Abstract
Background
Metastasis directed treatment (MDT) is increasingly performed with the attempt to improve outcome in non-small cell lung cancer (NSCLC) patients receiving targeted- or immunotherapy (TT/IT). This study aimed to assess the safety and efficacy of metastasis directed stereotactic radiotherapy (SRT) concurrent to TT/IT in NSCLC patients.
Methods
A retrospective multicenter cohort of stage IV NSCLC patients treated with TT/IT and concurrent (≤ 30 days) MDT was established. 56% and 44% of patients were treated for oligoprogressive disease (OPD) or polyprogressive disease (PPD) under TT/IT, polyprogressive respectively. Survival was analyzed using Kaplan–Meier and log rank testing. Toxicity was scored using CTCAE v4.03 criteria. Predictive factors for overall survival (OS), progression free survival (PFS) and time to therapy switch (TTS) were analyzed with uni- and multivariate analysis.
Results
MDT of 192 lesions in 108 patients was performed between 07/2009 and 05/2018. Concurrent TT/IT consisted of EGFR/ALK-inhibitors (60%), immune checkpoint inhibitors (31%), VEGF-antibodies (8%) and PARP-inhibitors (1%). 2y-OS was 51% for OPD and 25% for PPD. After 1 year, 58% of OPD and 39% of PPD patients remained on the same TT/IT. Second progression after MDT was oligometastatic (≤ 5 lesions) in 59% of patients. Severe acute and late toxicity was observed in 5.5% and 1.9% of patients. In multivariate analysis, OS was influenced by the clinical metastatic status (p = 0.002, HR 2.03, 95% CI 1.30–3.17). PFS was better in patients receiving their first line of systemic treatment (p = 0.033, HR 1.7, 95% CI 1.05–2.77) and with only one metastases-affected organ (p = 0.023, HR 2.04, 95% CI 1.10–3.79). TTS was 6 months longer in patients with one metastases-affected organ (p = 0.031, HR 2.53, 95% CI 1.09–5.89). Death was never therapy-related.
Conclusions
Metastases-directed SRT in NSCLC patients can be safely performed concurrent to TT/IT with a low risk of severe toxicity. To find the ideal sequence of the available multidisciplinary treatment options for NSCLC and determine what patients will benefit most, a further evaluated in a broader context within prospective clinical trials is needed continuation of TT/IT beyond progression combined with MDT for progressive lesions appears promising but requires prospective evaluation.
Trial registration: retrospectively registered
Keywords: Stereotactic, Radiotherapy, Immunotherapy, Targeted therapy, Concurrent, Oligometastases, NSCLC
Introduction
The development of targeted-, and immunotherapy (TT/IT) has improved the prognosis of stage IV non-small cell lung cancer (NSCLC) significantly. However, due to the development of resistance, most patients will eventually develop progressive disease. Since stereotactic radiotherapy (SRT) has emerged as a safe and locally effective modality for treatment of oligometastases, its use as part of the multimodality treatment in this situation is increasing [1], as radical local radiotherapy of persistent or progressive (oligo-) metastases could improve outcome [2–6].
Three randomized trials have shown that addition of a localized metastases-directed treatment (MDT) to systemic therapy improved progression free survival (PFS) as well as overall survival in NSCLC patients with oligometastatic disease [7–9]. However, the definition of oligometastatic disease varies in literature, while research on the identification of a biological oligometastatic state is ongoing [10]. This becomes obvious in a systematic review on oligometastatic NSCLC patients, where a variation of 5-year OS between 8.3% and 86% was observed [11]. The subcategorization of (oligo-)metastatic patients remains a challenge, but is important as the value of local treatments varies between these patients [3, 12].
Besides oligometastatic disease, SRT might also play a role as “salvage” treatment in the management of patients who have (oligo-)progressive metastatic disease while under TT/IT [12]. Once a stable oncological status is achieved under systematic therapy and no severe side effects develop, a patient preferably continues this drug for as long as possible, assuming that the further lines of systematic treatment are characterized by a worse therapeutic ratio. Unfortunately, intrinsic or acquired resistance to systematic drugs develops in nearly all patients [13]. Here, MDT could possibly prevent or delay the switch of systematic therapy by radical local treatment of all progressive metastatic sites. This study aimed to evaluate efficacy and safety of metastasis directed SRT (MDT) in NSCLC patients who are progressive under immuno- or targeted therapy.
Materials and methods
A retrospective international multicenter registry study was established by the German Society for Radiation Oncology (DEGRO) working group for radiosurgery and stereotactic radiotherapy to collect data on stage IV patients receiving SRT with concurrent targeted- or immunotherapy (TOaSTT study). The study was approved by the ethics committees at all participating sites (BASEC-Nr. 2016–01807). For this study, all patients with NSCLC were evaluated. Patients were treated with SRT between 07/2009 and 05/2018. Inclusion criteria were: ≥ 18 years of age, diagnosis of stage IV synchronous or metachronous metastatic disease, histological confirmation of NSCLC, SRT of any cranial or extracranial local recurrence or metastasis, treated concurrently (≤ 30 days) with any type of following systemic treatments: antibodies, tyrosine kinase inhibitors and/or immune-checkpoint inhibitors. Cranial SRT was defined as delivery of up to 5 fractions, or one fraction with a minimum of 16 Gy. Stereotactic body radiotherapy (SBRT) was defined as delivery of ≤ 10 fractions with a minimum total dose of 50 Gy (2 Gy equivalent, α/β of 10 Gy; the α/β-ratio of 10 represents the intrinsic radiosensitivity of NSCLC metastases [14]).
To further evaluate clinical scenarios in which MDT is currently often performed, the group of oligoprogressive disease patients (OPD, ≤ 5 metastases) and polyprogressive disease patients (PPD, > 5 metastases) was sub-analyzed. The OPD cohort consisted of patients where MDT of either all metastases or all oligoprogressive metastases was performed; the PPD cohort was characterized by patients, where polyprogressive disease was observed and only dominant lesions were treated with MDT, according to local interdisciplinary decision. Endpoints were overall survival (OS), time to therapy switch (TTS), progression free survival (PFS), local metastases control (LC), and toxicity. OS was defined as time of SRT to time of death, living patients were censored at the date of last follow-up. PFS and LC were defined as time of SRT to time of progression, which was determined by PET-CT/MRI, MRI, CT, ultrasound or X-ray imaging. PFS and LC were evaluated by censoring patients at their most recent imaging. TTS was defined as time of MDT to time of start of a new systemic therapy. Acute severe toxicity (grade ≥ 3 events, < 3 months after SRT) probably caused by MDT was analyzed using the Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Late severe (grade ≥ 3 events) toxicity was evaluated in patients with a follow-up of ≥ 3 months.
Descriptive statistical analysis was performed with SPSS v25.0 statistic software package (IBM Corp., Armonk, NY, USA). Kaplan–Meier survival curves with log-rank analysis for comparison of subgroups was used to evaluate OS, PFS, and TTS. The Fisher`s exact and Chi-square test was used to compare differences between two independent groups. Univariate and backward multivariate Cox regression analysis was performed to identify independent variables for OS, PFS and TTS. A p-value of less than 0.05 was deemed statistical significant.
Results
Patient characteristics
Data of 108 patients from 16 participating centers was included. Baseline patient, tumor and treatment characteristics are summarized in Table 1. Median patient age was 63 (range 33–80) years, 90% of patients had an ECOG performance score of ≤ 1, 41% had minimal co-morbidities (age-adjusted Charlson Comorbidity Index ≤ 3). The most frequent histological NSCLC subtype was adenocarcinoma (80%). Multiorgan metastatic disease was present in 71% of patients, with a median of 2 (range 1–7) involved organs per patient. Reported reasons for MDT in PPD patients were palliation of symptoms (26%), prevention of future complications (79%), attempt to extend treatment with current systemic therapy (11%) and to induce a possible immunomodulation effect (9%).
Table 1.
Patient characteristics of all 108 NSCLC patients treated with SRT concurrent to TT/IT
| N (%), median (range) | |
|---|---|
| All patients (n = 108) | |
| Age (years) | 63 (33–80) |
| Histology subtype | |
| ADC | 80 (74) |
| LCNEC | 3 (3) |
| SqCC | 6 (6) |
| Adenosquamous | 3 (3) |
| Unknown | 16 (15) |
| Synchronous metastatic disease | |
| Yes | 81 (75) |
| No | 27 (25) |
| Ligand expression/driver mutation | |
| EGFR | 49 (45) |
| ALK | 16 (15) |
| ROS1 | 2 (2) |
| PD-L1 | 5 (5) |
| No | 30 (28) |
| Unknown | 5 (5) |
| Previous systemic treatment lines | 1 (1–5) |
| Present metastases | |
| ≤ 5 | 53 (49) |
| > 5 | 55 (51) |
| Involved organs | 2 (1–7) |
| Status of primary tumor | |
| Controlled | 75 (69) |
| Progressive | 26 (24) |
| Unknown | 7 (7) |
| SRT treated lesions | |
| Brain | 144 (75) |
| Lymph nodes | 3 (2) |
| Lung | 18 (9) |
| Liver | 6 (3) |
| Adrenal gland | 3 (2) |
| Bone | 17 (9) |
| Soft tissue | 1 (0.5) |
| SRT treated lesions per patient | |
| Cranial | 1 (1–5) |
| Extracranial | 1 (1–3) |
| Type of systemic therapy | |
| EGFR/ALK-inhibitor | 65 (60) |
| PD-1/PD-L1-inhibitor | 33 (31) |
| Anti-VEGF-antibody | 9 (8) |
| PARP-inhibitor | 1 (1) |
| Prescribed BED10 (Gy) | |
| Cranial | 75 (26.6–113.9) |
| Extracranial | 95.3 (53.1–180) |
| Total GTV volume (cc) | |
| Cranial | 1.2 (0.04–15.3) |
| Extracranial | 8.4 (0.5–86.1) |
ADC adenocarcinoma, LCNEC large cell neuroendocrine carcinoma, SqCC squamous cell carcinoma
Targeted therapy/immunotherapy
Overall, 56% of patients received first line systemic therapy, while 44% were under second-line therapy or more (Table 1). Systemic therapy concurrent to MDT consisted of EGFR/ALK-inhibitors (60%), immune checkpoint inhibitors (31%), VEGF-antibodies (8%) and PARP-inhibitors (1%). In 10% of patients, these were combined with chemotherapy. Sixty-seven percent of patient started TT/IT before MDT, with a median of 269 days (range 1–180 days), 8% started IT/TT at the same time of SRT and 25% started IT/TT a median of 14 days (range 1–30) after SRT. Overall, in 28% of patients their systemic therapy was paused during MDT with a median of 10 (range 2–42) days. Targeted therapy was paused in 35% of the patients, for a median of 3 days before and 3 days after MDT (range 1–21 days). Immune checkpoint inhibitors were paused in 15% of patients, for a median of 7 days before and after MDT (range 1–19 days). Bevacizumab was paused in 20% of patients for a median of 14 days before up to 14 days after MDT (range 7–21 days). The decision to pausing TT/IT as well as the length of the TT/IT interruption during SRT was at the discretion of the participating center.
Stereotactic radiotherapy
In total, 192 lesions were irradiated. Brain metastases were the most frequent location (68%), with a median number of 1 (range 1–5) brain metastasis treated per patient. Median total tumor volume of brain metastases was 1.2 cc (range 0.04–15.3). Median prescribed dose for brain metastases was 20 Gy in 1 fraction (BED10 = 75 Gy). SBRT was performed in 32% of patients, PPD patients received SBRT less often compared to OPD patients (21% vs. 41% respectively). A median of 1 (range 1–3) extracranial metastases were treated simultaneously per patient. Extracranial metastases were treated with a median dose of 95.3 Gy (BED10) in median 3 fractions (range 1–5). Median planning targed volume of SBRT was 8.37 cc (range 0.54–86.10 cc).
Efficacy and factors influencing survival
Median follow-up was 18.7 (range 1–102) months. Median OS was 18.1 months, 2 year OS was 39% (Fig. 1). Cause of death was tumor-related in 85% and never therapy-related. There was no significant difference in OS between patients with- or without brain metastases (p = 0.181). There was also no significant difference in OS between patients receiving IT, TT, AAT or PARPi (p = 0.765). In univariate analysis, metastatic status (OPD vs. PPD) and number of affected organs were significant predictors of OS (Table 2). In multivariate analysis, metastatic status remained the only independent factor predicting OS (p = 0.002, HR 2.03 (95% CI; 1.3–3.17). Local control after SRT was 84% after 2 years, there was no difference between OPD and PPD. Overall, median PFS was 8.7 months. In OPD, this was median 10.4 months and 7.4 months for PPD patients, or 25% and 8% after 2 years, respectively (Fig. 1). In the univariate analysis, metastatic status, previous lines of systemic therapy and affected organs were significant predictors of PFS. In multivariate analysis, the number of previous lines of systemic therapy [p = 0.033, HR 1.7 (95%CI 1.05–2.77)] and number of affected organs (p = 0.023, HR 2.04 (95% CI 1.10–3.79) remained independent factors predicting PFS (Table 2).
Fig. 1.
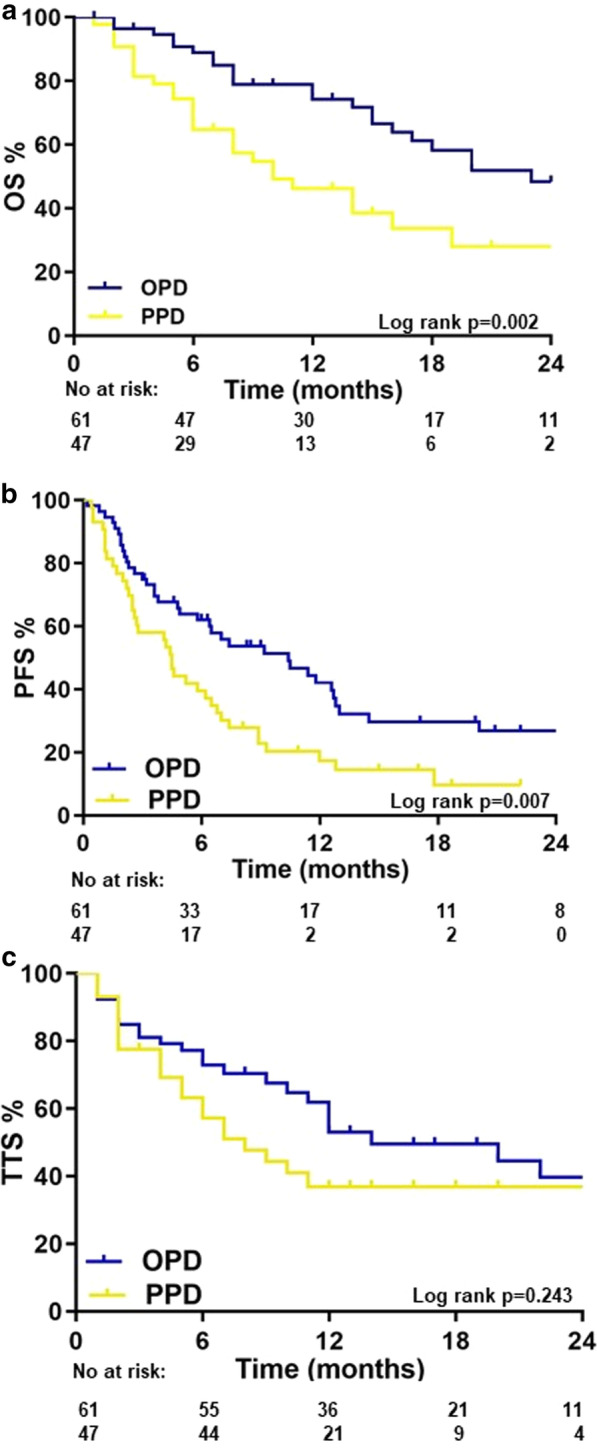
a Overall survival of metastatic NSCLC patients receiving metastasis directed therapy (MDT) concurrent to targeted- or immunotherapy (TT/IT). b Progression free survival (PFS) of oligoprogressive disease (OPD) NSCLC patients receiving MDT concurrent to targeted- or immunotherapy (TT/IT). c Time to systemic therapy change (TTS) after MDT in in NSCLC patients receiving concurrent SRT and TT/IT. Blue line = OPD patients where a MDT of all present metastases (≤ 5 metastases) was performed. Yellow line = OPD patients where MDT of all progressive lesions was performed and all other metastases are controlled by TT/IT
Table 2.
Uni- and multivariate Cox regression analysis
| Variables | OS | PFS | TTS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | ||||
| p value | p value | HR (95% CI) | p value | p value | HR (95% CI) | p value | p value | HR (95% CI) | |
| Cinical metastatic status (OPD, palliative intent) | 0.006 | 0.002 | 2.03 (1.30–3.17) | 0.002 | 0.334 | 0.053 | 0.938 | 1.0 (0.56–1.81) | |
| Initial stage (I–IV) | 0.567 | – | – | 0.402 | 0.561 | ||||
| Metastatic development (synchronous vs. metachronous) | 0.802 | – | – | 0.895 | 0.653 | ||||
| Previous lines of systemic treatment (1 vs. > 1) | 0.166 | 0.109 | – | 0.048 | 0.033 | 1.7 (1.05–2.77) | 0.093 | 0.152 | 1.57 (0.84–2.92) |
| Metastatic burden (1, 2–5, 6–10, > 10) | 0.461 | – | – | 0.062 | 0.759 | 0.051 | 0.686 | 1.10 (0.741.64) | |
| Affected organs (1 vs. > 1) | 0.03 | 0.633 | – | 0.004 | 0.023 | 2.04 (1.10–3.79) | 0.026 | 0.031 | 2.53 (1.09–5.89) |
| Location of metastases (cranial vs. extracranial) | 0.201 | 0.827 | – | 0.155 | 0.757 | 0.48 | |||
| Histology subtype (SqCC, ADC, LCNEC, adenosquamous, unknown) | 0.952 | – | – | 0.554 | 0.447 | ||||
| Gene mutation (yes vs. no) | 0.834 | – | – | 0.992 | 0.696 | ||||
| Status of primary tumor (controlled vs. progressive) | 0.973 | – | – | 0.701 | 0.824 | ||||
| Targeted therapy (IT, TT, AAT) | 0.855 | – | – | 0.424 | 0.64 | ||||
| SRT location (cranial, extracranial, both) | 0.25 | – | – | 0.149 | 0.571 | 0.767 | |||
| Number of SRT treated metastases (1–5) | 0.642 | – | – | 0.993 | 0.509 | ||||
| Start targeted therapy (before, during, after SRT) | 0.239 | – | – | 0.436 | 0.299 | ||||
| Targeted therapy paused during SRT (yes vs no) | 0.645 | – | – | 0.734 | 0.104 | 0.393 | 1.32 (0.75–2.34) | ||
p < 0.05 is significant
HR hazard ratio, CI confidence interval, TT targeted therapy, IT immunotherapy, AAT antiangiogenic therapy, PARPi PARP-inhibitor
Patterns of disease progression
In case of progression, only one organ was affected (range 1–4) in 69% of OPD patients and 36% of PPD patients. These recurrences were oligometastatic (≤ 5 new lesions) in 59% of OPD patients, with no difference between concurrent systemic therapy (p = 0.765). Forty-three percent of patients that developed a new oligoprogression received a second course of MDT. Other local therapies consisted of surgery in 2 patients and conventionally fractionated radiotherapy in 5 patients.
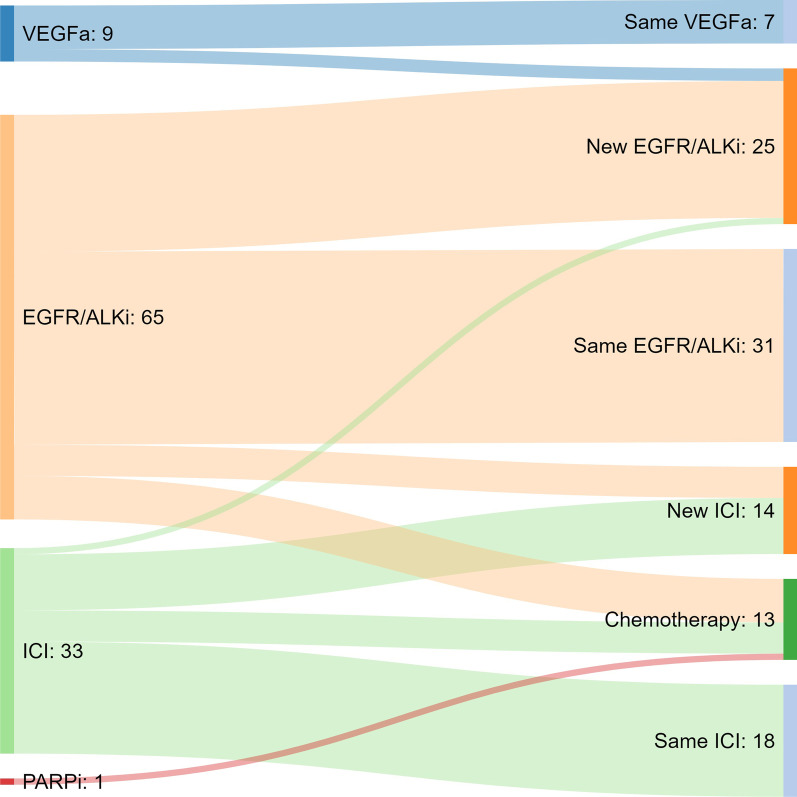
After one year, 58% of OPD patients and 39% of PPD patients were receiving the same systemic therapy as at the time of MDT. The median TTS was 14 months (range 5.7–22.3 months) for OPD patients and 8 months for PPD patients. There was no significant difference between administered systemic therapy (p = 0.220). In patients where systemic treatment was switched, the next line of treatment was usually a new targeted therapy in OPD patients (68%) and chemotherapy in PPD patients (52%) (Fig. 2). Patients receiving IT who had progressive disease after SRT switched to another IT (60%) or chemotherapy (33%), patients receiving TT who developed progressive disease after SRT most commonly switched to another TT (65%) (Fig. 2).
Fig. 2.
Flow chart of systemic therapy switch following SRT
Toxicity
Acute severe (≥ grade 3, < 30 days) toxicity likely caused or worsened by MDT was observed in 6 patients (5.5%), consisting of 7 grade 3 toxicities and 1 grade 4 toxicity (Table 3). Late severe toxicity (≥ grade 3, ≥ 30 days) was observed in 2 patients (1.9%); one patient with two 3 grade toxicities and 1 grade 4 toxicity, and one patient with grade 3 weight loss. Most severe toxicities occurred after SRT of brain metastases and in two cases after SBRT of lung metastases. All severe toxicities occurred in patients receiving immune checkpoint inhibition, VEGF-antibody or EGFR-inhibitors. There was no clear pattern of occurrence of severe toxicity observed (Table 3). No grade 5 toxicity occurred.
Table 3.
Observed infield acute and late severe (≥ grade 3) toxicity, graded by use of CTCAE v4.03
| Patient (n) | Toxicity | Grade | Location SRT | Treated metastases (n) | Treatment dose (Gy/fx) | Concurrent therapy | Start of concurrent therapy | Systemic therapy paused during SRT |
|---|---|---|---|---|---|---|---|---|
| Acute infield toxicity | ||||||||
| 1 | Headache | 3 | Brain | 3 | 24 Gy/1fx 100% Isodose) | Nivolumab | 8 days after SRT | – |
| 2 | Headache | 3 | Brain | 1 | 20 Gy/1fx (80% Isodose) | Bevacizumab | 365 days before SRT | No |
| 3 | Headache | 3 | Brain | 2 | 20 Gy/1fx (80% Isodose) | Nivolumab | 52 days before SRT | No |
| Gait disturbance | 3 | |||||||
| 4 | Headache | 4 | Brain | 3 | 20 Gy/1fx (80% Isodose) | Nivolumab | 11 days after SRT | – |
| Nausea | 3 | |||||||
| 5 | Dyspnea | 3 | Lung | 1 | 7 Gy/5fx (65% Isodose) | Gefitinib | 503 days before SRT | No |
| 6 | Thromboembolic event | 3 | Brain | 1 | 20 Gy/1fx (80% Isodose) | Osimertinib | 98 days before SRT | No |
| Late infield toxicity | ||||||||
| 7 | Radionecrosis | 3 | Brain | 5 | 20 Gy/1fx (80% Isodose) | Afatinib | 22 days before SRT | No |
| Nausea | 3 | |||||||
| Hemiparesis | 4 | |||||||
| 8 | Weight loss | 3 | Lung | 1 | 7 Gy/5fx (65% Isodose) | Erlotinib | 575 days before SRT | 4 days |
Discussion
This analysis of stage IV NSCLC patients receiving MDT for progressive or persistent metastases under targeted-, or immunotherapy showed survival rates in the OPD group that appear promising compared to literature on patients receiving TT/IT alone [15–17]. The concept of targeting OPD while continuing systemic therapy beyond progression is increasingly performed [18, 19]. This is based on the observation that (1) further lines of systematic treatment are characterized by a worse therapeutic effect, (2) MDT obtains good results with limited toxicity in the primary oligometastatic situation [7–9], and (3) whole genomic sequencing studies showing that through parallel evolution, subpopulations of metastatic clones are capable to metastasize themselves [20]. This may indicate that MDT of these lesions could possibly improve prognosis. Literature on the concept of MDT for OPD is still limited and includes, next to small retrospective studies [2–6], a phase II study, which showed that MDT of ≤ 6 progressive metastases in platinum-refractory NSCLC patients who received erlotinib resulted in a better PFS and OS than would be expected in patients receiving TT alone [21]. In all available studies, metastatic patients in varying phases of their treatment were included, which increases the difficulty to interpret their results and transfer the data to clinical practice. Furthermore, the increasing use of immunotherapy in this population has so far not been taken into account. Our study therefore analyzes real-life data of the efficacy and safety of MDT performed in metastatic NSCLC patients progressive under targeted-, as well as immunotherapy.
MDT resulted in a good OS in OPD patients, and allowed continuation of systemic therapy within the first year for many patients. The effect of MDT in the patient group treated with palliative intent was less pronounced in terms of OS and PFS. However, these patients were most frequently treated with SRT for brain metastases. Some TT/IT are characterized by good penetration of the blood–brain barrier; however, upfront MDT of cerebral metastases might improve patient outcome and quality of life [22–25].
When a new progression occurred after MDT, this was often again OPD and a repeat-irradiation was performed in most of these patients. The effectiveness of repeat-irradiations has been previously published for prostate cancer recurrences and treatment of brain metastases, generally resulting in an excellent local control with limited toxicity [22]. In our multivariate analysis, it was shown that the number of lines of systemic therapy influenced PFS. This reflects that the reducing efficacy of subsequent lines of therapy drives distant progression and appears to be more important than metastatic burden at time of MDT. Repeat-MDT instead of therapy-switch may therefore play an increasingly important role. However, experiences of repeat-irradiation remain limited and further studies need to investigate carefully the concept of repeat local treatment.
Although the concept of MDT for OPD under TT/IT is practiced with increasing frequency, several uncertainties remain. First of all, with the currently limited knowledge on the molecular background of resistant clones in NSCLC, it is not known whether preferably all metastases or just selected progressive metastases should be targeted, and what the threshold of the number and volume of targeted metastases should be [26]. Current decision-making is based on imaging and clinical criteria [11, 27], but studies on the biological status of metastatic NSCLC is urgently needed, including integration of liquid biopsy for staging and response assessment. Secondly, the timing of MDT currently remains a clinical decision, usually based on CT or PET-images. However, progression under TT/IT can be very slow, or after immunotherapy, a pseudoprogression could be observed. Thirdly, there might be different strategies in combining systemic therapies with MDT, as the effects of concurrent treatment may be diverse. For example, combining immunotherapy with MDT could possibly strengthen the antitumor immune response [28], whereas abscopal effects of radiotherapy are exceedingly unlikely in patients not receiving immunotherapy [26].
A limitation of this study is its retrospective nature which, however, is a way of a meaningful evaluation of a quickly changing clinical field and associated limitations in standardization of reporting of factors, such as toxicity and local control. Since there is a known risk of underreporting low grade toxicity in retrospective studies, only high grade toxicity was registered [29]. Furthermore, as all patients received a combined modality treatment, our study does not allow to evaluate the influence of the continued TT/IT alone on outcome. It may be possible, that patients with less metastatic sites have had a better prognosis irrespective of MDT. Especially under immunotherapy, residual sites potentially could remain stable for a longer time. However, for patients receiving targeted therapy a further progress of residual sites can be expected after several months. A first study comparing targeted therapy as monotherapy to a combined modality therapy with SRT indicated a benefit of combined therapy compared to targeted drugs alone [30] and will be further investigated in the randomized HALT (NCT03256981) and STOP-NSCLC NCT02756793 trials. Another limitation is the combined analysis of patients treated with IT/TT in one study population. This was done because the intention of MDT was similar irrespective of the systemic therapy, namely ablation of (oligo-) progressive metastases while the otherwise effective systemic therapy is continued.
In conclusion, metastases-directed SRT in NSCLC patients can be safely performed concurrent to TT/IT with a low risk of severe toxicity. To find the ideal sequence of the available multidisciplinary treatment options for NSCLC and determine what patients will benefit most, a further evaluated in a broader context within prospective clinical trials is needed continuation of TT/IT beyond progression combined with MDT for progressive lesions appears promising but requires prospective evaluation.
Acknowledgements
We would like to acknowledge Dr. Nicholas Bucknell, Dr. An Claes and Dr. Sabina Gerum for patient collection in the database and Dr. Alexander F.C. Hulsbergen for the support in the creation of Fig. 2.
Abbreviations
- AAT
Antiangiogenic therapy
- BED
Biologically effective dose
- CTCAE
Common terminology criteria for adverse events
- EGFRi
Epidermal growth factor receptor inhibitor
- IT
Immunotherapy
- MDT
Metastasis directed treatment
- NSCLC
Non small cell lung cancer
- OPD
Oligoprogressive disease
- PARPi
Poly(ADP-ribose)-polymerasen inhibitor
- PPD
Polyprogressive disease
- SBRT
Stereotactic body radiotherapy
- SRT
Stereotactic radiotherapy
- TT
Targeted therapy
- VEGFa
Vascular endothelial growth factor antibody
Authors' contributions
Conception/design of the work: MG, SK, CF. Acquisition and/or analysis: SK, JS, CF, DK, OB, KHK, FR, SS, JJCV, SA, MMS, MG, MS, MG, GK, IS, FL, FE. Interpretation of data: SK, MG, JJCV, SS, SA. Drafted the work or substantively revised it: SK, MG, JJCV, SS. All authors read and approved the final manuscript.
Funding
This work was financially supported by Varian Medical Systems. Varian Medical Systems was not involved in the study design, collection, analysis and interpretation of data, the writing of the report or in the decision to submit the article for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethics committees at all participating sites (BASEC-Nr. 2016-01807). A informed consent of all participants was obtained.
Consent for publication
Not applicable.
Competing interests
SA is supported by the German Cancer Consortium (DKTK), M Geier received a speaker fee from Roche and a speaker fee from Bristol-Myers-Squibb. SS is supported by a National Health and Medical Research Council (NHMRC) fellowship, and Cancer Council Victoria (CCV) Colebatch Fellowship. MS received a speaker fee from Merck Serono, AstraZeneca and Pierre Fabre. SK received a speaker fee from AstraZeneca.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 2.Gan GN, Weickhardt AJ, Scheier B, Doebele RC, Gaspar LE, Kavanagh BD, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–898. doi: 10.1016/j.ijrobp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merino Lara T, Helou J, Poon I, Sahgal A, Chung HT, Chu W, et al. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: Delaying the need to start or change systemic therapy? Lung Cancer. 2018;124:219–226. doi: 10.1016/j.lungcan.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Schmid S, Klingbiel D, Aeppli S, Britschgi C, Gautschi O, Pless M, et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: A Swiss cohort study. Lung Cancer. 2019;130:149–155. doi: 10.1016/j.lungcan.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Weickhardt AJ, Scheier B, Burke JM, Gan G, Lu X, Bunn PA, Jr, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez DR, Tang C, Zhang J, Blumenschein GR, Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palma DO, R.; Harrow, S.; Gaede, S.; Louie, A.; Haasbeek, C. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet Oncol. 2019;s0140–6736:32487–5. [DOI] [PubMed]
- 10.Pitroda SP, Khodarev NN, Huang L, Uppal A, Wightman SC, Ganai S, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018;9:1793. doi: 10.1038/s41467-018-04278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashworth A, Rodrigues G, Boldt G, Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 13.Lovly CM, Iyengar P, Gainor JF. Managing resistance to EFGR- and ALK-targeted therapies. Am Soc Clin Oncol Educ Book. 2017;37:607–618. doi: 10.14694/EDBK_176251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leeuwen CM, Oei AL, Crezee J, Bel A, Franken NAP, Stalpers LJA, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol. 2018;13:96. doi: 10.1186/s13014-018-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodeweges JE, Klinkenberg TJ, Ubbels JF, Groen HJM, Langendijk JA, Widder J. Long-term outcome of surgery or stereotactic radiotherapy for lung oligometastases. J Thorac Oncol. 2017;12:1442–1445. doi: 10.1016/j.jtho.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Russo A, Franchina T, Ricciardi GRR, Smiroldo V, Picciotto M, Zanghi M, et al. Third generation EGFR TKIs in EGFR-mutated NSCLC: where are we now and where are we going. Crit Rev Oncol Hematol. 2017;117:38–47. doi: 10.1016/j.critrevonc.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 18.Al-Halabi H, Sayegh K, Digamurthy SR, Niemierko A, Piotrowska Z, Willers H, et al. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1601–1607. doi: 10.1097/JTO.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 19.Campo M, Al-Halabi H, Khandekar M, Shaw AT, Sequist LV, Willers H. Integration of stereotactic body radiation therapy with tyrosine kinase inhibitors in stage IV oncogene-driven lung cancer. Oncologist. 2016;21:964–973. doi: 10.1634/theoncologist.2015-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32:3824–3830. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 22.Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro Oncol. 2016;18:1043–1065. doi: 10.1093/neuonc/now127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habets EJ, Dirven L, Wiggenraad RG, Verbeek-de Kanter A, Lycklama ANGJ, Zwinkels H, et al. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol. 2016;18:435–444. doi: 10.1093/neuonc/nov186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 25.Ni J, Li G, Yang X, Chu L, Wang J, Li Y, et al. Optimal timing and clinical value of radiotherapy in advanced ALK-rearranged non-small cell lung cancer with or without baseline brain metastases: implications from pattern of failure analyses. Radiat Oncol. 2019;14:44. doi: 10.1186/s13014-019-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunne EM, Fraser IM, Liu M. Stereotactic body radiation therapy for lung, spine and oligometastatic disease: current evidence and future directions. Ann Transl Med. 2018;6:283. doi: 10.21037/atm.2018.06.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiwert TY, Kiess AP. J Clin Oncol. 2020 Sept 28: Online ahead of print.
- 28.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Fukushi K, Narita T, Hatakeyama S, et al. Difference in toxicity reporting between patients and clinicians during systemic chemotherapy in patients with urothelial carcinoma. Int J Urol. 2017;24(5):361–366. doi: 10.1111/iju.13318. [DOI] [PubMed] [Google Scholar]
- 30.Elamin YY, Gomez DR, Antonoff MB, Robichaux JP, Tran H, Shorter MK, et al. Local consolidation therapy (LCT) after first line tyrosine kinase inhibitor (TKI) for patients with EGFR mutant metastatic non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2019;20:43–47. doi: 10.1016/j.cllc.2018.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.