Abstract
Background
Sepsis is a major health issue in preterm infants. Biomarkers are used to diagnose and monitor patients with sepsis, but C-reactive protein (CRP) is proven not predictive at onset of late onset neonatal sepsis (LONS) diagnosis. The aim of this study was to evaluate the association of interleukin-6(IL-6), procalcitonin (PCT) and CRP with subsequent sepsis severity and mortality in preterm infants suspected of late onset neonatal sepsis.
Methods
The study was conducted at the Erasmus University Medical Center–Sophia Children’s Hospital Rotterdam. Patient data from January 2018 until October 2019 were reviewed for all preterm neonates born with a gestational age below 32 weeks with signs and symptoms suggestive of systemic infection, in whom blood was taken for blood culture and for inflammatory biomarkers determinations. Plasma IL-6 and PCT were assessed next to CRP at the moment of suspicion. We assessed the association with 7-day mortality and sepsis severity (neonatal sequential organ failure assessment (nSOFA) score, need for inotropic support, invasive ventilation and thrombocytopenia).
Results
A total of 480 suspected late onset neonatal sepsis episodes in 208 preterm neonates (gestational age < 32 weeks) were retrospectively analyzed, of which 143 episodes were classified as sepsis (29.8%), with 56 (11.7%) cases of culture negative, 63 (13.1%) cases of gram-positive and 24(5.0%) cases of gram-negative sepsis. A total of 24 (5.0%) sepsis episodes resulted in death within 7 days after suspicion of LONS. Both IL-6 (adjusted hazard ratio (aHR): 2.28; 95% CI 1.64–3.16; p < 0.001) and PCT (aHR: 2.91; 95% CI 1.70–5.00; p < 0.001) levels were associated with 7-day mortality; however, CRP levels were not significantly correlated with 7-day mortality (aHR: 1.16; 95% CI (0.68–2.00; p = 0.56). Log IL-6, log PCT and log CRP levels were all significantly correlated with the need for inotropic support.
Conclusions
Our findings show that serum IL-6 and PCT levels at moment of suspected late onset neonatal sepsis offer valuable information about sepsis severity and mortality risk in infants born below 32 weeks of gestation. The discriminative value was superior to that of CRP. Determining these biomarkers in suspected sepsis may help identify patients with imminent severe sepsis, who may require more intensive monitoring and therapy.
Keywords: Interleukin-6, C-reactive protein, Procalcitonin, nSOFA, Sepsis, Late onset neonatal sepsis, Neonatology
Introduction
Neonatal sepsis is a major health issue, particularly in the preterm neonatal population, where low birth weight, immature immune system and other compromising factors make it a primary cause of morbidity and death [1–5]. Late onset neonatal sepsis (LONS) occurs after three days of life and may be caused by pathogens acquired at delivery or during the course of hospital care [6]. Although in most cases LONS onset is often inconspicuous, the clinical course may be alarmingly fulminant leading to septic shock and death within hours of onset [7, 8]. Infected neonates must therefore be promptly identified, and additionally, sepsis severity should ideally be assessed at moment of onset to intensify and adapt monitoring and therapy. Several chemical biomarkers are used to diagnose and monitor disease progression in patients suspected of sepsis. Three commonly used biomarkers are: C-reactive protein (CRP), procalcitonin (PCT) and interleukin-6 (IL-6) [9]. CRP, the most frequently used laboratory test for the diagnosis of neonatal sepsis, is extensively studied in routine practice and available as point of care test [10]. However, CRP level at onset is proven not predictive of LONS diagnosis [11]. PCT is an acute phase reactant and the PCT response is more rapid than the elevation of CRP [12, 13]. IL-6 is a pro-inflammatory cytokine and increases rapidly before PCT or CRP increases in neonatal septic patients [12].
For individual patient management, it is unclear which patients are most likely to have a severe sepsis or which are least likely to survive at moment of sepsis suspicion and thus would benefit from more intensive monitoring and from alternative treatment approaches. Risk assessment would greatly aid decision-making in individual patients. Recently, a new sepsis severity score (e.g., organ dysfunction) was adapted from adults for use in preterm neonates with LONS. This score, the neonatal Sequential Organ Failure Assessment (nSOFA) score, consists of respiratory, cardiovascular and hematological criteria [14]. A high score (> 4 points out of a maximum of 15) 12 h after onset is associated with mortality [14].
Little is known about the predictive value of inflammatory biomarker responses in preterm infants suspected of LONS. Nevertheless, CRP seems the most commonly used and widely implemented biomarker. Furthermore, few studies have purposefully attempted to evaluate biomarkers that could objectively reflect the risk of mortality and sepsis severity of neonatal sepsis in neonates suspected of LONS [15, 16]. The aim of the current study is to investigate the association of inflammatory biomarkers at moment of suspicion of LONS with subsequent risk of mortality and sepsis severity in preterm infants (< 32 weeks of gestation at birth).
Material and methods
Study design and population
The study was conducted at the Erasmus MC University Medical Center–Sophia Children’s Hospital Rotterdam, a level IV neonatal intensive care unit (NICU). Patient data from January 2018 until October 2019 were reviewed for all neonates with signs and symptoms suggestive of systemic infection, in whom blood was taken for blood culture and for inflammatory biomarkers determinations.
Ethical approval
The study was approved by the local ethical board of the Erasmus MC, University Medical Center. No explicit informed consent was obtained, the ethical board resumed responsibility. As part of standard care patients are asked for consent to use data for future medical research when admitted to our NICU.
Definition of sepsis
The Department of Microbiology provided all results of blood cultures, including timing of collection and identified micro-organisms. We excluded all control blood cultures. One patient could provide multiple cases of sepsis. Sepsis diagnosis was established by the criteria defined by the NICHD Neonatal Research Network [17]. LONS was defined as a positive blood culture obtained after 72 h of life and intent to treat with antibiotics for 5 days or more. An episode of culture proven sepsis was defined as a positive blood culture due to an identified bacterial organism (including coagulase-negative staphylococci), treated with antibiotics for 5 days or more or treated for a shorter duration if death occurred during treatment [17]. Culture negative sepsis was defined as (1) C-reactive protein level greater than 10 mg/L within two days after blood culture, (2) antibiotic treatment longer than 5 days (or intention to treat longer) and (3) clinical signs of sepsis assessed by treating physician.
Patient characteristics
The following patient characteristics were obtained from clinical charts: sex, gestational age, birthweight and postnatal age at suspicion. Data of the mother were collected from the Dutch Neonatology Register (LNR): age, hypertension, gestational diabetes, prolonged preterm rupture of membranes (pPROM), maternal fever, antenatal corticosteroid therapy and multiple birth.
Biomarkers
The biomarkers included in the analysis were serum IL-6, CRP and PCT. Biomarker levels were measured by the department of Clinical Chemistry and were retrospectively queried from the laboratory information system. Blood samples were taken at the time of the initial sepsis suspicion (0 h).
CRP levels were measured using a turbidimetric method (C502, Cobas 8000 system, Roche Diagnostics, Rotkreuz, Switzerland). PCT and IL-6 were both measured using Electro-Chemi Luminescent Immuno Assay (ECLIA) tests (E801, Cobas 8000 system, Roche Diagnostics, Rotkreuz, Switzerland).
Plasma levels of CRP, PCT and IL-6 were routinely determined as part of a diagnostic local workup protocol whenever sepsis was suspected in infants. No other cytokines were included in this protocol. At our center, we use heart rate observation (HeRO) monitoring in preterm infants as an early warning score for LONS [18]. According to local protocol, clinicians can consider to determine chemical biomarkers when the HeRO score is increased. Blood cultures are drawn and antibiotic treatment is started when the neonate shows evident clinical signs of sepsis or when CRP, PCT or IL-6 levels are increased.
Outcome measurements
The primary outcome was mortality within 7 days of suspicion of sepsis. The secondary outcomes were measures of sepsis severity; nSOFA score [14], need of inotropic therapy, need of mechanical ventilation and thrombocytopenia within 3 days of suspicion. The nSOFA parameters were obtained from clinical charts to calculate the nSOFA scores at time point, t = 12 h. A high nSOFA score was defined as more than 4 points out of a maximum of 15 at 12 h after onset. Inotropic support was defined as the start of inotropes after sepsis suspicion. Need for mechanical ventilation was defined as the need for intubation within 72 h after suspicion. If the patient was already intubated at moment of suspicion, this sepsis episode was excluded for analysis with this specific secondary outcome. Thrombocytopenia was defined as a platelet count < 50 × 109/L.
Statistical analysis
Statistical analysis was performed using R (R Core Team (2017), Vienna, Austria).
Categorical variables were described using absolute and relative frequencies. Continuous variables were described using medians and range (min–max) because of non-normal distributions.
A receiver operating characteristic (ROC) analysis was used to investigate the predictive value of each biomarker for 7-day mortality; a high nSOFA score at 12 h (nSOFA score > 4 [14]); inotropic support; intubation; and thrombocytopenia within 3 days. The exploratory optimal cutoff point for each biomarker was calculated using the R package ‘OptimalCutpoints.’ The Youden index was used to calculate the optimal cutoff points [19]. The Youden index calculates the cutoff point that optimizes the biomarker’s differentiating ability when equal weight is given to sensitivity and specificity. The cutoff point for predicting intubation was calculated by maximizing the specificity, to identify high risk patients to increase awareness in clinical practice. The effect of each biomarker on 7-day mortality was estimated using Cox regression models. We performed two sensitivity analyses; one additionally adjusting for previous sepsis episodes and the other for the type of infection. Due to the limited number of events, we were able to adjust for three possible confounders. All analyses were adjusted for sex, gestational age and birthweight. We investigated the 7-day survival using the Kaplan–Meier method. There is a lack of age specific reference values for these biomarkers. Based on the optimal cutoff analysis described before, the Kaplan–Meier method was used to estimate survival across groups with different levels of biomarkers at onset. The log-rank test was used to compare survival across groups with different levels of biomarkers.
The statistical analysis was performed on raw or logarithmically transformed data where appropriate. For all tests, a p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
In the study period, a total of 480 episodes of suspected LONS in 208 prematurely born patients (< 32 weeks of gestation at birth) occurred. Of these episodes, 143 (29.8%) were classified as sepsis. Gram-positive sepsis was most common (63 cases (13.1%), predominantly Coagulase-negative Staphylococci (CoNS) (48%) and Staphylococcus aureus (16%)). Gram-negative sepsis occurred less frequently (24 cases (5.0%), the most commonly found gram-negative organism was Escherichia coli (19%) (see Table 1)). Baseline maternal and patient characteristics are summarized in Table 1. The median gestational age of the neonates was 26 weeks and 3 days (range: 24 + 0–31 + 6), and the median birthweight was 850 (range: 445–2100) grams.
Table 1.
Patient and mother baseline characteristics
| Variable | Total episodes (n = 480) |
|---|---|
| Age of the mother (years) | 30 (18–43) |
| Hypertension Pregnancy Disorder (PIH/PE) | 107 (22.3%) |
| Gestational diabetes | 33 (6.9%) |
| pPROMa | 85 (17.7%) |
| Maternal Fever | 22 (4.6%) |
| Antenatal corticosteroid therapyb | |
| None | 64 (13.3%) |
| 1 dose of betamethasone | 113 (23.5%) |
| 2 doses of betamethasone | 295 (61.5%) |
| Singleton | 386 (80.4%) |
| Sex (male) | 249 (51.9%) |
| Gestational Age (weeks) | 26 + 3 (24 + 0 – 31 + 6) |
| Post menstrual age (weeks) | 29 + 1 (24 + 4 – 51 + 3) |
| Birthweight (grams) | 850 (445–2100) |
| Age at Evaluation (days) | 13 (4–165) |
| IL-6 level at onset (pg/mL) | 45 (2–1396700) |
| PCT level at onset (ng/mL) | 0.67 (0.02 – 100) |
| CRP level at onset (mg/L) | 3.45 (0.3 – 309.0) |
| Mortality | 24 (5%) |
| Type of infection | |
| No infection | 337 (70.2%) |
| Culture negative | 56 (11.7%) |
| Culture proven sepsis | 87 (18.1%) |
| Gram positive (% of total proven sepsis) | 63 (72%) |
| CoNSc | 42 (48%) |
| S. aureus | 14 (16%) |
| Others | 7 (8%) |
| Gram negative (% of total proven sepsis) | 24 (28%) |
| E.Coli | 17 (19%) |
| Klebsiella | 4 (5%) |
| Others | 3 (4%) |
| nSOFA score | |
| nSOFA score at t = 12 h | 0 (0–11) |
| Number of suspected episode | |
| 1 suspected episode | 84 |
| 2 suspected episode | 46 |
| > 2 suspected episodes | 78 |
aProlonged premature rupture of membranes
bNumber of doses of antenatal betamethasone therapy
cCoagulase negative staphylococci
Values are medians (min–max range) or percentages
Figure 1 shows the plasma concentrations of IL-6, PCT and CRP at the time of sepsis suspicion (0 h). Biomarker levels in patients with sepsis differed significantly compared with patients without sepsis (p < 0.001 for all biomarkers). Excluding culture negative sepsis episodes did not affect the differences in biomarker levels when comparing patients with sepsis and without sepsis (see Additional file 1).
Fig. 1.
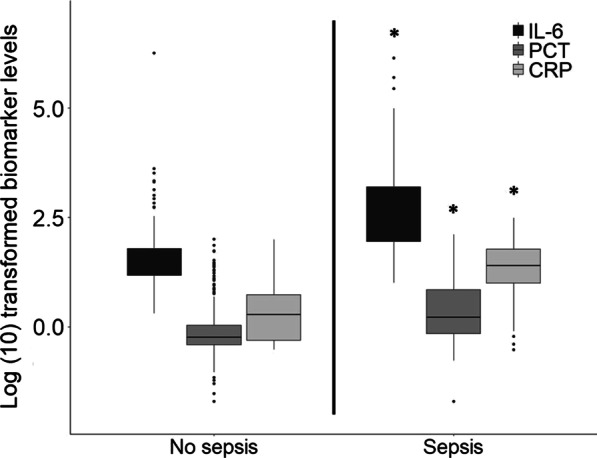
Biomarker levels at moment of suspicion. Log transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L) at the time of sepsis evaluation across patients with sepsis (both culture negative and culture positive sepsis) and without sepsis. Data are presented as Log(10) transformed data because of the wide range in biomarker levels and comparability. * P < 0.001
Mortality
In the entire study population, a total of 24 sepsis episodes in 22 patients resulted in death within 7 days (5.0%). Log IL-6 levels were associated with 7-day mortality (adjusted hazard ratio (aHR): 2.28; 95% CI (1.64–3.16; p < 0.001), in a model corrected for birth weight, gestational age and sex. Similarly, log PCT levels were associated with 7-day mortality (aHR: 2.91; 95% CI (1.70–5.00; p < 0.001). Contrarily, log CRP levels were not associated with 7-day mortality (aHR ratio: 1.16; 95% CI (0.68–2.00; p = 0.56). Sensitivity analyses showed that these associations are independent of previous sepsis episodes and the type of infection (see Additional files 2 and 3).
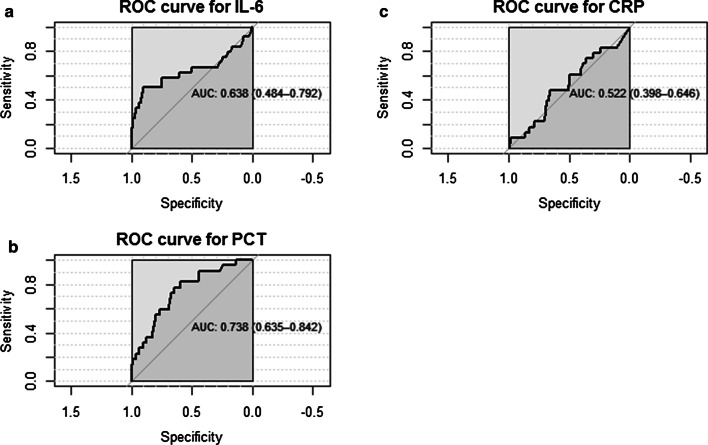
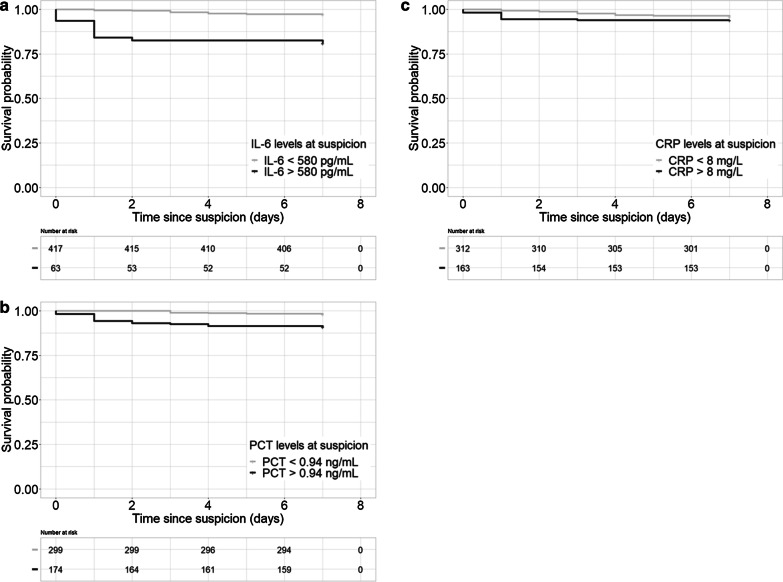
The area under the curve for predicting mortality was 0.64 (95% CI 0.48–0.79) for IL-6, and an exploratory cutoff point could be established at 580 pg/mL, with a sensitivity of 50.0% and a specificity of 89.9% (see Fig. 2). The area under the curve (AUC) for PCT was 0.74 (95% CI 0.64–0.84), and an exploratory PCT cutoff point could be established at 0.94 ng/mL. At this value, PCT reached a sensitivity of 77.3% and a specificity of 64.9%. The AUC for CRP was 0.52 (95% CI 0.40–0.65), and an exploratory CRP cutoff point could be established at 7.9 mg/L, with a sensitivity of 47.8% and a specificity of 65.8% (see Fig. 2). The Kaplan–Meier survival curves of the 7-day survival significantly differed across the two IL-6 categories with cutoff 580 pg/mL and the two PCT categories with cutoff 0.75 ng/mL, both p < 0.001 (see Fig. 3 panel A and B). Survival did not differ across the CRP categories with cutoff 8 mg/L at onset of sepsis, p = 0.33 (see Fig. 3 panel C).
Fig. 2.
ROC curves for IL6 (a), PCT (b) and CRP (c) with respect to predicting 7-day mortality
Fig. 3.
Kaplan–Meier survival curves. Primary end point of 7-day mortality according to IL-6 (a), PCT (b) and CRP (c) category at moment of sepsis suspicion
nSOFA score
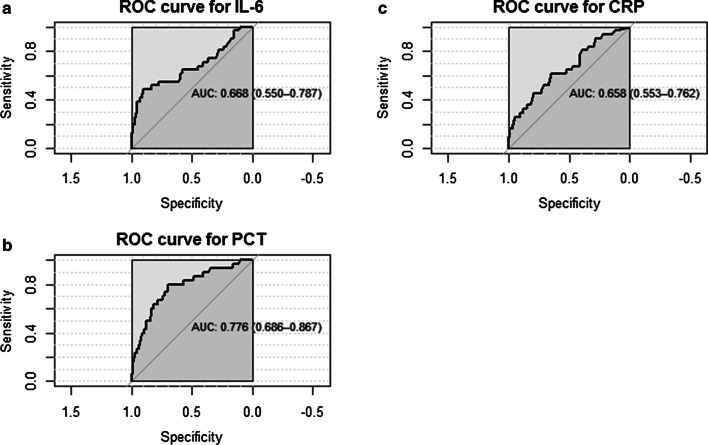
ROC curves and AUCs for IL-6, PCT and CRP were calculated with respect to predicting a nSOFA score above 4 at 12 h after onset. IL-6, PCT and CRP showed an AUC value of 0.668 (95% CI, 0.55–0.79), 0.776 (95% CI, 0.69–0.87) and 0.658 (95% CI, 0.55–0.76), respectively (see Fig. 4).
Fig. 4.
ROC curves for IL6 (a), PCT (b) and CRP (c) with respect to predicting a high nSOFA score (> 4) at t = 12 h after suspicion of sepsis
An exploratory IL-6 cutoff point could be established at 548 pg/mL. At this value, IL-6 reached a sensitivity of 48.3% and a specificity of 90.5%. An exploratory PCT cutoff point could be established at 1.06 ng/mL, with a sensitivity of 80.0% and a specificity of 70.2%. An exploratory CRP cutoff point could be established at 6.4 mg/L, with a sensitivity of 61.3% and a specificity of 65.3%.
Inotropic and respiratory support and thrombocytopenia
Inotropic support
Log IL-6, log PCT and log CRP levels were all significantly correlated with the need for inotropic support with an aHR of 2.41 (95% CI 1.93–3.00; p < 0.001); 4.24 (95% CI 3.02–5.96; p < 0.001) and 2.0 (95% CI 1.41–2.90; p < 0.001), respectively).
ROC curves and AUC values for IL-6, PCT and CRP were calculated with respect to predicting inotropic support need, intubation and thrombocytopenia within 3 days of suspicion. AUC values with their respective 95% confidence intervals, exploratory cutoff points with sensitivity and specificity are depicted in Table 2 for each of the biomarkers. In total, 79 patients were excluded from the respiratory support analysis, due to being already ventilated at moment of sepsis suspicion.
Table 2.
Prediction of inotropic and respiratory support and thrombocytopenia
| Total population (n = 480) | Interleukin-6 | Procalcitonin | C-reactive protein | |
|---|---|---|---|---|
| Inotropic support | N = 67 cases (14%) | |||
| AUC | 0.71 | 0.79 | 0.65 | |
| 95% CI | 0.63–0.80 | 0.63–0.80 | 0.56–0.73 | |
| Exploratory cutoff | 118 pg/mL | 1.03 ng/mL | 6.3 mg/L | |
| Sensitivity | 63.1% | 79.6% | 58.9% | |
| Specificity | 77.8% | 71.1% | 65.7% | |
| Thrombocytopenia | N = 79 cases (16.5%) | |||
| AUC | 0.77 | 0.83 | 0.79 | |
| 95% CI | 0.67–0.88 | 0.72–0.93 | 0.69–0.89 | |
| Exploratory cutoff | 391 pg/mL | 1.89 ng/mL | 11.0 mg/L | |
| Sensitivity | 60.0% | 79.2% | 80.0% | |
| Specificity | 88.1% | 82.3% | 72.6% | |
| Total population (n = 401) | ||||
| Respiratory support | N = 68 cases (17%) | |||
| AUC | 0.67 | 0.72 | 0.59 | |
| 95% CI | 0.60–0.74 | 0.65–0.80 | 0.51–0.67 | |
| Exploratory cutoff | 201 pg/mL | 1.6 ng/mL | 27 mg/L | |
| Sensitivity | 39.7% | 45.3% | 23.9% | |
| Specificity | 85.6% | 85.8% | 86.9% |
Data represent AUC values with their respective 95% confidence intervals, exploratory cutoff points with sensitivity and specificity for each biomarker
Discussion
LONS is a relevant issue in every NICU, and an early assessment of sepsis severity has the potential to improve quality of care. In this large retrospective study, we assessed the association between IL-6, PCT and CRP at onset and subsequent sepsis severity and mortality. Up until now, little has been known about the prognostic value of IL-6 and PCT for disease severity in preterm neonates suspected of LONS. Our results show that serum IL-6 and PCT, but not CRP, are associated with 7-day mortality in preterm infants suspected of LONS. IL-6, PCT and CRP at onset of sepsis were associated with various clinical measures of sepsis severity, such as need for inotropic support and mechanical ventilation, with IL-6 and PCT showing the strongest associations, with a higher discriminative value compared to CRP. To our knowledge, no other study investigated the association of these biomarkers with measures of sepsis severity. Determining these biomarkers in suspected LONS can help clinicians to identify neonates with possible imminent severe sepsis at moment of suspicion. The investigated biomarkers can be determined rapidly, usually within the matter of an hour and a half, and while the biomarker levels were related to the type of infection (e.g., gram-negative infections) they independently predicted the subsequent mortality risk. This risk assessment increases awareness and may lead to personalized monitoring. For example, peripheral arterial blood pressure measurement could be considered when high risk patients are identified, to monitor possible occurring of hypotension closely. Furthermore, they could enable personalized treatment, such as starting pentoxifylline in high risk patients [20]. Also, these biomarkers could provide a key for selecting patients at the highest risk for severe sepsis, in future clinical studies.
CRP is the most commonly available test in hospitals for neonatal sepsis [10, 11, 21, 22]. It is both used for diagnosing sepsis and monitoring disease progression. However, a recent meta-analyses by Brown and colleagues in 2255 infants showed that CRP is insensitive and nonspecific for LONS diagnosis [11]. Based on the kinetics of CRP, it is not preferred as an early prognostic and diagnostic marker; however, it has been shown that serial measurements of CRP could reliably rule-out infection 24 h after suspicion [23]. In the present study, we confirmed that CRP at onset of sepsis yields limited information on subsequent sepsis severity and risk of mortality. This is also in line with one small study by Romagnoli et al., which did not find a significant correlation of CRP levels at onset of sepsis and mortality in 39 preterm infants suspected of sepsis [24].
Little has previously been known about the prognostic value of IL-6 in neonates suspected of sepsis. We show that IL-6 can offer valuable information about the subsequent sepsis severity and risk of mortality among premature neonates suspected of LONS. Two other smaller studies showed inconsistent results of IL-6 and risk of mortality. Boskabandi et al. showed in 84 preterm and term patients that IL-6 might be valuable in predicting sepsis mortality [25], while Romagnoli et al. did not find a statistical significant difference in IL-6 levels in 39 septic preterm infants in survivors and non-survivors [24]. Studies in older pediatric patients and adults are in line with our findings; in these patients with sepsis, higher levels of IL-6 at the onset of sepsis are associated with a poorer outcome [26–28].
Serum PCT is a promising biomarker of increasing interest for detecting serious bacterial infections [29] and can be used for guiding duration of antibiotic therapy in early onset sepsis [30]. Our results suggest that PCT might also be a useful marker in predicting disease severity in LONS. However, caution is required when interpreting PCT levels [31] in an individual patient. Hahn et al. found that gestational age at birth and postnatal age had a significant effect on normal PCT levels, with more immature patients within early postnatal life having a wide range of PCT levels in a non-septic situation [31]. To the best of our knowledge, no other studies evaluated the value of PCT in predicting mortality or sepsis severity in LONS. Our study highlights a possible role of PCT in guiding clinical management.
Recently, Wynn et al. developed the nSOFA score, a sepsis severity score consisting of respiratory, cardiovascular and hematological criteria [14] and showed that a high score 12 h (> 4 out of 15) after onset of sepsis was associated with mortality. Biomarker levels of IL-6, PCT and CRP were associated with this composite outcome of sepsis severity, in which PCT had the highest diagnostic accuracy. Also, the biomarker levels were associated with each component of the nSOFA score (need for inotropic/mechanical support and thrombocytopenia). This highlights that these biomarkers at the moment of suspicion could provide information on the subsequent clinical course of the sepsis in a specific individual. To our knowledge, no other studies investigated the association of these biomarkers with measures of sepsis severity. These findings could help clinicians differentiate patients with possible severe sepsis at moment of suspicion, increase awareness for imminent clinical deterioration and also enable to personalize sepsis therapy.
There are some remarks for interpreting the study results. First, in our center we use heart rate observation (HeRO) monitoring [32] as an early warning system of sepsis. Based on changes in heart rate characteristics, we consider determining inflammatory biomarkers, possibly early in sepsis, when clinical signs are absent or non-specific. In centers without such monitoring, biomarkers could be determined later in the course of sepsis, which could affect the associations we found. For instance, after a rapid increase of IL-6 the levels also decrease rapidly and provide less information on future clinical course compared to determining IL-6 early in sepsis. This may limit generalizability of the results of our study. Second, if a patient had multiple suspected sepsis episodes we included all unique episodes independent of previous proven, culture negative or non-septic episodes. We hypothesized that the association between biomarker levels and subsequent mortality risk or sepsis severity is not modified by previous sepsis periods which a patient survived. Sensitivity analyses adjusting for previous sepsis episodes did not change the results materially. Furthermore, in daily clinical practice we would also not interpret a biomarker taking into account previous episodes. Our study also has limitations. We performed a retrospective cohort study including all patients with a gestational age < 32 weeks admitted to our hospital. We obtained many variables and outcome data from clinical charts, which may lead to misclassification due to mistakes in data entry. If misclassification would occur, we would expect this random misclassification decreasing precision of the effect estimates. We tried to limit missing data and misclassification by using automated registries for biomarker levels and blood cultures from the departments of clinical chemistry and microbiology respectively, and double data entry from the clinical charts to reduce data entry errors. Furthermore, despite being the largest cohort study investigating biomarkers levels in preterm neonates with suspected LONS, the number of events in our study is relatively small, limiting the ability to test model performance for calculated cutoff values in a validation sample.
Future research should focus on validating our findings in independent and larger populations. Furthermore, in this study we looked at short-term outcome measures; future research could focus on the relationship of these biomarkers with long-term (neurodevelopmental) outcomes measures.
Conclusion
In conclusion, our findings demonstrate that IL-6 and PCT offer valuable information about disease severity in subsequent clinical course and mortality risk in suspected preterm LONS. Both IL-6 and PCT are more informative than CRP and could guide personalized intensive monitoring and increase awareness for imminent clinical deterioration and timely intervention.
Supplementary information
Additional file 1. Figure: Biomarker levels at moment of suspicion. Description of data: Log(10) transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L) at the time of sepsis evaluation across patients with figure A) sepsis (only culture positive sepsis, excluding patients with culture negative sepsis) and without sepsis; figure B) sepsis (both culture negative and culture positive sepsis) and without sepsis. Data are Log(10) transformed for visualization purposes.
Additional file 2. Table: Estimates of hazard ratios of each biomarker for 7-day mortality with and without adjusting for previous sepsis episodes. Description of data: Effect estimates reflect the hazard ratio with their 95% confidence intervals. **p<0.001. Biomarker levels are Log(10) transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L).
Additional file 3. Table: Estimates of hazard ratios of each biomarker for 7-day mortality with and without adjusting for type of infection. Description of data: 1Type of infection defined in 4 categories: no – culture negative – gram-positive – gram-negative sepsis. Effect estimates reflect the hazard ratio with their 95% confidence intervals. *p< 0.05 ** p<0.001. Biomarker levels are Log(10) transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L).
Acknowledgements
Not applicable.
Abbreviations
- AUC
Area under the curve
- CRP
C-reactive protein
- IL-6
Interleukin-6
- LONS
Late onset neonatal sepsis
- nSOFA
Neonatal sequential organ failure assessment
- PCT
Procalcitonin
- ROC
Receiver operating characteristic
Authors’ contributions
HT conceptualized and designed the study, supervised data collection and reviewed and revised the manuscript. SK collected data, carried out the initial analyses, and drafted, reviewed and revised the manuscript. CR collected data and reviewed and revised the manuscript. SS, IR, YR and RK critically reviewed the manuscript for important intellectual content and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
The project was done with no specific support. The authors have no financial relationships relevant to this article to disclose.
Availability of data and materials
The datasets generated during the current study are not publicly available due to privacy legislation but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the local ethical board of the Erasmus MC, University Medical Center (Reference Number: MEC-2020-0111). No explicit informed consent was obtained, the ethical board resumed responsibility. As part of standard care patients are asked for consent to use data for future medical research when admitted to our NICU.
Consent for publication
Not applicable (no individual data).
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1186/s13054-020-03423-2.
References
- 1.Ganatra HA, Stoll BJ, Zaidi AK. International perspective on early-onset neonatal sepsis. Clin Perinatol. 2010;37(2):501–523. doi: 10.1016/j.clp.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35(3):706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 5.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Human Dev. 2012;88:S69–S74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng PC. Diagnostic markers of infection in neonates. Arch Dis Childh Fetal Neonatal Ed. 2004;89(3):F229–F235. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng PC, Cheng SH, Chui KM, Fok TF, Wong MY, Wong W, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Childh Fetal Neonatal Ed. 1997;77(3):F221–F227. doi: 10.1136/fn.77.3.f221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meem M, Modak JK, Mortuza R, Morshed M, Islam MS, Saha SK. Biomarkers for diagnosis of neonatal infections: a systematic analysis of their potential as a point-of-care diagnostics. J Global Health. 2011;1(2):201–209. [PMC free article] [PubMed] [Google Scholar]
- 10.Hofer N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasks. Neonatology. 2012;102(1):25–36. doi: 10.1159/000336629. [DOI] [PubMed] [Google Scholar]
- 11.Brown JVE, Meader N, Wright K, Cleminson J, McGuire W. Assessment of C-reactive protein diagnostic test accuracy for late-onset infection in newborn infants: a systematic review and meta-analysis. JAMA Pediatrics. 2020;174(3):260–268. doi: 10.1001/jamapediatrics.2019.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah BA, Padbury JF. Neonatal sepsis. Virulence. 2014;5(1):170–178. doi: 10.4161/viru.26906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stocker M, Hop WCJ, van Rossum AMC. Neonatal procalcitonin intervention study (NeoPInS): effect of Procalcitonin-guided decision making on duration of antibiotic therapy in suspected neonatal early-onset sepsis—a multi-centre randomized superiority and non-inferiority Intervention Study. BMC Pediatrics. 2010;10(1):89. doi: 10.1186/1471-2431-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatric Res. 2019;88:85. doi: 10.1038/s41390-019-0517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng PC, Li K, Leung TF, Wong RPO, Li G, Chui KM, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–1189. doi: 10.1373/clinchem.2005.062075. [DOI] [PubMed] [Google Scholar]
- 16.Ng PC, Li K, Wong RPO, Chui K, Wong E, Li G, et al. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch Dis Childh Fetal Neonatal Ed. 2003;88(3):F209–F213. doi: 10.1136/fn.88.3.F209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boghossian NS, Page GP, Bell EF, Stoll BJ, Murray JC, Cotten CM, et al. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatrics. 2013;162(6):1120–1124. doi: 10.1016/j.jpeds.2012.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatrics. 2011;159(6):900–906. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Schüller SS, Kempf K, Unterasinger L, Strunk T, Berger A. Intravenous pentoxifylline is well tolerated in critically ill preterm infants with sepsis or necrotizing enterocolitis. Eur J Pediatrics. 2020;2020:1–6. doi: 10.1007/s00431-020-03612-9. [DOI] [PubMed] [Google Scholar]
- 21.Ismail AQT, Gandhi A. Using CRP in neonatal practice. J Maternal Fetal Neonatal Med. 2015;28(1):3–6. doi: 10.3109/14767058.2014.885499. [DOI] [PubMed] [Google Scholar]
- 22.Hedegaard SS, Wisborg K, Hvas A-M. Diagnostic utility of biomarkers for neonatal sepsis: a systematic review. Inf Dis. 2015;47(3):117–124. doi: 10.3109/00365548.2014.971053. [DOI] [PubMed] [Google Scholar]
- 23.Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics. 1998;102(4):e41-e. doi: 10.1542/peds.102.4.e41. [DOI] [PubMed] [Google Scholar]
- 24.Romagnoli C, Frezza S, Cingolani A, De Luca A, Puopolo M, De Carolis MP, et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001;160(6):345–350. doi: 10.1007/pl00008445. [DOI] [PubMed] [Google Scholar]
- 25.Boskabadi H, Maamouri G, Tavakol Afshari J, Mafinejad S, Hosseini G, Mostafavi-Toroghi H, et al. Evaluation of serum interleukins-6, 8 and 10 levels as diagnostic markers of neonatal infection and possibility of mortality. Iran J Basic Med Sci. 2013;16(12):1232–1237. [PMC free article] [PubMed] [Google Scholar]
- 26.Patel RT, Deen KI, Youngs D, Warwick J, Keighley MRB. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Bjs. 1994;81(9):1306–1308. doi: 10.1002/bjs.1800810914. [DOI] [PubMed] [Google Scholar]
- 27.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215(4):356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan JS, Kilpatrick L, Costarino AT, Lee SC, Harris MC. Correlation of plasma cytokine elevations with mortality rate in children with sepsis. J Pediatrics. 1992;120(4, Part 1):510–515. doi: 10.1016/s0022-3476(05)82476-x. [DOI] [PubMed] [Google Scholar]
- 29.Vouloumanou EK, Plessa E, Karageorgopoulos DE, Mantadakis E, Falagas ME. Serum procalcitonin as a diagnostic marker for neonatal sepsis: a systematic review and meta-analysis. Intensive Care Med. 2011;37(5):747–762. doi: 10.1007/s00134-011-2174-8. [DOI] [PubMed] [Google Scholar]
- 30.van Rossum AMC, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004;4(10):620–630. doi: 10.1016/S1473-3099(04)01146-6. [DOI] [PubMed] [Google Scholar]
- 31.Hahn WH, Song JH, Park IS, Kim H, Park S, Oh MH. Reference intervals of serum procalcitonin are affected by postnatal age in very low birth weight infants during the first 60 days after birth. Neonatology. 2015;108(1):60–64. doi: 10.1159/000381330. [DOI] [PubMed] [Google Scholar]
- 32.Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics. 2001;107(1):97–104. doi: 10.1542/peds.107.1.97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure: Biomarker levels at moment of suspicion. Description of data: Log(10) transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L) at the time of sepsis evaluation across patients with figure A) sepsis (only culture positive sepsis, excluding patients with culture negative sepsis) and without sepsis; figure B) sepsis (both culture negative and culture positive sepsis) and without sepsis. Data are Log(10) transformed for visualization purposes.
Additional file 2. Table: Estimates of hazard ratios of each biomarker for 7-day mortality with and without adjusting for previous sepsis episodes. Description of data: Effect estimates reflect the hazard ratio with their 95% confidence intervals. **p<0.001. Biomarker levels are Log(10) transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L).
Additional file 3. Table: Estimates of hazard ratios of each biomarker for 7-day mortality with and without adjusting for type of infection. Description of data: 1Type of infection defined in 4 categories: no – culture negative – gram-positive – gram-negative sepsis. Effect estimates reflect the hazard ratio with their 95% confidence intervals. *p< 0.05 ** p<0.001. Biomarker levels are Log(10) transformed plasma concentrations of IL-6 (pg/mL), PCT (ng/mL) and CRP (mg/L).
Data Availability Statement
The datasets generated during the current study are not publicly available due to privacy legislation but are available from the corresponding author on reasonable request.