Abstract
Background
Though flow diverter is a safe and efficient modality, some patients can experience delayed aneurysmal rupture. The mechanism of delayed rupture is still obscure to us.
Methods
We performed a systematic search in the PubMed database for patients with delayed rupture of intracranial aneurysms after flow diverter placement.
Results
A total of 36 articles reporting on 60 patients were included in the final analysis. Of the 49 patients with description of presenting symptoms, six (12.2%) patients were incidentally diagnosed, 39 (87.8%) patients were admitted for aneurysmal rupture or mass effect. Multiple flow diverters were used in 38.3% (18/47) of the patients. Coil assistance was applied in 13.0% (7/54) of the patients. Delayed aneurysmal rupture led to intracranial hemorrhage or carotid–cavernous sinus fistula (CCF) in 76.8% (43/56) and 23.2% (13/56) of the patients, respectively. Of the 55 patients with description of outcome, 14 (25.5%) patients achieved good recovery, one (1.8%) patient was severely disabled, 40 (72.7%) patients died. All of the patients in the CCF group survived and experienced good recovery.
Conclusion
Increased intra-aneurysmal pressure, destabilization of the aneurysm wall by intra-aneurysmal thrombus, persistent residual intra-aneurysmal flow, characteristics of the specific aneurysm, and mechanical injury by the flow diverter might conjointly contribute to the final delayed rupture. There has been no established preventive measure to decrease the incidence of delayed rupture yet. The treatment and outcome depend on the presentation of delayed rupture. Patients presenting with aneurysm-related intracranial hemorrhage have a dismal outcome. Those presenting with CCFs usually have a satisfactory recovery.
Keywords: Intracranial aneurysm, flow diverter, intracranial hemorrhage, delayed rupture
Introduction
Intra-luminal flow diverter has been demonstrated to be a groundbreaking innovation in the treatment of intracranial aneurysms. It has shifted the treatment concept in endovascular management of intracranial aneurysms from direct coiling to blood flow diverting.1,2 The indications and off-label use of flow diverter have been continuously expanding, which range from giant to small, proximal to peripheral, anterior circulation to posterior circulation, unruptured to ruptured and saccular to non-saccular (fusiform/dissecting, pseudo and blood blister) aneurysms.3–8 Though flow diverter is a considerably safe and efficient modality, in rare circumstances, some patients can experience delayed aneurysmal rupture.9,10 However, the mechanism of delayed rupture is still obscure to us. To our knowledge, systematic review of delayed aneurysmal rupture after flow diverter placement is lacking. In this study, we perform a systematic literature review on this specific entity.
Methods
On 21 August 2019, we performed a systematic search in the PubMed database for patients with delayed rupture of intracranial aneurysms after flow diverter placement. The searching strategy was as follows: (flow diverter[Title/Abstract]) OR flow diversion[Title/Abstract]) OR flow diverting device[Title/Abstract]) OR pipeline[Title/Abstract]) OR pipeline flex[Title/Abstract]) OR surpass[Title/Abstract]) OR surpass streamline[Title/Abstract]) OR fred[Title/Abstract]) OR silk[Title/Abstract]) OR leo[Title/Abstract]) OR pipeline shield[Title/Abstract]) OR tubridge[Title/Abstract]) OR p64[Title/Abstract]) AND aneurysm[Title/Abstract]. The inclusion criteria for the identified articles were: 1) full text could be obtained or 2) sufficient data could be obtained from the abstract if the full text is inaccessible. Studies without sufficient description of the demographic, clinical and radiological data of the individual patient were excluded from the final analysis. In order to identify potentially missed articles, manual searching of the reference lists of the included articles was also performed. Modified Rankin Scale (mRS) was used for outcome assessment. A mRS score ≤ 3 was defined as good recovery. With respect to the criteria of delayed aneurysmal rupture, the following two points must be fulfilled: 1) rupture of the treated aneurysm after placement of the flow diverter, 2) demonstration of no contrast extravasation on the last angiogram before termination of the procedure.
Results
General information
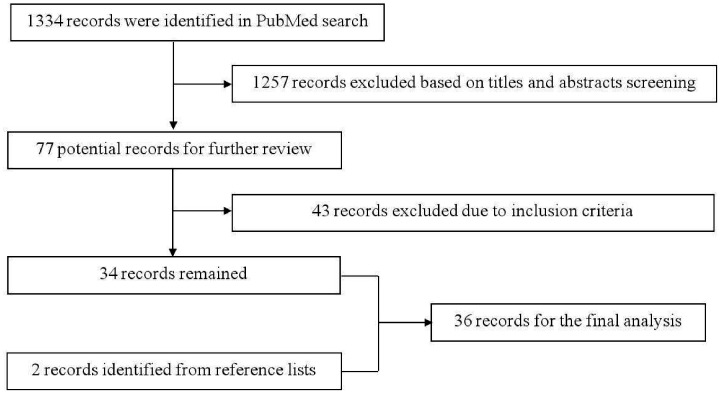
The PubMed search identified 1334 records, of which 1257 were excluded based on title and abstract screening. Forty-three records were further excluded after reading the full text. We manually searched the reference lists of the remaining 34 articles and two additional articles were further identified. Finally, 36 articles reporting on 60 patients were included in the final analysis (Table 1).8,11–45 The flowchart of searching strategy is illustrated in Figure 1. The patients were aged from 29 to 86 (55.31 ± 13.98) (n = 42) years, with a male to female ratio of 1:3 (11:33).
Table 1.
Clinical data of the patients with delayed aneurysmal rupture after flow diverter treatment.
| No. | First author/year | Age/sex | Presenting symptom | Aneurysm characteristics |
Antithrombotic regimen | Intraoperative details |
Immediate flow alteration | Immediate post-procedural complication | Partial formation of thrombus after FD | Interval to rupture | Findings after rupture | Further treatment | Speculated mechanism of rupture | Autopsy and finding | Outcome (mRS) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ruptured or unruptured | Location | Morphology | Intra-aneurysmal thrombosis | Size (mm) | Type and number of FDs | Coils assisting | ||||||||||||||
| 1 | Byrne/201011 | NA/NM | NA/NM | Unruptured | MCA | Fusiform | NA/NM | 24 | Clopidogrel and aspirin before and after treatment, heparin during treatment, warfarin and aspirin after ceasing clopidogrel | Silk | No | NA/NM | No | Yes | Six months | Acute hemorrhage, aneurysm enlargement | Surgical bypass | Warfarin | NA/NM | Death |
| 2 | Mustafa/201012 | 39/F | Hemifacial pain | Unruptured | ICA–cavernous | Fusiform | No | 17.6 | 300 mg Plavix before treatment, Plavix and aspirin after treatment | One Silk (4 mm×35 mm) | No | Significant stagnation of contrast within aneurysm | No | Yes | Two weeks | CCF | TVE of CCF with coils | Unknown | NA/NM | 0 |
| 3 | Lubicz/201013 | 71/F | Bitemporal hemianopsia | Unruptured | ICA–ophthalmic | Saccular | No | 28 | Systemic heparinization for 24 h. Aspirin and clopidogrel before treatment and continued for 3–6 months (aspirin 160 mg/day, clopidogrel 75 mg/day) | One Silk (4 mm×30 mm) | No | Significant flow reduction | No | NA/NM | 13 days | Massive SAH, stent migration | NA/NM | Migration into sac | NA/NM | Death |
| 4 | Turowski/201114 | 69/F | Incidental | Unruptured | ICA–ophthalmic | Saccular | No | 18.2 | Aspirin 100 mg/day and clopidogrel 75 mg/day for five days before treatment, and the same regimen after treatment | One Silk (4mm×30 mm) | No | Significant contrast stasis | No | Yes | 20 days | Extensive SAH | NA/NM | Unknown | NA/NM | Death |
| 5 | Kulcsár/201115 | 50/M | Acute severe headache | Unruptured | ICA–supraclinoid | Saccular | Yes | 21 | Aspirin and clopidogrel before treatment and continue for 2–3 months after FD placement | One Silk | Yes | Stagnation of contrast within aneurysm | Thromboembolism | Yes | Two days | SAH and ICH | NA/NM | Unknown | NA/NM | Good recovery |
| 6 | 74/F | Incidental | Unruptured | ICA–cavernous | Saccular | NA/NM | 20 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Silk | No | Stagnation of contrast within aneurysm | No | Yes | Three days | CCF | PAO | Unknown | NA/NM | NA/NM | |
| 7 | 44/F | Incidental | Unruptured | ICA–ophthalmic | Saccular | No | 18 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Silk | No | Inertia-driven inflow jet, stagnation of contrast within aneurysm | No | Yes | Five days | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 8 | 45/F | Visual loss | Unruptured | ICA–ophthalmic | Fusiform | NA/NM | 20 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Silk | No | Stagnation of contrast within aneurysm | No | Yes | Five days | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 9 | 70/M | Bilateral visual deficit | Unruptured | ICA–supraclinoid | Fusiform | No | 34 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | Two Leo, one Silk | No | Persisting inflow jet, stagnation of contrast within aneurysm | Thromboembolism | Yes | 12 days | Diffuse SAH | NA/NM | Unknown | Mural thinning and necrosis, loss of fibrous tissue and medial smooth muscle | Death | |
| 10 | 54/M | Visual problems | Unruptured | Basilar trunk | Fusiform | No | 17 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Leo, two Silk | No | Significant contrast stasis | No | Yes | 18 days | Diffuse SAH | NA/NM | Unknown | Mural thinning and necrosis, loss of fibrous tissue and medial smooth muscle | Death | |
| 11 | 69/F | Acute severe headache | Unruptured | ICA–ophthalmic | Saccular | NA/NM | 15 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Silk | No | Stagnation of contrast within aneurysm | Thromboembolism | Yes | 20 days | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 12 | 69/M | Diplopia, headache, unsteadiness | Unruptured | Basilar trunk | Fusiform | NA/NM | 25 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Leo, two Silk | No | Stagnation of contrast within aneurysm | No | Yes | 28 days | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 13 | 49/F | Visual deficit | Unruptured | ICA–supraclinoid | Saccular | NA/NM | 25 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement | One Silk | No | Stagnation of contrast within aneurysm | No | Yes | 48 days | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 14 | 48/F | Diplopia | Unruptured | ICA–cavernous | Saccular | NA/NM | 24 | Aspirin and clopidogrel before treatment and continue for 2-3 months after FD placement, aspirin till rupture | One Silk | No | Stagnation of contrast within aneurysm | No | Yes | 110 days | CCF | PAO | Unknown | NA/NM | NA/NM | |
| 15 | 66/F | Visual deficit | Unruptured | ICA–supraclinoid | Saccular | NA/NM | 18 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement, aspirin until rupture | One Silk | No | Stagnation of contrast within aneurysm | Thromboembolism | Yes | 135 days | Diffuse SAH | NA/NM | Unknown | NA/NM | Severe disability | |
| 16 | 67/F | Brainstem symptom | Unruptured | Basilar trunk | Fusiform | NA/NM | 31 | Aspirin and clopidogrel before treatment and continued for 2–3 months after FD placement, aspirin until rupture | One Leo, one Silk | No | Stagnation of contrast within aneurysm | No | Yes | 150 days | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 17 | Cebral/201116 | 62/F | NA/NM | Unruptured | ICA–supraclinoid | Saccular | No | 26 | NA/NM | One Pipeline | No | Significant contrast stasis | NA/NM | NA/NM | Four days | NA/NM | NA/NM | Unknown | NA/NM | NA/NM |
| 18 | 60/F | NA/NM | Unruptured | ICA–ophthalmic | Saccular | No | 38 | NA/NM | Two Pipeline | No | Significant contrast stasis | NA/NM | NA/NM | Seven days | NA/NM | NA/NM | Unknown | NA/NM | NA/NM | |
| 19 | Chow/201217 | NA/NM/F | Impairment of ambulation and swallowing | Unruptured | VA | Saccular | No | 23 | Aspirin and clopidogrel before and after treatment, heparin during procedure | One Pipeline (4mm×20 mm) | No | Significant contrast stasis | No | Yes | 20 days | SAH and ICH | NA/NM | Unknown | Mural necrosis | Death |
| 20 | Kan/201218 | 50/F | Vision loss | Unruptured | ICA–ophthalmic | Saccular | No | 22.46 | Aspirin (325 mg/day) and clopidogrel (75 mg/day) before treatment, aspirin and clopidogrel after treatment | Three Pipeline (4mm×18mm, 4.25mm×16mm, 4.25mm×14 mm) | No | Significant contrast stasis | No | NA/NM | Six days | SAH and ICH | NA/NM | Unknown | NA/NM | Death |
| 21 | Cirillo/2012 19 | NA/NM/M | NA/NM | Unruptured | Basilar artery | Saccular | No | >25 | Ticlopidine (twice/day) and aspirin (300 mg/day) for before (four days) and after treatment, heparin and aspirin during procedure | One Silk (5.5mm×60 mm) | No | Significant contrast stasis | Acute hydrocephalus | Yes | One week | ICH and IVH | NA/NM | Unknown | NA/NM | Death |
| 22 | Siddiqui/201220 | 42/M | Hemiparesis, facial weakness | Unruptured | Basilar artery | Fusiform | No | 35.6 | Aspirin and clopidogrel before treatment, heparin during procedure, eptifibatide after treatment | Three Pipeline (4 mm× 20mm, 4 mm× 12mm, 3.75mm ×12 mm) | No | Intra-aneurysmal flow is evident | Dysarthria, hemiparesis | NA/NM | One day | SAH and brainstem hemorrhage | NA/NM | Unknown | NA/NM | Death |
| 23 | 42/F | Vertigo, ataxia | Unruptured | Basilar artery | Fusiform | No | 37.1 | Aspirin and clopidogrel before treatment, eptifibatide after treatment | Three Pipeline (4 mm× 18mm, 4 mm× 12mm, 3.75mm ×16 mm) | Yes | Significant contrast stasis | Hemiparesis | Yes | Nine weeks | Diffuse SAH | NA/NM | Unknown | NA/NM | Death | |
| 24 | Velioglu/201221 | 37/F | NA/NM | Unruptured | ICA terminus | Saccular | No | 23 | Aspirin (100–300 mg/day) and clopidogrel (75 mg/day) before and after treatment, heparin during procedure | Leo, Silk | Yes | Slow flow in sac | Hemiparesis | Yes | Three days | SAH and diffuse brain edema | NA/NM | Unknown | NA/NM | Death |
| 25 | 47/F | CN III palsy | Unruptured | ICA–ophthalmic | Saccular | No | 20 | Aspirin (100–300 mg/day) and clopidogrel (75 mg/day) before and after treatment, heparin during procedure | Silk | No | Slow flow in sac | No | Yes | Five days | SAH and diffuse brain edema | NA/NM | Unknown | NA/NM | Death | |
| 26 | 40/F | Mass effect | Unruptured | ICA–ophthalmic | Saccular | No | 18 | Aspirin (100–300 mg/day) and clopidogrel (75 mg/day) before and after treatment, heparin during procedure | Silk, Leo | No | Significant slow flow in sac | Intra-stent thrombosis | Yes | Five days | SAH and infarction | One additional Silk | Unknown | NA/NM | 2 | |
| 27 | 35/M | CN III palsy | Unruptured | ICA–supraclinoid | Fusiform | No | 50 | Aspirin (100–300 mg/day) and clopidogrel (75 mg/day) before and after treatment, heparin during procedure | Three Leo, two Silk | No | Slow flow in sac | No | Yes | 30 days | ICH and IVH | NA/NM | Unknown | NA/NM | Death | |
| 28 | 40/F | Headache and ophthalmic symptoms | Unruptured | ICA–ophthalmic | Saccular | No | 50 | Aspirin (100–300 mg/day) and clopidogrel (75 mg/day) before and after treatment, heparin during procedure | Silk | No | Significant slow flow in sac | No | NA/NM | 25 days | SAH | NA/NM | Unknown | NA/NM | Death | |
| 29 | McAuliffe/201222 | 56/F | SAH | Ruptured | ICA–superior hypophyseal | Saccular | No | 21 | Loading dose aspirin (300 mg) and clopidogrel (600 mg) before treatment, heparin during procedure, aspirin and clopidogrel after treatment | Three Pipeline | No | Significant contrast stasis without discernible inflow jet | No | Yes | Eight days | SAH, aneurysm refilling with a new rupture point locule | One additional Pipeline | Unknown | NA/NM | Death |
| 30 | Colby/201323 | NA/NM | NA/NM | Unruptured | ICA–ophthalmic | Saccular | No | 3 | Aspirin (325 mg/day) and clopidogrel (75 mg/day) for seven days before treatment, heparin during procedure | One Pipeline (4mm×16 mm) | No | Persistent filling of contrast | No | No | 5 h | Diffuse SAH | NA/NM | Unknown | SAH from the aneurysm | Death |
| 31 | Darsaut et al./201324 | 69/F | Paraesthesias, hemiparesis | Unruptured | ICA–cavernous | Saccular | No | 60 | Aspirin (325 mg/day) and clopidogrel (75 mg/day) for seven days before treatment, aspirin and clopidogrel after treatment | Two Silk | No | Significant contrast stasis | Dysphasia, hemiparesis | Yes | One day | ICH | NA/NM | Unknown | Neutrophils infiltration | Death |
| 32 | Chalouhi/201325 | 46/F | Homonymous hemianopsia | Unruptured | ICA–supraclinoid | Saccular | No | 28 | Aspirin and clopidogrel before treatment | One Pipeline (4.25mm×30 mm) | No | Significant contrast stasis | Pipeline migration into sac | Yes | Three days | Extensive SAH, stent migration | Ventriculostomy, PAO with coils and Onyx | Pipeline migration into sac | NA/NM | Death |
| 33 | Toma/201326 | 60/F | Brainstem compression | Unruptured | VA | Saccular | No | 25 | Aspirin and clopidogrel before (300 mg/day) and after (75 mg/day) treatment, heparin for 5 days after treatment | Three Pipeline | No | Significant contrast stasis | No | Yes | One week | Extensive SAH and IVH | NA/NM | Unknown | NA/NM | Death |
| 34 | 42/F | NA/NM | Unruptured | ICA–ophthalmic | Saccular | NA/NM | 29 | Aspirin and clopidogrel before (300 mg/day) and after (75 mg/day) treatment, heparin for five days after treatment | NA/NM | NA/NM | Significant contrast stasis | NA/NM | Yes | Four days | NA/NM | NA/NM | Unknown | NA/NM | Death | |
| 35 | 38/F | NA/NM | Unruptured | Basilar artery | Saccular | NA/NM | 38 | Aspirin and clopidogrel before (300 mg/day) and after (75 mg/day) treatment, heparin for five days after treatment | NA/NM | NA/NM | Significant contrast stasis | NA/NM | Yes | 20 days | NA/NM | NA/NM | Unknown | NA/NM | Death | |
| 36 | Kuzmik/201327 | 48/NA/NM | Nausea and vomiting | Unruptured | ICA–paraclinoid | Saccular | No | 19 | Heparin for 48 h after treatment and then aspirin and clopidogrel | Two Silk | No | Minimal residual neck filling | No | Yes | One week | Extensive SAH, partial recanalization of aneurysm | NA/NM | Unknown | NA/NM | Death |
| 37 | Piano/201328 | 66/F | NA/NM | Unruptured | Basilar artery | Saccular | No | >25 | Aspirin and ticlopidine before (3–7 days) and after treatment | Pipeline | No | Significant contrast stasis without discernible inflow jet | No | Yes | Three weeks | SAH | NA/NM | Unknown | NA/NM | Death |
| 38 | Cruz/201329 | NM | SAH | Ruptured | ICA–supraclinoid | Saccular | No | >25 | Aspirin (325 mg) and clopidogrel (600 mg) before treatment, heparin during procedure, aspirin and clopidogrel after treatment | One Pipeline | Yes | Significant contrast stasis | No | NA/NM | Within 24 h | SAH | NA/NM | Unknown | NA/NM | Death |
| 39 | Chitale/2014 30 | 54/F | Headache | Unruptured | ICA–paraclinoid | Saccular | Yes | 5.5 | NA/NM | Pipeline | No | Significant contrast stasis | No | NA/NM | Within 1 h | Diffuse SAH | Ventriculostomy | Unknown | NA/NM | Death |
| 40 | Briganti/201531 | 41/F | Visual loss, ophthalmoplegia | Unruptured | ICA–supraclinoid | Saccular | No | 35 | Aspirin (150 mg) and clopidogrel (75 mg) before (five days) treatment, heparin during procedure | Two Pipeline | Yes | Significant contrast stasis | No | NA/NM | 12 h | Diffuse SAH | NA/NM | Unknown | NA/NM | Death |
| 41 | Ikeda/201532 | 67/F | Decreased eye vision | Unruptured | ICA–supraclinoid, ICA–paraclinoid | Saccular | No | 16 | Aspirin (100 mg/day, 14 days), clopidogrel (75 mg/day, 14 days), and cilostazol (200 mg/day, two days) before and after treatment, heparin during procedure | One Pipeline (3.75mm×18 mm) | No | Significant contrast stasis | No | Yes | 34 days | SAH | No | Unknown | Macrophage infiltration and wall degeneration | Death |
| 42 | Nerva/201533 | 30s/NA/NM | Headache and confusion | Ruptured | ICA–supraclinoid | BBA | No | 5 | Loading dosage aspirin (325 mg) and clopidogrel (75 mg) before treatment, heparin during procedure | One Pipeline | No | Contrast extravasation, without contrast stasis in the aneurysm | No | No | Five days | SAH and ICH | One additional Pipeline | Unknown | NA/NM | Death |
| 43 | Lin/201534 | Middle-aged/NA/NM | Headache and diplopia | Unruptured | ICA–cavernous | Saccular | No | 10 | Aspirin (325 mg/day) and clopidogrel (75 mg/day) for before (seven days) and after treatment | One Pipeline (5mm×18 mm) | No | Significant contrast stasis without inflow jet | No | NA/NM | Six weeks | CCF | TVE of the aneurysm sac and CS with coils | Unknown | NA/NM | 0 |
| 44 | Middle-aged/NA/NM | CN III palsy | Unruptured | ICA–cavernous | Saccular | No | 17 | Aspirin (325 mg/day) and clopidogrel (75 mg/day) before (seven days) and after treatment | One Pipeline (4.5mm×20 mm) | No | Significant contrast stasis | No | NA/NM | Three days | CCF | TVE of the aneurysm sac and CS with coils | Unknown | NA/NM | 0 | |
| 45 | Fox/201535 | Elderly/M | Gait ataxia, hyperflexia | Unruptured | Basilar artery | Saccular | NA/NM | 27 | Dual antiplatelets before and after treatment | One Pipeline | No | Significant contrast stasis | No | Yes | One week | SAH | NA/NM | Unknown | Organized thrombosis, linear tear | Death |
| 46 | Mazur/201636 | 29/M | Headache | Ruptured | ICA-supraclinoid | BBA | No | 4.3 | Abciximab during procedure, aspirin and clopidogrel after treatment | One Pipeline (5mm×14 mm) | No | Without significant contrast stasis | No | Yes | Nine days | SAH and ICH | One additional Pipeline | Unknown | NA/NM | Death |
| 47 | Raychev/201637 | Middle-aged/NA/NM | Hemiparesis and CN III palsy | Unruptured | ICA–PcomA | Saccular | NA/NM | >25 | Aspirin (325 mg/day) and clopidogrel (75 mg/day) before treatment | One Pipeline | No | Significant contrast stagnation | No | NA/NM | Five months | SAH and ICH | Sacrifice of ICA | Unknown | NA/NM | NA/NM |
| 48 | Roy/201738 | NA/NM | Incidental | Unruptured | ICA–cavernous | Saccular | NA/NM | 17.4 | NA/NM | Pipeline | NA/NM | NA/NM | NA/NM | NA/NM | 11 days | CCF | ICA sacrifice | Unknown | NA/NM | 0 |
| 49 | NA/NM | Incidental | Unruptured | ICA–cavernous | Saccular | NA/NM | 14.5 | NA/NM | Pipeline | NA/NM | NA/NM | NA/NM | NA/NM | 11 days | CCF | ICA sacrifice | Unknown | NA/NM | 1 | |
| 50 | NA/NM | Incidental | Unruptured | ICA–cavernous | Saccular | NA/NM | 31 | NA/NM | Pipeline | NA/NM | NA/NM | NA/NM | NA/NM | Three days | CCF | ICA sacrifice | Unknown | NA/NM | 0 | |
| 51 | Elderly patient/NA/NM | CN IV palsy | Unruptured | ICA–cavernous | Saccular | No | 19 | Aspirin and clopidogrel before treatment | One Pipeline | No | Significant contrast stasis | No | NA/NM | Six days | CCF | ICA sacrifice | Unknown | NA/NM | 1 | |
| 52 | Elderly patient/NA/NM | CN III palsy | Unruptured | ICA–cavernous | Saccular | No | 18.8 | Aspirin and clopidogrel before treatment | One Pipeline | No | Significant contrast stasis | No | NA/NM | Six days | CCF | ICA sacrifice | Unknown | NA/NM | 0 | |
| 53 | Oishi/201839 | 86/F | CN III palsy | Unruptured | ICA–cavernous | Saccular | No | 20 | Aspirin 105 mg/day and clopidogrel 50 mg/day for before (10 days) and after treatment, heparin during procedure | Three Pipeline (4.5mm×30mm, 4.75mm×30mm, 5mm×30 mm) | No | Significant contrast stasis | No | NA/NM | Six weeks | CCF | TVE of the aneurysm sac and CS with coils | Unknown | NA/NM | 0 |
| 54 | Hampton/201840 | NA/NM | Headache, hemianopsia | Unruptured | ICA terminus | Saccular | No | 35 | Aspirin 75 mg/day and clopidogrel 75 mg/day for one week before treatment, heparin, aspirin and clopidogrel after treatment | One Pipeline (2.75mm ×20 mm) | No | Significant contrast stasis | No | Yes | Five days | SAH and IVH | NA/NM | Unknown | NA/NM | Death |
| 55 | Sami/20188 | Young/NA/NM | NA/NM | Unruptured | ICA–cavernous | Pseudoaneurysm | No | 6 | Aspirin 325 mg/day and clopidogrel 75 mg/day before (one week) and after treatment, heparin during procedure | One Pipeline | No | Significant contrast stasis | No | Yes | One week | CCF | One additional Pipeline | Unknown | NA/NM | 0 |
| 56 | Nakae/201841 | 81/F | Diplopia | Unruptured | ICA–cavernous | Saccular | No | 18 | Aspirin 100 mg/day and clopidogrel 75 mg/day before treatment, heparin during procedure | One Pipeline (4.75 mm×25 mm) | No | Significant contrast stasis | No | NA/NM | 10 days | SAH, CCF | TVE of the aneurysm sac and CS with coils | Unknown | NA/NM | 1 |
| 57 | Bhogal/201842 | 62/M | Progressive cognitive decline | Unruptured | MCA bifurcation | Dissecting | Yes | 28 | Aspirin 100 mg/day and clopidogrel 75 mg/day before (one week) and after treatment, heparin during procedure | One Pipeline at first admission, a second Pipeline six months later | No | Significant contrast stasis | No | Yes | Eight months | ICH | Decompressive craniectomy | Unknown | NA/NM | Death |
| 58 | Griessenauer/201943 | 69/F | SAH | Ruptured | VA | Dissecting | NA/NM | 8 | Loading dose of aspirin (650 mg) and clopidogrel (600 mg) before treatment, heparin during procedure, aspirin and clopidogrel after treatment | Pipeline | NA/NM | NA/NM | NA/NM | NA/NM | The same day | SAH | NA/NM | Unknown | NA/NM | Death |
| 59 | Piano/201944 | 70/M | NA/NM | Unruptured | Basilar trunk | Dissecting | NA/NM | > 25 | Dual antiplatelet medication before and after treatment | One FRED | Yes | NA/NM | NA/NM | NA/NM | 35 days | SAH | NA/NM | Unknown | NA/NM | Death |
| 60 | Sirakov/201945 | NA/NM | Mass effect | Unruptured | ICA–ophthalmic | Saccular | No | > 25 | Aspirin 100 mg/day and clopidogrel 75 mg/day before (≥3 days) and after treatment, heparin during procedure | One p64 | Yes | Significant contrast stasis | No | NA/NM | Nine days | ICH | Conservative | Unknown | NA/NM | 3 |
BBA: blood blister aneurysm; CCF: carotid-cavernous fistula; CN: cranial nerve; CS: cavernous sinus; F: female; FD: flow diverter; ICA: internal carotid artery; ICH: intracerebral hemorrhage; IVH: intraventricular hemorrhage; M: male; MCA: middle cerebral artery; mRS: modified Rankin Scale; NA/NM: not applicable/not mentioned; PAO: parent artery occlusion; PcomA: posterior communicating artery; SAH: subarachnoid hemorrhage; TVE: transvenous embolization; VA: vertebral artery
Figure 1.
Flow chart of the searching strategy.
Before flow diverter placement
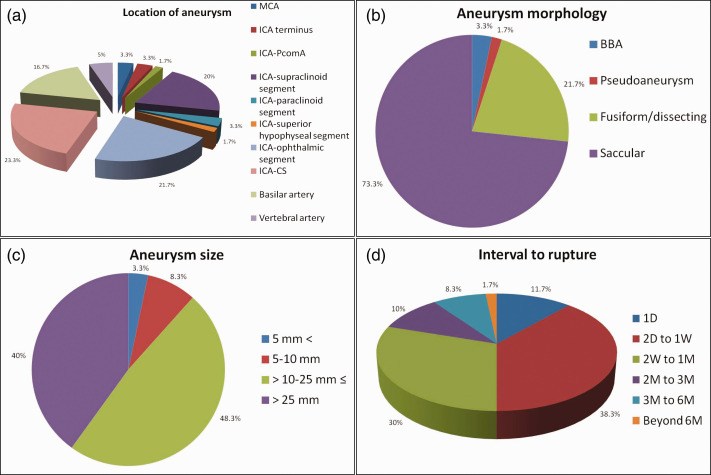
Of the 49 patients with description of presenting symptoms, six (12.2%) patients were incidentally diagnosed, five (10.2%) patients were admitted for aneurysmal rupture, two (4.1%) patients presented with non-subarachnoid hemorrhage-related headache and 36 (73.5%) patients presented with symptoms of mass effect by the responsible aneurysm. The treated aneurysms were unruptured in 91.7% (55/60) of the patients. The intracranial locations of the aneurysms were at middle cerebral artery, internal carotid artery (ICA) terminus, ICA-posterior communicating artery, ICA-supraclinoid segment, ICA-paraclinoid segment, ICA-superior hypophyseal segment, ICA-ophthalmic segment, ICA-cavernous sinus segment, basilar artery, vertebral artery in two (3.3%), two (3.3%), one (1.7%), 12 (20%), two (3.3%), one (1.7%), 13 (21.7%), 14 (23.3%), 10 (16.7%) and three (5%) patients, respectively (Figure 2(a)). The morphologies were blood blister aneurysm, pseudoaneurysm, fusiform/dissecting aneurysm, saccular aneurysm in two (3.3%), one (1.7%), 13 (21.7%) and 44 (73.3%) patients, respectively (Figure 2(b)). Three (6.8%, 3/44) aneurysms were demonstrated to be partially thrombosed before flow diverter placement. The sizes of the aneurysms were <5 mm, 5–10 mm, >10–≤25 mm, and >25 mm in two (3.3%), five (8.3%), 29 (48.3%) and 24 (40%) patients, respectively (Figure 2(c)).
Figure 2.
Statistical diagrams of the ruptured aneurysms after flow diversion.
(a) Intracranial location of the delayed ruptured aneurysms. (b) Morphology of the delayed ruptured aneurysms. (c) Maximal size of the delayed ruptured aneurysms. (d) The interval from successful deployment of flow diverter to delayed aneurysmal rupture.
CS: cavernous sinus; ICA: internal carotid artery; MCA: middle cerebral artery; PcomA: posterior communicating artery; BBA: blood blister aneurysm; D: day; M: month; W: week
Peri-flow diverter placement
As for the type of flow diverters used, FRED, Silk, p64 and Pipeline were solely used in one (1.7%, 1/58), 17 (29.3%, 17/58), one (1.7%, 1/58) and 32 (55.2%, 32/58) patients, respectively. Different flow diverters were conjointly used in 12.1% (7/58) of the patients. Multiple flow diverters were used in 38.3% (18/47) of the patients. Coil assistance was applied in 13.0% (7/54) of the patients. Immediate intra-aneurysmal contrast stagnation was demonstrated in 92.6% (50/54) of the patients. Other procedure-related complications occurred in 21.6% (11/51) of the patients immediately after flow diverter placement.
Outcome of flow diverter placement
Before post-procedural rupture, formation of intra-aneurysmal thrombus was demonstrated in 97.3% (36/37) of the patients after flow diverter placement. With respect to the timeline of delayed rupture, 11.7% (7/60) of the patients experienced aneurysmal rupture in 1 day post flow diverter placement, 38.3% (23/60) of the patients between the second day and first week, 30% (18/60) of the patients between the second week and first month, 10% (6/60) of the patients between the second and third month, 8.3% (5/60) of the patients between the third and sixth month and 1.7% (1/60) of the patients beyond the sixth month, respectively (Figure 2(d)). Delayed aneurysmal rupture led to intracranial hemorrhage and carotid–cavernous sinus fistula (CCF) in 76.8% (43/56) and 23.2% (13/56) of the patients, respectively. In the patients with intracranial hemorrhage after delayed aneurysmal rupture, only four (9.5%, 4/42) survived following further treatment. However, all of the patients with CCF survived and experienced good recovery. The definite cause of delayed rupture was identified in two (3.3%, 2/60) patients as flow diverter migration. Of the 55 patients with description of outcome, 14 (25.5%) patients achieved good recovery, one (1.8%) patient was severely disabled, 40 (72.7%) patients died.
Antithrombotic regimen
Preoperative antiplatelet regimen could be identified in 54 patients, of whom two (3.7%) received single antiplatelet agent (including one receiving abciximab), 51 (94.4%) received dual antiplatelet agents and one (1.9%) received triple antiplatelet agents. No patient received preoperative anticoagulation agent. Postoperatively, antithrombotic regimen could be identified in 46 patients, of whom six experienced aneurysmal rupture during receiving single antiplatelet agents (13.0%, including one receiving additional anticoagulation agent), 39 during receiving dual antiplatelet agents (84.8%, including two receiving additional anticoagulation agent), one (2.2%) during receiving triple antiplatelet agents.
Discussion
Mechanism of delayed rupture
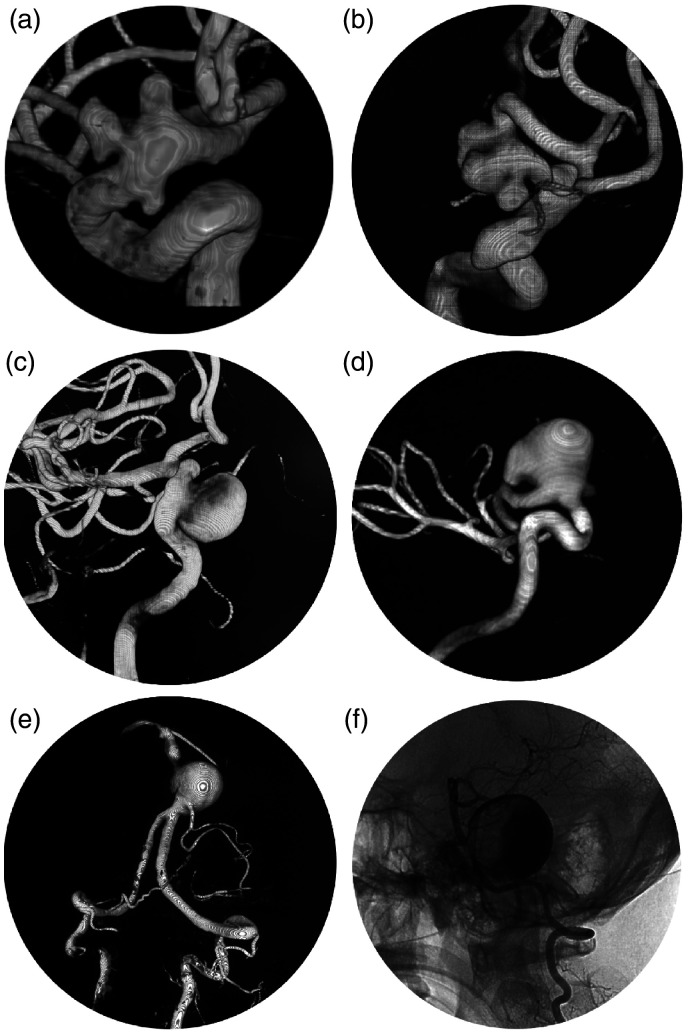
Flow diverter has become a more and more popular option, especially for the treatment of complex intracranial aneurysms. Some illustrative cases suited for flow diverter placement are presented in Figure 3. Delayed aneurysmal rupture is a severe complication after flow diverter placement, which could lead to a catastrophic consequence (Figure 4). The reported incidence varies greatly between different centers. In a single-center study of the 44 patients with cavernous carotid aneurysms treated with Pipeline, 11.4% (5/44) of the patients developed delayed CCF.38 However, in another international multi-center retrospective study of intracranial aneurysms treated with Pipeline, the reported incidence of delayed rupture was 0.6% (5/793).9 The mechanism is still unknown. According to the past studies, several factors might conjointly contribute to this severe consequence.
Figure 3.
Illustrative cases suitable for flow diverter placement.
(a) and (b) Multiple aneurysms at the paraclinoid segment of internal carotid artery. Large or giant aneurysm at the cavernous (c) and ophthalmic (d) segment. Large (e) and giant (f) aneurysms at the vertebra-basilar artery.
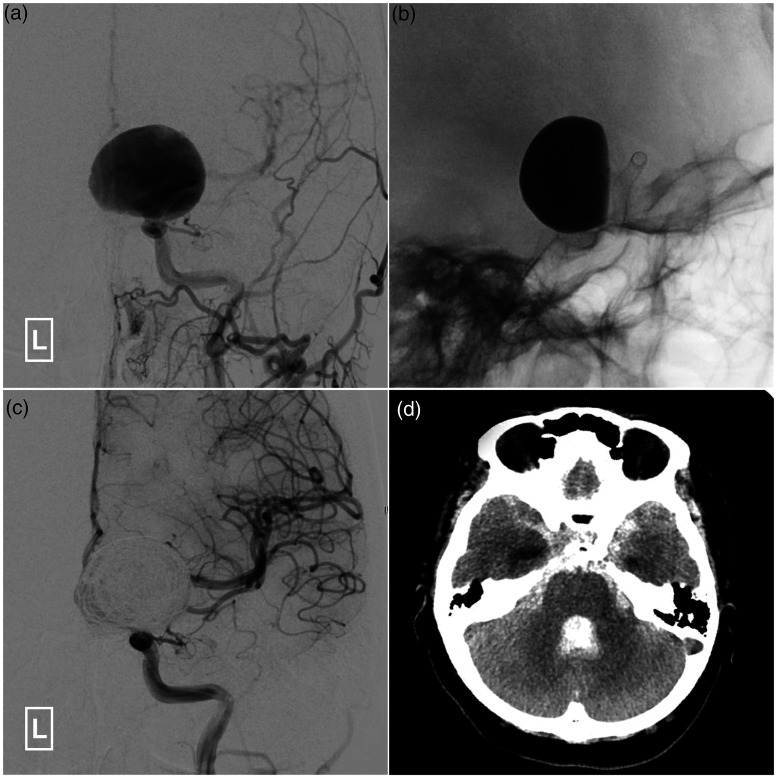
Figure 4.
Illustrative case of delayed aneurysmal rupture after flow diverter placement.
A 39-year-old woman is admitted for visual deterioration of the left eye. (a) Angiogram of the left ICA in AP view shows a giant aneurysm at the left ophthalmic segment of ICA. (b) X-ray after placement of a 3.5mm × 30mm Pipeline Flex shows stagnation of the contrast agent in the aneurysm. (c) Angiogram of the left ICA in AP view shows successful obliteration of the aneurysm after introducing several coils. (d) The patient experiences a sudden decline in mental state one day after flow diverter placement. Computed tomography shows extensive subarachnoid hemorrhage due to delayed aneurysmal rupture.
AP: anteroposterior; ICA: internal carotid artery
First, flow diverter placement leads to increased intra-aneurysmal pressure. Compared with the successfully occluded aneurysms, delayed ruptured aneurysms endure more intra-saccular pressure after flow diverter placement by computational hemodynamic analysis.16 This phenomenon can be explained in two possible ways. On one hand, successful deployment of the flow diverter stent could eliminate the pre-existing proximal stenosis of the parent artery. Though it is not the aim, reduction in proximal stenosis leads to reduced proximal resistance and subsequent increased pressure in the aneurysm segment. On the other hand, placement of flow diverter results in an increased resistance in the aneurysm segment of the parent artery, which would cause reduction in distal vascular resistance and increase in systemic blood pressure by complex autoregulation to maintain blood flow. The intra-aneurysmal pressure would increase with the increase in flow rate.
Second, though a key step in permanent aneurysm repair, intra-aneurysmal thrombus formation following flow diverter placement could also provoke a cascade of local inflammation and autolysis, leading to delayed aneurysm rupture.15,17,32 This process is a known source of protease secretion that can weaken the aneurysmal wall.35 Due to the destabilization of the aneurysm wall by intra-aneurysmal thrombus, any residual filling of the aneurysm sac after flow diverter deployment may have the potential for causing subsequent rupture.32,46 This deduction is based on clinical observation and postmortem histopathological investigations of the ruptured cases and previous study of abdominal aortic aneurysms with intra-luminal thrombus.17 In this study, before post-procedural rupture, partial formation of intra-aneurysmal thrombus was demonstrated in 97.3% (36/37) of the patients after flow diverter placement. However, it is still a mystery why intra-aneurysmal thrombosis may result in permanent cure in some patients but trigger autolysis and future rupture in other patients.
Other risk factors of delayed rupture include: 1) large and giant aneurysm; 2) symptomatic aneurysms; 3) saccular aneurysm with an aspect ratio of > 1.6; 4) delayed flow diverter migration into the aneurysm sac; 5) mechanical injury by flow diverter.13,15,25,35 In this study, we found that 88.8% and 97.8% of the aneurysms experiencing delayed rupture were large/giant and symptomatic aneurysms, respectively. In addition, delayed flow diverter migration into the aneurysm sac was also identified in two patients, which accounts for a rare cause of delayed aneurysm rupture.13,25 Linear tear of the aneurysm wall was identified in one patient during autopsy.35 Histopathological examination showed that the aneurysmal wall was extremely thin and microfissures were also noted. No inflammatory cells were detected within the wall and thrombus. Hence, in view of the aforementioned reasons, mechanical stretch was considered the cause of delayed rupture in this patient. Besides, due to the disruption in all layers of aneurysm wall, blood blister aneurysm or pseudoaneurysm is at higher risk of delayed rupture.33,36 Antithrombotic regimen might present another factor in delayed aneurysmal rupture. However, on one hand, the antithrombotic regimen varies greatly among different institutions with regard to the dosage, duration and agent selection. On the other hand, no study with large sample size focusing on antithrombotic regimen on delayed aneurysmal rupture has been published. Statistical analysis with regard to the antithrombotic regimen on delayed aneurysmal rupture could not be conducted at present. Of note, there were three patients who experienced delayed aneurysmal rupture during receiving single antiplatelet after ceasing the previous dual antiplatelet regimen.15 Hence, the effect of antiplatelet regimen on aneurysmal rupture is a complicated issue to be investigated. Flow diverter coated with antithrombotic shield presented to be a new technology in the treatment of intracranial aneurysms.47,48 As patients using flow diverter with shield technology adopt only single antiplatelet regimen, the risk of delayed aneurysmal rupture might be lower in theory. However, its effectiveness and safety need to be verified by further study.
Preventive measures
As was demonstrated by our study, delayed rupture meant a catastrophic event to most of the afflicted patients. Preventive measures to avoid this fatal complication are of great importance. Regrettably, to our knowledge, there has been no well-established suggestion to prevent delayed aneurysm rupture after flow diverter placement. All of the available measures are based on the speculated mechanism of delayed rupture. As hemodynamic study has demonstrated increased intra-aneurysmal pressure after flow diverter placement, some authors advocated careful post-procedural blood pressure control.16,32 However, no comparative study on blood control in reducing delayed aneurysm rupture has ever been published until now. The effectiveness is to be verified. To achieve complete and stabilized aneurysm thrombosis and early isolation from the blood flow, coil assistance or multiple stent-in-stent flow diverters were also suggested.15,16,32,49 But, according to our study, multiple flow diverters were used in 38.3% (18/47) of the patients who experienced delayed rupture. Coil assistance was applied in 13.0% (7/54) of the patients. Of note, multiple stent-in-stent flow diverters carry the risk of branch artery occlusion. To stabilize the already thrombosed aneurysm after flow diverter placement, some authors proposed reducing the post-procedural dose of antiplatelet agent.32 However, the dose and duration of antiplatelet agent post flow diverter placement has always been a question of debate. With respect to the different brands of flow diverters on the occurrence of delayed aneurysm rupture, no comparative study has ever been reported. In this review, FRED, Silk, p64 and Pipeline were solely used in one (1.7%, 1/58), 17 (29.3%, 17/58), one (1.7%, 1/58) and 32 (55.2%, 32/58) patients, respectively. Different generation of flow diverters might also affect the incidence of post-procedural aneurysm rupture. According to the preliminary reports, the second-generation flow diverters seem safer with regard to the issue of delayed rupture.44,50–52 But, as the second-generation flow diverters were just used in a relatively short period time and a limited population, prospective and comparative study with their first-generation counterparts is needed. Besides, as a majority of the delayed ruptured aneurysms were large/giant and symptomatic ones, the decision of flow diverter treatment should be prudent and under sufficient pre-procedural evaluation.
Treatment and outcome
In general, delayed aneurysm rupture after flow diverter placement can present with acute intracranial bleeding (subarachnoid hemorrhage and/or intracerebral hemorrhage) or CCF. Acute intracranial bleeding always means a fatal event. In our review, only four (9.5%, 4/42) patients survived following further treatment. Because of the rapidity of deterioration in general state, most of the patients in this subgroup have no opportunity for further treatment. Even with aggressive management, most of the cases would also experience inevitable deterioration and death.11,22,25,30,36 Of note, there was one patient who survived and experienced complete thrombosis of the aneurysm with just conservative management.45
Patients presented with CCF after flow diverter placement would experience a relatively more favorable outcome. According to this review, all of the patients with CCF survived and experienced good recovery. The treatment for this subgroup of patients was diverse and included transvenous embolization with coils, placement of an additional flow diverter, and ICA occlusion.8,12,15 Due to the higher metal coverage of flow diverter, it is difficult to advance the microcatheter through the flow diverter to the carvernous sinus. And embolization of the cavernous sinus via transarterial approach is always impossible.38,39 Hence, transvenous approach is the preferred route. But attention should be paid to the possibility of cranial nerve palsy by mass effect of coils. If transvenous approach is inaccessible, ICA sacrifice could be considered under prudent evaluation with balloon occlusion test. There was one report that the CCF was successfully treated with an additional flow diverter,8 but this case presented with a low-flow CCF. The CCF was gradually occluded in a delayed manner. Hence, the efficacy and safety of placing additional flow diverters warrants further investigation.
Conclusion
Delayed rupture of intracranial aneurysm after flow diverter treatment is a rare but usually fatal complication, the mechanism of which is still obscure to us. Increased intra-aneurysmal pressure, destabilization of the aneurysm wall by intra-aneurysmal thrombus, persistent residual intra-aneurysmal flow, characteristics of the specific aneurysm and mechanical injury by the flow diverter might conjointly contribute to the final delayed rupture. There has been no established preventive measure to decrease the incidence of delayed rupture yet. The proposed existing measures need to be verified by future study. The treatment and outcome depend on the presentation of delayed rupture. Patients presenting with aneurysm-related intracranial hemorrhage have a dismal outcome. Those presenting with CCFs usually have a satisfactory recovery. Flow diverter represents groundbreaking progress in the treatment of intracranial aneurysms, but is not an infallible method. Patients receiving flow diverter treatment should be evaluated individually.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Xianli Lv https://orcid.org/0000-0001-8270-8464
References
- 1.Karsy M, Guan J, Brock AA, et al. Emerging technologies in flow diverters and stents for cerebrovascular diseases. Curr Neurol Neurosci Rep 2017; 17: 96. [DOI] [PubMed] [Google Scholar]
- 2.Lv X, Jiang C, Wu Z, et al. Complex cerebral aneurysms: intra-luminal reconstruction using Pipeline flow-diverting stent and the obliteration mechanism. Neuroradiol J 2020; 33: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oishi H, Teranishi K, Yatomi K, et al. Flow diverter therapy using a Pipeline Embolization device for 100 unruptured large and giant internal carotid artery aneurysms in a single center in a Japanese population. Neurol Med Chir (Tokyo) 2018; 58: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang F, Zhang Y, Yan P, et al. Outcomes and complications after the use of the Pipeline embolization device in the treatment of intracranial aneurysms of the posterior circulation: A systematic review and meta-analysis. World Neurosurg 2019; 127: e888–e895. [DOI] [PubMed] [Google Scholar]
- 5.Schob S, Hoffmann KT, Richter C, et al. Flow diversion beyond the circle of Willis: Endovascular aneurysm treatment in peripheral cerebral arteries employing a novel low-profile flow diverting stent. J Neurointerv Surg 2019; 11: 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagnazzo F, Limbucci N, Nappini S, et al. Flow-diversion treatment of unruptured saccular anterior communicating artery aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2019; 40: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer S, Perez MA, Kurre W, et al. Pipeline embolization device for the treatment of intra- and extracranial fusiform and dissecting aneurysms: initial experience and long-term follow-up. Neurosurgery 2014; 75: 364–374; discussion 374. [DOI] [PubMed] [Google Scholar]
- 8.Sami MT, Gattozzi DA, Soliman HM, et al. Use of Pipeline embolization device for the treatment of traumatic intracranial pseudoaneurysms: Case series and review of cases from literature. Clin Neurol Neurosurg 2018; 169: 154–160. [DOI] [PubMed] [Google Scholar]
- 9.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: A multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu JL, Shi L, Yuan YJ, et al. Research progress on complications of intracranial aneurysms with flow-diverting stents. Int J Clin Exp Med 2016; 9: 13340–13350. [Google Scholar]
- 11.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: A multicentre prospective study. PLoS One 2010; 5: e12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustafa W, Kadziolka K, Anxionnat R, et al. Direct carotid-cavernous fistula following intracavernous carotid aneurysm treatment with a flow-diverter stent. A case report. Interv Neuroradiol 2010; 16: 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: A prospective study in 29 patients with 34 aneurysms. Stroke 2010; 41: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 14.Turowski B, Macht S, Kulcsar Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): Do we need to rethink our concepts? Neuroradiology 2011; 53: 37–41. [DOI] [PubMed] [Google Scholar]
- 15.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011; 32: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: Computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 2011; 32: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow M, McDougall C, O’Kelly C, et al. Delayed spontaneous rupture of a posterior inferior cerebellar artery aneurysm following treatment with flow diversion: A clinicopathologic study. AJNR Am J Neuroradiol 2012; 33: E46–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan P, Siddiqui AH, Veznedaroglu E, et al. Early postmarket results after treatment of intracranial aneurysms with the pipeline embolization device: A U.S. multicenter experience. Neurosurgery 2012; 71: 1080–1087; discussion 1087–1088. [DOI] [PubMed] [Google Scholar]
- 19.Cirillo L, Leonardi M, Dall’olio M, et al. Complications in the treatment of intracranial aneurysms with silk stents: An analysis of 30 consecutive patients. Interv Neuroradiol 2012; 18: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui AH, Abla AA, Kan P, et al. Panacea or problem: Flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg 2012; 116: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 21.Velioglu M, Kizilkilic O, Selcuk H, et al. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology 2012; 54: 1355–1365. [DOI] [PubMed] [Google Scholar]
- 22.McAuliffe W, Wenderoth JD. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the Pipeline embolization device. AJNR Am J Neuroradiol 2012; 33: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colby GP, Lin LM, Gomez JF, et al. Immediate procedural outcomes in 35 consecutive pipeline embolization cases: a single-center, single-user experience. J Neurointerv Surg 2013; 5: 237–246. [DOI] [PubMed] [Google Scholar]
- 24.Darsaut TE, Rayner-Hartley E, Makoyeva A, et al. Aneurysm rupture after endovascular flow diversion: The possible role of persistent flows through the transition zone associated with device deformation. Interv Neuroradiol 2013; 19: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalouhi N, Tjoumakaris SI, Gonzalez LF, et al. Spontaneous delayed migration/shortening of the pipeline embolization device: Report of 5 cases. AJNR Am J Neuroradiol 2013; 34: 2326–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toma AK, Robertson F, Wong K, et al. Early single centre experience of flow diverting stents for the treatment of cerebral aneurysms. Br J Neurosurg 2013; 27: 622–628. [DOI] [PubMed] [Google Scholar]
- 27.Kuzmik GA, Williamson T, Ediriwickrema A, et al. Flow diverters and a tale of two aneurysms. J Neurointerv Surg 2013; 5: e23. [DOI] [PubMed] [Google Scholar]
- 28.Piano M, Valvassori L, Quilici L, et al. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: A single-center experience. J Neurosurg 2013; 118: 408–416. [DOI] [PubMed] [Google Scholar]
- 29.Cruz JP, O’Kelly C, Kelly M, et al. Pipeline embolization device in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2013; 34: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitale R, Zanaty M, Chalouhi N, et al. Immediate aneurysm rupture after pipeline embolization: A new complication of flow diversion. Clin Neurol Neurosurg 2014; 124: 188–191. [DOI] [PubMed] [Google Scholar]
- 31.Briganti F, Leone G, Napoli M, et al. Early fatal hemorrhage after endovascular treatment of a giant aneurysm with flow diverter device and coils. Clin Neuroradiol 2015; 25: 201–205. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda H, Ishii A, Kikuchi T, et al. Delayed aneurysm rupture due to residual blood flow at the inflow zone of the intracranial paraclinoid internal carotid aneurysm treated with the Pipeline embolization device: Histopathological investigation. Interv Neuroradiol 2015; 21: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nerva JD, Morton RP, Levitt MR, et al. Pipeline Embolization Device as primary treatment for blister aneurysms and iatrogenic pseudoaneurysms of the internal carotid artery. J Neurointerv Surg 2015; 7: 210–216. [DOI] [PubMed] [Google Scholar]
- 34.Lin LM, Colby GP, Jiang B, et al. Transvenous approach for the treatment of direct carotid cavernous fistula following Pipeline embolization of cavernous carotid aneurysm: A report of two cases and review of the literature. J Neurointerv Surg 2015; 7: e30. [DOI] [PubMed] [Google Scholar]
- 35.Fox B, Humphries WE, Doss VT, et al. Rupture of giant vertebrobasilar aneurysm following flow diversion: Mechanical stretch as a potential mechanism for early aneurysm rupture. J Neurointerv Surg 2015; 7: e37. [DOI] [PubMed] [Google Scholar]
- 36.Mazur MD, Taussky P, MacDonald JD, et al. Rerupture of a blister aneurysm after treatment with a single flow-diverting stent. Neurosurgery 2016; 79: E634–E638. [DOI] [PubMed] [Google Scholar]
- 37.Raychev R, Tateshima S, Vinuela F, et al. Predictors of thrombotic complications and mass effect exacerbation after pipeline embolization: The significance of adenosine diphosphate inhibition, fluoroscopy time, and aneurysm size. Interv Neuroradiol 2016; 22: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy AK, Grossberg JA, Osbun JW, et al. Carotid cavernous fistula after Pipeline placement: A single-center experience and review of the literature. J Neurointerv Surg 2017; 9: 152–158. [DOI] [PubMed] [Google Scholar]
- 39.Oishi H, Teranishi K, Yatomi K, et al. Transvenous aneurysm sac and rupture point coil embolization of direct carotid cavernous fistula after Pipeline embolization. NMC Case Rep J 2018; 5: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hampton T, Walsh D, Tolias C, et al. Mural destabilization after aneurysm treatment with a flow-diverting device: A report of two cases. J Neurointerv Surg 2018; 10: i51–i55. [DOI] [PubMed] [Google Scholar]
- 41.Nakae R, Nagaishi M, Takano I, et al. Transvenous coil embolization for the treatment of carotid cavernous fistula after Pipeline placement: A case report. J Stroke Cerebrovasc Dis 2018; 27: e65–e69. [DOI] [PubMed] [Google Scholar]
- 42.Bhogal P, Chudyk J, Bleise C, et al. The use of flow diversion in vessels </=2.5 mm in diameter – A single-center experience. World Neurosurg 2018; 118: e575–e583. [DOI] [PubMed] [Google Scholar]
- 43.Griessenauer CJ, Ogilvy CS, Adeeb N, et al. Pipeline embolization of posterior circulation aneurysms: A multicenter study of 131 aneurysms. J Neurosurg 2018; 130: 923–935. [DOI] [PubMed] [Google Scholar]
- 44.Piano M, Valvassori L, Lozupone E, et al. FRED Italian registry: A multicenter experience with the flow re-direction endoluminal device for intracranial aneurysms. J Neurosurg 2019 May 10; 1–8. DOI: 10.3171/2019.1.JNS183005. [DOI] [PubMed] [Google Scholar]
- 45.Sirakov S, Sirakov A, Bhogal P, et al. The p64 flow diverter-mid-term and long-term results from a single center. Clin Neuroradiol Epub ahead of print 11 August 2019. DOI: 10.1007/s00062-019-00823-y. [DOI] [PubMed]
- 46.Hampton T, Walsh D, Tolias C, et al. Mural destabilization after aneurysm treatment with a flow-diverting device: A report of two cases. J Neurointerv Surg 2011; 3: 167–171. [DOI] [PubMed] [Google Scholar]
- 47.Pasarikovski CR, Waggass G, Cardinell J, et al. Pipeline embolisation device with shield technology for the treatment of ruptured intracranial aneurysm. Neuroradiol J 2019; 32: 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning NW, Cheung A, Phillips TJ, et al. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: Multicentre experience. J Neurointerv Surg 2019; 11: 694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siddiqui AH, Kan P, Abla AA, et al. Complications after treatment with pipeline embolization for giant distal intracranial aneurysms with or without coil embolization. Neurosurgery 2012; 71: E509–E513; discussion E513. [DOI] [PubMed] [Google Scholar]
- 50.Trivelato FP, Abud DG, Ulhoa AC, et al. Derivo embolization device for the treatment of intracranial aneurysms. Stroke 2019; 50: 2351–2358. [DOI] [PubMed]
- 51.Bhatia KD, Kortman H, Orru E, et al. Periprocedural complications of second-generation flow diverter treatment using Pipeline Flex for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg 2019; 11: 817–824. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Galdamez M, Biondi A, Kalousek V, et al. Periprocedural safety and technical outcomes of the new Silk Vista Baby flow diverter for the treatment of intracranial aneurysms: Results from a multicenter experience. J Neurointerv Surg 2019; 11: 723–727. [DOI] [PubMed] [Google Scholar]