Abstract
A wide range of neurological complications of coronavirus disease 2019 (COVID-19) is increasingly recognised. Although the majority of these remain ischaemic and haemorrhagic events, various disorders are being reported. In particular, several cases of diffuse acute leukoencephalopathy have been observed in critically ill patients with COVID-19 disease. We report the case of a 59-year-old man with multiple comorbidities and severe COVID-19 pneumonia who developed a diffuse leukoencephalopathy with microhaemorrhages and extensive associated white matter necrosis. Although this is the first documented case of extensive COVID-19-associated white matter necrosis, we highlight the relatively constant features of this injury similar to previously reported cases, including symmetrical involvement of the supratentorial white matter, sparing of the peripheral subcortical regions except in the precentral gyri, frequently associated microhaemorrhages, relative sparing of the deep gray matter structures and infratentorial structures, and lack of enhancement.
Keywords: COVID-19, leukoencephalopathy, microhaemorrhages, cytokine release syndrome, hypoxia, necrosis
Introduction
As the coronavirus disease 2019 (COVID-19) pandemic continues to evolve, a wider range of neurological complications is increasingly recognised.1 Although the majority of these remain ischaemic and haemorrhagic events, various disorders are being reported, presumably in relation to the cytokine release syndrome and/or neurotropism of the virus.1–3 Several cases of diffuse acute leukoencephalopathy have been observed, typically in critically ill patients, portending a poor prognosis.1,4–6 We report the case of a 59-year-old man with multiple comorbidities and severe COVID-19 pneumonia who developed a diffuse leukoencephalopathy with microhaemorrhages and extensive associated white matter necrosis. This is the first documented case of extensive COVID-19-associated white matter necrosis.
Case report
The patient is a 59-year-old man with a history of diabetes mellitus, hypertension and end-stage renal disease on dialysis, who was referred to our hospital on 4 June 2020 with severe COVID-19 pneumonia for intensive care support.
He was diagnosed on 30 May in the referring hospital with hypoxic respiratory failure complicated by cardiac arrest, for which he was resuscitated and revived after 3 minutes. COVID-19 infection was confirmed with reverse transcriptase polymerase chain reaction. On admission to our hospital, he was afebrile, requiring inotropes to maintain his blood pressure and on mechanical ventilation with fractional inspired oxygen (FIo2) of 35%.
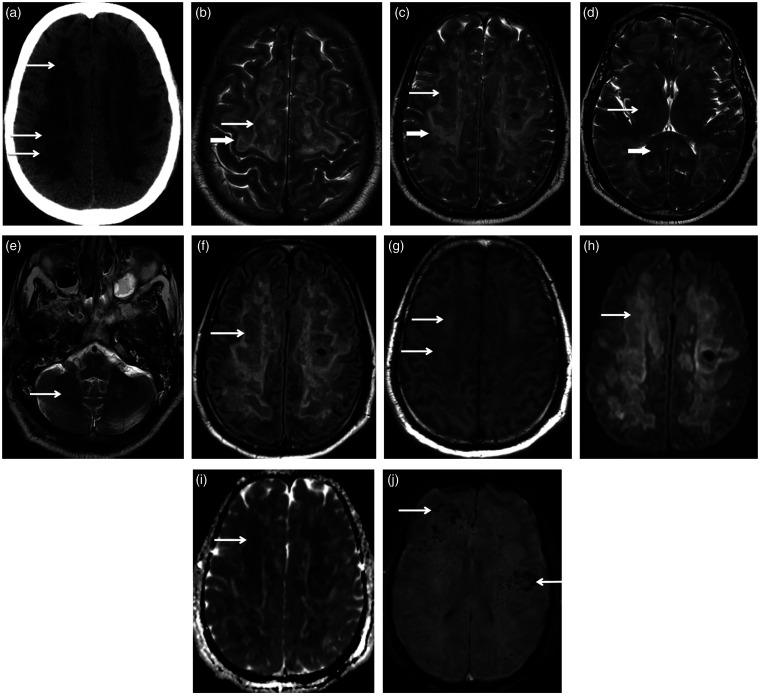
The patient was started on hydroxychloroquine and azithromycin. Chest X-ray and subsequent computed tomography (CT) confirmed bilateral peripherally predominant ground-glass densities in keeping with COVID pneumonia. Two days after admission, he developed a fever and required greater inotropic support, which raised concern for sepsis. His blood culture grew ampicillin-sensitive Enterococcus faecium and Candida dubliniesis, so he was treated with piperacillin-tazobactam and anidulafungin. CT scan of the abdomen and pelvis did not demonstrate a source of infection. Although the patient was not on sedation from admission, he had persistently depressed and worsening neurological status. A head CT performed on 7 June (Figure 1) demonstrated extensive pronounced hypoattenuation in the bilateral hemispheric white matter with intervening small areas of heterogeneous density, compatible with severe leukoencephalopathy. On 9 June, brain magnetic resonance imaging (MRI) (Figure 1) confirmed extensive T2 hyperintense bilateral symmetrical injury of the supratentorial white matter, predominantly involving the periventricular and deep white matter with sparing of the subcortical white matter, except in the precentral gyri. There were large areas of central T2 hypointensity, mild T1 hyperintensity and corresponding diffusion restriction and decreased fluid-attenuated inversion recovery (FLAIR) signal, consistent with white matter necrosis. Susceptibility weighted imaging (SWI) revealed numerous microhaemorrhages, most pronounced in the juxtacortical white matter. No enhancement was noted after contrast administration. Mild injury of the basal ganglia and infratentorial structures was present.
Figure 1.
(a) Axial head computed tomography (CT) demonstrates extensive bilateral pronounced white matter hypoattenuation with intervening small areas of intermediate density (arrows), suggestive of a severe likely destructive white matter injury. (b), (c), (d) and (e) Serial axial T2-weighted images. (b) Bilateral T2 hyperintense signal abnormality extends to the precentral subcortical white matter (short thick arrow), a feature noted in previous reports of coronavirus disease 2019 (COVID-19) leukoencephalopathy. Irregular central T2 hypointense signal (long thin arrow) is compatible with central necrosis. (c) At the level of the bilateral centrum semiovale, there is extensive symmetrical T2 hyperintense white matter injury (short thick arrow) sparing the most peripheral subcortical white matter. Large areas of centrally hypointense T2 signal (long thin arrow) are most suggestive of large areas of central necrosis. (d) Involvement of the internal capsules with relative sparing of the basal ganglia (thin long arrow) and mild involvement of the corpus callosum (thick short arrow) are demonstrated. (e) Injury of the infratentorial white matter is mild (arrow). Axial fluid-attenuated inversion recovery (FLAIR) (f), axial T1 (g), axial diffusion (h) and axial ADC map (i) at the level of the centrum semiovale show centrally hypointense FLAIR signal in the areas of white matter injury (arrow in (f)), corresponding mild T1 hyperintensity (arrows in (g)) and restricted diffusion in the same distribution (arrows in (h) and (i)), all features compatible with necrosis. (j) Axial susceptibility weighted imaging (SWI) shows hypointense microhaemorrhages, most numerous in the juxtacortical regions (arrows). Only a few foci of microhaemorrhage are seen in the corpus callosum.
The patient had worsening sepsis and neurological status despite maximal antibiotic and inotropic support. He died on 15 June 2020.
Discussion
The extensive white matter injury in our patient, involving the bilateral supratentorial white matter in a symmetrical distribution only sparing the most peripheral subcortical regions, is similar to what was observed in the available prior reports of COVID-associated leukoencephalopathy.1,4–6 Another feature is bilateral injury of the subcortical white matter of the precentral gyri, which was a relatively constant feature in the case series reported by Radmanesh et al.,4 suggesting selective vulnerability of this region. Contrary to what was previously noted,4,5 the white matter signal changes did not show a posteroanterior gradient in our patient, who had equally severe injury of the anterior and posterior white matter. The lack of enhancement, mild infratentorial involvement and relative sparing of the deep gray matter structures are consistent features among the patients reported previously4 and our patient. All patients as well as ours were critically ill on mechanical ventilation. Overall, the pattern of injury appears relatively constant, with diffuse symmetrical injury of the supratentorial white matter, sparing of the peripheral subcortical regions except in the precentral gyri, frequently associated microhaemorrhages, relative sparing of the deep gray matter structures and infratentorial structures and lack of enhancement.
The pathogenesis of the brain injury in our patient is uncertain and potential aetiologies include hypoxia, viral encephalitis, post-infectious demyelination, post-infectious vasculitis or COVID-associated cytokine release syndrome.2,3 The distribution of the white matter injury is very similar to what is seen in delayed posthypoxic leukoencephalopathy (DPHL), which supports a hypoxic aetiology. DPHL is a rare hypoxic brain injury, most frequently seen in relation to drug overdose7 or carbon monoxide poisoning,8 with fewer cases secondary to cardiopulmonary arrest.9 It manifests as a neurological relapse one to several weeks after initial recovery from an acute hypoxic episode.10 Features common with what was seen on our patient’s imaging is involvement of the deep white matter, relative sparing of the juxtacortical white matter, basal ganglia and infratentorial structures, as well as lack of enhancement.10 Histopathologically, DPHL exhibits extensive demyelination with axonal preservation11 and classically absent necrosis on imaging studies.10 The presence of extensive white matter necrosis in our patient and the lack of an initial neurological recovery are not in favour of DPHL secondary to his cardiac arrest, as the patient had persistent and worsening depressed consciousness since admission in the absence of sedation.
Angiotensin-converting enzyme 2 (ACE2) expressed in human vascular endothelium and respiratory epithelium is the primary functional receptor for COVID-19 entry into the host cells.12 The endotheliopathy caused by the virus is likely to be the result of direct viral invasion of endothelial cells and/or the immune response (cytokine storm) it induces after cellular invasion. It is responsible for COVID-19-associated coagulopathy with arterial, venous and microvascular thrombotic complications.13 Hypertension and diabetes are known to potentiate the endothelial injury caused by the virus,14 and probably contributed to the severity of the white matter damage in our patient.
The large areas of necrosis within the injured white matter in our patient were not seen in the previously reported cases of COVID-19-associated leukoencephalopathy.1,4,5 A single case report6 of a COVID-19-associated necrotising leukoencephalopathy is available, in which the necrotic features are mild, significantly less extensive than in our case. The diffuse necrosis in our patient suggests a more severe insult, likely to be related to the compound effect of the respiratory hypoxemia, prior cardiorespiratory arrest and concurrent comorbidities.
Microhaemorrhages are usually absent in DPHL10 but are an increasingly recognised complication of severe COVID-19 infection,1,4,5 which further supports the theory that our patient’s leukoencephalopathy is likely to be COVID-19 related. The predominant juxtacortical and callosal distribution in some prior reports of COVID-19-associated leukoencephalopathy4,5 is similar to what is seen in critical illness-associated microbleeds, a rare complication of acute respiratory failure.15 The distribution in our patient was predominantly juxtacortical, with less pronounced involvement of the corpus callosum. A diffuse distribution was also reported by Kremer et al.,1 who found white matter microhaemorrhages in 24% of their patient cohort. The pathophysiology remains unknown. They might represent a coexistent but distinct injury from leukoencephalopathy as simultaneous different patterns of COVID-related brain injuries were noted in 24% of the patient cohort reported by Kremer et al.1
Conclusion
Acute leukoencephalopathy is an increasingly recognised complication of severe COVID-19 infection and typically portends a poor prognosis. Its imaging features are very similar to DPHL, suggestive of a hypoxic aetiology. We report the first case of COVID-19-associated acute leukoencephalopathy with extensive necrosis, presumably reflecting a more severe form of the same pathology, aggravated by the patient’s concurrent comorbidities.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not obtained as there is no information included in the submitted material that allows identification of the patient.
ORCID iD: Manal Nicolas-Jilwan https://orcid.org/0000-0002-5701-5997
References
- 1.Kremer S, Lersy F, de Sèze J, et al. Brain MRI Findings in Severe COVID-19: a retrospective observational study. Radiology Epub ahead of print 16 June 2020. DOI: 10.1148/radiol.2020202222 [DOI] [PMC free article] [PubMed]
- 2.Zhou Z, Kang H, Li S, et al. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol 2020; 26: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore BJB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368: 473–474. [DOI] [PubMed] [Google Scholar]
- 4.Radmanesh A, Derman A, Lui YW, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology Epub ahead of print 21 May 2020. DOI: 10.1148/radiol.2020202040 [DOI] [PMC free article] [PubMed]
- 5.Sachs JR, Gibbs KW, Swor DE, et al. COVID-19-associated leukoencephalopathy. Radiology 2020; 296: E184–E185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radmanesh A, Derman A, Ishida K. COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy. J Neurol Sci 2020; 415: 116945. DOI: 10.1016/j.jns.2020.116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Betts AM, Ritter JL, Kubal WS. Reversible delayed posthypoxic leukoencephalopathy after drug overdose: MRI findings in a collection of patients. Emerg Radiol 2012; 19: 165–173. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Chang KH, Song IC, et al. Delayed encephalopathy of acute carbon monoxide intoxication: diffusivity of cerebral white matter lesions. AJNR Am J Neuroradiol 2003; 24: 1592–1597. [PMC free article] [PubMed] [Google Scholar]
- 9.Lou M, Jing CH, Selim MH, et al. Delayed substantia nigra damage and leukoencephalopathy after hypoxic-ischemic injury. J Neurol Sci 2009; 277: 147–149. [DOI] [PubMed] [Google Scholar]
- 10.Zamora CA, Nauen D, Hynecek R, et al. Delayed posthypoxic leukoencephalopathy: a case series and review of the literature. Brain Behav 2015; 5: e00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottfried JA, Mayer SA, Shungu DC, et al. Delayed posthypoxic demyelination. Association with arylsulfatase A deficiency and lactic acidosis on proton MR spectroscopy. Neurology 1997; 49: 1400–1404. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amraei R, Rahimi N. COVID-19, renin–angiotensin system and endothelial dysfunction. Cells 2020; 9: 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fanou EM, Coutinho JM, Shannon P, et al. Critical illness-associated cerebral microbleeds. Stroke 2017; 48: 1085–1087. [DOI] [PubMed] [Google Scholar]