Abstract
Background
White-matter lesions (WMLs) are frequently found in magnetic resonance imaging (MRi), and the WML load tends to be higher in patients affected by cervical internal carotid artery (cICA) stenosis.
Purpose
This study aimed to investigate whether and how WMLs influence cerebral networking in patients with asymptomatic cICA stenosis eligible for carotid endarterectomy (CEA) by exploiting the connectometry technique.
Methods
The study was designed as a cross-sectional exploratory investigation, and 28 patients with cICA stenosis eligible for CEA were enrolled. All patients received an MRI scan, including a T1-weighted, a FLAIR and a diffusion-weighted (DW) sequence. The T1 and FLAIR sequences were analysed for quantification of WML burden (WMLB) and total number of WMLs (TNWMLs). The DW data were reconstructed in the MNI space using q-space diffeomorphic reconstruction, and were grouped to create a connectometry database. The connectometry analysis evaluated the influence of both the WMLB and TNWMLs to local connectivity in a multiple regression model that included age, WMLB and TNWMLs, adopting three different T-score thresholds (1, 2 and 3). A p-value corrected for false discovery rate of <0.05 was adopted as a threshold to identify statistically significant results.
Results
The connectometry analysis identified several white-matter bundles negatively correlated with WMLB; no statistically significant correlation was found for TNWMLs.
Conclusion
Results of our study suggest that WMLs influence brain connectivity measured by the connectometry technique in patients with cICA stenosis eligible for CEA. Further studies are warranted to understand the role of WMLs better as a marker of disease in patients with cICA stenosis.
Keywords: Connectometry, white-matter lesions, carotid endarterectomy
Introduction
Stroke is one of the principal causes of disability and death worldwide. It is estimated that the incidence of stroke in Europe will be around 1.5 million people per year by 2025,1 and cervical internal carotid artery (cICA) stenosis is associated with stroke in 10–20% of cases.2 It is also note that cICA stenosis is associated with cognitive impairment.3
White-matter lesions (WMLs), also called white-matter hyperintensities, are sporadic small cerebral lesions of presumed vascular origin that appear hyperintense on T2-weighted sequences on magnetic resonance imaging (MRI) examinations.4 WMLs are common findings in healthy subjects, and their number tends to increase with age.5 However, the burden of WMLs increases in several medical conditions,4 included patients with cICA stenosis.6–8 WMLs are associated with different adverse clinical outcomes, including cognitive dysfunction, depression and functional disability.9,10 Different studies have shown that WMLs influence brain networks, as studied by resting-state functional connectivity in healthy subjects,11–13 but less is known in other medical conditions, including patients with cICA.
A deeper knowledge of the relationships between WMLs, brain networks and cognitive effects could help to define the role of WMLs better as a further parameter to be taken into account in the evaluation of patients with cICA stenosis for the choice of the therapeutic strategies. A first step in this direction was made by Porcu et al.14 who analysed the effects of WMLs by resting-state functional connectivity, evidencing that the total number of WMLs (TNWMLs) and the WML burden (WMLB) influence cerebral networking differently.
In recent years, MRI connectometry has been introduced in clinical practice as a technique for the analysis of the local connectome, quantifying the degree of connectivity between neighbouring voxels of the white matter measured by the density of the diffusing spins.15,16
In this cross-sectional exploratory study, we analysed whether and how the WMLs influence cerebral networks in asymptomatic patients with cICA stenosis eligible for carotid endarterectomy (CEA). In particular, we hypothesised that the TNWMLs and WMLB influence the local connectome differently analysed by the connectometry technique, with no regards for their localisation.
Methods
Study design and patient enrolment
The study design was cross-sectional, and it was approved by the local ethical committee. Further, the study was designed to be exploratory, and we planned to recruit at least 12 patients, as indicated by Julious.17
Participants were recruited from consecutive patients hospitalised in the vascular surgery department of our university hospital. The inclusion criteria for the study patients were: (a) a diagnosis of asymptomatic mono- or bilateral cICA stenosis between 60% and 99% according to the North American Symptomatic Carotid Endarterectomy Trial score18 as evidenced by computed tomography angiography, and (b) surgical indication for CEA according to the recent guidelines of the European Society for Vascular Surgery.19 For ‘asymptomatic patients’, we referred to patients with cICA stenosis ≥50% in the absence of retinal/cerebral ischaemia in the preceding six months.19 The exclusion criteria were: (a) medical history of severe systemic, inherited or acquired diseases (in particular severe psychiatric and oncologic disorders); (b) contraindications to MRI examinations; and (c) incidental evidence of severe intracranial pathologies on MRI, including acute, subacute or chronic territorial stroke.
All participants gave their informed consent to be included in the study.
MRI examination protocol
Similar to Porcu et al.,20 the MRI examinations were performed on an 1.5 Tesla Philips ‘Achieva dStream’ scanner (peak amplitude 33 mT/m, slew rate 160 mT/m/ms; Philips Healthcare, Eindhoven, NL) and a 16-channel head coil. The MRI protocol included the following three sequences: (a) isotropic 3D T1-weighted Turbo Field Echo (3D-T1TFE), with the parameters echo time (ET) = 3.43 ms, repetition time (RT) = 7.5 ms, flip angle (FA) = 8°, slice thickness = 1 mm and spacing between slices = 1 mm; (b) 3D Fluid Attenuated Inversion Recovery (3D-FLAIR) with the parameters TE = 292.283 ms, TR = 4800 ms, inversion time (IT) = 1660 ms, FA = 90°, slice thickness = 1 mm and spacing between slices = 0.57 mm; and (c) a diffusion-weighted (DW) sequence with the parameters 32 diffusion sampling directions, TE = 83.147 ms, TR = 3250.15 ms, B-values = 0 and 800 s/mm2, in-plane resolution = 1.75 mm and slice thickness = 2.5 mm.
All the MRI scans were checked by two expert neuroradiologists (M.P. and P.G., 7 and 4 years of experience, respectively). All the MRI scans were judged of acceptable quality, without any significant artefacts or incidental intra-cranial pathologies. None of the patients was excluded from the study.
Volumetric analysis of WMLs
The 3D-T1TFE and 3D-FLAIR sequences were anonymised and analysed by lesionBrain21,22 – an online tool for the segmentation and identification of the WMLB based on the use of a group of shallow neural networks.
The two above-mentioned expert neuroradiologists checked the results and considered them as correct. For each subject, we extracted TNWMLs (expressed as an absolute number), and the WMLB according to the formula:
The WMLs total volume represents the sum of the volume of all WMLs identified, and WMV expresses the global white-matter volume.
Correlations between age, TNWMLs and WMLB
Pearson’s correlation analysis was conducted in order to establish if there was a correlation between age and TNWMLs, between age and WMLB, and between WMLs and TNWMLs. Pearson’s correlation coefficient (ρXY) and the coefficient of determination (R2) were calculated for each analysis.
Connectometry analysis
The group connectometry analysis was performed using DSI studio v2019_05 (http://dsi-studio.labsolver.org/).15,16 DW data from all the study participants were included in the connectometry database. The B-table accuracy was checked by an automatic quality-control routine.23 The DW data were reconstructed in the MNI space using q-space diffeomorphic reconstruction24 obtaining the spin diffusivity function (SDF).16 The Human Connectome Project 1021 (HCP-1021) template was used as the DW atlas.24 A diffusion sampling length ratio of 1.25 was used with 2 mm of output resolution. The restricted diffusion was quantified using the model-free method called restricted diffusion imaging,25 and SDF values were used in the connectometry analysis.
The connectometry analysis studied the influence of TNWMLs and WMLB by applying a multiple regression model that included age, TNWMLs and WMLB to the connectometry database. Three different T-score threshold (T-score) values (1, 2 and 3) were used in three consecutive analyses for both TNWMLs and WMLB in order to select local connectomes. The tracking of the local connectomes was obtained applying a deterministic fibre-tracking algorithm.26 Topology-informed pruning27 was performed by using 10 iterations to remove false connections. All tracks generated from bootstrap resampling were included, and a p-value corrected for false discovery rate (p-FDR) of <0.05 was applied to identify statistically significant tracks. For each permutation, the seeding number was 100,000. A total of 10,000 randomised permutations was applied to the group label in order to obtain the null distribution of the track length and to estimate the FDR.
Results
Over an 18-month period (January 2018–June 2019), 28 patients (21 males and 7 females; age range = 61–84 years; mean age = 75 years) were enrolled in the study. Demographic data, MMSE scores and data derived from the volumetric analysis of the WMLs (TNWMLs and WMLB) are reported in Table 1.
Table 1.
Demographic data, clinical data and results derived from the volumetric analysis of the WMLs (TNWMLs and WMLB).
| Number | Sex | Age | MMSE | Mono- or bilateral critical ICA stenosis (60–99% according to NASCET) | TNWMLs | WMLB |
|---|---|---|---|---|---|---|
| Subject 1 | Male | 72 | 21.3 | Monolateral | 33 | 0.43% |
| Subject 2 | Male | 72 | 27.7 | Monolateral | 13 | 0.25% |
| Subject 3 | Male | 65 | 18.9 | Bilateral | 13 | 0.24% |
| Subject 4 | Female | 83 | 16.5 | Bilateral | 31 | 6.83% |
| Subject 5 | Female | 75 | 24.3 | Bilateral | 20 | 0.45% |
| Subject 6 | Female | 76 | 26 | Bilateral | 7 | 0.51% |
| Subject 7 | Male | 77 | 22 | Monolateral | 26 | 1.72% |
| Subject 8 | Male | 83 | 10.5 | Monolateral | 50 | 3.12% |
| Subject 9 | Male | 76 | 24 | Monolateral | 19 | 0.48% |
| Subject 10 | Male | 71 | 21.3 | Bilateral | 35 | 4.45% |
| Subject 11 | Female | 72 | 16.3 | Monolateral | 23 | 2.17% |
| Subject 12 | Male | 80 | 22.4 | Bilateral | 20 | 0.34% |
| Subject 13 | Male | 79 | 21.7 | Monolateral | 19 | 1.11% |
| Subject 14 | Male | 78 | 24.3 | Monolateral | 30 | 4.06% |
| Subject 15 | Male | 82 | 11.4 | Monolateral | 33 | 3.51% |
| Subject 16 | Male | 74 | 14.7 | Monolateral | 17 | 1.05% |
| Subject 17 | Male | 80 | 11.3 | Monolateral | 20 | 0.26% |
| Subject 18 | Female | 61 | 20 | Bilateral | 27 | 0.59% |
| Subject 19 | Male | 72 | 11.3 | Bilateral | 33 | 6.65% |
| Subject 20 | Male | 66 | 16 | Bilateral | 8 | 0.02% |
| Subject 21 | Male | 84 | 14.7 | Monolateral | 17 | 1.38% |
| Subject 22 | Male | 81 | 18.4 | Bilateral | 28 | 0.68% |
| Subject 23 | Female | 79 | 18 | Bilateral | 54 | 1.99% |
| Subject 24 | Male | 73 | 22.3 | Monolateral | 70 | 3.26% |
| Subject 25 | Male | 70 | 20.4 | Monolateral | 28 | 2.67% |
| Subject 26 | Male | 82 | 14.4 | Bilateral | 33 | 3.51% |
| Subject 27 | Male | 76 | 22.3 | Bilateral | 13 | 1.28% |
| Subject 28 | Female | 61 | 26 | Monolateral | 13 | 0.18% |
MMSE: Mini-Mental State Examination; ICA: internal carotid artery; NASCET: North American Symptomatic Carotid Endarterectomy Trial; TNWMLs: total number of white-matter lesions; WMLB: white-matter lesion burden.
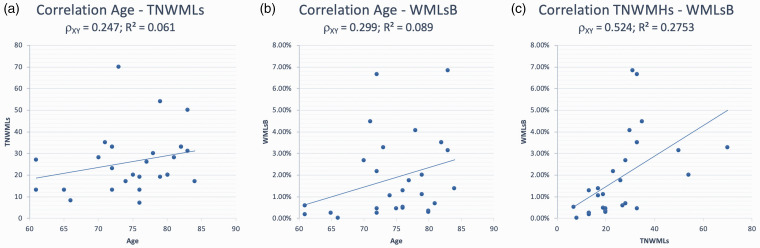
Pearson’s correlation analyses identified a weak positive correlation between age and TNWMLs (ρXY = 0.247; R2 = 0.061) and between age and WMLB (ρXY = 0.299; R2 = 0.089), as well as a moderate positive correlation between TNWMLs and WMLB (ρXY = 0.524; R2 = 0.2753). Results are reported in Figure 1.
Figure 1.
Pearson’s correlation tests. (a) Correlation between age and TNWMLs. (b) Correlation between age and WMLB. (c) Correlation between TNWMLs and WMLB. ρXY = Pearson correlation coefficient; R2 = coefficient of determination. TNWMLs: total number of white-matter lesions; WMLB: white-matter lesion burden.
The connectometry analysis that tested the influence of the TNWMLs did not find any statistically significant results for any of the T-scores tested.
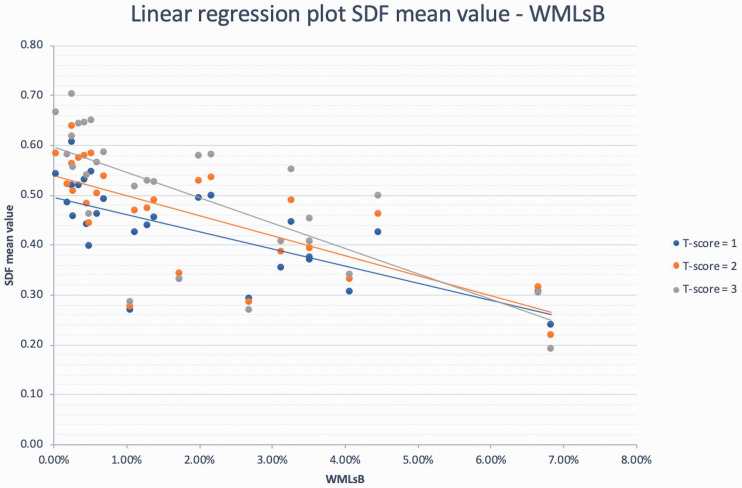
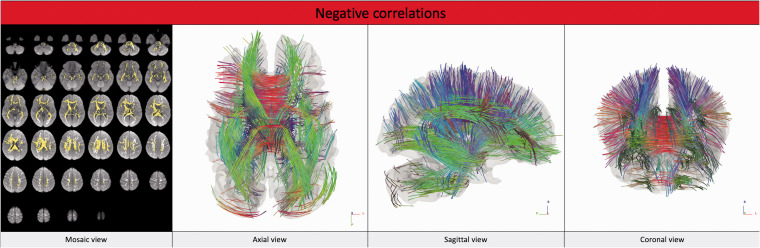
The connectometry analysis that tested the influence of WMLB revealed no statistically significant positive correlation with any of the above-mentioned variables. On the contrary, different tracts showed statistically significant negative correlations (p-FDR <0.05) with WMLB for each given T-score (1, 2 and 3). The analysis that identified the larger number of tracts negatively correlated with WMLB was the one conducted with a T-score of 2 (number of tracts identified = 9744). The white-matter tracts negatively correlated with WMLB are reported in Table 2 and Figure 2 (analysis conducted using a T-score of 2). The statistics are reported in Table 3 (the individual SDF mean values are reported in Supplemental Table S1), and the linear regression plots are presented in Figure 3.
Table 2.
White-matter tracts negatively correlated with WMLB according to the T-score adopted and to their related structures.
| Tracts identified | T-score = 1 | T-score = 2 | T-score = 3 |
|---|---|---|---|
| Right corticothalamic pathway | 23.56% | 16.83% | 24.49% |
| Right corticostriatal pathway | 10.05% | 15.12% | 16.34% |
| Corpus callosum | 27.06% | 12.86% | 11.07% |
| Left corticostriatal pathway | 4.64% | 8.78% | 4.82% |
| Left U-fibre | 1.66% | 7.01% | 3.36% |
| Left corticothalamic pathway | 5.89% | 6.72% | 5.62% |
| Right U-fibre | 1.51% | 6.63% | 3.17% |
| Right cingulum | 2.32% | 5.06% | 8.72% |
| Middle cerebellar peduncles | 4.01% | 4.57% | 6.50% |
| Left cingulum | 3.46% | 4.25% | 5.58% |
| Right acoustic radiation | 1.77% | 2.22% | 3.78% |
| Left superior longitudinal fasciculus | 4.05% | 1.62% | 0.70% |
| Left acoustic radiation | 0.55% | 1.01% | 0.86% |
| Right optic radiation | 1.99% | 0.77% | 0.44% |
| Right cerebellum | 1.14% | 0.70% | 0.10% |
| Others | 6.34% | 5.87% | 4.44% |
The results are reported as percentage (%) of the number of tracts identified. p-FDR <0.05.
Reference atlas: HCP-842_tractography.18
p-FDR: p-value corrected for false discovery rate.
Figure 2.
White-matter tracts that showed negative correlations with WMLB (T-score = 2; p-FDR <0.05). The images are presented according to the radiological orientation. In the mosaic view, the tracts are yellow; in the axial, sagittal and coronal view, the colour of the tracts varies according to their direction (red for right–left, blue for cranio–caudal, green for anterior–posterior). p-FDF: p-value corrected for false discovery rate.
Table 3.
Statistics of the tracts identified in the connectometry analysis that evaluated the influence of WMLB.
| T-score 1 | T-score 2 | T-score 3 | |
|---|---|---|---|
| Number of tracts identified | 4075 | 9744 | 6021 |
| Tract length mean (mm) | 66.8633 | 37.7833 | 33.3174 |
| Tract volume (mm3) | 121,216 | 192,336 | 125,096 |
| QA mean value | 0.609181 | 0.651934 | 0.706681 |
| NQA mean value | 0.392552 | 0.420096 | 0.455393 |
| GFA mean value | 0.0985736 | 0.104759 | 0.114427 |
| ISO mean value | 1.30388 | 1.29442 | 1.26228 |
p-FDR <0.05.
QA: quantitative anisotropy; NQA: normalised quantitative anisotropy; GFA: generalised fractional anisotropy; ISO: isotropic value of the orientation distribution function.
Figure 3.
Linear regression plots showing the negative relationships between WMLB and SDF for different T-score thresholds (1, 2 and 3). SDF: spin diffusivity function.
Among the tracts identified to be negatively correlated with WMLB in the analyses, it is noteworthy that the majority of them belong to the corticothalamic and corticostriatal pathways, the U-fibres and the middle cerebellar peduncles on both sides and the corpus callosum, independently of the T-score used.
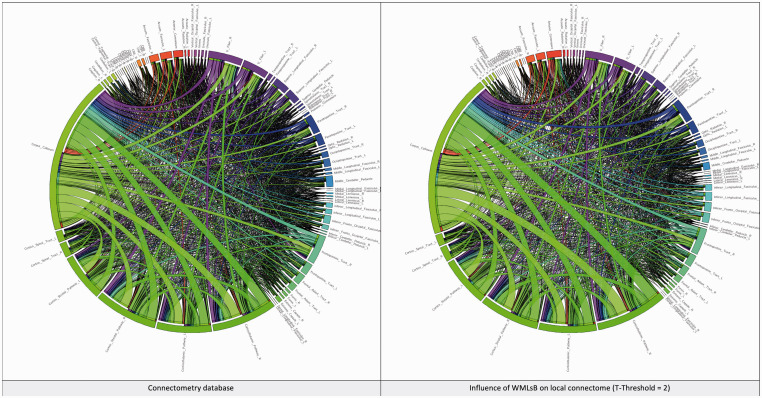
Network analysis using graph theory revealed that WMLB is also able to influence the global network properties, albeit differently, depending on the T-score value adopted. The network characteristic path length increases concordantly with WMLB (range 1.62–4.19%), whereas other properties of the network were negatively correlated with the WMLB, in particular density (between –3.99% and –5.00%), global efficiency (between –0.84% and –1.49%), average clustering coefficient (between –2.24% and –5.11%), small worldness (between –1.61% and –3.48%), transitivity (between –1.01% and –1.53%) and assortativity coefficient (between –2.82% and –3.17%).28–31 The network properties are reported in Table 4, and the network connectograms of the connectometry database, including the one influenced by WMLB (a T-score of 2), are shown in Figure 4.
Table 4.
Influence of WMLB on network properties according to the selected T-score.
| Network properties changesHR/ | |||
|---|---|---|---|
| Network properties | T-score 1 | T-score 2 | T-score 3 |
| Density | –3.29% | –5.00% | –4.40% |
| Average clustering coefficient | –2.24% | –4.66% | –5.11% |
| Transitivity | –1.01% | –1.73% | –1.53% |
| Network characteristic path length | 1.62% | 4.19% | 2.66% |
| Small-worldness | –1.61% | –3.48% | –3.43% |
| Global efficiency | –0.84% | –1.49% | –1.23% |
| Assortativity coefficient | –3.17% | –3.11% | –2.82% |
Figure 4.
These two connectograms represent the connectometry database (on the left) and the influence of the WMLB on the network for a T-score value of 2 (on the right).
Discussion
The results of this study show that the burden of WMLs influence the local connectome in patients with cICA stenosis eligible for CEA. It is reasonable to hypothesise that WMLs interfere with the normal transmission of the white-matter bundles, in particular at the level of the above-mentioned regions.
The analyses included age, WMLB and TNWMLs in the multiple regression model. This choice was justified by the fact that WMLs normally tend to increase with ageing31 and that the three Pearson’s correlation analyses did not find any strong correlation between these three variables.
The analysis that focused on the effects of WMLB found that different white-matter tracts showed reduced local connectivity correlated with WMLB, and no positive correlations were found. The tracts found to be negatively correlated with WMLB included the corticothalamic and corticostriatal pathways, the U-fibres, the middle cerebellar peduncles and the corpus callosum. These regions play a central role in different functions. For example, the corticostriatal pathway is implicated in appropriate goal-directed behaviours such as motivation and cognition to develop appropriate strategies in order to obtain a specific outcome.32 U-fibres are implicated in short-distance connections for sensorimotor and higher cognitive brain functions,33 and the corpus callosum is important for information transfer between cerebral hemispheres for sensory, motor and high-level cognitive functions.34 The influence of WMLB on cerebral networks using graph theory to the connectometry analyses revealed that the main properties of the network show a trend of the nodes of the network to be less connected to each other, as reflected by the degree and global efficiency values. Furthermore, they tend to be less organised in clusters, as revealed in particular by the increased values of average path length and reduced values in clustering coefficient and small worldness.
An apparently controversial aspect to be noted is that WMLs influence local connectivity in terms of WMLB but not in terms of TNWMLs. Actually, no white-matter tracts were positively or negatively correlated with the TNWMLs. One explanation for these results may be the relative low number of subjects recruited in the study. The effects of WMLB were evident, even with a small number of patients, whereas the effects of the TNWMLs could be evidenced in a larger cohort of patients. To the best of our knowledges, no similar studies have been conducted to date on this topic, except for the one by Porcu et al.14 mentioned earlier. In that study, the authors analysed the effects of WMLs on patients with cICA stenosis eligible for CEA by using resting-state functional connectivity and applying graph theory in order to study the effects of both WMLB and TNWMLs.14 In both the cases, statistically significant positive and negative correlations were found in both the two ROI-to-ROI analyses, and different nodes of the network showed changes in their properties. In particular, the global efficiency (i.e. the ability of a node to share information at a global level) of the precuneus – a pivotal region of the Default Mode Network – resulted in being inversely correlated with TNWMLs, whereas the local efficiency (i.e. the ability of a node to share information at a local level) resulted in being reduced in the right accumbens, and the right planum polare resulted in patients with higher WMLB. These findings could appear to contradict those of our study, but it should be considered that the technique used was different. It is, however, reasonable to affirm that our study can represent further proof in support of the fact that WMLs influence brain activity, at the level of both the global and local connectome, and that the effects are different if we consider WMLB and TNWMLs.
We acknowledge several limitations to our study: (a) the low number of recruited patients, (b) the lack of neurological and psychological tests (except MMSE), (c) the lack of a control group, (d) the use of a 1.5 Tesla MRI scanner and (e) the use of a DW sequence with 32 directions. Furthermore, the results did not take into account the localisation of the WMLs (supratentorial or infratentorial; subcortical, deep or periventricular), which could represent another important confounding factor.
We must remember that this study was designed as exploratory and not confirmatory. Our main goal was to investigate whether and how the WMLs influenced the cerebral networking in asymptomatic patients with cICA, a medical condition characterised by cognitive impairment8,9 and an increased number of WMLs,10–12 which are increasingly recognised to be associated with each other.3 The results of our study are statistically significant, and they allow us to speculate that further studies could help us in better understanding the impact of WMLs in these patients. The inclusion of a bigger cohort size, the use of a scanner with a higher static magnetic field (≥3.0 Tesla) and a more elaborate DW sequence (>32 directions), the use of a comprehensive battery of tests that investigate motor, behaviour and neurocognitive status, and the comparison of the results with a control group could help better define the role and the clinical impact of WMLs in this category of patients. These studies could be important from a clinical point of view in helping to understand the role of WMLs as a marker of disease in patients with cICA stenosis in an era in which the paradigm of carotid revascularisation for the prevention of stroke is changing.35
Conclusions
WMLs influence local connectivity of brain networks in patients with mono- or bilateral cICA stenosis eligible for CEA. Further studies will help to clarify the clinical impact of WMLs in patients affected by this medical condition, and the role of WMLs as a marker of disease.
Supplemental Material
Supplemental material, sj-pdf-1-neu-10.1177_1971400920959323 for White-matter hyperintensities in patients with carotid artery stenosis: An exploratory connectometry study by Michele Porcu, Roberto Sanfilippo, Roberto Montisci, Antonella Balestrieri, Jasjit S Suri, Max Wintermark and Luca Saba in The Neuroradiology Journal
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Michele Porcu https://orcid.org/0000-0002-3090-8541
Supplemental material: Supplemental material for this article is available online.
References
- 1.Béjot Y, Bailly H, Durier J, et al. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med 2016; 45: e391–e398. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty ML, Kissela B, Khoury JC, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013; 40: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutra AP. Cognitive function and carotid stenosis: review of the literature. Dement Neuropsychol 2012; 6: 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18: 684–696. [DOI] [PubMed] [Google Scholar]
- 5.De Leeuw FE, De Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saba L, Pascalis L, Sanfilippo R, et al. Carotid artery wall thickness and leukoaraiosis: preliminary results using multidetector row CT angiography. AJNR Am J Neuroradiol 2011; 32: 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saba L, Sanfilippo R, Pascalis L, et al. Carotid artery abnormalities and leukoaraiosis in elderly patients: evaluation with MDCT. AJR Am J Roentgenol 2009; 192: W63–70. [DOI] [PubMed] [Google Scholar]
- 8.Lu T, Liang J, Wei N, et al. Extracranial artery stenosis is associated with total MRI burden of cerebral small vessel disease in ischemic stroke patients of suspected small or large artery origins. Front Neurol 2019; 10: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien JT. Clinical significance of white matter changes. Am J Geriatr Psychiatry 2014; 22: 133–137. [DOI] [PubMed] [Google Scholar]
- 10.Paradise MB, Shepherd CE, Wen W, et al. Neuroimaging and neuropathology indices of cerebrovascular disease burden: a systematic review. Neurology 2018; 91: 310–320. [DOI] [PubMed] [Google Scholar]
- 11.Keřkovský M, Stulík J, Dostál M, et al. Structural and functional MRI correlates of T2 hyperintensities of brain white matter in young neurologically asymptomatic adults. Eur Radiol 2019; 29: 7027–7036. [DOI] [PubMed] [Google Scholar]
- 12.Reijmer YD, Schultz AP, Leemans A, et al. Decoupling of structural and functional brain connectivity in older adults with white matter hyperintensities. Neuroimage 2015; 117: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langen CD, Zonneveld HI, White T, et al. White matter lesions relate to tract-specific reductions in functional connectivity. Neurobiol Aging 2017; 51: 97–103. [DOI] [PubMed] [Google Scholar]
- 14.Porcu M, Garofalo P, Craboledda D, et al. Carotid artery stenosis and brain connectivity: the role of white matter hyperintensities. Neuroradiology 2020; 62: 377–387. [DOI] [PubMed] [Google Scholar]
- 15.Yeh FC, Badre D, Verstynen T. Connectometry: a statistical approach harnessing the analytical potential of the local connectome. Neuroimage 2016; 125: 162–171. [DOI] [PubMed] [Google Scholar]
- 16.Yeh FC, Wedeen VJ, Tseng WY. Generalized q-sampling imaging. IEEE Trans Med Imaging 2010; 29: 1626–1635. [DOI] [PubMed] [Google Scholar]
- 17.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Statist 2005; 4: 287–291. [Google Scholar]
- 18.Donnan GA, Davis SM, Chambers BR, et al. Surgery for prevention of stroke. Lancet 1998; 351: 1372–1373. [DOI] [PubMed] [Google Scholar]
- 19.Aboyans V, Ricco JB, Bartelink MEL, et al. Editor’s choice – 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 305–368. [DOI] [PubMed] [Google Scholar]
- 20.Porcu M, Craboledda D, Garofalo P, et al. Connectometry evaluation in patients undergoing carotid endarterectomy: an exploratory study. Brain Imaging Behav 2019; 13: 1708–1718. [DOI] [PubMed] [Google Scholar]
- 21.Manjón JV, Coupé P. volBrain: an online MRI brain volumetry system. Front Neuroinform 2016; 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coupé P, Tourdias T, Linck P, et al. LesionBrain: an online tool for white matter lesion segmentation In: Bai W, Sanroma G, Wu G, Munsell B, Zhan Y, Coupé P. (eds) Patch-based techniques in medical imaging. Patch-MI 2018. Lecture Notes in Computer Science, Vol. 11075. Cham, Switzerland: Springer. [Google Scholar]
- 23.Schilling KG, Yeh FC, Nath V, et al. A fiber coherence index for quality control of B-table orientation in diffusion MRI scans. Magn Reson Imaging 2019; 58: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh FC, Tseng WY. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 2011; 58: 91–99. [DOI] [PubMed] [Google Scholar]
- 25.Yeh FC, Liu L, Hitchens TK, et al. Mapping immune cell infiltration using restricted diffusion MRI. Magn Reson Med 2017; 77: 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh FC, Verstynen TD, Wang Y, et al. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 2013; 8: e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh FC, Panesar S, Barrios J, et al. Automatic removal of false connections in diffusion MRI tractography using topology-informed pruning (TIP). Neurotherapeutics 2019; 16: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10: 186–198. [DOI] [PubMed] [Google Scholar]
- 29.Bassett DS, Sporns O. Network neuroscience. Nat Neurosci 2017; 20: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett DS, Zurn P, Gold JI. On the nature and use of models in network neuroscience. Nat Rev Neurosci 2018; 19: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 2016; 139: 1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci 2016; 18: 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shukla DK, Keehn B, Smylie DM, et al. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia 2011; 49: 1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein A, Mesfin FB. Neuroanatomy, corpus callosum. In: StatPearls Treasure Island, FL: StatPearls Publishing, 2019. [PubMed]
- 35.Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol 2019; 18: 559–572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-neu-10.1177_1971400920959323 for White-matter hyperintensities in patients with carotid artery stenosis: An exploratory connectometry study by Michele Porcu, Roberto Sanfilippo, Roberto Montisci, Antonella Balestrieri, Jasjit S Suri, Max Wintermark and Luca Saba in The Neuroradiology Journal