Abstract
Background
Short-chain fatty acids (SCFAs) and serotonin (5-hydroxytryptamine, 5-HT) may be associated with the pathogenesis of irritable bowel syndrome (IBS). There are some reports of alterations in SCFAs and 5-HT in IBS, but their results are inconsistent. We aimed to perform a meta-analysis to assess alterations in SCFAs and 5-HT in IBS patients and their potential role in the abnormal brain-gut-microbiota (BGM) axis.
Methods
Case–control studies detecting SCFAs and 5-HT in IBS patients were identified from PubMed, Web of Science, Cochrane Library, and Scopus databases to identify relevant articles up to September 2018. The standardized mean differences (SMDs) with 95% confidence intervals (CIs) of SCFAs and 5-HT were calculated by REVIEW MANAGER 5.3 to evaluate the alterations of 5-HT and SCFAs in IBS.
Results
Five studies on SCFAs and 5 on 5-HT in IBS patients were included. As compared to healthy controls (HCs), the SMDs of 5-HT in IBS patients was 2.35 (95% CI 0.46–4.24) and the SMDs of total SCFAs, acetic acid, propionic acid, and butyric acid in IBS patients were − 0.01 (95% CI − 0.57–0.55), − 0.04 (95% CI − 0.55–0.47), 0.07 (95% CI − 0.45–0.60), and − 0.00 (95% CI − 0.49–0.49), respectively.
Conclusions
There was an increase in 5-HT in blood of IBS patients, indicating the increased 5-HT in blood may be involved in IBS pathogenesis. However, there were no significant differences in SCFAs in feces between IBS patients and HCs. But the study did not differentiate between subgroups of IBS. These findings might provide insight for future studies of the BGM axis in the pathogenesis of IBS.
Mei Luo and Xiaojun Zhuang contributed equally to the writing of this article
Keywords: Irritable bowel syndrome, Short-chain fatty acids, Serotonin, Brain-gut-microbiota axis, Meta-analysis
Background
Irritable bowel syndrome (IBS) is one of the most common functional gastrointestinal disorders with uncertain pathogenesis, and serious clinical symptoms (such as abdominal pain, diarrhoea or constipation) will have a significant impact on the quality of life in IBS patients [1, 2]. Visceral hypersensitivity, altered gastrointestinal permeability, immune dysfunction or psychological disorder have been generally accepted as potential contributors to the pathogenesis of IBS [3]. Accumulating evidence has demonstrated that alterations in gut microbiota and dysregulation of the brain-gut axis may be associated with IBS pathogenesis [4–7]. Moreover, the gut microbiota may be involved in IBS pathogenesis through the production of metabolites such as short-chain fatty acids (SCFAs) [8]. In the brain-gut axis, the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT), associated with visceral hypersensitivity and gastrointestinal permeability, has attracted much attention [9, 10]. Mounting evidence detailing the bidirectional interactions between the gut microbiota and the brain supports the concept of the brain-gut-microbiota (BGM) axis, integrating the central nervous system (CNS), gastrointestinal tract (GIT), and microbiota [11, 12].
The brain-gut axis is the interactional two-way regulating axis of the CNS and gastrointestinal function which comprises the CNS, enteric nervous system (ENS), neuroendocrine and immune system [13]. 5-HT, as an important neurotransmitter in the brain-gut axis, may regulate gastrointestinal motility, visceral sensing, and mucosal secretion by activating both intrinsic excitatory and inhibitory enteric motor neurons. While cholinergic neurons can be stimulated by 5-HT to release acetylcholine and further contract smooth muscle, 5-HT can also irritate inhibitory nitrergic neurons to produce nitric oxide to relax smooth muscle [7]. An excess of 5-HT may trigger primary neuronal afferents, leading to visceral hyperalgesia [14]. Thus, in recent years, 5-HT has attracted considerable attention due to its potential effects on gastrointestinal motility, visceral sensory function, and psychophysiology in IBS [15].
Studies worldwide have provided convincing evidence that gut microbiota alterations may be associated with IBS [3, 4, 16]. However, the causal relationship remains unclear. Interactions between the gut microbiota and host cells on the gut mucosal surface could affect the intestinal microenvironment. Conversely, an altered intestinal microenvironment could modify the gut microbiota [17]. SCFAs are the main metabolites of the gut microbiota and may stabilize the intestinal microenvironment and modulate the gut microbiota [18]. Chassard et al. [19] reported dysbiosis of multiple microbes in IBS patients, and a reduction in Rhodobacillus coli may directly lead to SCFA deficiency, ultimately resulting in the visceral pain of IBS. In addition, with elevated concentrations of SCFAs, the pH of the intestinal tract could be reduced. At low pH, the proliferation of probiotic bacteria may be promoted while some pathogenic bacteria may be inhibited [20].
It has been reported that SCFAs and 5-HT might be involved in the bidirectional interactions of the BGM axis [21, 22]. Alterations in SCFAs and 5-HT in IBS patients have been demonstrated in some studies, and these alterations may influence gut motor activity and induce various physical symptoms [10, 23, 24]. However, there remains no consensus regarding the role of alterations in SCFAs and 5-HT in the pathogenesis of IBS. Therefore, we aimed to compare alterations in SCFAs and 5-HT in IBS patients and healthy controls (HCs) to explore their potential involvement in the BGM axis in IBS. We performed a meta-analysis to provide more definitive information on whether alterations in SCFAs and 5-HT between IBS patients and HCs contribute to IBS. Our findings provide insight for further explorations of the roles of SCFAs and 5-HT in the BGM axis and their participation in the pathogenesis of IBS.
Methods
Information sources and search strategy
In this meta-analysis, we searched the PubMed, Web of Science, Cochrane Library, and Scopus databases to identify relevant articles up to September 2018. We used “irritable bowel syndrome”, “IBS”, “short-chain fatty acids”, “SCFAs”, “serotonin”, “5-hydroxytryptamine”, and “5-HT” as the search terms. We further retrieved the references from included studies to search potential articles. Finally we selected articles according to the following inclusion and exclusion criteria.
Inclusion and exclusion criteria
Studies were included if they were case–control studies detecting SCFAs and 5-HT in IBS patients and HCs. Below are the inclusion criteria to our meta analysis: (a) studies about IBS patients and HCs; (b) the participants are adults (both IBS patients and HCs); (c) studies measuring SCFAs or 5-HT; (d) available data in the articles and can eventually be expressed as mean ± SD; and (e) sufficient data to calculate the standardized mean difference (SMD) with 95% confidence interval (CI) between the IBS patients and HCs. Two researchers selected the studies that met the predetermined inclusion criteria independently. All potentially relevant papers were obtained and evaluated in detail. Any disagreement between researchers was resolved through discussion until consensus.
Below are the exclusion criteria to our meta analysis: (a) not a case–control study; (b) not adult participants; (c) no full text; and (d) no available or enough data. According to the inclusion and exclusion criteria, 10 articles (5 articles on SCFAs and 5 on 5-HT) were selected for our meta-analysis.
Data extraction
We extracted the following data after reviewed every article: title of the article, name of the first author, year of publication, country, diagnostic criteria of IBS, age and sex of participants, number of IBS patients, number of HCs, methods by which levels of SCFAs and 5-HT were analyzed, available data expressed as mean ± SD (Tables 1, 2, 3, 4, 5), and study samples of the article. Disagreements regarding data extraction were resolved through discussion until consensus.
Table 1.
The total SCFAs in IBS patient and HC
| References | IBS patient | HC | ||||
|---|---|---|---|---|---|---|
| Mean (mmol/l) |
SD | Number | Mean (mmol/l) |
SD | Number | |
| Kopecny and Simunek [27] | 88.15 | 207.44 | 16 | 108.95 | 60.01 | 5 |
| Mortensen et al. [8] | 96.89 | 3.64 | 18 | 114.00 | 19.00 | 9 |
| Ringel-Kulka et al. [26] | 92.10 | 44.10 | 114 | 92.00 | 33.30 | 33 |
| Tana et al. [25] | 107.10 | 31.10 | 26 | 85.80 | 28.60 | 26 |
| Vernia et al. [28] | 131.40 | 62.60 | 38 | 108.50 | 58.30 | 50 |
SCFAs, short-chain fatty acids; IBS, irritable bowel syndrome; HC, healthy control
Table 2.
The acetic acid in IBS patient and HC
| References | IBS patient | HC | ||||
|---|---|---|---|---|---|---|
| Mean (mmol/l) |
SD | Number | Mean (mmol/l) |
SD | Number | |
| Kopecny and Simunek [27] | 60.16 | 126.08 | 16 | 73.26 | 44.70 | 5 |
| Mortensen et al. [8] | 62.67 | 3.14 | 18 | 73.00 | 11.00 | 9 |
| Ringel-Kulka et al. [26] | 58.00 | 25.80 | 114 | 56.60 | 18.50 | 33 |
| Tana et al. [25] | 67.00 | 18.80 | 26 | 56.70 | 18.00 | 26 |
| Vernia et al. [28] | 83.20 | 43.70 | 38 | 70.00 | 49.80 | 50 |
IBS, irritable bowel syndrome; HC, healthy control
Table 3.
The propionic acid in IBS patient and HC
| References | IBS patient | HC | ||||
|---|---|---|---|---|---|---|
| Mean (mmol/l) |
SD | Number | Mean (mmol/l) |
SD | Number | |
| Kopecny and Simunek [27] | 18.06 | 45.00 | 16 | 26.08 | 13.53 | 5 |
| Mortensen et al. [8] | 18.11 | 2.05 | 18 | 21.00 | 5.00 | 9 |
| Ringel-Kulka et al. [26] | 18.20 | 9.80 | 114 | 20.00 | 8.00 | 33 |
| Tana et al. [25] | 20.50 | 9.20 | 26 | 15.30 | 6.60 | 26 |
| Vernia et al. [28] | 23.70 | 13.10 | 38 | 17.10 | 8.00 | 50 |
IBS, irritable bowel syndrome; HC, healthy control
Table 4.
The butyric acid in IBS patient and HC
| References | IBS patient | HC | ||||
|---|---|---|---|---|---|---|
| Mean (mmol/l) |
SD | Number | Mean (mmol/l) |
SD | Number | |
| Kopecny and Simunek [27] | 9.93 | 23.92 | 16 | 9.16 | 4.94 | 5 |
| Mortensen et al. [8] | 10.00 | 1.67 | 18 | 12.00 | 3.00 | 9 |
| Ringel-Kulka et al. [26] | 15.80 | 12.10 | 114 | 15.40 | 8.90 | 33 |
| Vernia et al. [25] | 18.60 | 15.10 | 38 | 13.00 | 8.50 | 50 |
IBS, irritable bowel syndrome; HC, healthy control
Table 5.
The 5-HT in IBS patient and HC
| References | IBS patient | HC | ||||
|---|---|---|---|---|---|---|
| Mean (nmol/l) |
SD | Number | Mean (nmol/l) |
SD | Number | |
| Thijssen et al. [32] | 10.99 | 22.75 | 154 | 10.84 | 13.44 | 137 |
| Keszthelyi et al. [33] | 26.20 | 18.20 | 15 | 1.90 | 1.36 | 15 |
| Yu et al. [30] | 35.31 | 2.15 | 30 | 10.55 | 1.35 | 30 |
| Park et al. [29] | 85.75 | 76.67 | 21 | 31.49 | 23.49 | 13 |
| Dunlop et al. [31] | 29.53 | 7.35 | 15 | 41.00 | 6.90 | 15 |
5-HT, 5-hydroxytryptamine; IBS, irritable bowel syndrome; HC, healthy control
Quality assessment of included studies
The quality of each study was assessed by our study team according to the Newcastle–Ottawa Scale for case–control studies, which includes three aspects: selection, comparability, and exposure or outcome evaluation in the study population. The selection criteria comprise four items: (a) is the case definition adequate? (b) representativeness of the cases, (c) selection of the controls, and (d) determination of controls. The comparability criteria include comparability of cases and controls on the basis of the design or analysis. The exposure criteria comprise three items: (a) ascertainment of exposure, (b) same method of ascertainment for cases and controls, and (c) non-response rate. Disagreements were resolved through discussion until consensus.
Statistical analysis
We performed a meta-analysis of alterations in SCFAs and 5-HT in IBS patients. REVIEW MANAGER 5.3, developed by staff at the Australasian Cochrane Centre in 2008 and revised in June 2014 to incorporate new features, was used for statistical analysis of continuous data. When the necessary continuous data were available, we calculated the SMD with 95% CI for the data. The I2 statistic was used to examine heterogeneity across studies. An I2 value close to 0% implies low heterogeneity, while an I2 value close to 100% implies high heterogeneity. In cases of obvious heterogeneity (I2 value > 50%), a random effects meta-analysis model was used.
Funnel plot analysis for publication bias
A funnel plot was used to assess publication bias. Publication bias leads to asymmetry of the funnel plot, as the dispersion of smaller samples is larger and scatters more widely at the bottom of the graph, while the dispersion of larger sample is smaller, resulting in a narrower spread at the top of the plot.
Results
Study selection
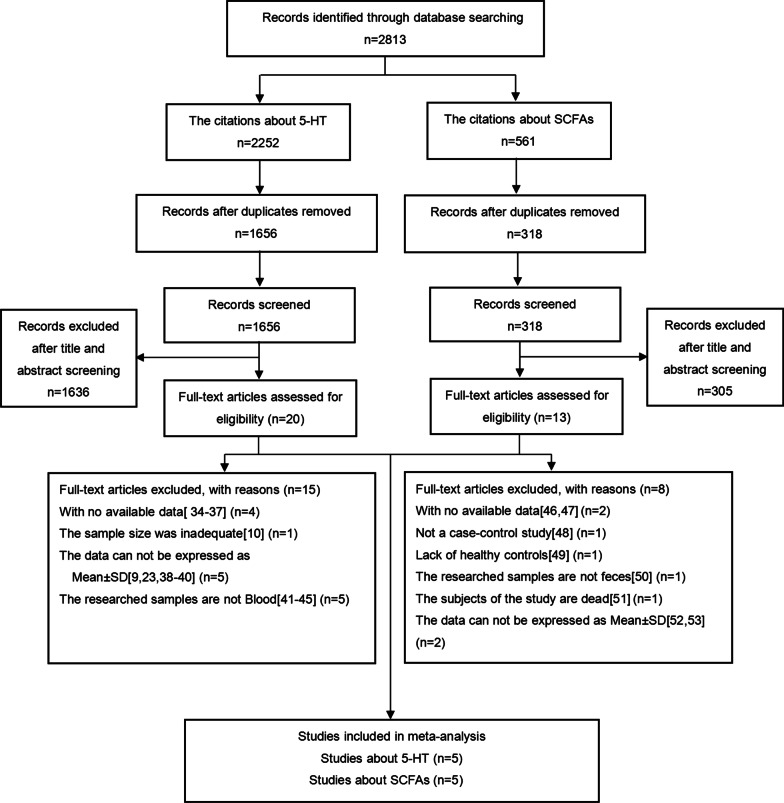
As shown in Fig. 1, a total of 2813 citations were obtained for review of titles and abstracts. After excluding duplicate and irrelevant articles, the remaining 13 articles on SCFAs and 20 articles on 5-HT was retrieved for review. Twenty-three studies were excluded for various reasons, as described in the flowchart (Fig. 1). Finally, five articles [8, 25–28] on SCFAs and five articles [29–33] on 5-HT were included in our meta-analysis. The two reviewers from our team were in full agreement in selecting these 10 studies.
Fig. 1.
Flow diagram of assessment of studies identified in the meta-analysis
Study characteristics
The characteristics of the studies are summarized in Tables 6 and 7. A total of 212 IBS patients and 123 HCs were included in the 5 studies on SCFAs. In all studies, SCFAs were analyzed from fecal samples. Four studies measured SCFA levels by gas chromatography, while one used high-pressure liquid chromatography (HPLC). Data from four studies were expressed in the form of mean ± SD and from one study as mean ± SEM. We then converted the mean ± SEM into the mean ± SD. SCFA levels are expressed as concentrations in mmol L−1. We analyzed alterations in total SCFAs, acetic acid, propionic acid, and butyric acid in IBS patients.
Table 6.
Characteristics of the five studies of SCFAs in the meta-analysis
| References | Year | Location | n, IBS | n, Control | IBS diagnosis | Control composition | Age, IBS (range, ) |
Female IBS, n |
Age, HC (range, ) |
Female HC, n |
Sample | Technique |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ringel-Kulka et al. [26] | 2015 | America | 114 | 33 | Rome III | Healthy controls | 35.4 ± 11.3 | 98 | 33.9 ± 13.0 | 29 | Feces | Gas chromatography |
| Tana et al. [25] | 2010 | Japan | 26 | 26 | Rome II | Healthy controls | 21.7 ± 2.0 | 13 | 21.9 ± 3.9 | 13 | Feces | HPLC |
| Kopecny and Simunek [27] | 2002 | Czech | 16 | 5 | Not reported | Healthy controls | Not reported | Not reported | Not reported | Not reported | Feces | Gas chromatography |
| Vernia et al. [28] | 1987 | Italy | 38 | 50 | Not reported | Healthy controls | Not reported | Not reported | Not reported | Not reported | Feces | Gas liquid chromatograph |
| Mortensen et al. [8] | 1987 | Denmark | 18 | 9 | Not reported | Healthy controls | 26–68 | 14 | Not reported | Not reported | Feces | Gas liquid chromatography |
SCFAs, short-chain fatty acids; IBS, irritable bowel syndrome; HC, healthy control; HPLC, high-pressure liquid chromatography
Table 7.
Chracteristics of the included studies of 5-HT in this meta-analysis
| References | Year | Location | n, IBS | n, Control | IBS diagnosis | Control Composition |
Age, IBS (range, ) |
Female IBS, n |
Age, HC (range, ) |
Female HC, n |
Sample | Technique |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yu et al. [30] | 2016 | China | 30 | 30 | Not reported | Healthy controls | 39 ± 21 | 14 | 42.5 ± 19.5 | 15 | Serum | HPLC |
| Dunlop et al. [31] | 2005 | Britain | 15 | 15 | Rome II | Healthy controls | 35.1 ± 2.9 | 15 | 35.9 ± 2.6 | 10 | Plasma | HPLC |
| Thijssen et al. [32] | 2015 | Netherlands | 154 | 137 | Rome III | Healthy controls | 44.5 ± 16.3 | 108 | 44.2 ± 19.3 | 84 | Plasma | HPLC |
| Park et al. [29] | 2009 | Korea | 21 | 13 | Rome II | Healthy controls | 24–78 | 13 | 29–68 | 6 | Plasma | HPLC |
| Keszthelyi et al. [33] | 2013 | Netherlands | 15 | 15 | Rome III | Healthy controls | 44 ± 13 | 8 | 33 ± 17 | 10 | Plasma | HPLC |
5-HT, 5-hydroxytryptamine; IBS, irritable bowel syndrome; HC, healthy control; HPLC, high-pressure liquid chromatography
For the five studies on 5-HT, 235 IBS patients and 210 HCs were included in our study. In all studies, 5-HT was analyzed in blood samples (four in plasma samples and one in serum sample) and measured by HPLC. The data from three studies were expressed as the mean ± SD, one study as the mean ± SEM, and one study as the median and range. We converted the available data to the form of mean ± SD where necessary. Concentrations of 5-HT are expressed as nmol L−1. Among the 10 articles included in our study, IBS patients and HCs were age- and sex-matched in 7 studies.
Assessment of study quality
As shown in Table 8, we used Newcastle–Ottawa Scale to evaluate the quality of each case–control study. All studies included were of moderate quality but did not influence the eligibility of the studies.
Table 8.
Quality assessment of the included studies
| References | Year | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|---|
| Ringel-Kulka et al. [26] | 2015 | 4 | 1 | 2 | 7 |
| Tana et al. [25] | 2010 | 4 | 1 | 2 | 7 |
| Kopecny and Simunek [27] | 2002 | 2 | 1 | 2 | 5 |
| Vernia et al. [28] | 1987 | 2 | 1 | 2 | 5 |
| Mortensen et al. [8] | 1987 | 2 | 1 | 2 | 5 |
| Yu et al. [30] | 2016 | 2 | 1 | 2 | 5 |
| Dunlop et al. [31] | 2005 | 4 | 1 | 2 | 7 |
| Thijssen et al. [32] | 2015 | 4 | 1 | 2 | 7 |
| Park et al. [29] | 2009 | 4 | 1 | 2 | 7 |
| Keszthelyi et al. [33] | 2013 | 4 | 1 | 2 | 7 |
Selection includes four criteria: (1) is the determination of case adequate? (2) the representation of the cases, (3) the selection of the controls, (4) The determination of the controls; Comparability criteria include the Comparability of cases and controls is taken into account in design and statistical analysis; Exposure contains three criteria: (1) determination of exposure factors, (2) use the same method to determine the exposure factors in case and control group, (3) non-response rate
Meta-analysis of SMD
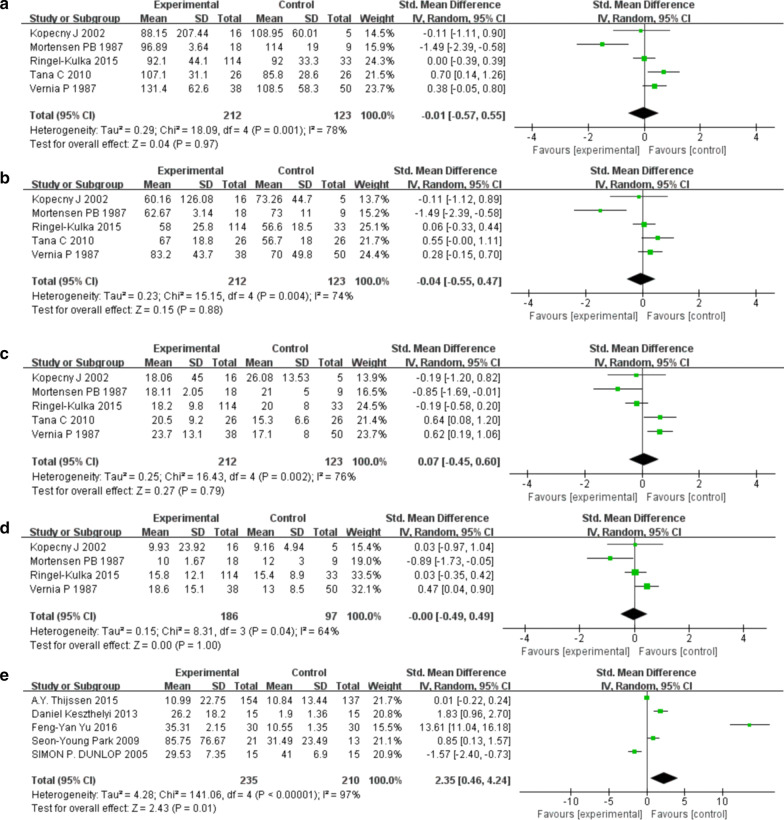
Our meta-analysis analyzed alterations in SCFAs (total SCFAs, acetic acid, propionic acid, and butyric acid) and 5-HT in IBS patients from feces and blood respectively. As compared to HCs, the SMD of total SCFAs was − 0.01 (95% CI − 0.57–0.55), that of acetic acid was − 0.04 (95% CI − 0.55–0.47), that of propionic acid was 0.07 (95% CI − 0.45–0.60), and that of butyric acid was − 0.00 (95% CI − 0.49–0.49), as shown in Fig. 2a–d. There was significant heterogeneity in the studies included, with I2 values over 50%.
Fig. 2.
Forest plots of alterations of SCFAs and 5-HT in IBS patients versus HCs: a total SCFAs, b acetic acid, c propionic acid, d butyric acid, e 5-HT
As shown in Fig. 2e, the SMD of 5-HT in IBS patients was 2.35 (95% CI 0.46–4.24). There was also significant heterogeneity in the studies included, with an I2 value of 97%.
Analysis of funnel plots for publication bias
As shown in Fig. 3a–e, funnel plots were used to assess publication bias among the studies included. However, as the number of included studies was below 10, the ability of these funnel plots to detect publication bias is limited.
Fig. 3.
Funnel plots of alterations of SCFAs and 5-HT in IBS patients versus HCs: a total SCFAs, b acetic acid, c propionic acid, d butyric acid, e 5-HT
Discussion
In recent years, alterations in bidirectional brain-gut interactions have been considered to play a vital role in IBS and some related functional gastrointestinal diseases [18, 54]. Alterations in the gut microbiota have also been confirmed as mechanisms of IBS pathogenesis worldwide [3]. It has been generally accepted that a stable gut microbiota is essential for normal gut physiology and contributes to appropriate signaling along the brain-gut axis [55]. SCFAs, as the main metabolites of the gut microbiota, and 5-HT, as an important regulatory factor of the brain-gut axis, may be involved in the abnormal BGM axis of IBS. Alterations in SCFAs in feces and 5-HT in blood have been found in IBS patients in some studies [27, 28, 30–32]. This is the first meta-analysis to explore the alterations in SCFAs and 5-HT in IBS, and some interesting findings were observed in our meta-analysis. We found that 5-HT levels in the blood in IBS patients were increased compared to those in HCs. However, we found no significant differences in SCFAs in feces between IBS patients and HCs.
Ninety-five percent of human 5-HT comes from the intestines, mainly the intestinal EC cells of the mucous layer, which contains tryptophan hydroxylase (TPH), necessary for the synthesis of 5-HT. The release of 5-HT in EC cells is sensitive to the pressure and chemical stimulation in the lumen [56]. In our study, we found that 5-HT levels were increased in the blood in IBS patients compared to those in HCs. Only one study by Dunlop et al. [31] reported lower 5-HT levels in IBS patients. In the 5 studies on 5-HT in our meta-analysis, all researchers used high-pressure liquid chromatography (HPLC) to measure 5-HT levels in subjects. However, their findings were inconsistent, which may imply that the HPLC method is unstable and has poor repeatability. We did not analyze 5-HT levels among different subtypes of IBS patients in our study. However, some studies have found that differential alterations in 5-HT in various IBS subtypes. Atkinson et al. [9] reported that 5-HT levels were reduced in patients with constipation-predominant IBS (IBS-C). Bearcroft et al. [10] and Houghton et al. [24] found increased 5-HT levels in patients with diarrhea-predominant IBS (IBS-D). These inconsistent findings indicate that alterations in 5-HT may be related to the symptoms of constipation or diarrhea in IBS patients. Houghton et al. [24] also reported that food intake could stimulate colonic motor activity, alter visceral sensitivity, increase blood 5-HT levels, and lead to abdominal pain or diarrhea in IBS patients. Hence, we speculate that alterations in 5-HT may be related to IBS pathogenesis. Our findings support the idea that 5-HT may be a therapeutic target for IBS. In fact, some drugs that act on 5-HT signaling are currently used to treat IBS. Tegaserod, a 5-HT4 partial agonist and alosetron, a 5-HT3 antagonist is used in IBS-C and IBS-D to relieve symptoms, respectively. Other agents regulating the levels of 5-HT, such as tricyclic antidepressants and serotonin selective reuptake inhibitors, have also been used in some patients with IBS [56]. Although we found differences in 5-HT levels in blood between IBS patients and HCs, further studies to explore the correlation between altered 5-HT levels and IBS symptoms are warranted.
SCFAs are a type of saturated fatty acids which including acetic acid, propionic acid, butyric acid, isobutyric acid, isopentoic acid, caproic acid, and isocaproic acid. The main SCFAs distributed in the intestines are acetic acid, propionic acid, and butyric acid. Most SCFAs are absorbed by the intestines, while only low levels of SCFAs escape absorption and can be detected in fecal samples [57]. In our study, we found there were no significant differences in total SCFAs in the feces between the IBS patients and HCs from the available data. We then explored differences in acetic acid, propionic acid, and butyric acid between IBS patients and HCs. Similarly, there were no significant differences in acetic acid, propionic acid, or butyric acid between IBS patients and HCs. Although there were no significant alterations in SCFAs in IBS patients, some studies have reported that SCFAs are altered in the feces of IBS patients [25, 27, 28]. Tana et al. [25] found that levels of acetic acid, propionic acid, and total SCFAs were significantly higher in IBS patients than in controls. These findings were similar to the results reported by Vernia et al. [28]. However, Kopecny et al. [27] found that the fecal SCFAs of IBS patients were characterized by lower levels of total SCFAs, acetic acid, and propionic acid and by higher levels of butyric acid. Although there is no consensus regarding alterations in SCFAs in IBS patients, the altered SCFAs reported in the abovementioned studies may imply their involvement in the pathogenesis of IBS. Interestingly, some studies have reported that SCFAs are associated with symptoms in IBS patients. Soret et al. [58] found that SCFAs could induce plasticity of intestinal myometrial neurons and promote colonic motility, which may cause diarrhea in IBS patients. In addition, Cherbut et al. [59] and Grider et al. [60] reported that the activity of SCFAs in regulating colonic motility may be affected by their concentrations. Thus, a low concentration (10–100 mmol L−1) of SCFAs could increase colonic motility or have no effect, while a high concentration (> 100 mmol L−1) of SCFAs could inhibit colonic motility. Maintenance of gut microbiota homeostasis could release SCFAs to maintain intestinal mucosal permeability [61]. Dysbiosis of the gut microbiota may lead to insufficient SCFA intake in IBS patients, which directly constrains the distribution of compact connexin and then increases intestinal mucosal permeability [62]. Regulating SCFAs through the intestinal microenvironment may be a potential therapeutic target for IBS. Clinical trials have found that relevant SCFA products can improve IBS symptoms; for example, sodium butyrate reduced visceral pain in IBS patients [53, 63]. Although some studies have reported alterations in SCFAs in feces of IBS patients and the association of these alterations with symptoms in IBS patients, we found no significant differences in SCFA levels in feces in IBS patients and HCs in our meta-analysis. This implies that the gut microbiota may be involved in the pathogenesis of IBS through pathways other than SCFAs, such as alterations in immune profiles, effects on the CNS, or modulation of the gut neuromuscular function [62], or most SCFAs are absorbed in the colon and the analyzed results from feces are not representative for SCFAs in other parts of the gastrointestinal tract. However, the lack of significant differences in SCFAs between IBS patients and HCs in our meta-analysis may also be due to inadequate sample sizes in the original studies. The analytical methods or samples used to measure SCFA levels also need improvement and optimization in future studies.
As our study indicated that the 5-HT was increased in blood with IBS patients. 5-HT is the significant signaling molecule in the BGM axis and Clarke et al. [64] found the gut microbiota regulated the synthesis of 5-HT in CNS, which might further suggest that the disorder of the BGM axis may be the pathogenesis of IBS.
There are some limitations to our study. First, the statistical heterogeneity was significant among the included studies. This could be explained by differences in analytical methods, sample size, and diagnostic criteria for IBS in the included studies. Second, some studies used intestinal mucosal tissues as samples to analyze 5-HT levels in IBS patients and HCs [33, 40–45]. Data from these studies could not be processed effectively and were not included in our meta-analysis. This may lead to a bias in our meta-analysis results. Lastly, there are four subtypes of IBS, including IBS-C and IBS-D. However, our meta-analysis did not analyze the various subtypes of IBS owing to the limited number of samples but just analyze the IBS-D, the validity of the results could be questioned. More complete profiles of alterations in fecal, blood, or mucosal metabolites in various IBS subtypes are need for a better understanding of their roles in the BGM axis of IBS.
Conclusions
In conclusion, we found increased levels of 5-HT in IBS patients in our meta-analysis. However, there was no significant alteration of SCFAs between IBS patients and HCs. Our findings imply that alterations in 5-HT may be associated with the pathogenesis of IBS and affect the symptoms of IBS patients. Although we found no significant differences in SCFAs between IBS patients and HCs in our meta-analysis, some studies have reported that SCFAs are altered in IBS; such alterations in SCFAs may be a potential mechanism for the development or maintenance of symptoms in IBS patients. The findings of our study warrant further exploration of the relationship between the BGM axis and IBS. Therefore, further studies involving advanced molecular techniques and more strict experimental design are needed to explore alterations in SCFAs in IBS patients, which study samples would not just confined to feces.
Acknowledgements
None.
Abbreviations
- SCFAs
Short-chain fatty acids
- 5-HT
5-Hydroxytryptamine
- IBS
Irritable bowel syndrome
- BGM
Brain-gut-microbiota
- SMDs
Standardized mean differences
- CIs
Confidence intervals
- HCs
Healthy controls
- CNS
Central nervous system
- GIT
Gastrointestinal tract
- ENS
Enteric nervous system
- SMD
Standardized mean difference
- CI
Confidence interval
- TPH
Tryptophan hydroxylase
- IBS-C
Patients with constipation-predominant IBS
- IBS-D
Patients with diarrhea-predominant IBS
Authors’ contributions
ML, XZ and LX conceived and designed the study; ML and XZ collected the data, refined the acquired data for inclusion and analysed the data; ZT assisted in data interpretation; ML, XZ and LX drafted the manuscript. All the authors have approved the final version of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (81970471).
Availability of data and materials
All data and materials during this study are presented within the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mei Luo and Xiaojun Zhuang contributed equally to the writing of this article
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217–1224. doi: 10.1111/j.1365-2036.2004.01939.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang XJ, Xiong LS, Li L, Li M, Chen M. Alterations of gut microbiota in patients with irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:28–38. doi: 10.1111/jgh.13471. [DOI] [PubMed] [Google Scholar]
- 4.Zhuang XJ, Tian ZY, Li L, Zeng ZR, Chen MH, Xiong LS. Fecal microbiota alterations associated with diarrhea-predominant irritable bowel syndrome. Front Microbiol. 2018;9:1600. doi: 10.3389/fmicb.2018.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moraes-Filho JP, Quigley MM. The intestinal microbiota and the role of probiotics in irritable bowel syndrome: a review. Arq Gastroenterol. 2015;52:331–338. doi: 10.1590/S0004-28032015000400015. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery IB, Otoole PW, Ohman L, Claesson MJ, Deane J, Quigley EMM, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 7.Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 8.Mortensen PB, Andersen JR, Arffmann S, Krag E. Short-chain fatty acids and the irritable bowel syndrome: the effect of wheat bran. Scand J Gastroenterol. 1987;22:185–192. doi: 10.3109/00365528708991878. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–46. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20(39):14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenham S, Clarke G, Cryan J, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbara G, Cremon C, Giorgio RD, Dothel G, Zecchi L, Bellacosa L, et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep. 2011;13:308–315. doi: 10.1007/s11894-011-0195-7. [DOI] [PubMed] [Google Scholar]
- 15.Bixquert JM. Treatment of irritable bowel syndrome with probiotics: an etiopathogenic approach at last? Rev Esp Enferm Dig. 2009;101:553–564. doi: 10.4321/s1130-01082009000800006. [DOI] [PubMed] [Google Scholar]
- 16.Lyra A, Rinttila T, Nikkila J, Kurikka LK, Kajander K, Malinen E, et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15:5936–5945. doi: 10.3748/wjg.15.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohman L, Tornblom H, Simren M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12:36–49. doi: 10.1038/nrgastro.2014.200. [DOI] [PubMed] [Google Scholar]
- 18.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chassard C, Dapoigny M, Scott KP, Crouzet L, Delhomme C, Marquet P, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 20.Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:718–727. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- 21.Dass NB, John AK, Bassil AK, Crumbley CW, Whehee WR, Maurio FP, et al. The relationship between the effects of short chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19:66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 22.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin N Am. 2017;46:77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 23.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–1189. doi: 10.1016/S0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 24.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–e115. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 26.Ringel-Kulk T, Choi CH, Temas D, Kim A, Maier DM, Scott K, et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol. 2015;110:1339–1346. doi: 10.1038/ajg.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopecny J, Simunek J. Cellulolytic bacteria in human gut and irritable bowel syndrom. Acta Vet Brno. 2002;71:421–427. doi: 10.2754/avb200271040421. [DOI] [Google Scholar]
- 28.Vernia P, Latella G, Magliocca FM, Mancuso G, Caprilli R. Seeking clues for a positive diagnosis of the irritable bowel syndrome. Eur J Clin Investig. 1987;17:189–193. doi: 10.1111/j.1365-2362.1987.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 29.Park SY, Park MH, Yoon KW, Cho SB, Lee WS, Park CH, et al. Plasma 5-hydroxytryptamine concentration and its correlation with psychopathology in patients with irritable bowel syndrome. Gut Liver. 2009;3:26–30. doi: 10.5009/gnl.2009.3.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu FY, Huang SG, Zhang HY, Ye H, Chi HG, Zou Y, et al. Comparison of 5-hydroxytryptophan signaling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J Gastroenterol. 2016;22:3451–3459. doi: 10.3748/wjg.v22.i12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/S1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 32.Thijssen AY, Mujagic Z, Jonkers DM, Ludidi S, Keszthelyi D, Hesselink MA. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther. 2016;43:272–282. doi: 10.1111/apt.13459. [DOI] [PubMed] [Google Scholar]
- 33.Keszthelyi D, Troost FJ, Jonkers DM, Kruimel JW, Leue C, Masclee AM. Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: Relation to serotonin and psychological state. J Psychosom Res. 2013;74:501–504. doi: 10.1016/j.jpsychores.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Cheung CKY, Lee YY, Chan Y, Cheong PK, Law WT, Lee SF, et al. Decreased basal and postprandial plasma serotonin levels in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2013;11:1125–1129. doi: 10.1016/j.cgh.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–258. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Ranjan P, Mittal B, Ghoshal UC. Serotonin transporter gene (SLC6A4) polymorphism in patients with irritable bowel syndrome and healthy controls. J Gastrointest Liver Dis. 2012;21:31–38. [PubMed] [Google Scholar]
- 38.Houghton LA, Atkinson W, Lockhart S, Fell C, Whorwell PJ, Keevil B. Sigmoid-colonic motility in health and irritable bowel syndrome: a role for 5-hydroxytryptamine. Neurogastroenterol Motil. 2007;19:724–731. doi: 10.1111/j.1365-2982.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 39.Stasi C, Bellini M, Gambaccini D, Duranti E, Bortoli ND, Fani B, et al. Neuroendocrine dysregulation in irritable bowel syndrome patients: a pilot study. J Neurogastroenterol. 2017;23:428–434. doi: 10.5056/jnm16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuo XL, Li YQ, Yang XZ, Guo M, Guo YT, Lu XF, et al. Plasma and gastric mucosal 5-hydroxytryptamine concentrations following cold water intake in patients with diarrhea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2007;22:2330–2337. doi: 10.1111/j.1440-1746.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 41.Cremon C, Carini G, Wang BX, Vasina V, Cogliandro RF, Giorgio RD, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290–1298. doi: 10.1038/ajg.2011.86. [DOI] [PubMed] [Google Scholar]
- 42.Kerckhoffs AP, Terlinde JJ, Akkermans LM, Samsom M. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–907. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang SH, Dong L, Luo JH, Gong J, Li L, Lu XL, et al. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 45.El-salhy M, Wendelbo I, GundersenU D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 46.Morken MH, Valeur J, Norin E, Midtvedt T, Nysaeter G, Berstad A. Antibiotic or bacterial therapy in post giardiasis irritable bowel syndrome. Scand J Gastroenterol. 2009;44:1296–1303. doi: 10.3109/00365520903274401. [DOI] [PubMed] [Google Scholar]
- 47.Treem WR, Ahsan N, Kastoff G, Hyams JS. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23:280–286. doi: 10.1097/00005176-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Valeur J, Roseth AG, Knudsen T, Malmstrom GH, Fiennes JT, Midtvedt T, et al. Fecal fermentation in irritable bowel syndrome: influence of dietary restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols. Digestion. 2016;94:50–56. doi: 10.1159/000448280. [DOI] [PubMed] [Google Scholar]
- 49.Kajander K, Krogius-kurikka L, Rinttila T, Karjalainen H, Palva A, Korpela R. Effects of multispecies probiotic supplementation on intestinal microbiota in irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:463–473. doi: 10.1111/j.1365-2036.2007.03391.x. [DOI] [PubMed] [Google Scholar]
- 50.Undseth R, Jakobsdottir G, Nyman M, Berstad A, Valeur J. Low serum levels of short-chain fatty acids after lactulose ingestion may indicate impaired colonic fermentation in patients with irritable bowel syndrome. Clin Exp Gastroenterol. 2015;8:303–308. doi: 10.2147/CEG.S94084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cummings JH, Pomare EW, Branch HW, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eastwood MA, Walton BA, Brydon WG, Anderson JR. Faecal weight, constituents, colonic motility, and lactose tolerance in the irritable bowel syndrome. Digestion. 1984;30:7–12. doi: 10.1159/000199085. [DOI] [PubMed] [Google Scholar]
- 53.Kang DW, DiBaise JK, Ilhan ZE, Crowell MD, Rideout JR, Caporaso JG, et al. Gut microbial and short-chain fatty acid profiles in adults with chronic constipation before and after treatment with lubiprostone. Anaerobe. 2015;33:33–41. doi: 10.1016/j.anaerobe.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 56.Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 57.Besten GD, Eunen KV, Groen AK, Venema K, Reijngoud DJ, Bakkeret BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the en-teric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 59.Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:1415–1422. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 60.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 2007;292:429–437. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 62.Hyland NP, Quigley EM, Brint E. Microbiotahost interactions in irritable bowel syndrome: epithelial barrier, immune regulation and braingut interactions. World J Gastroenterol. 2014;20:8859–8866. doi: 10.3748/wjg.v20.i27.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banasiewicz T, Krokowicz L, Stojcev Z, Kaczmarek BF, Kaczmarek E, Maik J, et al. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis. 2013;15:204–209. doi: 10.1111/j.1463-1318.2012.03152.x. [DOI] [PubMed] [Google Scholar]
- 64.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatr. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials during this study are presented within the manuscript.