Abstract
Chronic health conditions are commonplace in older populations. The process of aging impacts many of the world's top health concerns. With the average life expectancy continuing to climb, understanding patterns of morbidity in aging populations has become progressively more important. Cancer is an age-related disease, whose risk has been proven to increase with age. Limited information is published about the epidemiology of cancer and the cancer contribution to mortality in the 85+ age group, often referred to as the oldest-old. In this review, we perform a comprehensive assessment of the most recent (2011–2016) literature on cancer prevalence, incidence and mortality in the oldest-old. The data shows cancer prevalence and cancer incidence increases until ages 85–89, after which the rates decrease into 100+ ages. However the number of overall cases has steadily increased over time due to the rise in population. Cancer mortality continues to increase after age 85+. This review presents an overview of plausible associations between comorbidity, genetics and age-related physiological effects in relation to cancer risk and protection. Many of these age-related processes contribute to the lowered risk of cancer in the oldest-old, likewise other certain health conditions may “protect” from cancer in this age group.
Keywords: Oldest-old, Ageing, Neurodegeneration, Cancer incidence, Cancer mortality
1. Introduction
Cancer is the leading cause of death in developed countries and the 2nd leading cause in developing countries (Torre et al., 2015a). Presently 57% of the global cancer burden and 65% of cancer deaths are from less developed regions in Africa, Asia (excluding Japan), Latin America and the Caribbean (Torre et al., 2015a; Jemal et al., 2011). Cancer incidence is expected to increase 75% by 2030 due to risk-associated lifestyle behaviors and the westernization of economically developing countries (Torre et al., 2015a). However, most important is the attributable risk of cancer associated with aging and the growth of the world's population (Torre et al., 2015a; Bray et al., 2012). The World Health Organization (WHO) estimates the 2015 average global life expectancy at birth is 71.4 years. That is a 5-year increase from the year 2000 and marks the fastest increase since the 1960's. Life expectancy is lowest in the WHO African regions (60.0 years) where incidence of all cancers is lower, and highest in the WHO European regions (76.8 years) where incidence of all cancers is higher. Regional differences in cancer incidence indicate aging is essential to understanding cancer epidemiology.
Cancer risk increases with advancing age. Individuals aged 65 years and older make up 58% of newly diagnosed cancers in developed countries and 40% in developing countries (Torre et al., 2015b). Within the older population, the group referred to as the oldest-old, which include individuals aged 85 years and older are considered the fastest growing segment of the population in developed countries (Gardner et al., 2013; Ortman et al., 2014). Within this group are smaller subgroups of nonagenarians (90–99 years) and centenarians (≥ 100 years). The oldest-old exceed the current human life expectancy by 20–25 years. They represent an adequate model of human longevity in which to study the beneficial and adverse ramifications of progressive aging on cancers. Studies of this nature will have sufficient implications on public health strategies.
In 2012 Nicholas Pavlidis et al. published the first systemic review on cancer prevalence and mortality in centenarians (Pavlidis et al., 2012). At that time the oldest-old and the subgroup of centenarians had historically been under-studied, primarily due to inherently small sample sizes and difficulties in recruitment. Their examination of the limited literature on cancer epidemiology in centenarians from 1918 to 2012 in 10 separate countries revealed six important conclusions. (1) There is a decreased prevalence of cancer in the oldest-old, with incidence rates dropping off at age 80 and reaching close to zero around age 100. (2) There is an increased incidence of incidental tumors at advanced ages. (3) Centenarians are characterized by low metastatic rates. The metastatic rate is almost double in younger populations then at older ages. This can be a result of slow growing less aggressive tumors at older ages or the assumption that older adults may die from something else before their tumor has had time to spread. (4) Centenarians have an apparent higher frequency of multiple primary tumors. (5) Cancer mortality or cancer as a cause of death decreases with age. Mortality rates were around 25–46% in ≤80 years of age, 21% for 90–99 years of age and 4% for 100+ years of age. The cause of death in centenarians was more attributed to other complications and causes than tumor spread. (6) The etiology is unclear as to how centenarians and the oldest-old preserve protection against cancer. Multiple mechanisms related to aging, immune response, cell survival and signaling, stress and frailty have been implicated as potential explanations for this phenomenon.
New information pertaining to cancer in the oldest-old has become available since the completion of the 2012 review. Therefore the current review will determine if their conclusions have sustained their validity overtime as the age and growth of the population have increased. We reviewed recent literature between 2011–2016 on cancer incidence, prevalence, and mortality in the oldest-old. We also looked at literature evidence of genes, comorbidities, and other health conditions, which could “protect” or lower the risk of cancer at older ages.
2. Methods
Several literature searches for English language articles on human subjects written in the last 5 years were conducted using PubMed in June 2016. The electronic database identified 272 articles matching the criteria “Cancer incidence” AND “oldest-old” OR “centenarians.” We then substituted “incidence” for “prevalence” and “mortality” separately and searched the database, identifying 24 other studies. An extended search using multiple keywords such as “nonagenarians,” “old age,” “morbidity, ‘cancer registries,’ and “age-specific” identified a few hundred more. All studies with stratified age-specific cancer incidence, prevalence, or mortality were considered for evaluation. Articles pertaining to single cancers were included, but evaluated separately from all cancer studies. Articles pertaining to a singular gender or ethnic group were also included, but evaluated separately from all-inclusive studies. Articles listed without an age category of 85+ or above were excluded.
In September 2016, several additional literature searches were conducted on PubMed to identify potential factors associated with cancer risk in older adults. 4 articles on cancer incidence and multiple morbidities were found using the same search criteria mentioned above. Another search using “cancer,” “dementia,” “Alzheimer's disease,” and “oldest-old” identified 164 published articles addressing neurodegeneration and tumorigenesis. We identified multiple other articles using the keywords “inverse cancer mortality” “frailty,” “genetics” “cancer risk,” “elderly” and “oldest-old.”
The articles we reviewed reported data from autopsy reports, vital statistics, cancer registries, questionnaires and surveys across all continents. Secondary searches were conducted for articles with data from countries with high life expectancy and a more prominent oldest-old population. These places included but were not limited to Australia, Japan, New Zealand, North America and many countries in Europe.
3. Results
3.1. Data collection
17 articles were identified from 4 different countries and 2 separate regions of Europe (see Tables 1–3): United States, Denmark, United Kingdom (UK), Korea, Western Europe and Northern Europe. Subjects in studies were 85+ years. The data ranged from 1920 to 2012. There were 8 USA articles (1973–2010), 6 Denmark (1978–2012), 1 Korea (1999–2011) and 2 combined articles from the US, UK, and parts of Europe (1995–2010). 10 articles only reported information on a singular cancer or group of related cancers, whereas 6 articles reported on all cancers. Multiple datasets from different sources were used in individual articles to construct a more inclusive study design. Articles using data from the Surveillance, Epidemiology and End Results (SEER) Program 9 included 9.5% of the U.S., SEER 13 included 14% of the U.S. and SEER 18 included 28% of the U.S. Articles using United States Cancer Statistics (USCS) included 91.3% of the U.S. and data from the Center for Disease Control (CDC) National Program of Cancer Registries (NPCR) included 96% of the U.S. The Indian Health Service (IHS) data includes 62% of AI/AN people in the U.S and the NORDCAN data includes close to 100% of cancer cases in all Nordic countries. Some articles from Tables 1–3 did not comment on information pertaining to the included study population for the sources of data used in the study.
Table 1.
Selected Primary Research Studies on Cancer Prevalence in the Oldest-old.
| Reference | Source of Data | Age | Cancer Type | Cancer Prevalence |
|---|---|---|---|---|
| USA Harding et al. (2012) | SEER 9 and 2000 US Census (1998–2002) | 85–99; 100+ | I. All sites II. Breast III. Lung IV. Colorectal V. Prostate |
I. Female: ↓85+ Male: ↓85+ II. Female: ↓85+ Male: ↓100+ III. Female: ↓85+ Male: ↓85+ IV. Female: stable at 85+ Male: ↓100+ V. Female: N/A Male: ↓85+ |
| USA Joseph et al. (2014) | SEER 13 (1973–2007) | 100–115 | I. Lung, Prostate, Breast, Urinary and Colorectal cancers II. Breast III. Lung IV. Colorectal V. Prostate VI. Urinary |
I. Female: ↓100+ Male: ↓100+ II. Female: ↓100+ Male: ↓100+ III. Female: ↓100+ Male: ↓100+ IV. Female: ↓100+ Male: ↓100+ V. Female: N/A; Male: ↓100+ VI. Female: ↓100+ Male: ↓100+ |
| USA a Gundrum and Ronald (2012) | SEER 18 – Medicare Linked Database (2000–2007) | 85+ | I. Lung, Prostate, Breast and Colorectal cancers II. Breast III. Lung IV. Colorectal V. Prostate |
I. Female: ↓85+ Male: ↓85+ II. All: ↓85+ III. All: ↓85+ IV. All: ↓85+ V. Male: ↑85+ |
| USA, UK Dianne Pulte et al. 2014 Pulte et al. (2014) | SEER 13 and National Cancer Registry (1996–2010) | 85+ | I. Chronic lymphocytic leukemia (CLL) | I. All: ↓85+ |
| Denmark Pedersen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1978–2012) | 85+ | I. All sites (excluding non-melanoma skin cancer) | I. Female: stable at 85+ Male: ↓90+ |
| Denmark Ewertz et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. All sites (excluding non-melanoma skin cancer) | I. Female: ↓85+ Male: ↓85+ |
| Denmark Song and Jeon (2015) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. Breast | I. Female: ↓85+ Male: ↓85+ |
| Denmark Kristiansen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. Colon II. Rectum and Anus |
I. Female: ↓85+ Male: ↓85+ II. Female: ↓85+ Male: ↓85+ |
| Denmark Brændegaard Winther et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. Prostate | I. Female: N/A Male: ↓85+ |
| USA Andersen et al. (2012) | SEER, NPCR, USCS (2001–2010) | 85+ | I. Renal Cell Carcinoma (RCC) | I. All: ↓85+ |
| USA b Thakkar et al. (2014) | SEER, UCR, US Census, NCHS (1973–2002) | 85–99 | I. All sites | I. Female: ↓85+ Male: ↓85+ |
Abbreviations: (SEER) Surveilance, Epidemiology and End Results Program, (NPCR) National Program of Cancer Registries, (USCS) United States Cancer Statistics, (UCR) Utah Cancer Registry, (NCHS) National Center for Health Statistics.
Stage IV cancers only, no multiple primary cancer diagnoses.
State of Utah only.
Table 3.
Selected Primary Research Studies on Cancer Mortality in the Oldest-old.
| Reference | Source of Data | Age | Cancer Type | Cancer Mortality |
|---|---|---|---|---|
| USA Harding et al. (2012) | SEER 9 and 2000 US Census (1998–2002) | 85–99; 100+ | I. All sites II. Breast III. Lung IV. Colorectal V. Prostate |
I. Female: ↓100+ Male: ↑100+ II. Female: ↓85+ Male: ↓100+ III. Female: ↓85+ Male: ↓85+ IV. Female: ↓100+ Male: ↓100+ V. Female: N/A Male: ↓100+ |
| USA a Wong et al. (2014) | SEER 18 and CBTRUS (2000–2010, 2004–2008) | 85+ | I. All sites (excluding non malignant meningiomas) II. Colorectal |
I. All: ↑85+ II. All: ↑85+ |
| USA Moller et al. (2011) | SEER 9, 13 and 18, NCHS (1973–2009) | 85+ | I. Breast II. Lung III. Colorectal IV. Prostate |
I. All: ↑85+ II. All: ↑85+ III. All: ↑85+ IV. Male: ↑85+ |
| Denmark Pedersen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1978–2012) | 85+ | I. All sites (excluding non-melanoma skin cancer) | I. Female: stable at 90+ Male: ↑90+ |
| Denmark Ewertz et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. All sites (excluding non-melanoma skin cancer) | I. Female: ↑90+ Male: ↑90+ |
| Denmark Song and Jeon (2015) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Breast | I. Female: ↑90+ Male: ↓90+ | |
| Denmark Jensen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Lung | I. Female: 90+ Male: 90+ | |
| Denmark Kristiansen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Colon II. Rectum and Anus |
I. Female: ↑90+ Male: ↑90+ II. Female: ↑90+ Male: ↑90+ |
|
| Denmark Brændegaard Winther et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Prostate | I. Female: N/A Male: ↑90+ | |
| UK, USA, Western Europe, Northern Europe Poulsen et al. (2016) | WHO Mortality Database (1995–97, 2003-05) | 85+ | I. All sites II. Breast III. Lung IV. Colorectal V. Prostate |
UK I. All: ↑85+ Female: ↑85+ Male: ↑85+ II. Female: ↑85+ Male: N/A III. All: ↓85+ Female: ↓85+ Male: ↑85+ IV. All: ↑85+ Female: ↑85+ Male: ↑85+ V. Female: N/A Male: ↑85+ USA I. All: ↑85+ Female: ↑85+ Male: ↑85+ II. Female: ↑85+ Male: N/A III. All: ↓85+ Female: ↓85+ Male: ↓85+ IV. All: ↑85+ Female: ↑85+ Male: ↑85+ V. Female: N/A Male: ↑85+ Western Europe I. All: ↑85+ Female: ↑85+ Male: ↑85+ II. Female: ↑85+ Male: N/A III. All: ↓85+ Female: ↓85+ Male: ↓85+ IV. All: ↑85+ Female: ↑85+ Male: ↑85+ V. Female: N/A Male: ↑85+ Northern Europe I. All: ↑85+ Female: ↑85+ Male: ↑85+ II. Female: ↑85+ Male: N/A III. All: ↓85+ Female: ↓85+ Male: ↓85+ IV. All: ↑85+ Female: ↑85+ Male: ↑85+ V. Female: N/A Male: ↑85+ |
Abbreviations: (SEER) Surveillance, Epidemiology and End Results Program, (CBTRUS) Central Brain Tumor Registry of the United States, (NVSS) National Vital Statistics System, (NDI) National Death Index, (NCHS) National Center for Health Statistics, (WHO) World Health Organization.
Invasive cases only, increasing cancers only.
The WHO released reports of the 2015 life expectancy estimates for 195 countries. The countries in our study were ranked in the top 15% for highest life expectancy. The life expectancy and each countries rank compared to other regions are as follows: Republic of Korea 82.3 years (11), UK 81.2 years (20), Denmark 80.6 years (27), and United States 79.3 years (31).
3.2. Cancer prevalence
The global prevalence of cancer and the overall number of reported cases have continued to increase over time with the growth of the population. The largest increase has been exhibited in the 90+ age group, 8 likely a result of the rise in global life expectancy. Evidence shows higher cancer prevalence at older ages in more recent time periods compared to earlier time periods (Ewertz et al., 2016; Pedersen et al., 2016). When measured for age-specific rates, the trend of cancer prevalence appears to decrease with increasing age, peaking at ages 65–85 years and declining after age 90 (Pedersen et al., 2016; Joseph et al., 2014; Harding et al., 2012; Pulte et al., 2015). At more recent time periods cancer prevalence peaks later in age, also likely due to global life expectancy shifting these peaks forward into older ages.
Certain types of cancers are more prevalent in older age groups. The most common cancer types amongst the oldest-old are breast, colorectal, prostate, and lung/bronchus cancers (Gundrum and Ronald, 2012). In our review of the literature colorectal cancer had the highest prevalence in individuals 85–99 from SEER 9 data (Harding et al., 2012), whereas a separate study on individuals with Medicare from SEER 18 data reported lung cancer (47%) prevalence as the highest followed by colorectal (29%), prostate (16%), breast (8%) cancers in 85+ (Wong et al., 2014). The differences between study populations could account for the difference we see in prevalence rates. Amongst centenarians breast cancer (29.24%) had the highest rate of prevalence followed by colorectal (19.28%), prostate (18.34%), and lung/bronchus (17.83%) cancers (Joseph et al., 2014). The transition from high prevalence of lung and colorectal cancers for 85+ to high prevalence of breast cancer in 100+ is likely due to the women to male ratio of centenarians. Women live longer than men, therefore more women with cancer will survive to centenarian age and the high majority of those women will have breast cancer than any other cancer.
Figs. 1 and 2 show the pattern of cancer prevalence is decreasing in the oldest-old populations for all cancers. The only change we see is for prostate cancer in 1 USA study (Wong et al., 2014), which only reported on stage IV cancers in individuals with Medicare from the SEER data, and therefore excludes a selection of the population at risk. We conclude that cancer prevalence in the oldest-old continues to rise with the growth of the population and the rise in life expectancy, but within the population the oldest-old have the lowest prevalence of cancer than any other age group.
Fig. 1.
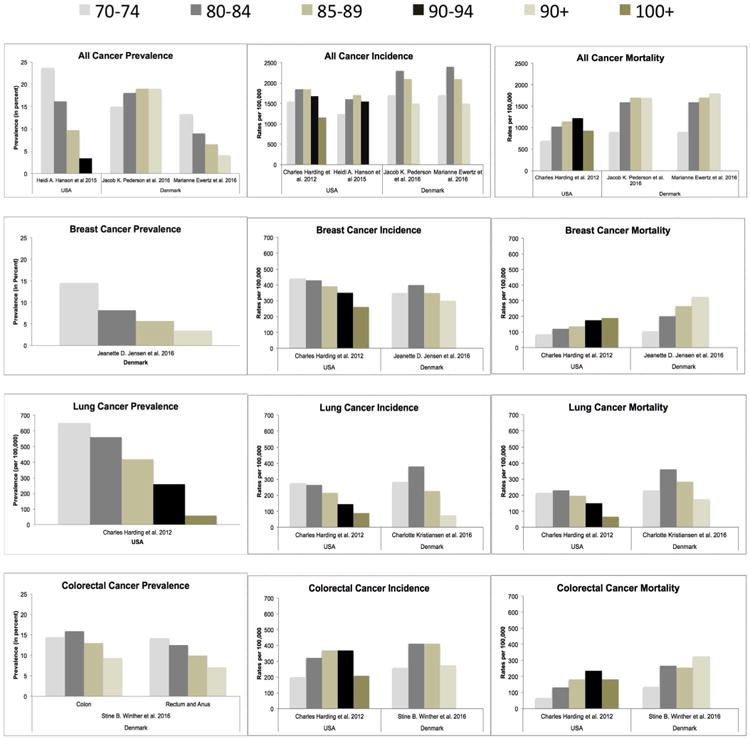
Cancer prevalence, incidence and mortality rates in females (5-year age groups).
Rates of cancer prevalence, incidence and mortality in females by 5-year age categories, from articles selected from the review. Figure shows all-cancer, prostate cancer, lung cancer and colon cancer rates. Prevalence rates are reported as percents, except lung cancer prevalence which is per 100, 000. Incidence and mortality rates are all per 100, 000.
Fig. 2.
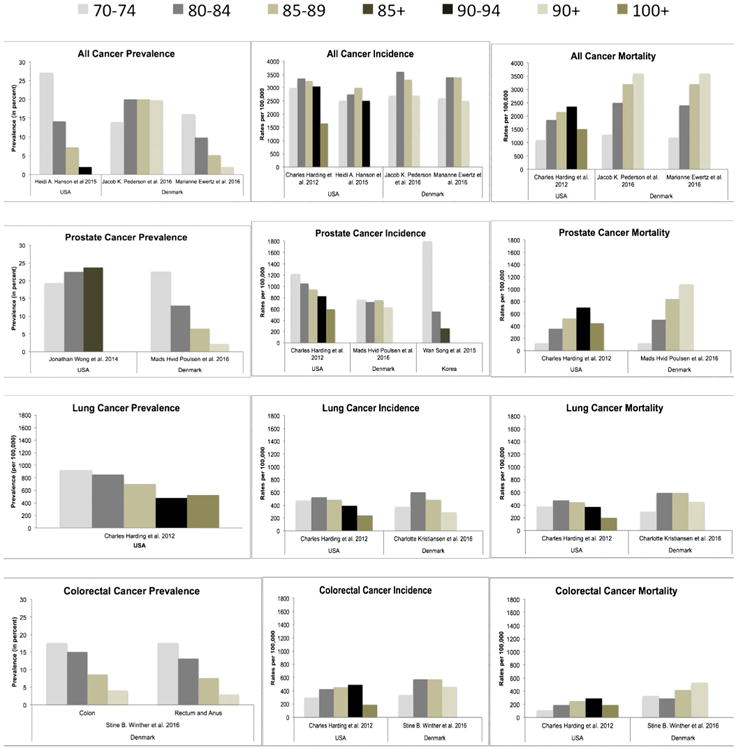
Cancer prevalence, incidence and mortality rates in males (5-year age groups).
Rates of cancer prevalence, incidence and mortality in males seperated by 5-year age categories, from articles selected from the review. Figure shows all-cancer, breast cancer, lung cancer and colon cancer rates. Prevalence rates are reported as percents, except lung cancer prevalence which is per 100, 000. Incidence and mortality rates are all per 100, 000.
3.3. Cancer incidence
Literary evidence suggests that cancer incidence is characterized by delayed onset of disease and decreased cancer risk as age increases (Andersen et al., 2012). Current studies show that cancer incidence peaks closer to age 90 before decreasing at advanced ages (Ewertz et al., 2016; Pedersen et al., 2016; Harding et al., 2012), as compared to previously reported work that showed cancer incidence increased with age until about the 8th decade of life and then plateaued after age 90, trending towards zero in centenarians (Harding et al., 2012). This is again the likely result of the rise in life expectancy pushing the incidence peak forward into older ages.
Rates of cancer incidence can be type-, region-, and sex- specific. Subtypes of cancer can have higher or lower incidence rates that peak at different age groups than is reported for all-cancer incidence. In the US, incidence of urological cancers like kidney, peak at much earlier ages before starting to decrease with increasing age (King et al., 2014), whereas for invasive cases of cancers such as colorectal, pancreatic and stomach, incidence continues to rise with increasing age (Thakkar et al., 2014). In Figs. 1 and 2, incidence rates of colorectal cancer peaks later in age compared to prostate, breast, or lung cancers.
Different geographical areas show various rates of cancer incidence (Figs. 1 and 2). Denmark had a higher rate of all-cancer incidence compared to the US. For both countries the lowest rates of all-cancer incidence were recorded in the 90+. The US had higher rates of prostate cancer in the oldest-old than in Korea or Denmark. Prostate cancer was fairly stable across all age groups in Denmark (Poulsen et al., 2016), but steadily declined in the US and Korea after ages 70–74 (Harding et al., 2012; Song and Jeon, 2015). The lowest rates of breast and lung cancers were in the 90+, with peak rates of incidence being in the 80–84 age range before decreasing at 85+ in Denmark and US (Harding et al., 2012; Jensen et al., 2016; Kristiansen et al., 2016). Colorectal cancer incidence peaked at later ages in both Denmark (85–89) and US (90–94) before decreasing at older ages.
Aging changes the makeup of the population. There are gender differences in the oldest-old that inherently effect cancer incidence rates. In 2012, a study using SEER data determined all-cancer incidence was higher in males than females for 85+ (Gundrum and Ronald, 2012). Our review of the literature determined the same. For males (Fig. 2) prostate cancer incidence is the highest for 85+. In the US, lung cancer rates are 2nd highest in 85–89 and 100+ age groups. Colorectal cancer is 2nd highest for ages 90–94 and presumably ages 95–99. In Denmark, colorectal cancer is 2nd highest for all males 85+. For females (Fig. 1), breast and colorectal cancers are similar at ages 85–89 in the US. Colorectal cancer is however the highest in the 90–94 group, whereas breast is the highest in the 100+ group. In Denmark, rates of colorectal cancer are the highest in the 85–89 groups, but breast cancer incidence is higher in the 90+.
Notably, cancer incidence in the oldest-old has been decreasing overtime as a result of increased colorectal screening in the general population (Gundrum and Ronald, 2012). The gap between male and female cancer incidence rates in the oldest-old has been steadily declining since the introduction of the prostate specific antigen test (psa) in 1990 and is projected to continue decreasing until, at minimum, year 2030 (Gundrum and Ronald, 2012). Overall the evidence shows that cancer incidence has type-, region- and sex-specific differences in rates for the oldest-old, who have the lowest rates of newly diagnosed cancers than any other age group and a higher rate of late-onset disease.
3.4. Cancer mortality
Cancer mortality refers to cancer as the cause of death. It does not include individuals with cancer who die from other causes. The most recent literature (Figs. 1–3) show all cancer mortality is the highest in the oldest-old (Ewertz et al., 2016; Pedersen et al., 2016; Thakkar et al., 2014; Moller et al., 2011), with rates steadily increasing until age 100+, and then declining at centenarian ages (Harding et al., 2012). Mortality rates were higher in men than women. Prostate cancer mortality was highest in men and colorectal cancer mortality was highest in women, except in the UK where breast cancer mortality was highest. The pattern of mortality for prostate and colorectal cancers are similar to all cancer mortality. However, breast cancer rates continue to increase into centenarian ages. Rates of lung cancer mortality were mixed based on sex and geographical location. Lung cancer decreased after age 85 in the US, Western Europe, northern Europe and in women from the UK and Denmark. In Denmark the decline in men was after age 90 and the UK showed an increase in lung cancer mortality for men 85+.
Fig. 3.
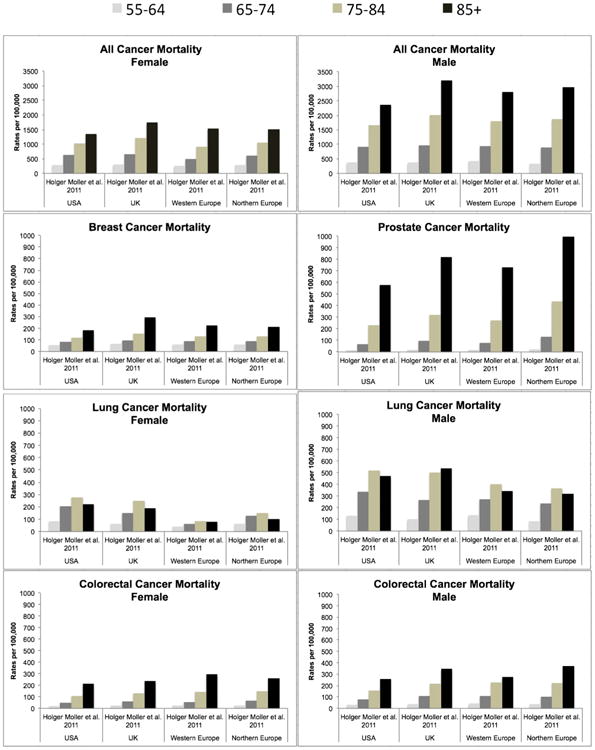
Cancer mortality rates in male and females (10-year age groups).
Rates of male and female cancer mortality by 10-year age categories from 1 article selected from the review. Mortality rates reported as per 100, 000.
In 2008 the distribution of cancer deaths amongst the oldest-old were approximately 20% lung, 13% colorectal, 8% prostate, 6% breast and 6% pancreatic (Anand et al., 2014). Death rates since 2000 showed a substantial increase in lung cancer and drastic declines in colorectal cancer. Several other cancers had consistent rates. Time trends show that overall cancer mortality is increasing over time in the oldest-old and decreasing in younger age groups (Ewertz et al., 2016; Moller et al., 2011). It is projected that by 2030 the absolute number of cancer deaths may rise by 90% annually and the top 5 causes of cancer death will be lung (22%), prostate (10%), breast (10%), bladder (8%) and colorectal (8%) (Gundrum and Ronald, 2012). This suggests that treatments for cancer are ineffective in the oldest old or they are not being treated for their disease at all. This could explain why we see mortality going down in younger groups and not in older groups. Currently men are more likely to die from cancer than woman (Ewertz et al., 2016; Pedersen et al., 2016; Kristiansen et al., 2016; Brændegaard Winther et al., 2016; Moller et al., 2011). Stage at diagnosis also plays a significant role in cancer mortality for 85+, with later stage disease causing more mortality in this group (Becker et al., 2014) likely due to the absence of treatment and the tumors aggressive and invasive behavior. Cancer mortality increases at advancing ages compared to younger groups and do not plateau like rates for prevalence or incidence.
4. Discussion
4.1. Why is cancer incidence/prevalence decreased in oldest-old?
The absence of cancer in this population could be a result of fundamental age-related physiological changes, involving rates of metabolism, cell proliferation, and information processing. Aging is characterized by decreased cellular proliferation and cellular metabolism, both of which impact cancer's ability to evade the body (Ukraintseva and Yashin, 2003; Anisimov, 2003). With increasing age tumor cells double at a much slower rate decreasing the rate of tumor growth (Ukraintseva and Yashin, 2003), as a result slow growing cancers are more common in older adults with late onset tumor growth. Furthermore, cells have a finite lifespan of limited cellular division that decreases with age until they reach a level of irreversible growth arrest where senescent cells have little to no probability of malignant transformation (Ukraintseva and Yashin, 2003; Anisimov, 2003). Essentially, extreme aging can result in age-related declines of physiological processes and decreased risk of cancer at older ages.
On the contrary, aging and senescent cells may play a secondary role in tumorigenesis, favoring tumor growth, reoccurrence, and metastasis through changes in the tissue microenvironment (Remi-Martin et al., 2015). As aging occurs senescent cells accumulate, secreting cytokines, chemokines and proteases in a process called senescence-associated secretory phenotype (SASP). SASP is characterized by low-level chronic inflammation referred to as inflammaging, which has been shown to increase the risk of age-related physiologies, including cancer and degenerative disorders in the elderly (Remi-Martin et al., 2015; Barajas-Gómez et al., 2017). Elements of SASP, interleukin 6 (IL-6) and 8 (IL-8) are proven pathogenic factors in tumor development (Remi-Martin et al., 2015; Barajas-Gómez et al., 2017).
Several studies on cancer models done in vitro and in mice provide evidence that SASP stimulates angiogenesis and activates cancer stem sells and epithelial-mesenchymal transition (Ghosh and Capell., 2016). A 2016 study showed the removal of senescent cells delayed tumorigenesis and increased longevity in normal mice models, which could be a result of decreased SASP (Baker et al., 2016). Similar results were found in a study on Rapamycin, a mammalian TORC1 (MTOR) inhibitor that suppresses SASP in senescent cells (Barajas-Gómez et al., 2017). The use of Rapamycin decreased prostate tumor growth and increased longevity in mice.
Natural aging is not the only process to stimulate senescent cell production. Ultraviolet radiation (sunlight), stress and anti-cancer therapies can cause genotoxicity and DNA damage similar to that of the aging process (Ghosh and Capell., 2016). This can have deleterious effects on cells, pushing them into senescence and causing further SASP. A study in Glioblastoma (GBM) patients, who have a high rate of tumor reoccurrence, showed an increase number of senescent cells and SASP after radiotherapy (Bonafè et al., 2002). This is evidence of the potential role of genotoxicity and SASP in cancer reoccurrence and resistance to treatment.
The role of SASP and senescent cells is not fully understood in the oldest-old. Barajas-Gomez et al. performed a study in Mexico using a geriatric cohort between 60 and 83 years of age (Barajas-Gómez et al., 2017). Blood samples from 64 people were converted into individual Elderly Patient Serum (EPS) separated by age. The EPS was then treated against MCF-7 breast cancer cell lines and tested for cellular proliferation. The results showed some EPS (23.4%) increased MCF-7 proliferation. Similar to the response of MCF-7 cells treated with SASP. The concentration of cytokines in EPS when adjusted for age did not differ by age group in the study cohort. However, there was a higher concentration of pro-inflammatory cytokines present in EPS than what is reported in serum from younger populations. Furthermore, an examination of proliferation by age group showed significantly higher numbers between ages 66–75. Proliferation was low for ages under 65 and over 76. When adjusted for gender, men and women were similar.
There is still not enough available information to suggest SASP alone is responsible for increased tumorigenesis during extreme aging. The results of the Mexico study warrant a closer look at the process in the oldest-old. Some evidence suggests the oldest-old may be genetically inclined to protect or delay against the deleterious effects of inflammaging. Which could explain why incidence and prevalence are declining in this population. More information on the mechanisms of SASP in the oldest-old is needed.
A developing hypothesis regarding trends in cancer incidence in the oldest-old, refer to restructuring of the immune system caused by germ-line variability of the genes that control deoxyribonucleic acid (DNA) repair, stress response, apoptosis and other fundamental cellular activities (Anisimov, 2003). One proposed point of view is that the oldest-old lack the genes that predispose them to chronic diseases (Bonafè et al., 2002; Miyaishi et al., 2000). Literary evidence shows the oldest-old have unique protective genetic variations, which induce a genetic predisposition to prolonged aging, demonstrated by centenarian offspring who survive longer with less health complications (Ruiz et al., 2012; Terry et al., 2004). Polymorphisms of tumor protective genes like tumor protein p53 and the lack of deleterious genes like breast cancer 1 (BRCA1) and transforming protein p21 (HRAS1) in this population may make them cancer resistant (Jeon et al., 2016; Bonafè et al., 2002). Tumor suppressor gene p53 plays a major role in cell division, apoptosis, malignant transformation, and DNA repair. Current data speculates that p53 polymorphisms impact individual risk of cancer when associated with environmental stress and other factors (Anisimov, 2003). In the oldest-old only a minute portion of the population would have been subjected to enough environmental factors to exert selective loss of the p53 genotype (Anisimov, 2003). BRCA1 is a breast cancer susceptibility gene involved in recombinational repair. Data in the oldest-old show slight differences in BRCA1 genotype frequencies compared to younger populations, but not a big enough sample size to show significance or suggest its role in cancer risk (Perls et al., 2007). HRAS1 gene regulates various mechanisms involved with proliferation, stress response, energy metabolism, and apoptosis. More recently it has been identified in processes of immunosenescence, insulin resistance and neurodegeneration. Its a3 allele is decreased in centenarians in respect to younger populations, indicating that a3 allele carriers are potentially at a disadvantage for human longevity, although the mechanism is not yet clear (Anisimov, 2003; Vijg et al., 2001). This allele has been characterized as a frailty allele. Furthermore HRAS1 has a familial relationship with RAS2, a gene that controls yeast lifespan via modulation of stress response. It has been theorized that HRAS1 could potentially regulate lifespan in humans using the same evolutionary pathway to conserve stress response (Anisimov, 2003; Vijg et al., 2001).
Information on human longevity identified age-related cytokine changes that influence functionality of the immune system. Centenarians exhibit higher levels of cytoxic T cells CD8 and CD28 – and an increased percent of natural killer (NK) cells (Anisimov, 2003). In this environment high amounts of interferon gamma (INF-) and interleukin 4 (IL-4) are produced, which is not conducive for tumor growth (Anisimov, 2003). Researchers hypothesize that the age-related changes that restructure the immune system creating an adverse environment for cancer cells to accumulate may explain the decreased cancer incidence in the oldest-old.
Another explanation is that there is decreased cancer screening or detection bias in the elderly and thus many diagnoses are missed in this population. The oldest-old are at a greater risk of falling, cardiovascular disease, stroke, etc. associated with their age. They are more likely to suffer side effects from procedures and anesthesia, and may not benefit from identification of cancer if they are not expected to live for decades after cancer diagnosis. A health care provider will most likely opt out of cancer screening in the elderly to avoid complications resulting from screening techniques. Screening methods have also played an important role in determining cancer incidence. Reports on cancer incidence during terms of heavy cancer screening show a decrease in incidence for breast, colorectal and prostate cancer at ages 85+ after the introduction of screening tools. For cancers with screening tools, the higher incidence rates are found at younger ages because it can be detected sooner. On the contrary, conditions like lung cancer have higher incidence rates in older ages because they do not screen for it early (Becker et al., 2014). A recent study looking at cervical cancer in American Indian and Alaska Native (AI/AN) women compared to white women found that the differences in incidence rates for those 85+ had moderately to do with higher screening practices in white women than in AI/AN women (Meg et al., 2014). White women showed decreased incidence at age 85+ compared to ages 65–84 and AI/AN women had an increase in incidence at age 85+ compared to ages 65–84. Access to cancer screening resources may contribute to the patterns of incidence in different age populations.
4.2. All cause mortality and cancer as a cause of death
Our review is consistent with the overall findings of the previous 2012 review on centenarians, which found that cancer mortality decreases at 100+ ages. However, because we found only 1 article where separate rates for centenarians were included more information is needed to determine a definite conclusion. An earlier article from 2000 on cancer mortality under age 100, reported that age-specific cancer mortality rates increased with age with the exception being in lung and pancreatic cancer for both men and women, which decreased in those ≥95 years (de Rijke et al., 2000). Our review supports the previous findings.
It has become commonplace to combine information on individuals 85+ to compensate small sample sizes but evidence of their differences should push future researchers to aim to separate the 85+ into 5- or 10-year age categories. Surveillance programs and census data should also aim to standardize the categories so information can be shared and compared. Additionally, differing results of age-specific cancer mortality could be a result of discrepancies between the definition of cancer death by region and how each individual country determines cause of death. The cause of death on a death certificate or as determined by an autopsy report can be inaccurate.
The risk of cancer mortality is low compared to the risk of death from other causes in the oldest-old. 85+ individuals are more likely to die from old age/frailty (28.1%) and pneumonia (17.7%) than cancer (4.4%) (Evans et al., 2014). Prognoses for the oldest-old after a cancer diagnosis should consider complications due to age and comorbidity as major risk factors for death. Younger people have less contributing health factors to consider and it should be easier to determine the actual cause of death in a young person than in someone who is older. As a result cancer mortality can vary as age increases and people have more health problems.
4.3. Cancer and neurodegeneration
Inverse cancer comorbidity is the phenomenon that demonstrates the protective effects of other diseases to reduce cancer risk. A recent review has described multiple studies demonstrating an inverse relationship between dementia and cancer in the elderly (Ganguli, 2014). In particular, Alzheimer's disease has shown evidence of not only confounding cancer incidence rates but also lowering the risk for cancer in older age individuals (Driver, 2012). This same phenomenon is not observed in vascular dementia (Roe et al., 2010) where the cancer risk is higher in people with vascular dementia (Driver, 2014), and therefore points to biological mechanisms that are not just a consequence of cognitive dysfunction (Roe et al., 2010; Driver, 2014; Benito-León et al., 2014; Driver et al., 2012). In fact the relationship was stronger when non-Alzheimer's and mixed dementias were excluded (Driver et al., 2012).
Limited data compiled from a few sources point to cellular processes involved with shared similar pathways between neurodegeneration and tumorigenesis as a potential explanation (Driver, 2012). A substantially large cohort study of 1 million northern Italy residents showed the risk of cancer was 50% less in patients with AD Dementia and AD Dementia risk was 35% in patients with cancer (Musicco et al., 2013). When adjusted for age, the 85+ group had lower incidence of cancer in the AD cohort and lower incidence of dementia in the cancer cohort compared to the general population. A similar study done in Taiwan showed that patients with dementia had significantly lower rates of cancer over a 7-year period compared to matched controls (Lin et al., 2016). They stratified for ages 80–89 and 90–99, the results were not reported. The Framingham Heart study conducted in the US found cancer survivors had a 33% decreased risk of Alzheimer's compared to those without cancer (Driver et al., 2012). Participants with probable Alzheimer's had a 61% decreased risk of incident cancer compared to those without Alzheimers. To eliminate the possibility that the relationship between cancer and dementia was due to selective mortality, initial analysis of participants was restricted to those surviving to at least age 80. The relationship did not change (Driver et al., 2012) – indicating the pattern could apply to the oldest-old if examined separately. Studies on cancer and neurodegenerative disorders could partly be explained by selective mortality or under diagnosis of either disease in elderly populations. Selective mortality is less likely due to work on vascular dementia, which disputes this idea. Moreover, the inverse relationship between cancer and neurodegeneration is better explained by mechanisms of genetics, biology and physiology.
Extreme aging is a primary risk factor for cancer and neurodegeneration. Their strong association with age suggests multiple phases in the biology of aging are linked to shared pathways and genes in tumorigenesis and neurodegeneration. However, these genes and path-ways are controlled in opposing directions for each disease. Although age-related changes in metabolic regulation may act as an initiating event for both tumorigenesis and neurodegeneration, cancer is still defined as unrestricted cellular proliferation, whereas neurodegeneration is a system of premature cell death. Each individual neuron is invaluable and is required to survive and sustain connections with different neurons for as long as it can. But in tissues with proliferative potential apoptosis is important to regulate cellular growth, and no cell is invaluable, another can easily replace it. Researchers have proposed that increased apoptosis may lower cancer risk but aid neurodegeneration, while increased cell survival may decrease neurodegeneration but stimulate tumor growth (Benito-León et al., 2014). Other physiological processes shared between cancer and neurodegeneration, induced by extreme aging, are mitochondrial dysregulation and oxidative stress.
Metabolic dysregulation can contribute to age-related sporadic forms of AD and cancer. The role of the Warburg effect in cellular growth and neuronal cell death explains an inverse relationship between tumorigenesis and neurodegeneration (Demetrius and Simon., 2013). Aging causes a decrease in the homeostatic stability of mature cells. This creates competition between cells for energy resources and they will undergo either an increase in glycolysis or an increase in oxidative phosphorylation (OxPhos), metabolic alterations to compensate for any loss in energy production (Demetrius and Simon., 2013). Cancer cells thrive in conditions of increased glycolysis because it causes an upregulation of the glycolytic enzyme 6-phospho-fructo- 2-kinase/fructose-2, 6-biphosphatase-3 (PFKFB3), which suppresses apoptosis, promotes angiogenesis and cellular proliferation (Yalcin et al., 2014). The metabolization of glucose by glycolysis in cancer cells is the Warburg effect (Demetrius and Simon., 2013). Neurodegeneration is induced in conditions of increased OxPhos which down regulates (PFKB3), contributing to neuronal cell death. The catabolization of glucose by OxPhos in normal cells and neurons is the inverse Warburg effect (Demetrius and Simon., 2013). The metabolic activity that characterizes cancer and AD, functions in opposing directions for tumorigenesis and neurodegeneration demonstrating an inverse relationship. Overall an increase in glycolysis (upregulation of PFKFB3) increases the risk of cancer and an increase in OxPhos (downregulation of PFKFB3) increases the risk for AD (Yalcin et al., 2014).
Literature on the genetics of age-related diseases has identified several genes and protein–protein interactions shared between cancer and AD pathways (Bonafè et al., 2002; Driver, 2012; Driver, 2014; Driver et al., 2012). Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1) plays a role in cell division and is overexpressed in many human tumors. Its function is inactivated in the brain tissue of AD patients (Driver, 2014; Driver et al., 2012). Inhibition of Pin1 reverses tumor expression, decreasing the risk of cancer (Driver et al., 2012). Upregulation of Pin1 in neurons reverses neurodegeneration and decreases the risk of AD (Driver et al., 2012). Pin1 works simultaneously in both diseases but in different directions. The tumor suppressor gene p53 induces apoptosis in cells with DNA damage. Upregulation of p53 reduces cancer risk but induces aging through cellular senescence and cell death (Driver, 2014). As a result some p53 polymorphisms protect from cancer but may increase the risk of neurodegeneration (Driver, 2014). The wingless-type mouse mammary tumor virus (Wnt) signaling pathway, responsible for cell survival and proliferation, is also inactive in AD, but upregulated in many cancers (Driver, 2014). Finally, human Apolipoprotein E (ApoE) is a genetic risk factor for AD. Carriers of the gene have increased metabolic dysfunction compared to non-carriers (Capri et al., 2006). Reports of associations between cancer and ApoE have been inconclusive (Anand et al., 2014). A recently conducted meta-analysis found no overall association between cancer and ApoE (Anand et al., 2014). However, there was a weak but significant negative association between the ApoE4+ genotype and cancer risk (Anand et al., 2014). ApoE4 has significantly lower frequencies in centenarians compared to younger cohorts and the presence of ApoE4 reduces life expectancy (Capri et al., 2006). Much of the available data on neurodegeneration and cancer is not available on the oldest-old. Future studies should examine the oldest-old because the age structure of the world's population is changing.
4.4. Age-related medical conditions and cancer
At older ages the prevalence of the clinical phenotype of frailty increases and is characterized by functional limitations, reduced physiologic reserve, adverse outcomes and a high risk of mortality (Kanapuru et al., 2013). It is possible that the presence of frailty results in lower cancer incidence. A recent article identified a negative association between frailty and cancer incidence in elderly men (Kanapuru et al., 2013). The women were found to have a higher prevalence of frailty and an overall lower rate of cancer incidence comparative to men, however it was insignificant (Kanapuru et al., 2013). The results support the notion of frailty as a protective mechanism against cancer, but it may be more true for men than women. It is hypothesized that cellular senescence could potentially inhibit tumorigenesis in frail populations (Evans et al., 2014). The prevalence of frailty in the oldest-old increases with advancing age (Lee et al., 2016) and the existence of frailty in the oldest-old suggests the possibility that biological implications of frailty may impact cancer incidence.
Type 2 diabetes (T2DM) is an adult-onset diabetes whose risk increases with age. The process of aging has been linked to lower insulin levels and decreasing islet function, which are common indications of T2DM in patients (Driver et al., 2012). According to the CDC, the prevalence and incidence of T2DM increases until age 65 and levels off at older ages (US Data & Trends Redirect, 2012). Although, there is some evidence to suggest there is a slight decrease in prevalence after age 85 (Dunning et al., 2014). Rates of T2DM in the older age groups may impact cancer occurrence in the oldest-old. Diabetes has been shown to be inversely associated with cancer risk (Akushevich et al., 2013; Tabarés-Seisdedos et al., 2011; Hu et al., 2016). The literature proposes many biological mechanisms characteristic of T2DM (Lin et al., 2013) that could be responsible for the inverse association. A mutation in the transcription factor 7-like 2 (TCF7L2) is a known risk factor for T2DM (Chen et al., 2013). TCF7L2 is activated in the Wnt pathway, which is associated with tumorigenesis. A recent meta-analysis on cancer risk and the TCF7L2 gene showed no association with overall cancer risk (Chen et al., 2013). When stratified by specific cancers, there was a positive association with colon, prostate, and breast cancers. Specifically, the T allele of the TCF7L2 gene showed an inverse association with prostate cancer. The literature on TCF7L and its association with T2DM and cancer is inconsistent and more information is needed in the future. Moreover, certain anti-diabetic drugs such as thiazolidinediones (Bosetti et al., 2013) and metformin (Noto et al., 2012) may also reduce the risk of all-site and type-specific cancers like liver (Bosetti et al., 2013), colorectal (Bosetti et al., 2013; Noto et al., 2012), and lung cancer (Tabarés-Seisdedos et al., 2011; Hu et al., 2016; Noto et al., 2012). However, there are some type-specific cancers like pancreatic cancer whose risk is increased by diabetes (Akushevich et al., 2013).
An acute cerebrovascular accident (CVA) or stroke may potentially reduce cancer risk at older ages. The Framingham study measured the probability of staying cancer free in males and females with and without stroke. No observed differences were found for women, however in men there was a higher probability of being cancer free for 80+ individuals (Ukraintseva et al., 2010). Suggesting that aging could be influencing cancer risk after stroke occurrence. A similar study using the National Long Term Care Survey looked at the incidence of cancer in males and females with and without stroke. They found similar results in females as the Framingham study, but in males they reported a higher risk of cancer among men with stroke at age 90, but do not say if the data is statistically significant.
In a recent study they reported the occurrence of site-specific cancers in individuals with health conditions more prominent in older ages. Breast cancer risk was reduced in people with angina pectoris and hip fractures (Akushevich et al., 2013). Colon cancer risk decreased in patients with arthritis (Akushevich et al., 2013). More information on these conditions in the oldest-old and whether they protect from certain cancers should be studied further.
4.5. Genetics of longevity and reduced cancer burden in the oldest-old
Genetic profiles that slow the aging process also impede the development of age-related diseases (Kenyon, 2010). It has been estimated that 25 percent of the variability associated with longevity is explained by genetic contributions (Hjelmborg et al., 2006; Deelen et al., 2014). The genetic profiles associated with longevity have increasing influence with advanced age (Hjelmborg et al., 2006; Tan et al., 2012). A recent meta-analytic GWAS study confirmed the role of the previously identified APOE locus in longevity and identified an additional region on chromosome 5q33.3 associated with survival beyond 90 years of age (Deelen et al., 2014). APOE encodes for apolipoprotein E (APOE), an essential component of lipoproteins that is involved in cholesterol and triglyceride transport in the blood. APOE has three different isoforms identified in humans (E2, E3 and E4) (Mahley and Rall, 2000). The APOE4 allele has been associated with reduced longevity, while the APOE2 allele has been associated with prolonged longevity (Schächter et al., 1994; Deelen et al., 2011). In addition to being associated with serum cholesterol levels, SNPs in linkage disequilibrium with the APOE locus have been associated with reduced serum IGF-1 levels (Deelen et al., 2011). Other human studies have supported this finding by demonstrating that centenarians have higher expression of deactivating mutations of IGF-1 (Suh et al., 2008). Further, genetic variants in the insulin receptor are associated with longevity in humans (Kojima et al., 2004). Hence, it seems likely that aberrant metabolic profiles, similar to those of calorie restriction, are important for postponing the cellular aging process. Polymorphisms in the downstream FOXO3 transcription factor in the Insulin/IGF-1 signaling pathway, have also been associated with increased longevity (Kenyon, 2010; Willcox et al., 2008; Anselmi et al., 2009). Reduced IGF-1 levels following calorie restriction have also been associated with reduced cancer burden (Juul, 2003) and subsequently may partially mediate the relationship between extreme longevity and reduced cancer risk.
Recent pathway network analysis performed in the largest GWAS study to date, identified genetic polymorphisms associated with longevity to be clustered in the following four general pathways: 1) metabolism, 2) immunity, 3) calcium signaling and 4) MAPK signaling (Zeng et al., 2016). This GWAS analysis also confirmed previously identified APOE (discussed above) and IL6 polymorphisms to be implicated in extreme longevity (Zeng et al., 2016; Soerensen et al., 2013). It is hypothesized for immune regulators to be implicated in the aging process since cellular hallmarks of aging include the decline in certain immune functions (immunosenescence) and activation of others (inflammaging). Since IL-6 regulates chronic inflammation, it could play a role not only in aging by also in establishing a microenvironment conducive to cancer growth (Heikkilä et al., 2008). IL6 has also been observed to be a growth factor for various cancers (Aggarwal et al., 2006). Therefore, studying genetic variations in IL6 contributing to longevity may deeper our understanding of the inverse relationship between advanced age and reduced cancer incidence and prevalence.
Inhibition of the mTOR pathway most closely mimics the physiological mechanism of calorie restriction that has been observed to extend lifespan across different species (Kenyon, 2010; Fontana et al., 2010). mTOR, the mechanistic target of Rapamycin, is a serine/threonine kinase that serves as a major regulator of cell growth and metabolism in response to metabolic and hormonal signals (Stanfel et al., 2009). Upon reduced metabolic demands, mTOR is down-regulated leading to the down-regulation of DNA translation, which has been associated with increased longevity in model organisms including worms, flies and mice (Johnson et al., 2013). Recent GWAS analyses support the role of mTOR signaling in longevity. Certain MAPK (an upstream regulator in the mTOR pathway) polymorphisms were more commonly observed in the oldest old (Zeng et al., 2016). Recent drug trials have demonstrated that Rapamycin may extend longevity by reducing cancer burden (Ehninger et al., 2014). Hence, mTOR genetic polymorphisms may increase longevity by increasing cancer resistance.
5. Conclusion
The literature reviewed here suggests three main conclusions about aging and measures of cancer risk or burden. First, after age 85 incidence and prevalence decline, compared to younger groups. Prevalence appears to peak between age 85 and 90, but approaches zero by age 100. Second, the specific trajectory of incidence across age groups can vary by gender, geographic regions, and cancer type, but when all cancer types are pooled risk seems to decline after age 85. Third, cancer mortality increases with age, although cancers may not be the leading cause of death among the oldest old.
The current literature offers several, not unrelated, classes of notions toward explaining a reduced risk of cancer among the elderly. One set of ideas is that tumorigenesis and neurodegeneration share cellular and genetic pathways, but in opposing, perhaps mutually exclusive, directions. Thus an active neurodegenerative process could preclude tumorigenesis, and vice versa. Another view is that the immune system fluctuates among the very old, such that, tumor surveillance and control is better than among younger people. Other possible explanations involve the interaction of certain genes with accumulated environmental and biologic stressors, in such a way as to prevent cancers and other morbidities. The majority of research focusing on genetics of age-related conditions has been focused on genetic heterogeneity related to specific disease etiology. An exciting new avenue is focused on pleiotropic effects, where certain genetic variants are associated with protection from various diseases of aging, including cancer (Kulminski et al., 2016). Further examination of these genes could provide a clearer understanding of genetic components of reduced cancer incidence and prevalence in the oldest-old. Clearly there is overlap among these ideas, and much potential for important research. However, at present there are significant obstacles to medical research on the oldest old, including underreporting of conditions.
There are very few resources to evaluate illness in the oldest-old. It is particularly difficult to study cancer in this population. A substantial amount of information is unreported because patients and their caregivers may choose to defer treatment. Much of the data comes from a variety of sources including, but not limited to cancer registries, autopsies, death certificates, and patient reported surveys. However no standardized method has been established to detect or study cancer in this population. It can be difficult to compare results across studies over various regional and demographic areas if the sources of information are incomplete or too dissimilar.
Due to the current make-up and the inherent socio-economic disparities of the U.S. population of people aged 85 and over, current epidemiological studies on the oldest-old contain small sample sizes encompassing mostly whites, women, and highly educated individuals (Ukraintseva et al., 2010). These 3 groups also encompass characteristics of countries with the highest life expectancy. Therefore any conclusions from current studies may have limited applications to the changing demographics of the United States, specifically the increased numbers of ethnicities that are gaining better access to healthcare and are likely to be more represented in the future. Our findings may not be generalizable to other countries – particularly in the developing world where there are more people of color and life expectancy is lower. We should also consider the differences in environmental health and public health policy in different geographical regions, which could contribute to the reduction of cancer risk or increased human longevity in specific areas around the world. However despite the many challenges to study cancer in the oldest-old, we can continue to build a clearer understanding of the issue by looking at more detailed information available on prevalence, incidence and mortality. Future investigations targeting risk/protective factors related to successful aging with and without cancer in this population may provide unique information on how the oldest-old appear to escape cancer. Further prospective research on these topics is needed in the future.
Table 2.
Selected Primary Research Studies on Cancer Incidence in the Oldest-old.
| Reference | Source of Data | Age | Cancer Type | Cancer Incidence |
|---|---|---|---|---|
| USA Harding et al. (2012) | SEER 9 and 2000 US Census (1998–2002) | 85–99; 100+ | I. All sites II. Breast III. Lung IV. Colorectal V. Prostate |
I. Female: ↓85+ Male: ↓85+ II. Female: ↓85+ Male: ↓85+ III. Female: ↓85+ Male: ↓85+ IV. Female: stable at 85+ Male: ↓100+ V. Female: N/A Male: ↓85+ |
| USAa Wong et al. (2014) | SEER 18 and CBTRUS (2000–2010, 2004–2008) | 85+ | I. All sites (excluding non malignant meningiomas) II. Colorectal III. Pancreas IV. Stomach |
I. All: ↓85+ II. All: ↑85+ III. All: ↑85+ IV. All: ↑85+ |
| USA Daniel Becker et al. 2014 Moller et al. (2011) | SEER 9, 13 and 18 (1973–2009) | 85+ | I. Breast II. Lung III. Colorectal IV. Prostate |
I. All: ↑85+ II. All: ↑85+ III. All: ↑85+ IV. Male: ↑85+ |
| Denmark Pedersen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1978–2012) | 85+ | I. All sites (excluding non-melanoma skin cancer) | I. Female: ↓85+ Male: ↓85+ |
| Denmark Ewertz et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. All sites (excluding non-melanoma skin cancer) | I. Female: ↓85+ Male: ↓90+ |
| Denmark Song and Jeon (2015) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | 85+ | I. Breast | I. Female: ↓85+ Male: ↓90+ |
| Denmark Jensen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Lung | I. Female: ↓85+ Male: ↓85+ | |
| Denmark Kristiansen et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Colon II. Rectum and Anus |
I. Female: ↓90+ Male: ↓90+ II. Female: ↓85+ Male: ↓85+ |
|
| Denmark Brændegaard Winther et al. (2016) | NORDCAN, Danish Cancer Register, Danish Cause of Death register (1980–2012) | I. Prostate | I. Female: N/A Male: ↓90+ | |
| USA Andersen et al. (2012) | SEER, NPCR, USCS (2001–2010) | 85+ | I. Renal Cell Carcinoma (RCC) | I. All: ↓85+ |
| USAb Thakkar et al., (2014) | SEER, UCR, US Census, NCHS (1973–2002) | 85–99 | I. All sites | I. Female: ↓90+ Male: ↓90+ |
| Korea King et al. (2014) | Korea National Cancer Incidence Database (1999–2011) | 85+ | I. Prostate II. Kidney III. Bladder |
I. Female: N/A Male: ↓85 + II. All: ↓85+ Female: ↓85+ Male: ↓85+ III. All: ↓85+ Female: ↓85+ Male: ↓85+ |
Abbreviations: (SEER) Surveillance, Epidemiology and End Results Program, (CBTRUS) Central Brain Tumor Registry of the United States, (NPCR) National Program of Cancer Registries, (USCS) United States Cancer Statistics, (UCR) Utah Cancer Registry, (NCHS) National Center for Health Statistics.
Invasive cases only, increasing cancers only.
State of Utah only.
Acknowledgments
Funding: This study was supported by the National Institute for Neurological Diseases and Stroke Award (NINDS/NIH) [NS072234[; UCI Cancer Center Award [P30CA062203[; The NIH IMSD training grant [R25GM055246[; UCI Provost PhD Fellowship; UCI Diversity Recruitment Fellowship; and NIH grantR01AG021055.
Footnotes
Author contribution: Shantell C. Nolen: conducted final literature review, wrote the manuscript.
Marcella Evans: conducted initial literature review.
Avital Fischer: wrote Section 4.5 on genetics and longevity.
Maria Corrada: critically edited manuscript.
Claudia Kawas: reviewed the manuscript.
Daniela A. Bota: supervised and critically edited the manuscript.
Disclosures: The authors (Shantell C. Nolen, Marcella A. Evans, Avital Fischer, Maria M. Corrada, Claudia H. Kawas and Daniela A. Bota) have no financial relationships relevant to this manuscript. Neither of the authors has any conflict of interests.
References
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Kulminski A, Yashin AI. Morbidity risks among older adults with pre-existing age-related diseases. Exp Gerontol. 2013;48(12):1395–1401. doi: 10.1016/j.exger.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, et al. Association between apolipoprotein E genotype and cancer susceptibility: a meta-analysis. J Cancer Res Clin Oncol. 2014;140(7):1075–1085. doi: 10.1007/s00432-014-1634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol Ser A: Biol Sci Med Sci. 2012 doi: 10.1093/gerona/glr223. glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN. The relationship between aging and carcinogenesis: a critical appraisal. Crit Rev Oncol Hematol. 2003;45(3):277–304. doi: 10.1016/s1040-8428(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Anselmi CV, Malovini A, Roncarati R, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12(2):95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- Baker Darren J, et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Gómez Bertha Alicia, et al. Relationship of inflammatory profile of elderly patients serum and senescence-associated secretory phenotype with human breast cancer cells proliferation: role of IL6/IL8 ratio. Cytokine. 2017;91:13–29. doi: 10.1016/j.cyto.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Becker D, Ryemon S, Gross J, Levy B, Grossbard M, Ennis R. Cancer trends among the extreme elderly in the era of cancer screening. J Geriatr Oncol. 2014;5(4):408–414. doi: 10.1016/j.jgo.2014.06.050. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Romero JP, Louis ED, Bermejo-Pareja F. Faster cognitive decline in elders without dementia and decreased risk of cancer mortality NEDICES Study. Neurology. 2014;82(16):1441–1448. doi: 10.1212/WNL.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafè M, Barbi C, Storci G, Salvioli S, Capri M, Olivieri F, Franceschi C. What studies on human longevity tell us about the risk for cancer in the oldest old: data and hypotheses on the genetics and immunology of centenarians. Exp Gerontol. 2002;37(10):1263–1271. doi: 10.1016/s0531-5565(02)00137-7. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18(2):148–156. doi: 10.1634/theoncologist.2012-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brændegaard Winther S, Baatrup G, Pfeiffer P, Qvortrup C. Trends in colorectal cancer in the elderly in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl.1):29–39. doi: 10.3109/0284186X.2015.1114674. [DOI] [PubMed] [Google Scholar]
- Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- Capri Miriam, et al. The genetics of human longevity. Ann N Y Acad Sci. 2006;1067(1):252–263. doi: 10.1196/annals.1354.033. [DOI] [PubMed] [Google Scholar]
- Chen Jingxiang, et al. Association between TCF7L2 gene polymorphism and cancer risk: a meta-analysis. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Uh HW, et al. Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 2011;10(4):686–698. doi: 10.1111/j.1474-9726.2011.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Uh HW, et al. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum Mol Genet. 2014;23(16):4420–4432. doi: 10.1093/hmg/ddu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetrius Lloyd A, Simon David K. The inverse association of cancer and Alzheimer's: a bioenergetic mechanism. J R Soc Interface. 2013;10(82):20130006. doi: 10.1098/rsif.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Wolf PA. Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver JA. Understanding the link between cancer and neurodegeneration. J Geriatr Oncol. 2012;3(1):58–67. [Google Scholar]
- Driver JA. Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology. 2014;15(6):547–557. doi: 10.1007/s10522-014-9523-2. [DOI] [PubMed] [Google Scholar]
- Dunning T, Sinclair A, Colagiuri S. New IDF Guideline for managing type 2 diabetes in older people. Diabetes Res Clin Pract. 2014;103(3):538–540. doi: 10.1016/j.diabres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71(22):4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Ho Y, Daveson BA, Hall S, Higginson IJ, Gao W. Place and cause of death in centenarians: a population-based observational study in England, 2001–2010. PLoS Med. 2014;11(6):e1001653. doi: 10.1371/journal.pmed.1001653. GUIDE_Care project. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewertz M, Christensen K, Engholm G, Kejs AMT, Lund L, Matzen LE, Herrstedt J. Trends in cancer in the elderly population in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl. 1):1–6. doi: 10.3109/0284186X.2015.1114678. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span-from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. . (80-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M. Cancer and dementia: it's complicated. Alzheimer Dis Assoc Disord. 2014;29(2):177–182. doi: 10.1097/WAD.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Valcour V, Yaffe K. Dementia in the oldest old: a multi-factorial and growing public health issue. Alzheimer's Res Ther. 2013;5(4):1. doi: 10.1186/alzrt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh Kanad, Capell Brian C. The senescence-associated secretory phenotype: critical effector in skin cancer and aging. J Investig Dermatol. 2016;2 doi: 10.1016/j.jid.2016.06.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundrum Jacob D, Ronald S Go. Cancer in the oldest old in the United States: current statistics and projections. J Geriatr Oncol. 2012;3(4):299–306. [Google Scholar]
- Harding C, Pompei F, Wilson R. Peak and decline in cancer incidence, mortality, and prevalence at old ages. Cancer. 2012;118(5):1371–1386. doi: 10.1002/cncr.26376. [DOI] [PubMed] [Google Scholar]
- Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–945. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Hjelmborg J vB, Iachine I, Skytthe A, et al. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119(3):312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Hu JX, Thomas CE, Brunak S. Network biology concepts in complex disease comorbidities. Nat Rev Genet. 2016 doi: 10.1038/nrg.2016.87. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jensen JD, Cold S, Nielsen MH, Jylling AMB, Søe KL, Larsen LB, Ewertz M. Trends in breast cancer in the elderly in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl. 1):59–64. doi: 10.3109/0284186X.2015.1115118. [DOI] [PubMed] [Google Scholar]
- Jeon Hee-Young, et al. Irradiation induces glioblastoma cell senescence and senescence-associated secretory phenotype. Tumor Biol. 2016;37(5):5857–5867. doi: 10.1007/s13277-015-4439-2. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SC, Delcastilo E, Loukas M, Osiro S. Common cancers in centenarians. Med Sci Monit. 2014;20:18–23. doi: 10.12659/MSM.889877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul A. Serum Levels of Insulin-like Growth Factor I and Its Binding Proteins in Health and Disease. Growth Horm IGF Res. 2003;13 doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Kanapuru B, Simonsick EM, Ershler WB. Is cancer incidence decreased in the frail elderly? Evidence from a prospective cohort study. J Geriatr Oncol. 2013;4(1):19–25. doi: 10.1016/j.jgo.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001–2010. J Urol. 2014;191(6):1665–1670. doi: 10.1016/j.juro.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Kamei H, Aizu T, et al. Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp Gerontol. 2004;39(11):1595–1598. doi: 10.1016/j.exger.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kristiansen C, Schytte T, Hansen KH, Holtved E, Hansen O. Trends in lung cancer in elderly in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl. 1):46–51. doi: 10.3109/0284186X.2015.1114676. [DOI] [PubMed] [Google Scholar]
- Kulminski AM, He L, Culminskaya I, et al. Pleiotropic associations of allelic variants in a 2q22 region with risks of major human diseases and mortality. Barsh GS, editor. PLoS Genet. 2016;12(11):e1006314. doi: 10.1371/journal.pgen.1006314. [DOI] [PMC free article] [PubMed]
- Lee DR, Kawas CH, Gibbs L, Corrada MM. Prevalence of frailty and factors associated with frailty in individuals aged 90 and older: the 90+ study. J Am Geriatr Soc. 2016;64(11):2257–2262. doi: 10.1111/jgs.14317. [DOI] [PubMed] [Google Scholar]
- Lin CY, Lane HY, Chen TT, Wu YH, Wu CY, Wu VY. Inverse association between cancer risks and age in schizophrenic patients: a 12-year nationwide cohort study. Cancer Sci. 2013;104(3):383–390. doi: 10.1111/cas.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Hsiu-Li, et al. Inverse association between cancer and dementia: a population-based registry study in Taiwan. Alzheimer Dis Assoc Disord. 2016;30(2):118–122. doi: 10.1097/WAD.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC. A Polipoprotein E. Far more than a lipid transport protein. Annu Rev Genom Hum Genet. 2000;1(1):507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Meg Watson, et al. Cervical cancer incidence and mortality among American Indian and Alaska Native women. J Inf. 2014;104(S3):1999–2009. doi: 10.2105/AJPH.2013.301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaishi O, Ando F, Matsuzawa K, Kanawa R, Isobe KI. Cancer incidence in old age. Mech Ageing Dev. 2000;117(1):47–55. doi: 10.1016/s0047-6374(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Moller H, Flatt G, Moran A. High cancer mortality rates in the elderly in the UK. Cancer Epidemiol. 2011;35(5):407–412. doi: 10.1016/j.canep.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Musicco Massimo, et al. Inverse occurrence of cancer and Alzheimer disease A population-based incidence study. Neurology. 2013;81(4):322–328. doi: 10.1212/WNL.0b013e31829c5ec1. [DOI] [PubMed] [Google Scholar]
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7(3):e33411. doi: 10.1371/journal.pone.0033411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortman JM, Velkoff VA, Hogan H. An Aging Nation: the Older Population in the United States. US Census Bureau; Washington, DC: 2014. pp. 25–1140. [Google Scholar]
- Pavlidis N, Stanta G, Audisio RA. Cancer prevalence and mortality in centenarians: a systematic review. Crit Rev Oncol Hematol. 2012;83(1):145–152. doi: 10.1016/j.critrevonc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Pedersen JK, Engholm G, Skytthe A, Christensen K. Cancer and aging: epidemiology and methodological challenges. Acta Oncol. 2016;55(Suppl. 1):7–12. doi: 10.3109/0284186X.2015.1114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perls T, Kohler IV, Andersen S, Schoenhofen E, Pennington J, Young R, Elo IT. Survival of parents and siblings of supercentenarians. J Gerontol Ser A: Biol Sci Med Sci. 2007;62(9):1028–1034. doi: 10.1093/gerona/62.9.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen MH, Dysager L, Gerke O, Lund L. Trends in prostate cancer in elderly in Denmark, 1980–2012. Acta Oncol. 2016;55(Suppl.1):74–78. doi: 10.3109/0284186X.2015.1115120. [DOI] [PubMed] [Google Scholar]
- Pulte D, Redaniel MT, Bird J, Jeffreys M. Survival for patients with chronic leukemias in the US and Britain: age-related disparities and changes in the early 21 st century. Eur J Haematol. 2015;94(6):540–545. doi: 10.1111/ejh.12468. [DOI] [PubMed] [Google Scholar]
- Remi-Martin Laberge, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17(8):1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe CM, Fitzpatrick AL, Xiong C, Sieh W, Kuller L, Miller JP, Morris JC. Cancer linked to Alzheimer disease but not vascular dementia. Neurology. 2010;74(2):106–112. doi: 10.1212/WNL.0b013e3181c91873. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Fiuza-Luces C, Buxens A, Cano-Nieto A, Gómez-Gallego F, Santiago C, Lucia A. Are centenarians genetically predisposed to lower disease risk? Age. 2012;34(5):1269–1283. doi: 10.1007/s11357-011-9296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schächter F, Faure-Delanef L, Guénot F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nature. 1994;6(1):29–32. doi: 10.1038/ng1294-340. [DOI] [PubMed] [Google Scholar]
- Soerensen M, Dato S, Tan Q, et al. Evidence from case–control and longitudinal studies supports associations of genetic variation in APOE, CETP, and IL6 with human longevity. Age (Omaha) 2013;35(2):487–500. doi: 10.1007/s11357-011-9373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Jeon HG. Incidence of kidney, bladder, and prostate cancers in Korea: an update. Korean J Urol. 2015;56(6):422–428. doi: 10.4111/kju.2015.56.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The, T.O.R. pathway comes of age. Biochim Biophys Acta. 2009;1790(10):1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, et al. Functionally significant insulin-like growth factor I. receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105(9):3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarés-Seisdedos R, Dumont N, Baudot A, Valderas JM, Climent J, Valencia A, Rubenstein JL. No paradox, no progress: inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12(6):604–608. doi: 10.1016/S1470-2045(11)70041-9. [DOI] [PubMed] [Google Scholar]
- Tan Q, Jacobsen R, Sørensen M, Christiansen L, Kruse TA, Christensen K. Analyzing age-specific genetic effects on human extreme age survival in cohort-based longitudinal studies. Eur J Hum Genet. 2012;21(10):451–454. doi: 10.1038/ejhg.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry DF, Wilcox MA, McCormick MA, Pennington JY, Schoenhofen EA, Andersen SL, Perls TT. Lower all-cause, cardiovascular, and cancer mortality in centenarians' offspring. J Am Geriatr Soc. 2004;52(12):2074–2076. doi: 10.1111/j.1532-5415.2004.52561.x. [DOI] [PubMed] [Google Scholar]
- Thakkar JP, McCarthy BJ, Villano JL. Age-specific cancer incidence rates that continue to rise through the oldest age groups. Am J Med Sci. 2014;348(1):65. doi: 10.1097/MAJ.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015a;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Torre L, Siegel R, Jemal A. Global Cancer Facts & Figs. 3rd. American Cancer Society; Atlanta: 2015b. pp. 1–61. [Google Scholar]
- US Data & Trends Redirect. Centers for Disease Control and Prevention Web 01 Oct 2016. Centers for Disease Control and Prevention; 2012. Apr, [Google Scholar]
- Ukraintseva S, Yashin A. Individual aging and cancer risk: how are they related? Demogr Res. 2003;9:163–196. [Google Scholar]
- Ukraintseva SV, Arbeev KG, Akushevich I, Kulminski A, Arbeeva L, Culminskaya I, Yashin AI. Trade-offs between cancer and other diseases: do they exist and influence longevity? Rejuvenation Res. 2010;13(4):387–396. doi: 10.1089/rej.2009.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J, Perls T, Franceschi C, Orsouw NJ. BRCA1 gene sequence variation in centenarians. Ann N Y Acad Sci. 2001;928(1):85–96. doi: 10.1111/j.1749-6632.2001.tb05639.x. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Xu B, Yeung HN, Roeland EJ, Martinez ME, Le QT, Murphy JD. Age disparity in palliative radiation therapy among patients with advanced cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):224–230. doi: 10.1016/j.ijrobp.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin A, et al. 6-Phosphofructo-2-Kinase (PFKFB3) promotes cell cycle progression and suppresses apoptosis via cdk1-Mediated phosphorylation of p27. Cell Death Dis 5. 2014;5(7):e1337. doi: 10.1038/cddis.2014.292. PMC. Web. 29 Mar. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Nie C, Min J, et al. Novel loci and pathways significantly associated with longevity. Sci Rep. 2016;6(1):21243. doi: 10.1038/srep21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijke JM, Schouten LJ, Hillen HF, Kiemeney LA, Coebergh JWW, van den Brandt PA. Cancer in the very elderly Dutch population. Cancer. 2000;89(5):1121–1133. doi: 10.1002/1097-0142(20000901)89:5<1121::aid-cncr22>3.3.co;2-7. [DOI] [PubMed] [Google Scholar]


