Methotrexate is a gold standard among disease modifying antirheumatic drugs and is also extensively used clinically in combination with oncological therapies. Thus, it is not surprising that nuclear medicine found an interest in methotrexate in the search for diagnostic and therapeutic solutions. Numerous folate-related radiopharmaceuticals have been proposed for nuclear medicine purposes; however, methotrexate radioagents represent only a minority. This imbalance results from the fact that methotrexate has significantly weaker affinity for folate receptors than folic acid. Nevertheless, radiolabeled methotrexate agents utilized as a tool for early detection and imaging of inflammation in rheumatoid arthritis patients gave promising results. Similarly, the use of multimodal MTX-release nanosystems may find potential applications in radiosynovectomy and theranostic approaches in folate receptor positive cancers.
Keywords: methotrexate, anticancer drug, radiopharmaceuticals
1. Introduction
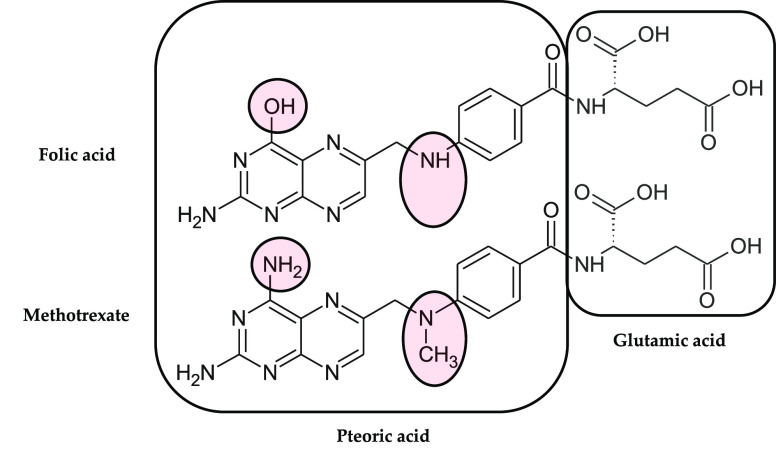
Folic acid (FA, vitamin B9, Figure 1) is a member of the folate family, known as a significant methyl-donor in the cellular formation of the essential amino acid methionine, and the syntheses of elements of nucleic acids—purines and thymidylates. However, even before the accurate determination of folates’ regulation of cell growth, function, and proper division, folate deficiency has been studied in the context of leukemia remission in humans.1 These beneficial effects have given the impulse to the search for compounds that are antagonists of FA action without causing FA deficiency. Indeed, the implementation of folate antagonists, known also as antifolates, provides similar and more selective effects on acute and subacute leukemia patients.2,3 Over time, methotrexate (MTX, formerly amethopterin, Figure 1) has been distinguished in clinical oncology among other initial antifolates, primarily due to its acceptable toxicity profile. Currently, MTX is one of the most widely applied chemotherapeutics, used alone or in combination therapy for different types of cancer, mainly in acute lymphoblastic leukemia, but also trophoblastic neoplasms, breast, lung, head and neck cancers, osteosarcomas, cutaneous T-cell lymphomas, or non-Hodgkin’s lymphomas.4,5 Furthermore, it has found great application in autoimmune pathologies and inflammatory disorders such as psoriasis and rheumatoid arthritis (RA).6,7 For most early and well diagnosed RA patients, MTX is the preferred initial disease-modifying antirheumatic drug.
Figure 1.
Structural differences between folic acid (top structure) and methotrexate (bottom).
MTX’s complex mechanism of action is involved in antitumor and immunosuppressive activity of this drug. First, MTX shows a folate antagonism by blocking multiple enzymes on the folate metabolic pathway and thereby disturbing the metabolic balance of the cell. After crossing the cell membrane, MTX follows rapid intracellular bioconversion into polyglutamate derivatives (MTX-pGlu), via folylpolyglutamyl synthase (FPGS).8 This bioactivation significantly enhances MTX pharmacological activity,9,10 namely, extending the intracellular retention and expanding inhibitory activity. Free MTX inhibits noticeably only dihydrofolate reductase (DHFR), while polyglutamated MTX provides greater inhibition of that enzyme. DHFR catalyzes the reduction of dihydrofolate into tetrahydrofolate—a significant proximal carbon atom donor in various transmethylation reactions, including synthesis of nucleosides. Furthermore, MTX-pGlu targets thymidylate synthase, amidophosphoribosyltransferase, and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase,11−14 other key enzymes in de novo thymidylates and purines syntheses. Consequently, the cellular syntheses of DNA and RNA building blocks are highly inhibited, whereby the cell loses its proliferative ability and undergoes apoptosis.15,16 This scenario applies primarily to highly proliferating cells that are most sensitive to the cytotoxic effect of MTX.17,18
Furthermore, AICAR transformylase blockage results in accumulation of AICAR, the inhibitor of adenosine and adenosine monophosphate deaminases.19,20 Therefore, intracellular elevation of AICAR promotes an adenosine increase followed by its extracellular efflux.21 Adenosine is a local signal transmitter, acting by its specific receptors (adenosine receptors A1, A2A, A2B, A3) present on the surface of the immune cell but also on the origin tissue. Activation of A2A and A3 receptors overexpressed on leucocytes and synoviocytes, specific macrophage-like and fibroblast-like cells in RA patients decreases production of inflammatory cytokines and downregulates the immune system.22,23 Adenosine through A2A also inhibits the action of activated neutrophils and macrophages including production of pro-inflammatory tumor necrosis factor and interleukins24,25 as well as promoting leukocyte production of anti-inflammatory interleukins 4 and 10.26 Thus, MTX indirectly induces an anti-inflammatory and immunosuppressive effect through adenosine-mediated action.
Pleiotropic action of MTX led to its wide application in clinical oncology and treatment of numerous inflammatory and autoimmune pathologies. In this review, we focus on the perspectives of MTX features for the needs of nuclear medicine. The overview of articles presented below concerns the application of MTX in the form of any radioactive agents to date. We will acquaint the reader with MTX’s ability to act as a leading vector (in terms of diagnostic radiopharmaceuticals) or the ability to support the apoptotic effect of radiation (with regard to therapeutic radiopharmaceuticals). Both of these aspects are key issues in the design of new receptor radiopharmaceuticals for the ever more intensive development in nuclear medicine.
2. Mechanisms of Intracellular Transport of Methotrexate
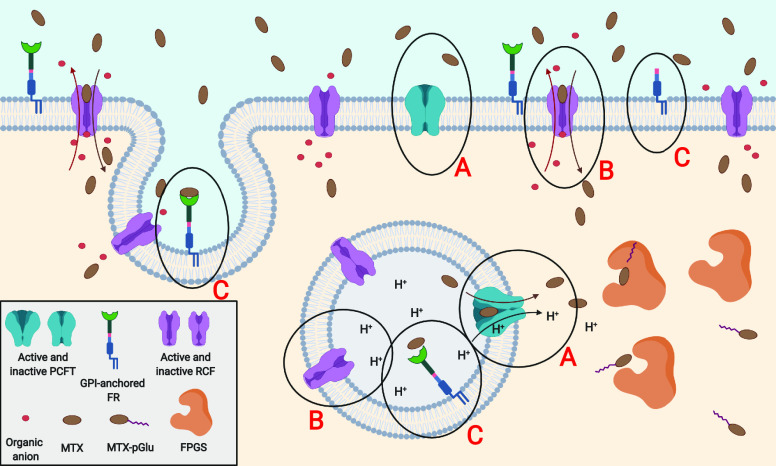
MTX is a small molecule that possesses two free carboxylic moieties from glutamate and two weak amino groups in the aromatic structure of 4-aminopteroic acid (Figure 1). It is slightly soluble in water, and if so, becomes strongly ionized and unable to penetrate biological membranes by itself. Thus, the distribution of MTX depends on bioavailability associated with an active transport system. First, MTX adsorption is clearly related to the route of drug administration, especially in the case of the oral form. The intestinal adsorption of the drug is mediated via proton-coupled folate transporters (PCFTs, more precisely, solute carrier family 46 member 1, SLC46A1),27 specifically, symporters that exhibit high density in the upper gastrointestinal tract, where at a preferred low pH participate in specific uptake of both oxidized and reduced folates.28 PCFTs also occur on many healthy tissues and cancer cells, which are barely active at physiological pH; however, during the internalization process they mediate active folate transport due to the acidic environment inside the endosome (Figure 2 and Figure 3).27,28
Figure 2.
Physiologic state of the cell: (A) Around neutral pH proton-coupled folate transporters are in an inactive state. To become activated, PCFTs require an extracellular acidic environment or local pH decrease, e.g., in lumen of lysosomes. (B) Reduced carrier folates are responsible for the main MTX influx to the cell, by exchanging MTX for organic phosphate anions. Reduction of pH by 1.5–2 units inactivates RCFs. (C) Low expression of membrane anchored folate receptors insignificantly transports MTX through endocytosis.
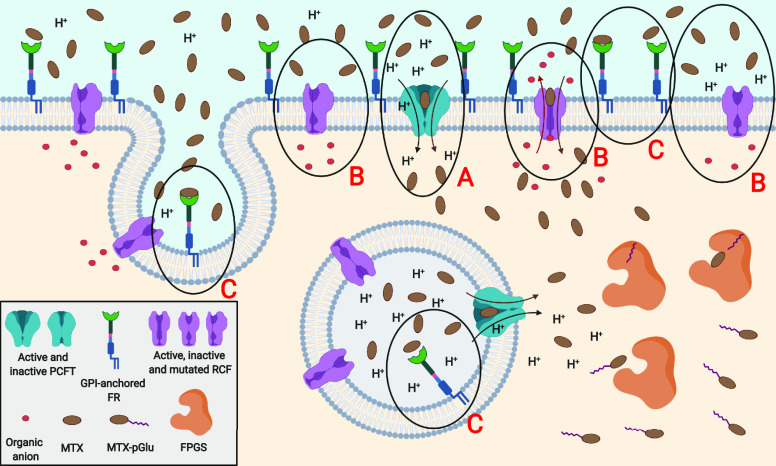
Figure 3.
Pathologic/cancer state of the cell: (A) Local extracellular decrease of pH may activate PCFTs on the cell surface and provide strong MTX influx. (B) At the same time, RCFs may be set up in an inactive state depending on the pH. Moreover, cancer cells are able to induce RCF impairment thereby acquiring MTX resistance. (C) Expression of FRs may significantly increase in some cancer cell lines due to increased sensitivity to folates; thus, MTX endocytic transport also improves.
Around neutral pH, cellular MTX transport is realized mainly by the reduced folate carrier 1 (RFC) or to some extent via endocytosis by the folate receptors (FRs) (Figure 2). RFC is a 12 transmembrane-domain exporter in the solute carrier transporter superfamily (solute carrier family 19 member 1, SLC19A1), ubiquitously expressed in human tissues and cancers.29 It mediates the influx of 5-methyl-tetrahydrofolate or the majority of DHFR antagonists in exchange for various organic anions. In comparison to PCFT, RFC demonstrates high specificity only for reduced folates (Km ≈ 1–10 μM for MTX),30,31 whereas affinity for FA is 100-fold less (Km ≈ 100–400 μM). Furthermore, the reduced folate carrier impairment is one of the most clinically important mechanisms responsible for cancer cell resistance to MTX therapy (Figure 3),32 since antifolates are predominantly delivered intracellularly by RFC.
However, MTX cellular uptake takes place also via FRs (formerly called the folate binding proteins), realized through receptor mediated endocytosis. This transport pathway is mostly dedicated to FA and oxidized folates (Kd ≈ 0.01 nM for FA), while MTX and other DHFR inhibitors show above 3-fold lower affinity to FRs (Kd ≈ 10–100 nM for MTX).31,33 Importantly, RFC and FRs operate independently and possess individual characteristics of action: different pH and temperature sensitivities, tissue expression, distinct chemical individuals causing transport blockade, and an inverse ratio of reduced/oxidized folates binding affinities.34,35 Moreover, compared to FRs, RFC creates a stronger complex with MTX36 and has a higher maximal velocity and Michaelis–Menten transport constant for MTX transport.35 Despite these facts, the contribution of both types of transport may provide similar influx rates of MTX. This is possible only when FR density is significantly elevated (e.g., on the surfaces of cancer cells) and drug concentration in the blood is fairly low (e.g., radiopharmaceutical application).
The most contemporary distinction of FRs is made between four isoforms: two glycosylphosphatidylinositol (GPI)-anchored receptor isoforms α and β, free soluble receptor isoform γ,37 and newly discovered isoform δ (also known as JUMO or IZUMO1R), which is also the GPI-anchored receptor.38,39 The noticeably up-regulation of folate receptor isoform α (FR-α) is observed in various epithelial cancers including breast and kidney carcinomas,40,41 also brain cancer,42 while significant FR-α overexpression is reported in lung and internal parts of female reproductive system carcinomas.43−48 FRs-α physiologically occur to a small extent on the choroid plexus, fallopian tubes, colorectal and pulmonary epithelial cells,43,44,48,49 and in particular on the renal proximal and distal tubules;48,50 however, they are hardly accessible to folate conjugates circulating in the bloodstream,51 and thus, folate imaging gives the only pronounced signal in the kidneys of healthy controls.52,53
Second FR isoform β (FR-β) is expressed on surfaces of activated macrophages during chronic inflammatory diseases,54 in spleen of patients with chronic and acute myelogenous leukemia,55,56 and also on healthy placental tissue.44 Both isoforms are beneficial in the clinical prognosis of cancer. FR-α is specified as reliable cancer marker,49,57−59 that even may allow for the possibility of defining a tumor grade and subtype55,57 or prognosis view for patient recovery.41,58 Similarly, elevated expression of FR-β can be detected in the microenvironment of particular tumors due to a specific receptor presence on tumor-associated macrophages.60 In addition, both isoforms show different binding affinities for some ligands that are folate analogues,34,61 which may give an opportunity to design a selective tracer for a specific FR isoform in order to diagnose a particular type of cancer or inflammatory disease.
FR isoform γ (FR-γ) is a soluble protein related principally to hematopoietic and lymphoid systems.37 Because of its inability to connect to GPI anchor, FR-γ is constantly secreted into the serum at the modest level; however, this secretion increases during dietary folate deficiency and especially in the case of leukemia. Therefore, the increase of FR-γ serum level predominantly indicates the presence of malignancies of bone marrow, spleen, or thymus.37
FR isoform δ (FR-δ, FOLR4), commonly known as JUNO, originally has been reported on CD4+CD25+ regulatory T-cells responsible for ongoing tumor resistance in the murine immune system.62 Moreover, mammalian JUNO serves as an egg cell plasma membrane receptor for IZUMO1 sperm-specific protein. Creation of JUNO-IZUMO1 complexes results from an egg cell–sperm fusion essential for the fertilization process.39
The foregoing ample description of MTX active transport shows the significance of folate action in mammalian physiology. The presence of so many pathways of FA derivative uptake cannot be accidental. Particular expression of presented receptors on healthy tissues and cancers or inflammation makes them attractive molecular targets. Simultaneously, this provides huge opportunities to search for new imaging and therapeutic solutions for laboratory diagnostics and nuclear medicine.
Molecular sciences provide numerous data on the correlation between FRs/RFC affinities or cellular influx capacities with structure of folates/MTX and theirs conjugates.31,33,36,63−65 While MTX has a superior affinity toward ubiquitous RFC than FRs, MTX conjugates should provide a molecular recognition of FRs at the specific place of receptor overexpression. The choice of FRs as a molecular target in nuclear medicine has significant advantages such as great tumor/inflammation targeting or the possibility of endocytic delivery across the membrane of various types of conjugated molecules (proteins, immune agents, nucleotides, chromophores, radionuclides, nanostructures, liposomes, etc.).
3. Radionuclide-Labeled MTX Agents
The widespread use of MTX in oncology and the development of quantitative radionuclide tumor imaging have given rise to further research of radioconjugates based on FA and MTX analogues. Currently, numerous folate-based radiopharmaceuticals have been proposed for nuclear medicine purposes;66,67 however, MTX radioagents represent only a minority in the field because MTX has more than three orders of magnitude weaker affinity toward folate receptors than FA.31,33 The conversion of oxygen into nitrogen in the pteridine ring (Figure 1) deprives MTX of a significant ability to form a hydrogen bond at the active center of folate receptors, which consequently decrease binding to FRs.36 Despite the weakened affinity of MTX for FRs, there have been numerous studies describing multiple applications of radionuclide labeled MTX agents.
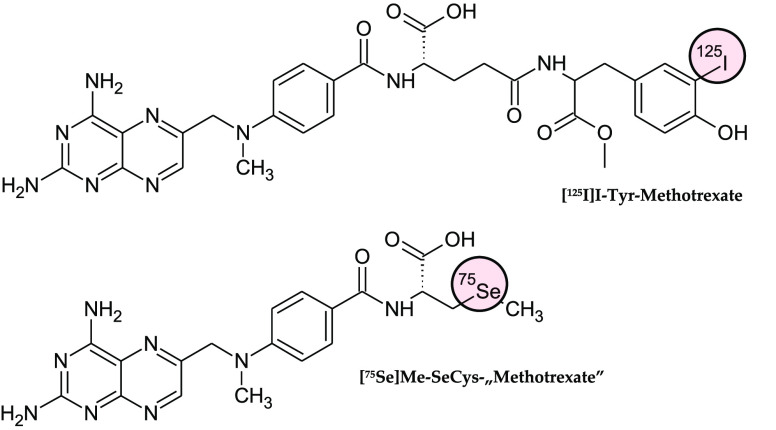
The earliest reports refer to tritiated MTX derivative 3′,5′,7-[3H]-MTX,68 which has been applied in MTX uptake studies of murine leukemia L210 cell line69−71 and in vivo on rodents.30,72 Subsequently, the natural evolution of this research has been in pharmacokinetic and metabolic studies of MTX in small mammalians, rhesus monkeys,73,74 and humans.75−77 In the meantime, the first [3H]-MTX radioimmunochemical methods for the determination of MTX in biological matrix were developed.78,79 In a short period of time, practical proposition of radioimmunoassay using MTX labeled with gamma emitters (selenium-75 and iodine-125) was reported for more convenient clinical monitoring of patients receiving MTX chemotherapy.80,81 [75Se]Methyl-l-selenocysteine amino-acid-exchanged MTX derivative ([75Se]Me-SeCys-“MTX”) and MTX conjugated with [125I]iodo-tyrosine methyl ester ([125I]I-Tyr-MTX) (Figure 4) enhance radioimmunoassays performance and time, allowing for radioactivities to be counted more efficiently, while maintaining precision, sensitivity, and reliability, as it may be used with β– emitting [3H]-MTX. For instance, MTX plasma levels of acute lymphoid leukemia in children under high dose MTX therapy was monitored using [125I]I-Tyr-MTX.82
Figure 4.
Structural formulas of MTX radioimmunoassay agents: 3-[125I]iodo-tyrosine methyl ester conjugated with MTX (top structure) and [75Se]methyl-selenocysteine conjugated with 4-amino-10-methyl-pteroic acid (bottom).
Generally, in most MTX cellular transport studies, tritiated derivative [3H]-MTX is the preferred reference compound. This was observed in affinity studies for FR-α, RFC, and PCFT of newly designed antifolate drugs,28,31,83,84 in PCFT-mediated transport research on mesothelioma cell lines,85 and FR-β-mediated transport investigation on synovial macrophages from RA patients.54 Similarly, [3H]-MTX was applied to monitor in vitro/in vivo enzymatic polyglutamylation rate and thereby the ability to inhibit the thymidylate synthase in murine leukemia cells,86 or DHFR inhibition in human nonsmall-cell lung carcinoma.87 Additionally, [3H]-MTX uptake studies were performed on MTX-resistant osteogenic sarcoma cell line variants.88
In parallel to cellular transport research, new methods for desirable MTX distribution have been investigated. For this purpose, a number of various types of liposomes (lipophilic forms of the delivery drug system) containing entrapped [3H]-MTX have been developed and tested due to their ability to modify tissue distribution or metabolic rate of MTX.89−93 Similarly, conjugates of [3H]-MTX with functionalized polypeptides (hydrophilic forms of drug carriers) were examined specifically for their analogical aspects.94,95 For instance, simple modification of the primary drug, namely, [3H]-MTX conjugation with individual peptide phenylalanine (via α-carboxyl group of glutamine moiety in MTX structure), produced a less toxic prodrug, capable of revealing its intrinsic cytotoxicity using tumor targeted monoclonal antibody conjugated with specific hydrolyzing enzyme.96 Additionally, a significantly larger hydrophilic drug carrier in the form of antitransferrin-receptor monoclonal antibody (anti-TFR-mAb) and [3H]-MTX conjugate has been proposed to enable migration of MTX across the blood–brain barrier (BBB).97 Another antibody-based carrier, [3H]-MTX conjugated with mAb against mouse mammary tumor antigen, demonstrated its ability toward selective tumor delivery of MTX.98
It is also worth noting the concept of conjugating MTX with modified serum albumin for more intended drug delivering. [125I]-Iodinated conjugate of MTX with maleylated-bovine serum albumin (BSA) was capable of increasing deposit of the radioagent in macrophages, which resulted in a superior antileishmanial effect compared to free MTX.99 [3H]-MTX conjugated with galactosylated or lactosylated BSA exhibits modulated hepatocyte uptake of [3H]-MTX followed by slow and long-acting release, as well as reduced kidney accumulation of [3H]-MTX than without conjugation with BSA.100,101 The assessment of organ distribution of [3H]-MTX was performed on mice using radioagent conjugated with human serum albumin (HSA) itself or entrapped in hydrophilic albumin microspheres.102 Small microspheres of 10 or 22.4 μm mean diameter were distributed mainly into the lungs and liver due to the limitation of organ vascularization clearance. Application of [3H]-MTX-HSA microspheres prolonged the biological half-life of [3H]-MTX but also the retention time of [3H]-MTX-HSA in all evaluated organs. Furthermore, simple conjugation of [3H]-MTX with HSA or MTX with [125I]I-HSA enabled more effective radioagent uptake compared to free [3H]-MTX on most studied human tumor xenografts.103 Authors showed that these MTX-HSA conjugates aimed at both FR and albumin receptors provided the ability to induce a significant antitumor effect, even in originally MTX-resistant tumors.
Reports on simple and ingenious possibilities of MTX targeting (to a specific target or selected systemic compartment) provided an insight into new possibilities in MTX therapy and the impetus for further development of MTX-based radiopharmaceuticals.
Recently, two MTX derivatives with two glutamine or lysine molecules attached to the amino groups at positions 2 and 4 of the pteridine ring have been presented.104,105 These MTX conjugates were characterized by significant increased permeability through BBB, in which both conjugates were capable of 99mTc-complexing through direct labeling method. In vivo distribution studies on mice confirmed increased cerebral distribution of [99mTc]Tc-MTX-(glutamine)2 and [99mTc]Tc-MTX-(lysine)2 radiocomplexes with the ability of enzymatic release of MTX in the central nervous system.
Despite the unfavorable binding to FRs, several radiolabeled methotrexate agents have been obtained and tested for their targeting ability toward FR positive tumor cells. [99mTc]Tc-MAG2-MTX and [99mTc]Tc-MAG3-MTX conjugates were evaluated and examined on breast cancer cell lines and murine xenografts, showing poor results.105−108 The outcome was similar to that of stealth liposomes containing MTX directly labeled with 99mTc109 or polyPEGylated [99mTc]Tc-MTX complexes, which were both examined on mice with Ehrlich ascites tumor.110 However, ex vivo biodistribution of [99mTc]Tc-MTX complex on natural tumor bearing mice showed explicit and stable over time uptake of the complex at the tumor sites.111 Moreover, clinical experiments of imaging breast cancer using [99mTc]Tc-MTX complex provided noticeable and stable over time uptake at the cancer lesions, but global targetability of radiopharmaceutical was poor and patient scans were difficult to assess.112 Further investigations on human regular and carcinoma breast cell lines with [99mTc]Tc-MTX complex and MTX-chitosan nanoparticles labeled with 99mTc exhibit [99mTc]Tc-MTX complex unspecific binding to both examined cell models, while MTX-chitosan nanoparticles showed specific binding toward breast cancer cell line in contrast to unloaded chitosan nanoparticles.113,114
Other investigated radioligands based on MTX vector also showed unsatisfactory traceability of FR-expressing tumors in rodent models. γ-Emitting [111In]In-DTPA-ethylenediamine-MTX115 showed very low uptake in breast tumor, but still superior than [111In]In-DTPA complex and sensitive to FA blockage. [68Ga]Ga- or [99mTc]Tc-chitosan-polyglutamate-MTX complexes,116 both obtained in direct labeling, showed negligible human breast tumor targetability (tumor-to-blood ratios below 1) throughout in vivo biodistribution. Bombesin analogue and MTX conjugate complexed with 99mTc117 was found to demonstrate high affinity and internalization grade toward FRs (FR-positive KB cell line Kd = 3.95 ± 0.80 nM; internalization at 31.0 ± 2.10%) or bombesin receptors expressed on tumor cell lines. However, further biodistribution on the xenograft murine models with the same cell lines revealed little tumor uptake of peptide-MTX radioconjugate and proved its inadequacy for intended imaging use.
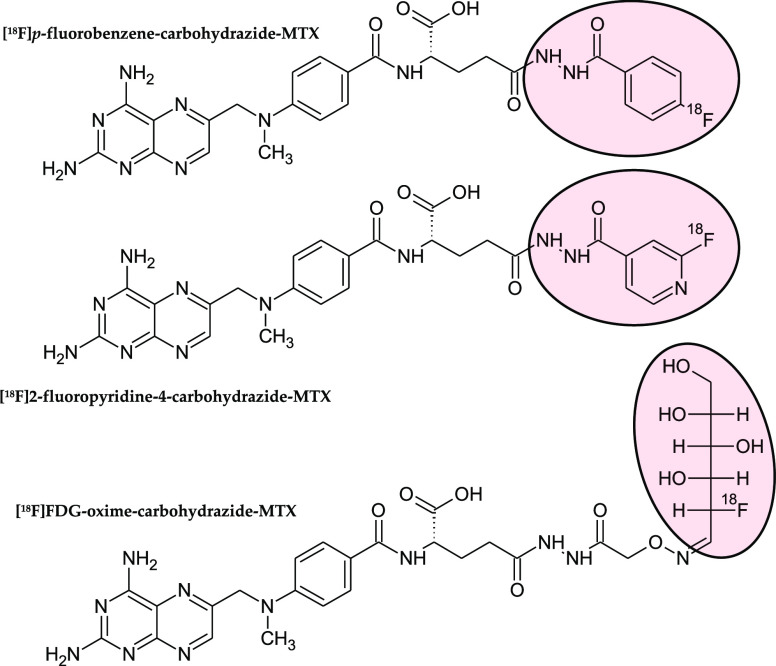
Furthermore, for positron emission tomography (PET) imaging few 18F radiotracers based on MTX have been developed. These include MTX derivatives conjugated with selected prosthetic groups already labeled with 18F, as p-fluorobenzene-carbohydrazide, 2-fluoropyridine-4-carbohydrazide,118 or 2-fluoro-2-deoxy-d-glucose (FDG)119 (Figure 5). [18F]p-Fluorobenzene-carbohydrazide-MTX and [18F]2-fluoropyridine-4-carbohydrazide-MTX in initial in vitro examination on FRs expressing tumor cell line showed about 2-fold lower binding affinity in comparison to equivalent folate radiotracers (FR-positive KB cell line Kd values 22.52 ± 1.79 and 31.42 ± 0.95 nM versus 13.08 ± 0.83 and 15.51 ± 1.80 nM, respectively).118 In further biodistribution studies on xenograft mice MTX radiotracers exhibited tumor uptake comparable with concentrations of the tracer in blood and sensitive to FA blocking, however, significantly worse uptake than that observed in the case of folate analogues. The third MTX-radioligand, [18F]FDG-oxime-carbohydrazide-MTX, proved to be the most promising.119 It had not only higher binding affinity than previous MTX-tracers (FR-positive KB cell line Kd = 4.74 ± 0.71 nM), but a similar in vivo imaging study demonstrated a noticeable tumor uptake at the same level as in other organs such as spleen, lungs, or intestines. At the same time, comparable [18F]FDG-oxime-carbohydrazide-folate demonstrated superior binding affinity (FR-positive KB cell line Kd = 1.83 ± 0.15 nM) than [18F]FDG-oxime-carbohydrazide-MTX as well as significant tumor uptake, 3-fold higher than in the case of MTX radioagent. These reports clearly indicated the superiority of folate-based radiotracers in the biodistribution on FR-positive tumor bearing rodents against MTX-based radioligands.
Figure 5.
Structural formulas of 18F radiotracers based on MTX: [18F]p-fluorobenzene-carbohydrazide-MTX (top structure), [18F]2-fluoropyridine-4-carbohydrazide-MTX (middle), and [18F]FDG-oxime-carbohydrazide-MTX (bottom). Fluorine-containing prosthetic groups are highlighted in light red.
[99mTc]Tc-MTX complex was also investigated as a diagnostic agent for RA and compared to [99mTc]Tc-MDP (methylene diphosphonate) complex. MDP complexed with 99mTc is one of the most commonly applied diphosphonate-based radiopharmaceutical for skeletal scintigraphy in patients with bone metastases.120,121 For decades, it has been considered as the standard single-photon emission computed tomography (SPECT) imaging agent with superior sensitivity compared to conventional radiography, as well as due to limited availability of PET facilities in numerous nuclear medicine departments. [99mTc]Tc-MDP skeletal uptake mechanism is based on the bone surface chemisorption associated with the local blood flow and metabolic activity of the osteoblasts, factors easily altered by the tumor presence. Clinical experiments conducted on 5 patients with RA and 19 healthy volunteers compared [99mTc]Tc-MTX and [99mTc]Tc-MDP complexes in RA lesions imaging.122 In reported results, the authors present scintigraphy evaluation of both radioagents only for two patients with regard to whole volunteer group. Thus, the obtained outcome for particular subjects indicated that [99mTc]Tc-MTX demonstrated effective detection of early and small articular rheumatoid nodules, while [99mTc]Tc-MDP exhibited noticeable differences in uptakes of healthy and severe inflammation altered joints only.
Another study investigated the ability of [99mTc]Tc-MTX complex to detect severe (chemically induced) inflammations.123 An ex vivo study performed on a small group of three mice with experimentally induced inflammation showed very high uptake of radiocomplex in kidneys and significant accumulation in bones. In parallel, [99mTc]Tc-MTX binding to hydroxyapatite at the level of about 45% after 10 min of incubation was determined. Furthermore, dynamic imaging of turpentine-irritated mouse pointed to a high instant uptake of [99mTc]Tc-MTX in the induced-inflammatory tissue and unfortunately also in liver. Simultaneously, control mice imaging showed first of all high bladder and kidney uptake, but also highlighted radiocomplex uptake in scull and in hind-limb joints. Despite authors demonstrating elevated uptake of [99mTc]Tc-MTX in the target inflamed tissue, MTX radiocomplex had limited usability of chemically induced inflammation imaging due to low selectivity. Nevertheless, at the same time, this very study provided firm evidence of [99mTc]Tc-MTX affinity to the hydroxyapatite present in skeletal tissue.
It is worth highlighting skeletal imaging performed on rats and rabbits using 99mTc radiocomplex of MTX-bisphosphonate conjugate.124 Although the precise structure of the complex was unknown in study, radioconjugate exhibited very high binding to bones and joints, most likely through a bisphosphonate fragment. The authors of the study indicated promising prognostic utility of researched radioconjugate, but also the potential application of a similar complex containing therapeutic radionuclide 186Re for the treatment of osteosarcoma or bone metastases.
Above reports111−119 provided limited uptake of MTX radioagents by FR-positive cancer cell lines especially in comparison to folate-based radioagents. However, results of in vivo or ex vivo biodistribution showed detectable MTX radioagents uptake at tumor sites, but also substantial uptake in spleen. On the other hand, reports on inflammatory lesions imaging122,123 indicated that [99mTc]Tc-MTX complex enabled clear detection of articular rheumatoid nodules or chemically induced inflammation. Interestingly, the favorable quality of these visualizations obtained using MTX radioagents may be due to pro-inflammatory M1 macrophages that highly express not only FR-β but also RFC on their surfaces.125 These macrophages were recognized as granulocyte macrophage colony-stimulating factor-driven macrophages and demonstrated substantial contribution in tumor microenvironment and in close proximity inflamed tissue.126 Moreover, it was determined that these pro-inflammatory M1 macrophages showed a specific response for methotrexate action.127
More recently, to improve the outcome of the well-established therapeutic strategies, novel nanomaterials that combine immune agent MTX and radionuclides (for diagnosis or therapy) were developed. For targeted treatment of rheumatoid-affected joints, radiolabeled biodegradable nanoparticles entrapped with MTX were proposed.128 These nanoparticles made of polylactic-co-glycolic acid (PLGA) polymer have the ability to release encapsulated drug in a controlled manner. Additionally, they possess hyaluronic acid (HA) molecules conjugated on their surface, which specifically guides molecules to the inflamed synovial tissue cells, and the macrocyclic chelator DOTA chelating cation of β– emitting radionuclide 177Lu, which is favorable for mild irritation of macrophages in inflamed synovial tissue. In vitro assay on murine macrophages cell line was proven to display controlled and specific cytotoxicity of this multifunctional nanopolymeric system that was superior than MTX alone or PLGA polymer without HA, MTX, or [177Lu]Lu-DOTA addition. Nevertheless, further in vivo examinations must be performed before these nanoparticles will find clinical application in radiosynovectomy of RA patients.
Another multimodal system based on multiwalled carbon nanotubes (MWCNTs) was developed for theranostic application in FR positive cancers.129 Carbon nanotubes were surface-functionalized with Alexa-fluor-488/647 fluorochrome, diagnostic radionuclide 99mTc, tumor-targeting FA, and MTX as anticancer agents. These nanotubes had the ability to release MTX under the lysosomal acidic conditions inside the cell, enabling MTX to exert its therapeutic action. Moreover, it was shown that these carbon nanotubes had superior binding affinity to FR positive human lung or breast cancer cell lines than MTX. Similarly, FR positive xenograft mice biodistribution studies showed that the microparticles demonstrated significant tumor uptake (however, also with very high uptake in liver, spleen and lungs) and tumor volume inhibition greater than free MTX. Proposed dual-imaging MWCNT system confirmed designed targeting and therapeutic functions; however, this still needs to improve the ability of tumor-specific delivery.
Presented reports on nanomaterial multimodal systems128,129 seem very perspective and are designed rationally with properly established functions of the nanosystems. The selected biovectors (hyaluronic acid and folic acid) turned out to be appropriate and crucial for research outcomes. Moreover, a simultaneous application of immune agent and radiation (in reference 129 γ-emitting 99mTc may be replaced by some β– emitter, as 186Re) produced a synergistic effect in anti-inflammatory action against cancer or/and immune cells.130
In another study, an efficient in vitro delivery of the radiolabeled MTX conjugate to the targeted erythroid leukemia cells line expressing the transferrin receptor (TFR) was performed using a two-stage targeting procedure.131 First, anti-TFR monoclonal antibody conjugated with recombinant DHFR enzyme (anti-TFR-mAb-rDHFR) was pretargeted to the tumor cells, followed by [111In]In-DTPA-hydrazide-MTX administration. In vitro cell binding study demonstrated high specific binding of MTX radioconjugate—about 14-fold higher compared to nonspecific binding observed in the case of nonconjugated anti-TFR-mAb application in the pretargeting stage. Without the pretargeting stage, [111In]In-DTPA-hydrazide-MTX demonstrated nonspecific binding to examine cell line model equally low as a negative control [111In]In-DTPA complex. Such a two-phase system of drugs delivery into target sites seems to be effective and beneficial for experimental applications and promising to use in similar in vivo targeting procedures.
Table 1. Collection of Reports Concerning Radioagents Based on MTX.
| MTX radioagent | research | refs |
|---|---|---|
| [3H]-MTX | Specific pharmacokinetic and metabolic studies of MTX, radioimmunoassay application; | (31, 36, 69−81, 83−88) |
| MTX delivery drug system studies | (89−103) | |
| [75Se]Me-SeCys-“MTX” | Radioimmunoassay for clinical monitoring of MTX chemotherapy | (80, 81) |
| [125I]I-Tyr-MTX | Radioimmunoassay for clinical monitoring of MTX chemotherapy | (81, 82) |
| [99mTc]Tc-MTX-(glutamine)2 | BBB permeable prodrug studies | (104, 105) |
| [99mTc]Tc-MTX-(lysine)2 | ||
| [99mTc]Tc-MAG2-MTX | FR-expressing tumor imaging | (106−108) |
| [99mTc]Tc-MAG3-MTX | ||
| 99mTc and 68Ga various complexes of MTX and its derivatives | FR-expressing tumor imaging | (109−114, 116, 117) |
| [111In]In-DTPA-CH2CH2-MTX | FR-expressing tumor imaging | (115) |
| [99mTc]Tc-MTX complex | Diagnostic application in RA and inflammation | (122, 123) |
| [99mTc]Tc-MTX-bisphosphonate complex | Skeletal imaging on rats and rabbits | (124) |
| [18F]p-fluorobenzene-carbohydrazide-MTX | PET tracers for FR positive cancers | (118, 119) |
| [18F]2-fluoropyridine-4-carbohydrazide-MTX | ||
| [18F]FDG-oxime-carbohydrazide-MTX | ||
| [111In]In-DTPA-hydrazide-MTX | In vitro targeting of erythroid leukemia cells after anti-TFR-mAb-rDHFR pretargeting | (131) |
| [177Lu]Lu-DOTA-HA-PLGA(MTX) | Potential radiosynovectomy application in RA patients | (128) |
| [99mTc]Tc-AF488/647-FA-MTX-MWCNTs | Multimodal multiwalled carbon nanotubes (MWCNTs) system for theranostic application in FR positive cancers | (129) |
4. Conclusion
Presented review has been prepared to comprehensively highlight any perspectives of methotrexate radioagents for application in nuclear medicine. First, the mechanisms of intracellular transport of methotrexate were presented to give the reader insight into the in vivo fate of free methotrexate. In the main part, strict emphasis was placed on methotrexate-based radioagents starting with a brief list of pharmacokinetic and metabolic research using radiolabeled methotrexate, radioimmunochemical methods for clinical monitoring of MTX during chemotherapy, as well as multiple studies of an MTX delivery drug system.
MTX ability to act as a leading vector in the form of simple [99mTc]Tc-MTX complex, various MTX derivatives complexed with 99mTc or 68Ga, DTPA-MTX conjugate chelated with 111In and 18F PET traces based on MTX has been examined in numerous reports. Only a few of these reports provided any promising results in terms of oncological imaging of folate receptors, where they most clearly showed the considerable superiority of folate-based radiotracers over those based on MTX. From the other point of view, application of [99mTc]Tc-MTX enabled early detection of inflammation in small articular rheumatoid nodules in comparison to [99mTc]Tc-MDP that differentiates only severe articular inflammations from healthy joints,122 even though the clinical results were confirmed on two of five examined subjects. Also, [99mTc]Tc-MTX complex provided clear visualization of chemically induced inflammation on a murine model, despite low selectivity of the radiocomplex.123 Moreover, the same study reported firm evidence that [99mTc]Tc-MTX exhibited high affinity to the bone-like hydroxyapatite.
We were surprised when we started our own research on [99mTc]Tc-MTX radiocomplex and found great difficulty in the radiosyntheses of [99mTc]Tc-MTX according to reports provided in this review (paper under preparation), which gives us food for thought related to the credibility of the presented above research.
On the other hand, the utilization of MTX in therapeutic radiopharmaceuticals is very limited to the component of multifunctional nanoparticles. Radiolabeled with 177Lu, biodegradable PGLA nanoparticles entrapped with MTX are an elaborate concept for radiosynovectomy,128 where decreased inflammation of the synovial tissue is induced synergistically by a disease-modifying antirheumatic drug and soft beta radionuclide guided by hyaluronic acid. Despite the promising results, the presented research is only the first step of the preclinical evaluation and requires further in vivo trials.
Acknowledgments
The contribution of P.K.H. has been done in the frame of the National Centre for Research and Development Project No. POWR.03.02.00-00-I009/17 (Radiopharmaceuticals for molecularly targeted diagnosis and therapy, RadFarm, Operational Project Knowledge Education Development 2014-2020 cofinanced by European Social Fund).
Glossary
Abbreviations
- A1, A2A, A2B, A3
adenosine receptors
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- BBB
blood–brain barrier
- BSA
bovine serum albumin
- DHFR
dihydrofolate reductase
- DTPA
diethylenetriaminepentaacetic acid
- FA
folic acid, vitamin B9
- FDG
2-fluoro-2-deoxy-d-glucose
- FR-α
folate receptor isoform α
- FR-β
folate receptor isoform β
- FR-γ
folate receptor isoform γ
- FR-δ
folate receptor isoform δ, FOLR4
- FRs
folate receptors, folate binding proteins
- GPI
glycosylphosphatidylinositol
- HA
hyaluronic acid
- HSA
human serum albumin
- KB
human papilloma cell line, folate receptor- positive
- Kd
dissociation constant
- Km
Michaelis constant
- mAb
monoclonal antibody
- MAG2
mercaptoacetyl-glycyl-glycine
- MAG3
mercaptoacetyl-glycyl-glycyl-glycine
- MDP
methylene diphosphonate
- MTX
methotrexate
- MTX-pGlu
polyglutamate derivatives of methotrexate
- MWCNTs
multiwalled carbon nanotubes
- PCFT
proton-coupled folate transporter
- PEG
polyethylene glycol
- PET
positron emission tomography
- PLGA
poly lactic-co-glycolic acid
- RA
rheumatoid arthritis
- rDHFR
recombinant dihydrofolate reductase
- RFC
reduced folate carrier
- SeCys
selenocysteine
- SPECT
single-photon emission computed tomography
- TFR
transferrin receptor
- Tyr
tyrosine
This research was funded by statutory activity of the Institute of Nuclear Chemistry and Technology, Warsaw, Poland.
The authors declare no competing financial interest.
References
- Heinle R. D.; Welch A. D.. Experiments with pteroylglutamic acid and pteroylglutamic acid deficiency in human leukemia J. Clin. Invest. 1948, 27, 539. [PubMed] [Google Scholar]
- Farber S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood 1949, 4, 160–167. 10.1182/blood.V4.2.160.160. [DOI] [PubMed] [Google Scholar]
- Dameshek W. The Use of Folic Acid Antagonists in the Treatment of Acute And Subacute Leukemia: A Preliminary Statement. Blood 1949, 4, 168–171. 10.1182/blood.V4.2.168.168. [DOI] [PubMed] [Google Scholar]
- Methotrexate Injection FDA PI. https://www.drugs.com/pro/methotrexate-injection.html (accessed February 01, 2020).
- Koźmiński P.; Halik P. K.; Chesori R.; Gniazdowska E. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020, 21, 3483–3522. 10.3390/ijms21103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. S.; Landewé R. B. M.; Bijlsma J. W. J.; Burmester G. R.; Dougados M.; Kerschbaumer A.; McInnes I. B.; Sepriano A.; van Vollenhoven R. F.; de Wit M.; Aletaha D.; Aringer M.; Askling J.; Balsa A.; Boers M.; den Broeder A. A.; Buch M. H.; Buttgereit F.; Caporali R.; Cardiel M. H.; De Cock D.; Codreanu C.; Cutolo M.; Edwards C. J.; van Eijk-Hustings Y.; Emery P.; Finckh A.; Gossec L.; Gottenberg J. E.; Hetland M. L.; Huizinga T. W. J.; Koloumas M.; Li Z.; Mariette X.; Müller-Ladner U.; Mysler E. F.; da Silva J. A. P.; Poór G.; Pope J. E.; Rubbert-Roth A.; Ruyssen-Witrand A.; Saag K. G.; Strangfeld A.; Takeuchi T.; Voshaar M.; Westhovens R.; van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- Wilsdon T. D.; Whittle S. L.; Thynne T. R. J.; Mangoni A. A. Methotrexate for psoriatic arthritis. Cochrane Database Syst. Rev. 2019, 1, CD012722. 10.1002/14651858.CD012722.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh C. M.; Krumdieck C. L.; Nair M. G. Polygammaglutamyl metabolites of methotrexate. Biochem. Biophys. Res. Commun. 1973, 52, 27–34. 10.1016/0006-291X(73)90949-2. [DOI] [PubMed] [Google Scholar]
- Jolivet J.; Schilsky R. L.; Bailey B. D.; Drake J. C.; Chabner B. A. Synthesis, retention, and biological activity of methotrexate polyglutamates in cultured human breast cancer cells. J. Clin. Invest. 1982, 70, 351–360. 10.1172/JCI110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner B. A.; Allegra C. J.; Curt G. A.; Clendeninn N. J.; Baram J.; Koizumi S.; Drake J. C.; Jolivet J. Polyglutamation of methotrexate: is methotrexate a prodrug?. J. Clin. Invest. 1985, 76, 907–912. 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra C. J.; Drake J. C.; Jolivet J.; Chabner B. A. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 4881–4885. 10.1073/pnas.82.15.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra C. J.; Chabner B. A.; Drake J. C.; Lutz R.; Rodbard D.; Jolivet J.. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates J. Biol. Chem. 1985, 260, 9720–9726. [PubMed] [Google Scholar]
- Baggott J. E.; Vaughn W. H.; Hudson B. B. Inhibition of 5-aminoimidazole4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem. J. 1986, 236, 193–200. 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant M. E.; Lyons S. D.; Phillips L.; Christopherson R. I.. Antifolates Induce Inhibition of Amido Phosphoribosyltransferase in Leukemia Cell. J. Biol. Chem. 1992, 267, 11038–11045. [PubMed] [Google Scholar]
- Genestier L.; Paillot R.; Fournel S.; Ferraro C.; Miossec P.; Revillard J. P. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J. Clin. Invest. 1998, 102, 322–328. 10.1172/JCI2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks L. D.; Rückermann K.; Qiu Y.; Hawrylowicz C. M.; Richards D. F.; Swaminathan R.; Kirschbaum B.; Simmonds H. A. Methotrexate inhibits the first committed step of purine biosynthesis in mitogen stimulated human T-lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis?. Biochem. J. 1999, 342, 143–152. 10.1042/bj3420143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniuk W. M.Purineless Death as a Link Between Growth Rate and Cytotoxicity by Methotrexate Cancer Res. 1972, 32, 1506–1511. [PubMed] [Google Scholar]
- Choudhury R. C.; Ghosh S. K.; Palo A. K. Cytogenetic Toxicity of Methotrexate in Mouse Bone Marrow. Environ. Toxicol. Pharmacol. 2000, 8, 191–196. 10.1016/S1382-6689(00)00041-7. [DOI] [PubMed] [Google Scholar]
- Baggott J. E.; Vaughn W. H.; Hudson B. B. Inhibition of 5-aminoimidazole4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem. J. 1986, 236, 193–200. 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koźmiński P.; Halik P. K.; Gniazdowska E.. Methotrexate—Mechanisms of Drug Action Encyclopedia 2020. 10.32545/encyclopedia202005.0014.v2. [DOI] [Google Scholar]
- Cronstein B. N.; Naime D.; Ostad E. The antiinflammatory mechanism of methotrexate: increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 1993, 92, 2675–2682. 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani K.; Vincenzi F.; Tosi A.; Targa M.; Masieri F. F.; Ongaro A.; De Mattei M.; Massari L.; Borea P. A. Expression and Functional Role of Adenosine Receptors in Regulating Inflammatory Responses in Human Synoviocytes. Br. J. Pharmacol. 2010, 160 (1), 101–115. 10.1111/j.1476-5381.2010.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp L. K.; Hazlett J.; Roberts R. L.; Frampton C.; Highton J.; Hessian P. A. Adenosine receptor expression in rheumatoid synovium: a basis for methotrexate action. Arthritis Res. Ther. 2012, 14, R138. 10.1186/ar3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N.; Levin R. I.; Belanoff J.; Weissmann G.; Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J. Clin. Invest. 1986, 78, 760–770. 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A.; Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Constantin A.; Loubet-Lescoulie P.; Lambert N.; Yassine-Diab B.; Abbal M.; Mazieres B.; de Preval C.; Cantagrel A. Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: Evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum. 1998, 41, 48–57. . [DOI] [PubMed] [Google Scholar]
- Qiu A.; Jansen M.; Sakaris A.; Min S. H.; Chattopadhyay S.; Tsai E.; Sandoval C.; Zhao R.; Akabas M. H.; Goldman I. D. Identification of an Intestinal Folate Transporter and the Molecular Basis for Hereditary Folate Malabsorption. Cell 2006, 127, 917–928. 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Zhao R.; Qiu A.; Tsai E.; Jansen M.; Akabas M. H.; Goldman I. D. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol. Pharmacol. 2008, 74, 854–862. 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R.; Goldman I. D. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1–3 and SLC46A1) and folate receptors. Mol. Aspects Med. 2013, 34, 373–385. 10.1016/j.mam.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M.; Goutas L. J.; Mines L. S.. Extent of the Requirement for Folate Transport by L1210 Cells for Growth and Leukemogenesis in Vivo Cancer Res. 1985, 45, 4732–4734. [PubMed] [Google Scholar]
- Westerhof G. R.; Schornagel J. H.; Kathmann I.; Jackman A. L.; Rosowsky A.; Forsch R. A.; Hynes J. B.; Boyle F. T.; Peters G. J.; Pinedo H. M.; Jansen G.. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: correlates of molecular-structure and biological activity Mol. Pharmacol. 1995, 48, 459–471. 459. [PubMed] [Google Scholar]
- Zhao R.; Goldman I. D. Resistance to antifolates. Oncogene 2003, 22, 7431–7457. 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- Wibowo A. S.; Singh M.; Reeder K. M.; Carter J. J.; Kovach A. R.; Meng W.; Ratnam M.; Zhang F.; Dann C. E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 15180–15188. 10.1073/pnas.1308827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W.; Feng S.; Freisheim J. H.; Gentry L. E.; Ratnam M. Differential stereospecificities and affinities of folate receptor isoforms for folate compounds and antifolates. Biochem. Pharmacol. 1992, 44, 1898–1901. 10.1016/0006-2952(92)90089-2. [DOI] [PubMed] [Google Scholar]
- Spinella M. J.; Brigle K. E.; Sierra E. E.; Goldman I. D. Distinguishing between folate receptor-alpha-mediated transport and reduced folate carrier-mediated transport in L1210 leukemia cells. J. Biol. Chem. 1995, 270, 7842–7849. 10.1074/jbc.270.14.7842. [DOI] [PubMed] [Google Scholar]
- Chen C.; Ke J.; Zhou X. E.; Yi W.; Brunzelle J. S.; Li J.; Yong E. L.; Xu H. E.; Melcher K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. 10.1038/nature12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F.; Wu M.; Ross J. F.; Miller D.; Ratnam M. Folate receptor type gamma is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification: Protein characterization and cell type specificity. Biochemistry 1995, 34, 5660–5665. 10.1021/bi00016a042. [DOI] [PubMed] [Google Scholar]
- Spiegelstein O.; Eudy J. D.; Finnell R. H. Identification of two putative novel folate receptor genes in humans and mouse. Gene 2000, 258, 117–125. 10.1016/S0378-1119(00)00418-2. [DOI] [PubMed] [Google Scholar]
- Juno is the egg Izumo receptor and is essential for mammalian fertilisation. Nature 2014, 508, 483–487. 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman S. D.; Lark R. H.; Coney L. R.; Fort D. W.; Frasca V.; Zurawski V. R. Jr.; Kamen B. A.. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues Cancer Res. 1992, 52, 3396–3401. [PubMed] [Google Scholar]
- Hartmann L. C.; Keeney G. L.; Lingle W. L.; Christianson T. J.; Varghese B.; Hillman D.; Oberg A. L.; Low P. S. Folate receptor overexpression is associated with poor outcome in breast cancer. Int. J. Cancer 2007, 121, 938–942. 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- Weitman S. D.; Frazier K. M.; Kamen B. A. The folate receptor in central nervous system malignancies of childhood. J. Neuro-Oncol. 1994, 21, 107–112. 10.1007/BF01052894. [DOI] [PubMed] [Google Scholar]
- Mantovani L. T.; Miotti S.; Menard S.; Canevari S.; Raspagliesi F.; Bottini C.; Bottero F.; Colnaghi M. I. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19. Eur. J. Cancer 1994, 30, 363–369. 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Ross J. F.; Chaudhuri P. K.; Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines: physiologic and clinical implications. Cancer 1994, 73, 2432–2443. . [DOI] [PubMed] [Google Scholar]
- Holm J.; Hansen S. I.; Hoier-Madsen M.; Helkjaer P. E.; Nichols C. W. Folate receptors in malignant and benign tissues of human female genital tract. Biosci. Rep. 1997, 17, 415–427. 10.1023/A:1027313502270. [DOI] [PubMed] [Google Scholar]
- Wu M.; Gunning W.; Ratnam M.. Expression of folate receptor type a in relation to cell type, malignancy, and differentiation in ovary, uterus and cervix Cancer Epidemiol. Biomarkers Prev. 1999, 8, 775–782. [PubMed] [Google Scholar]
- Bueno R.; Appasani K.; Mercer H.; Lester S.; Sugarbaker D. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2001, 121, 225–233. 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- Parker N.; Turk M. J.; Westrick E.; Lewis J. D.; Low P. S.; Leamon C. P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Weitman S. D.; Weinberg A. G.; Coney L. R.; Zurawski V. R.; Jennings D. S.; Kamen B. A.. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis Cancer Res. 1992, 52, 6708–6711. [PubMed] [Google Scholar]
- Holm J.; Hansen S. I.; Hoier-Madsen M.; Bostad L. A high-affinity folate binding-protein in proximal tubule cells of human kidney. Kidney Int. 1992, 41, 50–55. 10.1038/ki.1992.7. [DOI] [PubMed] [Google Scholar]
- Patrick T. A.; Kranz D. M.; van Dyke T. A.; Roy E. J. Folate receptors as potential therapeutic targets in choroid plexus tumors of SV40 transgenic mice. J. Neurooncol. 1997, 32, 111–123. 10.1023/a:1005713115147. [DOI] [PubMed] [Google Scholar]
- Wang S.; Luo J.; Lantrip D. A.; Waters D. J.; Mathias C. J.; Green M. A.; Fuchs P. L.; Low P. S. Design and Synthesis of [111In]DTPA-Folate for Use as a Tumor-Targeted Radiopharmaceutical. Bioconjugate Chem. 1997, 8, 673–679. 10.1021/bc9701297. [DOI] [PubMed] [Google Scholar]
- Mathias C. J.; Hubers D.; Low P. S.; Green M. A. Synthesis of [99mTc]DTPA-Folate and Its Evaluation as a Folate-Receptor-Targeted Radiopharmaceutical. Bioconjugate Chem. 2000, 11, 253–257. 10.1021/bc9901447. [DOI] [PubMed] [Google Scholar]
- Nakashima-Matsushita N.; Homma T.; Yu S.; Matsuda T.; Sunahara N.; Nakamura T.; Tsukano M.; Ratnam M.; Matsuyama T. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999, 42, 1609–1616. . [DOI] [PubMed] [Google Scholar]
- Ross J. F.; Wang H.; Behm F. G.; Mathew P.; Wu M.; Booth R.; Ratnam M. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer 1999, 85, 348–357. . [DOI] [PubMed] [Google Scholar]
- Lynn R. C.; Poussin M.; Kalota A.; Feng Y.; Low P. S.; Dimitrov D. S.; Powell D. J. Jr Targeting of folate receptor b on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood 2015, 125, 3466–3476. 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffoli G.; Cernigoi C.; Russo A.; Gallo A.; Bagnoli M.; Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 1997, 74, 193–198. . [DOI] [PubMed] [Google Scholar]
- Iwakiri S.; Sonobe M.; Nagai S.; Hirata T.; Wada H.; Miyahara R. Expression status of folate receptor alpha is significantly correlated with prognosis in non-small-cell lung cancers. Ann. Surg. Oncol. 2008, 15, 889–899. 10.1245/s10434-007-9755-3. [DOI] [PubMed] [Google Scholar]
- D’Angelica M.; Ammori J.; Gonen M.; Klimstra D. S.; Low P. S.; Murphy L.; Weiser M. R.; Paty P. B.; Fong Y.; DeMatteo R. P.; Allen P.; Jarnagin W. R.; Shia J. Folate receptor-α expression in resectable hepatic colorectal cancer metastases: patterns and significance. Mod. Pathol. 2011, 24, 1221–1228. 10.1038/modpathol.2011.82. [DOI] [PubMed] [Google Scholar]
- Puig-Kröger A.; Sierra-Filardi E.; Domínguez-Soto A.; Samaniego R.; Corcuera M. T.; Gómez-Aguado F.; Ratnam M.; Sánchez-Mateos P.; Corbí A. L. Folate Receptor β Is Expressed by Tumor-Associated Macrophages and Constitutes a Marker for M2 Anti-inflammatory/ Regulatory Macrophages. Cancer Res. 2009, 69, 9395–9403. 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- Maziarz K. M.; Monaco H. L.; Shen F.; Ratnam M. Complete mapping of divergent amino acids responsible for differential ligand binding of folate receptors alpha and beta. J. Biol. Chem. 1999, 274, 11086–11091. 10.1074/jbc.274.16.11086. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.; Hirota K.; Nagahama K.; Ohkawa K.; Takahashi T.; Nomura T.; Sakaguchi S. Control of Immune Responses by Antigen-Specific Regulatory T Cells Expressing the Folate Receptor. Immunity 2007, 27, 145–159. 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Li H.; Lee R. J. Targeted drug delivery via folate receptors. Expert Opin. Drug Delivery 2008, 5, 309–319. 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- Wong P. T.; Choi S. K. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. Int. J. Mol. Sci. 2015, 16, 1772–1790. 10.3390/ijms16011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira E.; Sarria M. P.; Azoia N. G.; Antunes E.; Loureiro A.; Guimaraes D.; Noro J.; Rollett A.; Guebitz G.; Cavaco-Paulo A. Internalization of Methotrexate Conjugates by Folate Receptor-α. Biochemistry 2018, 57, 6780–6786. 10.1021/acs.biochem.8b00607. [DOI] [PubMed] [Google Scholar]
- Müller C. Folate based radiopharmaceuticals for imaging and therapy of cancer and inflammation. Curr. Pharm. Des. 2012, 18, 1058–1083. 10.2174/138161212799315777. [DOI] [PubMed] [Google Scholar]
- Müller C. Folate-based radiotracers for PET imaging-update and perspectives. Molecules 2013, 18, 5005–5031. 10.3390/molecules18055005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert A. H.; Jarman M. Radiolabelled Methotrexate: A Warning. Lancet 1979, 313, 166–167. 10.1016/S0140-6736(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Goldman I. D.; Lichtenstein N. S.; Oliverio V. T.. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell J. Biol. Chem. 1968, 243, 5007–5017. [PubMed] [Google Scholar]
- Kamen B. A.; Cashmore A. R.; Dreyer R. N.; Moroson B. A.; Hsieh P.; Bertino J. R.. Effect of [3H]methotrexate impurities on apparent transport of methotrexate by a sensitive and resistant L1210 cell line J. Biol. Chem. 1980, 255, 3254–3257. [PubMed] [Google Scholar]
- Yang C. H.; Sirotnak F. M.; Dembo M. Interaction Between Anions and the Reduced Folate/Methotrexate Transport System in L1210 Cell Plasma Membrane Vesicles: Directional Symmetry and Anion Specificity for Differential Mobility of Loaded and Unloaded Carrier. J. Membr. Biol. 1984, 79, 285–292. 10.1007/BF01871067. [DOI] [PubMed] [Google Scholar]
- Ohata M.; Fredericks W. R.; Neuwelt E. A.; Sundaram U.; Rapoport S. I.. [3H]Methotrexate Loss from the Rat Brain following Enhanced Uptake by Osmotic Opening of the Blood-Brain Barrier Cancer Res. 1985, 45, 1092–1096. [PubMed] [Google Scholar]
- Henderson E. S.; Adamson R. H.; Denham C.; Oliverio V. T.. The Metabolic Fate of Tritiated Methotrexate I. Absorption, Excretion, and Distribution in Mice, Rats, Dogs and Monkeys Cancer Res. 1965, 25, 1008–1017. [PubMed] [Google Scholar]
- Jacobs S. A.; Stoller R. G.; Chabner B. A.; Johns D. G. 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J. Clin. Invest. 1976, 57, 534–538. 10.1172/JCI108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. S.; Adamson R. H.; Oliverio V. T.. The Metabolic Fate of Tritiated Methotrexate II. Absorption and Excretion in Man Cancer Res. 1965, 25, 1018–1024. [PubMed] [Google Scholar]
- Reich S. D.; Bachur N. R.; Goebel R. H.; Berman M. A Pharmacokinetic Model for High-Dose Methotrexate Infusions in Man. J. Pharmacokinet. Biopharm. 1977, 5, 421–433. 10.1007/BF01061726. [DOI] [PubMed] [Google Scholar]
- Calvert A. H.; Bondy P. K.; Harrap K. R.. Some observations on the human pharmacology of methotrexate Cancer Treat. Rep. 1977, 61, 1647–1656. [PubMed] [Google Scholar]
- Bohuon C.; Duprey F.; Boudene C. Radioimmunoassay of methotrexate in biological fluids. Clin. Chim. Acta 1974, 57, 263–267. 10.1016/0009-8981(74)90406-9. [DOI] [PubMed] [Google Scholar]
- Aherne G. W.; Piall E. M.; Marks V. Development and application of a radioimmunoassay for methotrexate. Br. J. Cancer 1977, 36, 608–617. 10.1038/bjc.1977.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne W.; Piall E.; Marks V. Radioimmunoassay of methotrexate: use of 75Se-labelled methotrexate. Ann. Clin. Biochem. 1978, 15, 331–334. 10.1177/000456327801500179. [DOI] [PubMed] [Google Scholar]
- Paxton J. W.; Rowell F. J.; Cree G. M. Comparison of three radioligands, selenium-75, iodine-125, and tritium, in the radioimmunoassay of methotrexate. Clin. Chem. 1978, 24, 1534–1538. 10.1093/clinchem/24.9.1534. [DOI] [PubMed] [Google Scholar]
- Bartyik K.; Turi S.; Orosz F.; Karg E. Methotrexate inhibits the glyoxalase system in vivo in children with acute lymphoid leukaemia. Eur. J. Cancer 2004, 40, 2287–2292. 10.1016/j.ejca.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Westerhof G. R.; Rijnboutt S.; Schornagel J. H.; Pinedo H. M.; Peters G. J.; Jansen G.. Functional Activity of the Reduced Folate Carrier in KB, MA104, and IGROV-I Cells Expressing Folate-Binding Protein Cancer Res. 1995, 55, 3795–3802. [PubMed] [Google Scholar]
- Wang Y.; Zhao R.; Goldman I. D. Characterization of a Folate Transporter in HeLa Cells with a Low pH Optimum and High Affinity for Pemetrexed Distinct from the Reduced Folate Carrier. Clin. Cancer Res. 2004, 10, 6256–6264. 10.1158/1078-0432.CCR-04-0645. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhao R.; Chattopadhyay S.; Goldman I. D.. A Novel Folate Transport Activity in Human Mesothelioma Cell Lines with High Affinity and Specificity for the New-Generation Antifolate, Pemetrexed Cancer Res. 2002, 62, 6434–6437. [PubMed] [Google Scholar]
- Mauritz R.; Peters G. J.; Kathmann I.; Teshale H.; Noordhuis P.; Comijn E. M.; Pinedo H. M.; Jansen G. Dynamics of antifolate transport via the reduced folate carrier and the membrane folate receptor in murine leukaemia cells in vitro and in vivo. Cancer Chemother. Pharmacol. 2008, 62, 937–948. 10.1007/s00280-008-0683-0. [DOI] [PubMed] [Google Scholar]
- Izbicka E.; Diaz A.; Streeper R.; Wick M.; Campos D.; Steffen R.; Saunders M. Distinct mechanistic activity profile of pralatrexate in comparison to other antifolates in in vitro and in vivo models of human cancers. Cancer Chemother. Pharmacol. 2009, 64, 993–999. 10.1007/s00280-009-0954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affleck K.; Embleton M. J. Monoclonal antibody targeting of methotrexate (MTX) against MTX-resistant tumour cell lines. Br. J. Cancer 1992, 65, 838–844. 10.1038/bjc.1992.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K. Differential distribution of liposome-entrapped [3H]methotrexate and labelled lipids after intravenous injection in a primate. Biochim. Biophys. Acta, Biomembr. 1976, 448, 531–550. 10.1016/0005-2736(76)90108-5. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K.; Tracy T. F.; Watson R. E.; Kung D.; Reiss F. L.; Bourke R. S.. Distribution of free and liposome-entrapped [3H]methotrexate in the central nervous system after intracerebroventricular injection in a primate Cancer Res. 1978, 38, 706–712. [PubMed] [Google Scholar]
- Kim C. K.; Choi Y. J.; Lim S. J.; Lee M. G.; Lee S. H.; Hwang S. J. Lymph node targeting and pharmacokinetics of [3H]methotrexate-encapsulated neutral large unilamellar vesicles and immunoliposomes. Int. J. Pharm. 1993, 98, 9–18. 10.1016/0378-5173(93)90035-E. [DOI] [Google Scholar]
- Kim C. K.; Lee M. K.; Han J. H.; Lee B. J. Pharmacokinetics and tissue distribution of methotrexate after intravenous injection of differently charged liposome-entrapped methotrexate to rats. Int. J. Pharm. 1994, 108, 21–29. 10.1016/0378-5173(94)90412-X. [DOI] [Google Scholar]
- Kim C. K.; Han J. H. Lymphatic delivery and pharmacokinetics of methotrexate after intramuscular injection of differently charged liposome-entrapped methotrexate to rats. J. Microencapsulation 1995, 12, 437–446. 10.3109/02652049509087256. [DOI] [PubMed] [Google Scholar]
- Shen W. C.; Du X.; Feener E. P.; Ryser H. J. P. The intracellular release of methotrexate from a synthetic drug carrier system targeted to fc receptor-bearing cells. J. Controlled Release 1989, 10, 89–96. 10.1016/0168-3659(89)90020-5. [DOI] [Google Scholar]
- Hudecz F.; Clegg J. A.; Kajtar J.; Embleton M. J.; Pimm M. V.; Szekerke M.; Baldwin R. W. Influence of carrier on biodistribution and in vitro cytotoxicity of methotrexate -branched polypeptide conjugates. Bioconjugate Chem. 1993, 4, 25–33. 10.1021/bc00019a004. [DOI] [PubMed] [Google Scholar]
- Vitols K. S.; Haag-Zeino B.; Baer T.; Montejano Y. D.; Huennekens F. M.. Methotrexate-a-Phenylalanine: Optimization of Methotrexate Prodrug for Activation by Carboxypeptidase A-Monoclonal Antibody Conjugate Cancer Res. 1995, 55, 478–481. [PubMed] [Google Scholar]
- Friden P. M.; Walus L. R.; Musso G. F.; Taylor M. A.; Malfroy B.; Starzyk R. M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 1991, 88, 4771–4775. 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo N.; Takeda Y.; Kishida K.; Kato Y.; Saito M.; Umemoto N.; Hara T. Target-selective cytotoxicity of methotrexate conjugated with monoclonal anti-MM46 antibody. Cancer Immunol. Immunother. 1987, 25, 1–6. 10.1007/BF00199293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri G.; Mukhopadhyay A.; Basu S. K. Selective delivery of drugs to macrophages through a highly specific receptor. An efficient chemotherapeutic approach against leishmaniasis. Biochem. Pharmacol. 1989, 38, 2995–3002. 10.1016/0006-2952(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Han J. H.; Oh Y. K.; Kim D. S.; Kim C. K. Enhanced hepatocyte uptake and liver targeting of methotrexate using galactosylated albumin as a carrier. Int. J. Pharm. 1999, 188, 39–47. 10.1016/S0378-5173(99)00206-9. [DOI] [PubMed] [Google Scholar]
- Han J.; Lim S. J.; Lee M. K.; Kim C. K. Altered Pharmacokinetics and Liver Targetability of Methotrexate by Conjugation with Lactosylated Albumins. Drug Delivery 2001, 8, 125–134. 10.1080/107175401316906883. [DOI] [PubMed] [Google Scholar]
- Kim C. K.; Hwang S. J.; Lee M. G. The organ targetability of small and large albumin microspheres containing free and HSA conjugated methotrexate. Int. J. Pharm. 1993, 89, 91–102. 10.1016/0378-5173(93)90109-S. [DOI] [Google Scholar]
- Wosikowski K.; Biedermann E.; Rattel B.; Breiter N.; Jank P.; Löser R.; Jansen G.; Peters G. J.. In Vitro and in Vivo Antitumor Activity of Methotrexate Conjugated to Human Serum Albumin in Human Cancer Cells Clin. Cancer Res. 2003, 9, 1917–1926. [PubMed] [Google Scholar]
- Singh V. K.; Subudhi B. B. Development of reversible glutamine conjugate of methotrexate for enhanced brain delivery. Med. Chem. Res. 2015, 24, 624–635. 10.1007/s00044-014-1172-0. [DOI] [Google Scholar]
- Singh V. K.; Subudhi B. B. Development and characterization of lysine-methotrexate conjugate for enhanced brain delivery. Drug Delivery 2016, 23, 2327–2337. 10.3109/10717544.2014.984369. [DOI] [PubMed] [Google Scholar]
- Okarvi S. Preparation and evaluation of Tc-99m-labeled folate and methotrexate analogs as tumor imaging agents. J. Nucl. Med. 2006, 47, 507P. [DOI] [PubMed] [Google Scholar]
- Okarvi S. M.; Jammaz I. A. Preparation and In Vitro and In Vivo Evaluation of Technetium-99m-Labeled Folate and Methotrexate Conjugates as Tumor Imaging Agents. Cancer Biother.Radiopharm. 2006, 21, 49–60. 10.1089/cbr.2006.21.49. [DOI] [PubMed] [Google Scholar]
- Ahmed N.; Fatima S.; Irfan J.; Saeed S. Modified method for methotrexate-Tc-99m labeled radiopharmaceutical, synthesis and evaluation. J. Nucl. Med. 2012, 53, 1754. [Google Scholar]
- Subramanian N.; Arulsudar N.; Chuttani K.; Mishra P.; Sharma R. K.; Murthy R. S. R. Radiolabeling, Biodistribution and Tumor Imaging of Stealth Liposomes Containing Methotrexate. Alasbimn J. 2003, 6, AJ22–26. [Google Scholar]
- Shukla G.; Tiwari A. K.; Kumar N.; Sinha D.; Mishra P.; Chandra H.; Mishra A. K. Polyethylene Glycol Conjugates of Methotrexate and Melphalan: Synthesis, Radiolabeling and Biologic Studies. Cancer Biother.Radiopharm. 2008, 23, 571–580. 10.1089/cbr.2008.0497. [DOI] [PubMed] [Google Scholar]
- Dar U. K.; Khan I. U.; Javed M.; Ahmad F.; Ali M.; Hyder S. W.. Preparation and biodistribution in mice of a new radiopharmaceutical-technetium-99m labeled methotrexate, as a tumor diagnostic agent Hell. J. Nucl. Med. 2012, 15, 120–124. [PubMed] [Google Scholar]
- Rasheed R.; Javed M.; Ahmad F.; Sohail A.; Murad S.; Masood M.; Rasheed S.; Rasheed S. Preparation of (99m)Tc-labelled methotraxate by a direct labeling technique as a potential diagnostic agent for breast cancer and preliminary clinical results. Hell. J. Nucl. Med. 2013, 16, 33–37. 10.1967/s002449910069. [DOI] [PubMed] [Google Scholar]
- Ekinci M.; Ilem-Ozdemir D.; Gundogdu E.; Asikoglu M. Methotrexate loaded chitosan nanoparticles: Preparation, radiolabeling and in vitro evaluation for breast cancer diagnosis. J. Drug Delivery Sci. Technol. 2015, 30, 107–113. 10.1016/j.jddst.2015.10.004. [DOI] [Google Scholar]
- Ozgenc E.; Ekinci M.; Ilem-Ozdemir D.; Gundogdu E.; Asikoglu M. Radiolabeling and in vitro evaluation of 99mTc-methotrexate on breast cancer cell line. J. Radioanal. Nucl. Chem. 2016, 307, 627–633. 10.1007/s10967-015-4210-6. [DOI] [Google Scholar]
- Ilgan S.; Yang D. Y.; Higuchi T.; Zareneyrizi F.; Kim E. E.; Podoloff D. A. Imaging tumor folate receptors using 111In-DTPA-methotrexate. Cancer Biother. Radiopharm. 1998, 13, 177–184. 10.1089/cbr.1998.13.177. [DOI] [PubMed] [Google Scholar]
- Tsao N.; Yang D.; Wei I. C.; Huang Y. H.; Huang C. C.; Chanda M.; Kurihara H.; Kohanim S.; Oh C. S.; Kim E. E. Biodistribution and imaging of Ga-68 and Tc-99m-glycopeptide-methotrexate (GP-MTX) in rodents. J. Nucl. Med. 2008, 49, 331P. [Google Scholar]
- Okarvi S. M.; Al Jammaz I. Synthesis and evaluation of a technetium-99m labeled cytotoxic bombesin peptide conjugate for targeting bombesin receptor-expressing tumors. Nucl. Med. Biol. 2010, 37, 277–288. 10.1016/j.nucmedbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Jammaz I. A.; Al-Otaibi B.; Amer S.; Okarvi S. M. Rapid synthesis and in vitro and in vivo evaluation of folic acid derivatives labeled with fluorine-18 for PET imaging of folate receptor-positive tumors. Nucl. Med. Biol. 2011, 38, 1019–1028. 10.1016/j.nucmedbio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Al Jammaz I.; Al-Otaibi B.; Amer S.; Al-Hokbany N.; Okarvi S. Novel synthesis and preclinical evaluation of folic acid derivatives labeled with 18F-[FDG] for PET imaging of folate receptor-positive tumors. Nucl. Med. Biol. 2012, 39, 864–870. 10.1016/j.nucmedbio.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Bombardieri E.; Aktolun C.; Baum R. P.; Bishof-Delaloye A.; Buscombe J.; Chatal J. F.; Maffioli L.; Moncayo R.; Mortelmans L.; Reske S. N. Bone scintigraphy: procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, BP99–BP106. 10.1007/s00259-003-1347-2. [DOI] [PubMed] [Google Scholar]
- Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J. Nucl. Med. 2005, 46, 1356–1367. [PubMed] [Google Scholar]
- Rasheed R.; Gillani J.; Jielani A.; Irum F.; Lodhi N.; Rasheed S.; Rasheed S. Tc99m Methotrexate (MTX) A Novel Complex for Imaging of Rheumatoid Arthritis (RA): First Clinical Trials. Gen. Med. (Los Angeles) 2016, S213. 10.4172/2327-5146.1000S213. [DOI] [Google Scholar]
- Papachristou M.; Kastis G. A.; Stavrou P. Z.; Xanthopoulos S.; Furenlid R. L.; Datseris I. E.; Bouziotis P. Radiolabeled methotrexate as a diagnostic agent of inflammatory target sites: A proof-of-concept study. Mol. Med. Rep. 2018, 17, 2442–2448. 10.3892/mmr.2017.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosain F.; Spencer R. P.; Couthon H. M.; Sturtz G.. Targeted Delivery of Antineoplastic Agent to Bone: Biodistribution Studies of Technetium-99m-Labeled Gem-Bisphosphonate Conjugate of Methotrexate J. Nucl. Med. 1996, 37, 105–107. [PubMed] [Google Scholar]
- Samaniego R.; Palacios B. S.; Domiguez-Soto Á.; Vidal C.; Salas A.; Matsuyama T.; Sánchez-Torres C.; de la Torre I.; Miranda-Carús M. E.; Sánchez-Mateos P.; Puig-Kröger A. Macrophage uptake and accumulation of folates are polarization-dependent in vitro and in vivo and are regulated by activin A. J. Leukocyte Biol. 2014, 95, 797–808. 10.1189/jlb.0613345. [DOI] [PubMed] [Google Scholar]
- Escribese M. M.; Sierra-Filardi E.; Nieto C.; Samaniego R.; Sánchez-Torres C.; Matsuyama T.; Calderon-Gómez E.; Vega M. A.; Salas A.; Sánchez-Mateos P.; Corbí A. L. The prolyl hydroxylase PHD3 identifies proinflammatory macrophages and its expression is regulated by activin A. J. Immunol. 2012, 189, 1946–1954. 10.4049/jimmunol.1201064. [DOI] [PubMed] [Google Scholar]
- Municio C.; Soler Palacios B.; Estrada-Capetillo L.; Benguria A.; Dopazo A.; García-Lorenzo E.; Fernández-Arroyo S.; Joven J.; Miranda-Carús M. E.; González-Álvaro I.; Puig-Kröger A. Methotrexate selectively targets human proinflammatory macrophages through a thymidylate synthase/p53 axis. Ann. Rheum. Dis. 2016, 75, 2157–2165. 10.1136/annrheumdis-2015-208736. [DOI] [PubMed] [Google Scholar]
- Trujillo-Nolasco R. M.; Morales-Avila E.; Ocampo-García B. E.; Ferro-Flores G.; Gibbens-Bandala B. V.; Escudero-Castellanos A.; Isaac-Olive K. Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019, 103, 109766–109776. 10.1016/j.msec.2019.109766. [DOI] [PubMed] [Google Scholar]
- Das M.; Datir S. R.; Singh R. P.; Jain S. Augmented Anticancer Activity of a Targeted, Intracellularly Activatable, Theranostic Nanomedicine Based on Fluorescent and Radiolabeled, Methotrexate-Folic Acid-Multiwalled Carbon Nanotube Conjugate. Mol. Pharmaceutics 2013, 10, 2543–2557. 10.1021/mp300701e. [DOI] [PubMed] [Google Scholar]
- Wang J.; Li Z.; Wang Z.; Yu Y.; Li D.; Li B.; Ding J. Nanomaterials for Combinational Radio–Immuno Oncotherapy. Adv. Funct. Mater. 2020, 30, 1910676. 10.1002/adfm.201910676. [DOI] [Google Scholar]
- Hawkins G. A.; McCabe R. P.; Kim C. H.; Subramanian R.; Bredehorst R.; McCullers G. A.; Vogel C. W.; Hanna M. G. Jr.; Pomato N.; Delivery of Radionuclides to Pretargeted Monoclonal Antibodies Using Dihydrofolate Redactase and Methotrexate in an Affinity System Cancer Res. 1993, 53, 2368–2373. [PubMed] [Google Scholar]