Abstract
Purpose
To assess whether an asymmetry exists in the prevalence of carotid artery intraplaque hemorrhage (IPH) between right- and left-sided arteries.
Materials and methods
The records of all patients with atherosclerotic carotid artery disease that underwent neck magnetic resonance angiography imaging with high-resolution plaque sequences between 2017 and 2020 at our institution were retrospectively reviewed. The prevalence of stenosis and IPH was determined for all patients and compared between the left and right carotid arteries of those with unilateral anterior circulation ischemic strokes. Multiple regression analysis was performed to determine potential independent associations of IPH laterality with ischemic strokes.
Results
A total of 368 patients were included overall and 241 were male (65.4%). There were a total of 125 asymptomatic patients and 211 patients with unilateral anterior circulation ischemic strokes. Of patients with ischemic strokes, 55.5% had left-sided strokes compared with 44.5% who had right-sided strokes (p = 0.03). Patients with left-sided strokes had a higher prevalence of ipsilateral IPH than those with right-sided strokes (64.1% versus 36.2%, p < 0.0001), despite similar degrees of stenosis. Both age (odds ratio (OR): 1.0; 95% confidence interval (CI): 1.0–1.1; p = 0.007) and the presence of left-sided IPH (OR: 3.2; 95% CI: 1.5–6.8; p = 0.003) were independently associated with unilateral ischemic strokes.
Conclusions
Left-sided plaques more frequently have IPH and may be more likely to result in ipsilateral ischemic strokes compared with right-sided plaques. The underlying mechanism of asymmetric distribution of IPH between right and left carotids remains unclear.
Keywords: Carotid artery, carotid plaque, intraplaque hemorrhage, stroke, asymmetric
Introduction
Ischemic stroke is a leading cause of death and disability worldwide.1 Prior reports have indicated that left-hemispheric strokes carry a higher prevalence than right-hemispheric strokes.2–4 A commonly held explanation for this phenomenon is that the majority of individuals have left-hemispheric dominance in terms of language functionality, and therefore hemispheric strokes on the left side are more readily recognized clinically than those on the right, even though magnetic resonance imaging (MRI)-based prevalence of left versus right strokes may be similar.5,6 Alternatively, a high prevalence of left-sided strokes may be the result of an increased prevalence of left-sided carotid artery atherosclerosis and a higher propensity for formation of vulnerable plaque phenotypes including intraplaque hemorrhage (IPH).7,8 Although evidence to support the latter theory has been provided in a prior report,7 confirmatory studies with large cohorts are absent from the literature. The purpose of this study was to therefore investigate the symmetrical prevalence of IPH in the right versus left carotid arteries in a large population of patients with atherosclerotic carotid artery disease.
Methods
Baseline patient characteristics and imaging characteristics
This was a cross-sectional retrospective study. Institutional review board approval was obtained prior to the initiation of this study. All patients included in this study provided written informed consent for involvement in research activities at our institution. The medical and radiographic records of all patients who presented to our institution and had atherosclerotic carotid artery disease as demonstrated on magnetic resonance angiography (MRA) images of the neck within the previous three years were retrospectively reviewed. Exclusion criteria were as follows: (a) the MRA protocol did not include magnetization prepared–rapid gradient echo (MPRAGE) sequences (used for evaluation of IPH); (b) the images that were obtained were degraded by artifact or were of poor quality and were judged to be clinically non-diagnostic (i.e. motion artifact); or (c) the patient did not have imaging evidence of atherosclerotic disease of at least one carotid artery on MRA imaging.
For all plaques, the degree of luminal stenosis was assessed using the North American Symptomatic Carotid Endarterectomy Trial criteria.9 The presence or absence of carotid IPH was noted on MPRAGE sequences by a single neuroradiologist with greater than five years’ experience at our institution who was blinded to the research question and laterality of symptomatic hemisphere. IPH was defined as hyperintense signal intensity within the plaque on the three-dimensional (3D) MPRAGE sequence greater than 150% of that seen in the adjacent sternocleidomastoid muscle as previously described.10
Clinical histories and relevant vascular comorbidities were abstracted via a review of the electronic medical record. The presence of comorbidities was determined on a yes or no basis and included coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus and smoking history. Whether or not each patient was taking a statin or anti-platelet (aspirin or clopidogrel) at the time of MPRAGE imaging was also determined.
Patient grouping strategy
Patients were first categorized into two groups: those with and those without a history of ischemic stroke. Patients without a history of ischemic stroke are heretofore referred to as “asymptomatic.” A history of ischemic stroke was determined based on review of each patient’s medical record which included a radiologist’s report of an ischemic stroke within six weeks of MPRAGE imaging. From the group of patients with a history of ischemic stroke, a more specific group of patients with unilateral anterior circulation ischemic stroke, likely secondary to carotid disease, was selected by excluding patients with the following: (a) bilateral ischemic events (likely secondary to non-carotid embolic source); (b) posterior circulation ischemic strokes; or (c) a history of atrial fibrillation.
The majority of asymptomatic patients underwent neck MRA imaging for symptoms thought to be potentially related to carotid artery pathology but were ultimately not found to have ischemic stroke. Other indications for obtaining neck MRAs in asymptomatic patients included follow-up imaging for connective tissue diseases, a history of head/neck neoplasia, a ruling out of arterial dissection or a history of fibromuscular dysplasia.
Subgroup analysis of patients with anterior circulation ischemic stroke
To determine the prevalence of IPH in right versus left carotid arteries, patients with unilateral anterior circulation ischemic strokes were compared. In this subgroup, the carotid artery ipsilateral to the ischemic stroke was analyzed. Variables compared between the two groups included the prevalence of each hemispheric stroke, demographics, comorbidities, ipsilateral degree of stenosis and prevalence of ipsilateral IPH.
Multiple regression analyses
In order to determine independent associations with ischemic stroke, multiple logistic regression analysis was performed. Independent variables included age, sex, degree of right and left-sided stenosis, presence of left or right-sided IPH and any other variables found to be significant on univariable analysis. All patients meeting criteria for inclusion into the study were included in the multiple regression analysis. To determine independent associations with right- and left-sided strokes specifically, two additional multiple regression analyses were performed, in which right- and left-sided strokes were used as dependent variables. Independent variables included in these analyses were the same as mentioned above.
Imaging protocol
MRI of the neck was performed as previously reported.11 The carotid vessel wall imaging was performed on a 3T MRI scanner (GE 750, GE Healthcare, Milwaukee, WI, USA) utilizing a 16‐channel head/neck/spine coil and consisted of at least these three sequences: (a) two-dimensional time of flight; (b) 3D fast spoiled gradient echo (3D‐IR‐FSPGR or 3D MPRAGE) acquired in the coronal plane; and (c) gadolinium bolus carotid MRA acquired in the coronal plane. MRA covered the entire course of the extracranial carotid artery from the aortic arch to the petro-cavernous segment of the internal carotid artery (ICA) to ensure coverage of the entire plaque. The 3D MPRAGE sequence was used as previously described12 with the following settings: TR/TE = 13.2 ms/3.2 ms, flip angle = 15°, in plane spatial resolution = 0.63 mm ×0.63 mm, reconstructed resolution = 0.31 mm × 0.31 mm, slice thickness = 1 mm, number of excitation = 2, TI = 304 ms, TR with respect to the non-selective inversion = 568 ms, acquisition time = 3 min 50 s.
Statistical analysis
The aims of this study were twofold: (a) to compare the prevalence of IPH between left and right carotid arteries in patients with unilateral anterior circulation ischemic strokes; and (b) to determine if the presence of right- and left-sided IPH were independently associated with ischemic strokes. Means and standard deviations (SDs) were calculated for continuous variables including age, laboratory values and degree of stenosis. Percentages were calculated for binary and categorical variables including sex, comorbidities, medication use, prevalence of carotid stenosis and prevalence of IPH. Fisher’s exact probability test was used to determine significance between binary variables when comparing right- and left-sided arteries in symptomatic patients. A two-tailed Student’s t-test was used to compare the degree of stenosis between right and left carotid arteries in symptomatic patients. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for variables involved in the multiple regression analysis. A finding was considered statistically significant for any p-value <0.05. We did not perform any corrections for multiple comparisons. All calculations were performed in Microsoft Excel and Stata 14.1 (StataCorp, College Station, Texas, USA).
Results
Patients and baseline characteristics
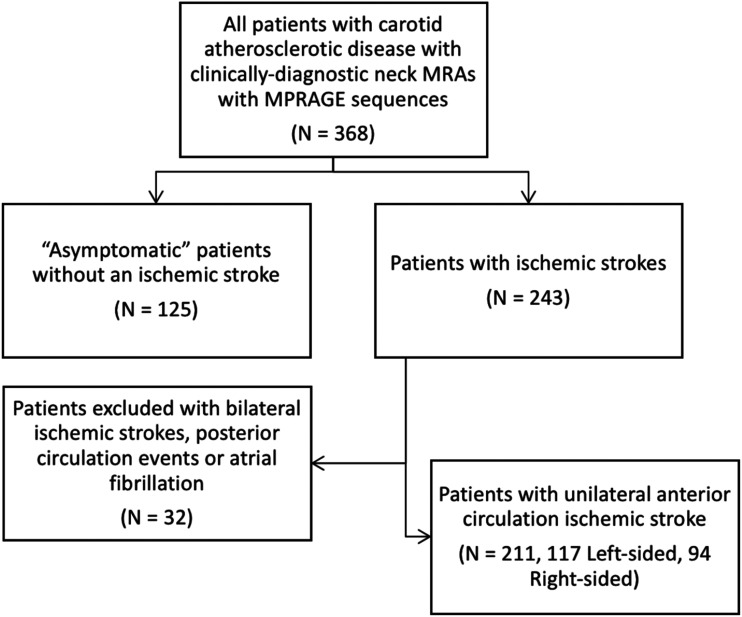
There were 368 patients with carotid atherosclerotic disease (736 arteries in total) that fitted the inclusion criteria with neck MRAs available for review. The mean age at the time of imaging was 67.8 (SD = 13.2); 241 patients were male (65.4%). There were 125 (34.0%) patients without ischemic strokes. From the 368 total patients, 243 had ischemic strokes (66.0%). Of these 243 patients, 32 were excluded as a result of bilateral ischemic strokes, posterior circulation strokes or a history of atrial fibrillation. There were a total of 211 patients with unilateral anterior circulation ischemic strokes for a total of 211 ipsilateral carotid arteries. Our patient selection process is outlined in Figure 1. All patients included in the study had information available regarding the presence of each comorbidity. Demographic and clinical characteristics of our study population and the details of the carotid arteries of included patients are summarized in Tables 1 and 2, respectively. Of the asymptomatic patients, 32 had IPH (25.6%): 10 patients had right-sided IPH (31.3%), 18 had left-sided IPH (56.3%), and four had bilateral IPH (12.5%). An example of carotid IPH on MPRAGE sequences is shown in Figure 2.
Figure 1.
Flowchart outlining patient selection strategy.
MPRAGE: magnetization prepared–rapid gradient echo; MRA: magnetic resonance angiography.
Table 1.
Baseline demographics and clinical characteristics of study patients.
| Total number of patients | 368 |
| Age (years), mean (SD) | 67.8 (13.2) |
| Male gender, n (%) | 241 (65.4) |
| Comorbidities, n (%) | |
| Coronary artery disease | 129 (35.1) |
| Hypertension | 285 (77.4) |
| Hyperlipidemia | 309 (84.0) |
| Ever smoker | 216 (58.7) |
| Diabetes mellitus | 100 (27.2) |
| Laboratory values, mean (SD) | |
| HDL (mg/dL) | 47.3 (14.6) |
| LDL (mg/dL) | 86.2 (35.5) |
| Triglycerides (mg/dL) | 145.4 (96.6) |
| Medications at the time of imaging, n (%) | |
| Statin | 394 (61.3) |
| Anti-platelet (aspirin or clopidogrel) | 296 (80.4) |
HDL: high density lipoprotein; LDL: low density lipoprotein; SD: standard deviation.
Table 2.
Carotid artery features of all patients.
| Total number of patients | 368 |
| Total number of arteries | 736 |
| Overall degree of stenosis, mean (SD) | 30.7 (34.0) |
| Degree of stenosis, n (%) | |
| <30% | 401 (54.4) |
| 30–49% | 68 (9.2) |
| 50–69% | 122 (16.6) |
| 70+% | 145 (19.8) |
| Arteries with intraplaque hemorrhage, n (%) | 128 (17.4) |
SD: standard deviation.
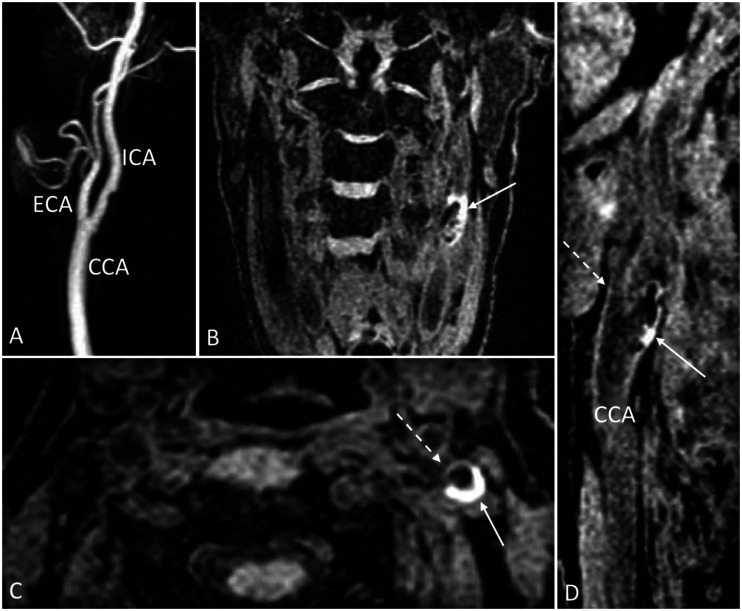
Figure 2.
Example of carotid IPH as evidence by hyperintensity on MPRAGE sequences. (a) 3D reformatted post-gadolinium MRA of the left common, external and internal carotid arteries demonstrating no significant areas of stenosis. (b) Oblique coronal and (c) axial fat-suppressed MPRAGE demonstrating an area of hyperintensity within the left ICA suggesting the presence of IPH (arrows). A normal-appearing ECA is visualized anterior to the area of IPH seen in (c) (dashed arrow). (d) Sagittal reformatted fat-suppressed MPRAGE again showing the presence of IPH in the ICA (solid arrow) with a normal-appearing ECA (dashed arrow) and CCA.
3D: three-dimensional; CCA: common carotid artery; ECA: external carotid artery; ICA: internal carotid artery; IPH: intraplaque hemorrhage; MPRAGE: magnetization prepared–rapid gradient echo; MRA: magnetic resonance angiography.
Analysis of patients with anterior circulation ischemic strokes
These results are summarized in Table 3. Of the patients with unilateral anterior circulation ischemic strokes, 117 (55.5%) and 94 (44.5%) had unilateral left- and right-sided ischemic strokes, respectively (p = 0.03). Patients with left-sided strokes had a higher prevalence of diabetes mellitus than those with right-sided strokes (35.9% versus 21.3%, p = 0.02). There were otherwise no significant differences between symptomatic patients with left- and right-sided strokes in regard to age, sex or comorbidities. Similarly, there were no significant differences between groups regarding the degree of stenosis, or the prevalence of each category of stenosis. However, patients with left-sided unilateral strokes had a significantly higher prevalence of ipsilateral IPH than those with right-sided strokes (64.1% versus 36.2%, p < 0.0001).
Table 3.
Analysis of patients with unilateral anterior circulation ischemic strokes.
| Patients with unilateral anterior circulation ischemic strokes (N = 211) |
|||
|---|---|---|---|
| Right side | Left side | p-value | |
| n (%) | 94 (44.5) | 117 (55.5) | 0.03 |
| Age (years), mean (SD) | 68.9 (12.9) | 69.5 (11.4) | 0.75 |
| Male, n (%) | 67 (71.2) | 94 (80.3) | 0.14 |
| Comorbidities, n (%) | |||
| Coronary artery disease | 36 (38.3) | 43 (36.8) | 0.89 |
| Hypertension | 70 (74.5) | 93 (79.5) | 0.41 |
| Hyperlipidemia | 83 (88.3) | 102 (87.2) | 0.83 |
| Diabetes mellitus | 20 (21.3) | 42 (35.9) | 0.02 |
| Obstructive sleep apnea | 16 (17.0) | 27 (23.1) | 0.30 |
| Ever smoker | 56 (59.6) | 79 (67.5) | 0.25 |
| Ipsilateral degree of stenosis, mean (SD) | 42.6 (35.2) | 43.2 (31.6) | 0.88 |
| Ipsilateral degree of stenosis, n (%) | |||
| <30% | 36 (38.3) | 40 (34.2) | 0.56 |
| 30–49% | 10 (10.6) | 13 (11.1) | 0.99 |
| 50–69% | 20 (21.3) | 34 (29.1) | 0.21 |
| 70+% | 28 (29.8) | 30 (25.6) | 0.54 |
| Ipsilateral intraplaque hemorrhage, n (%) | 34 (36.2) | 75 (64.1) | <0.0001 |
SD: standard deviation.
Multiple regression analyses
All patients who met inclusion criteria (368 in total) were included in the analysis. Independent variables included age, male sex, diabetes mellitus, degree of right or left carotid stenosis and the presence of right or left IPH. Of the independent variables, age (OR: 1.0; 95% CI: 1.0–1.1; p = 0.007) and the presence of left-sided IPH (OR: 3.2; 95% CI: 1.5–6.8; p = 0.003) were independently associated with unilateral ischemic strokes. These data are summarized in Table 4.
Table 4.
Multiple regression analysis of variables associated with ischemic strokes.
| Independent variables | Odds ratio (95% CI) | p-value |
|---|---|---|
| Age | 1.0 (1.0–1.1) | 0.007 |
| Male sex | 1.3 (0.76–2.5) | 0.29 |
| Diabetes mellitus | 1.7 (0.85–3.3) | 0.14 |
| Degree of right-sided stenosis | 1.0 (0.99–1.0) | 0.76 |
| Degree of left-sided stenosis | 1.0 (0.99–1.0) | 0.72 |
| Right-sided intraplaque hemorrhage | 1.9 (0.75–4.7) | 0.18 |
| Left-sided intraplaque hemorrhage | 3.2 (1.5–6.8) | 0.003 |
CI: confidence interval.
The same independent variables were also used in two additional multiple regression analyses, in which right- and left-sided strokes were used as separate dependent variables. Age (OR: 1.0; 95% CI: 1.0–1.1; p = 0.02), diabetes mellitus (OR: 2.1; 95% CI: 1.3–3.4; p = 0.004) and the presence of left-sided IPH (OR: 1.8; 95% CI: 1.0–3.2; p = 0.04) were independently associated with left-sided ischemic strokes. Age was the only variable with an independent association with right-sided ischemic strokes (OR: 1.0; 95% CI: 1.0–1.1; p = 0.04). These data can be found in Table 5.
Table 5.
Multiple regression analyses to determine independent associations with left and right-sided ischemic strokes.
| Side of ischemic stroke |
||||
|---|---|---|---|---|
| Left |
Right |
|||
| Independent variables | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| Age | 1.0 (1.0–1.1) | 0.02 | 1.0 (1.0–1.1) | 0.04 |
| Male sex | 1.5 (0.97–2.5) | 0.07 | 1.2 (0.73–1.9) | 0.50 |
| Diabetes mellitus | 2.1 (1.3–3.4) | 0.004 | 0.89 (0.54–1.5) | 0.66 |
| Degree of right-sided stenosis | 1.0 (0.99–1.0) | 0.20 | 1.0 (1.0–1.1) | 0.05 |
| Degree of left-sided stenosis | 1.0 (0.99–1.0) | 0.05 | 0.97 (0.98–1.1) | 0.16 |
| Right-sided intraplaque hemorrhage | 0.53 (0.25–1.1) | 0.10 | 1.7 (0.83–3.4) | 0.15 |
| Left-sided intraplaque hemorrhage | 1.8 (1.0–3.2) | 0.04 | 0.38 (0.20–1.7) | 0.30 |
CI: confidence interval.
Discussion
The current study suggests that, although atherosclerotic plaques cause similar degrees of luminal stenosis in both carotid arteries, IPH is more frequently observed in left-sided plaques and is associated with a higher prevalence of ipsilateral ischemic stroke. To our knowledge, only one prior study has similarly sought to assess for an asymmetry in vulnerable plaque features between the carotid arteries. This study, by Selwaness et al.,7 included a cohort of 1414 patients without stroke. The authors found that unilateral plaques were twice as prevalent on the left compared with the right. Carotid IPH was significantly more prevalent on the left, whereas right-sided plaques had higher prevalence of calcification suggesting greater vulnerability of left-sided plaques and greater stability of right-sided plaques. Plaque thickness was also greater on the left, although the degree of stenosis did not differ between sides, in agreement with our findings. Given that only stroke-free patients were included, this study controlled for the possible selection bias that patients with left-sided strokes are more likely to be symptomatic, and therefore undergo imaging of the carotids.
The current study supports this asymmetric pattern of IPH, suggesting that left carotid arteries are indeed more likely to develop IPH relative to the right. The reason for this is uncertain, although it is possible that the anatomic layout of the arterial network contributes to hemodynamic differences between the carotid arteries that make left-sided plaques more prone to hemorrhage. Even subtle variability resulting in more turbulent flow on the left and laminar flow on the right could increase the stress on left-sided plaques, ultimately resulting in the higher observed prevalence of IPH.
Anatomic differences have been shown on prior studies to have atherosclerotic consequences. Sitzer et al.,13 for example, demonstrated that a dorsal or dorsomedial origin of the ICA from the common carotid artery (CCA) was associated with the presence of both increased ICA wall thickness and atherosclerosis, suggesting that geometrical features of carotid artery anatomy may result in an increased risk of carotid disease. Similarly, Phan et al.14 found that the risk of carotid stenosis was independently associated with larger internal ICA angles, defined as the degree at which the ICA bifurcates from the vertical plane of the CCA. An independent association was also found between smaller ICA radius size and degree of carotid stenosis. Aortic pulse wave velocity is asymmetric between the carotid arteries, and we speculate that overall lower pulse wave velocity in the left internal carotid may predispose to preferential atheroma formation by an undefined mechanism.15 As such, it is also conceptually possible that left-sided carotid arteries are more prone to acquire such geometric alterations throughout life, thereby increasing the risk for further hemodynamic strain with subsequent plaque formation. In support of this theory, intima media thickness has previously been described to be greater in the left CCA.16 Hemispheric dominance leading to unilateral increases in ICA flow velocities may also play a role. Bogren et al.17 demonstrated that right-handed patients (suggesting left-hemispheric dominance) had higher flow rates in the left ICA, the opposite of which was true for left-handed patients. Given the higher populational prevalence of left-hemispheric predominance, this may contribute to higher prevalences of left-sided ICA plaques. Each of the listed theories is highly speculative and requires additional studies to investigate the underlying cause of increased prevalence of left-sided carotid artery disease.
Although it is possible that higher prevalence of IPH plays a role in the observed asymmetry in left-sided strokes, such an association remains yet to be confirmed. IPH plays an essential role in determining plaque vulnerability and is a risk factor for stroke regardless of the degree of carotid stenosis.18–20 Intriguingly, our results demonstrated that left-sided IPH is indeed independently associated with ipsilateral stroke, although this independence was not found for right-sided IPH. This suggests that left-sided IPH may be more likely to contribute to ischemic symptoms compared with right-sided. If such an association exists, this may have clinical implications in that left-sided plaques, in particular those with IPH, may be considered more strongly for interventional measures compared with right-sided plaques. It should be noted, however, that the differences found between left- and right-sided IPH, although significant, were marginal. Further investigation is therefore necessary in order to elucidate a potential association between left-sided IPH and risk of distal ischemic events before any definitive conclusions may be drawn.
The current study shares limitations of other retrospective, single-center studies. Our study may be biased as a result of patients not providing informed consent for inclusion; the impact of this on our results is unclear. Because patients with strokes were not excluded, it is possible that a selection bias exists in that patients with left-sided stroke were more likely to undergo imaging studies from the outset. However, this is unlikely because a similar vulnerable plaque distribution has been reported in a large cohort of patients without stroke.7 Other features relating to carotid artery disease including additional plaque morphological features, plaque thickness and anatomical and geometric features may play important roles but were not addressed in the current study. We utilized a definition of carotid IPH as being a hyperintensity that is greater than 150% of the adjacent sternocleidomastoid muscle. This definition has been utilized previously.10,21 However, other reports have utilized a hyperintensity threshold of 200%, which may increase the specificity of carotid IPH detection.22 Further study is required to identify the optimal threshold of relative hyperintensity to accurately identify the presence of carotid IPH.
Conclusions
IPH within carotid artery atherosclerotic plaque has a higher prevalence in left carotid arteries compared with right. Although this increased prevalence is likely associated with an increased risk for left-sided ischemic events, it is unlikely that any definitive conclusions may be drawn from our study. Further study is necessary to elucidate the underlying mechanism of asymmetric distribution of IPH between right and left carotids.
Footnotes
Consent for publication: No identifiable patient information is included in the present study and therefore consent was not required.
Consent to participate: All patients included in this study provided written informed consent for involvement in research activities at our institution.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Institutional review board approval was obtained prior to initiation of this study.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anthony S Larson https://orcid.org/0000-0001-6021-3452
References
- 1.Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133: e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.Hedna VS, Bodhit AN, Ansari S, et al. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J Clin Neurol 2013; 9: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foerch C, Misselwitz B, Sitzer M, et al. Difference in recognition of right and left hemispheric stroke. Lancet 2005; 366: 392–393. [DOI] [PubMed] [Google Scholar]
- 4.Naess H, Waje-Andreassen U, Thomassen L, et al. High incidence of infarction in the left cerebral hemisphere among young adults. J Stroke Cerebrovasc Dis 2006; 15: 241–244. [DOI] [PubMed] [Google Scholar]
- 5.Fink JN. Underdiagnosis of right-brain stroke. Lancet 2005; 366: 349–351. [DOI] [PubMed] [Google Scholar]
- 6.Portegies ML, Selwaness M, Hofman A, et al. Left-sided strokes are more often recognized than right-sided strokes: the Rotterdam study. Stroke 2015; 46: 252–254. [DOI] [PubMed] [Google Scholar]
- 7.Selwaness M, van den Bouwhuijsen Q, van Onkelen RS, et al. Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right. Stroke 2014; 45: 3226–3230. [DOI] [PubMed] [Google Scholar]
- 8.Hosseini AA, Kandiyil N, Macsweeney ST, et al. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol 2013; 73: 774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 10.Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 2008; 47: 337–342. [DOI] [PubMed] [Google Scholar]
- 11.Brinjikji W, DeMarco JK, Shih R, et al. Diagnostic accuracy of a clinical carotid plaque MR protocol using a neurovascular coil compared to a surface coil protocol. J Magn Reson Imaging 2018; 48: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 12.Zhu DC, Ferguson MS, DeMarco JK. An optimized 3D inversion recovery prepared fast spoiled gradient recalled sequence for carotid plaque hemorrhage imaging at 3.0 T. Magn Reson Imaging 2008; 26: 1360–1366. [DOI] [PubMed] [Google Scholar]
- 13.Sitzer M, Puac D, Buehler A, et al. Internal carotid artery angle of origin: a novel risk factor for early carotid atherosclerosis. Stroke 2003; 34: 950–955. [DOI] [PubMed] [Google Scholar]
- 14.Phan TG, Beare RJ, Jolley D, et al. Carotid artery anatomy and geometry as risk factors for carotid atherosclerotic disease. Stroke 2012; 43: 1596–1601. [DOI] [PubMed] [Google Scholar]
- 15.Dzeko M, Peters CD, Kjaergaard KD, et al. Aortic pulse wave velocity results depend on which carotid artery is used for the measurements. J Hypertens 2013; 31: 117–122. [DOI] [PubMed] [Google Scholar]
- 16.Chaubey S, Nitsch D, Altmann D, et al. Differing effect of modifiable cardiovascular risk factors on intima-media thickening and plaque formation at different sites of the arterial vasculature. Heart 2010; 96: 1579–1585. [DOI] [PubMed] [Google Scholar]
- 17.Bogren HG, Buonocore MH, Gu WZ. Carotid and vertebral artery blood flow in left- and right-handed healthy subjects measured with MR velocity mapping. J Magn Reson Imaging 1994; 4: 37–42. [DOI] [PubMed] [Google Scholar]
- 18.Saba L, Yuan C, Hatsukami TS, et al. Carotid artery wall imaging: perspective and guidelines from the ASNR Vessel Wall Imaging Study Group and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2018; 39: E9–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindler A, Schinner R, Altaf N, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: meta-analysis of individual patient data. JACC Cardiovasc Imaging 2020; 13: 395–406. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Yoshimura S, Shirakawa M, et al. Asymptomatic moderate carotid artery stenosis with intraplaque hemorrhage: progression of degree of stenosis and new ischemic stroke. J Clin Neurosci 2019; 63: 95–99. [DOI] [PubMed] [Google Scholar]
- 21.Singh N, Moody AR, Gladstone DJ, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology 2009; 252: 502–508. [DOI] [PubMed] [Google Scholar]
- 22.McNally JS, McLaughlin MS, Hinckley PJ, et al. Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke 2015; 46: 84–90. [DOI] [PubMed] [Google Scholar]