Abstract
The polycystic ovary syndrome is a common endocrine disorder that has profound implications for women throughout their reproductive years. A diagnosis of polycystic ovary syndrome is associated with reproductive challenges including a difficulty in conceiving as well as the pregnancy-related complications of miscarriage, hypertensive disorders, gestational diabetes and prematurity. Consequently, polycystic ovary syndrome has profound implications for women and their offspring with regard to reproductive function in the short term and in the longer term the risk of chronic illness and congenital anomalies, and health care resources should be directed accordingly to mitigate against these risks.
Keywords: complications, polycystic ovary syndrome, pregnancy, reproduction
Introduction
Polycystic ovary syndrome (PCOS) affects 5%–20% of women of reproductive age worldwide and is characterized by hyperandrogenism, ovulatory dysfunction and polycystic ovarian morphology.1 The 2003 Rotterdam criteria are currently the internationally accepted criteria by which PCOS is diagnosed. Patients are diagnosed with PCOS when two out of three criteria are satisfied: oligoovulation or anovulation, clinical and/or biochemical signs of hyperandrogenism and/or the presence of polycystic ovaries (PCO) and exclusion of other etiologies (congenital adrenal hyperplasia and androgen-secreting tumors).2 There also exists the Androgen Excess and PCOS (AE-PCOS) Society definition which recommends that clinical or biochemical hyperandrogenism should be essential for diagnosis, but also ovulatory dysfunction is required in the form of either oligo-anovulation or PCO.3
The pathophysiology of PCOS is multifactorial, and it is believed that a genetic predisposition exists that is exacerbated by excess adiposity. It is thought that the pathophysiology of PCOS involves the interaction between abnormal ovarian morphology, due to excess androgen production by the PCO—hyperinsulinemia, and elevated luteinizing hormone (LH) levels.4 It has been shown that ovarian androgen production in women with PCOS is accelerated due to the increased ovarian theca cell androgenic enzymatic activity of 3β-hydroxysteroid dehydrogenase (HSD) 17α-hydroxylase/C17, 20 lyase, a product of CYP17.5 Women affected by PCOS can present with a spectrum of signs and symptoms, ranging from having minimal to major systemic manifestations of hyperandrogenemia and may have profound implications for a woman with regard to her reproductive health and the long-term health outcomes of her offspring.6 The aim of this critical review is to highlight these potential implications and suggested interventions to reduce their impact.
Reproductive features in women with PCOS
Adolescents with PCOS often have earlier menarche, particularly if they are obese.7 Precocious puberty, early adrenarche presenting with early pubic hair development or oligomenorrhoea may also be presenting symptoms.8–12 With regard to the potential etiology of PCOS, it is thought that exposure to excess androgens in early fetal life may increase the likelihood of developing PCOS in later years.13
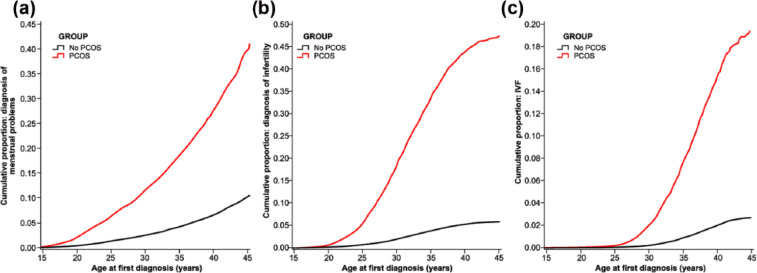
Various clinical and phenotypic presentations may precede the diagnosis of PCOS and include, but are not limited to, hyperandrogenism, menstrual irregularity, olio/anovulation and infertility.7 In total, 80% of women affected by anovulatory infertility have PCOS, which is further adversely affected by excess weight.14 Furthermore, women with anovulatory infertility have a longer interval until pregnancy, hence are more likely to require fertility investigations and treatment (Figure 1).14,15 Interestingly, in one observational study, women with PCOS are also more likely to have had their first pregnancy at a younger age and gave birth at least once; however, the total number of pregnancies in their lifetime tends to be lower than women without PCOS.14 Women diagnosed with PCOS have a lower natural conception rate, an increased rate of biochemical pregnancy and a reduced response to artificial reproductive technology (ART).7,16
Figure 1.
Incidence of menstrual disorder, infertility and need for IVF treatment by age.
Source: Reproduced with permission from Oxford University Press from Hart and Doherty.14
Reproductive outcomes
PCOS is associated with an increase in subfertility, ectopic pregnancy and early pregnancy loss (EPL).14,15 Potential causes are an altered endometrial environment and subsequent reduction in implantation success7 due to the hyperinsulinemic environment and concurrent hyperandrogenism.17 The rates of infertility and EPL have been estimated to be 15 times and three times greater, respectively, than women of similar demographics;18,19 however, it is unclear as to whether body mass index (BMI) or the use of fertility treatment (ovulation induction and/or in vitro fertilization (IVF)) had a role to play in the higher rates observed.
In one study, rates of EPL were no different between women with and without PCOS; however, the control group were comparatively older with lower ovarian reserve.16 A meta-analysis has reported a five times increased risk of EPL and reported a significant improvement of EPL in patients treated with metformin. It has been shown that treatment with the insulin sensitizer metformin, a synthetically derived biguanide, which leads to a reduction in serum insulin concentrations, may improve the features of PCOS. After IVF miscarriage, rates of 35.8% have been reported in women with PCOS in comparison to rates of 23.6% in women without PCOS; however, raised BMI, increased waist hip ratio and insulin resistance are also likely to play a part.
According to the World Health Organization (WHO), PCOS is the commonest cause of anovulatory infertility.20 For some women with PCOS undergoing IVF or in vitro maturation (IVM), increasing quantities of follicle-stimulating hormone (FSH) are required to stimulate ovulation, particularly in those women who are obese.15 Due to the polycystic nature of the ovaries in women with PCOS, they are at moderate risk of ovarian hyperstimulation syndrome (OHSS).16 Reported OHSS rates in the literature for women with PCOS who conceive after IVF are up to 7.5% compared to women without PCOS being in the order of 2.7%.21 Despite the increased oocytes retrieved during an IVF cycle, the oocytes are often of a poorer quality, and these cycles are characterized by lower fertilization rates and a higher incidence of embryo transfer cancellations due to failed fertilization or OHSS.15
Pregnancy complications
Several studies have consistently reported an increase in maternal complications for women with PCOS. There appears to be a trebling of the risk of maternal complications in women with PCOS when compared to healthy controls.22 However, this may be influenced by the fact that many women with PCOS have a greater chance of presenting in pregnancy with pre-existing medical issues.6 This is believed to be due to the hyperinsulinemia and hyperandrogenemia commonly seen in women with PCOS. This is further exacerbated by obesity, the greater recourse to ART, and potentially, the greater risk of multiple pregnancies derived from recourse to ART in women with PCOS.7
The extent of the increase in incidence of gestational diabetes (GDM), pregnancy-induced hypertension (PIH), preeclampsia (PET) and cesarean section (CS) differs slightly among studies, but are reproducibly increased even when data are adjusted for BMI. In one study, a twofold to threefold increase in GDM was seen,7 and PIH/PET were increased threefold to fourfold in women with PCOS,23–25 which corresponds to a 50% increase in PET.7 The need to perform an operative vaginal delivery, elective and non-elective CS was doubled,22 and this is likely to have been impacted by the increase in maternal obesity and fetal macrosomia seen in women with GDM and multiple pregnancies derived from ART. Increased gestational weight gain has also been evident in women with PCOS,7 as well as a five times increase in adverse pregnancy outcomes seen in women with PCOS and oligo-anovulation.7 A prolonged time to pregnancy is a known risk factor for obstetric complications and adverse perinatal outcomes and is often seen in women with PCOS.7 All of these factors make it essential that any treating doctor should endeavor to ensure that a woman with PCOS is as healthy as possible prior to conception and that if ART is required safe and appropriate intervention is performed to ensure she conceives a singleton pregnancy.
GDM rates are higher for women with PCOS and a greater number of these women are diagnosed in the first trimester, and this was thought to be due to insufficient pancreatic β-cell function to overcome the placental hormone-mediated exacerbation of pre-existing insulin resistance.16,26 Insulin resistance is also thought to have a direct effect on vascular function and is, therefore, implicated in the increased pregnancy complications seen in women with PCOS.7 In one study, no differences were found in the preconception period between women with PCOS diagnosed with GDM compared to women without GDM in respect to maternal age, fasting glucose, fasting insulin, homeostasis model assessment of insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI) or testosterone;26 however, patients with GDM had higher BMI and lower homeostasis model assessment of β-cell function (HOMA-β). Obese pregnant women with PCOS demonstrated a high incidence of GDM with severe insulin resistance, including high fasting insulin, HOMA-IR and HOMA-β at preconception compared with normal-weight patients.26 As such, measuring β-cell function, such as HOMA-β, at preconception may predict the risk of GDM in pregnant PCOS patients, and a reduction of BMI in the preconception period may reduce the chance of developing GDM. In Sterling et al.,16 the risk of being diagnosed with GDM in pregnant women with PCOS was 15.5% compared to 5% in the control group. The risk of developing GDM was not significantly increased in lean women with PCOS.
PIH and PET are both increased in women with PCOS, despite adjusting for BMI and ART.27 It is hypothesized that hypertensive disorders in pregnant women with PCOS are influenced by elevated serum androgen levels in the mother.28 Testosterone is thought to act on trophoblast invasion and therefore impacts on placental morphology and function.29–31 Pregnancy appears to enhance the low-grade inflammation typically seen in women with PCOS and is associated with higher low-density lipoprotein (LDL) and triglyceride levels, free radicals and inflammatory markers.32 These act to induce endothelial dysfunction, which alters remodeling of spiral arteries, which results in reduction of uterine artery impedance, reduced depth of endovascular trophoblast and abnormal placentation.29,33
Interestingly, placental signal transducer and activator of transcription 3 (P-STAT3) signaling are increased in placentas of women with PCOS, whereas mechanistic target of rapamycin (mTOR) signaling is unchanged.34 P-STAT3 and mTOR are responsible for the regulation of placenta nutrient transport and fetal growth, and alterations in STAT3 levels are seen in pregnant women with PCOS even in the absence of obstetric and perinatal complications. Higher levels of STAT3 have been reported in women with hyperandrogenism, anovulation and PCO compared with women who have anovulation and PCO only. Although circulating levels of androstenedione, androst-5-ene-3β, 17β-diol, testosterone and 5α-dihydrotestosterone were higher and estradiol lower in women with PCOS compared to controls; no correlation between STAT3 and sex steroid levels were found.34 Leptin, often increased in the presence of PCOS, increases levels of STAT3 by binding to its receptor.35
Increased P-STAT3 may represent a pro-inflammatory state and may be involved in upregulation of key amino acid transporters that influence fetal growth.36 Increased levels of STAT3 are seen in women with PCOS without pregnancy-related pathology despite reductions in placenta thickness, density and volume.30 These placenta features are consistent with changes associated with vascular lesions, chronic cillitis and intervillositis and abnormal villus maturity. They are more common in women with features of hyperandrogenism, anovulation and PCO31 and may result due to increased levels of circulating cytokines.34
Rates of preterm delivery (PTD) in women with PCOS are increased, particularly for those with hyperandrogemia.7 Doherty et al.6 reported rates of 21.1% compared to 12.5% in the control group (Table 1).7 The risk of stillbirth is elevated with rates reported in the order of 3.3% versus 1.6% in control groups.14 Elevated stillbirth rates are influenced by the effects of increased maternal pregnancy risk factors in women with PCOS and prematurity.6 In an older study, using a control group with a low ovarian reserve, Sterling et al.16 demonstrated adverse pregnancy outcomes in women with PCOS who conceived via IVF, compared to those women who conceived with IVF who did not have PCOS. Their findings were consistent with those published in the literature. GDM and hypertensive disorders in pregnancy were both increased. They also found an increase in antepartum hemorrhage (APH), cervical cerclage placement, preterm premature rupture of membranes (PPROMs) and fetal death in utero (FDIU).16 These findings were consistent with those of Wax37 who also recorded increased rates of cesarean wound complications.
Table 1.
Neonatal outcomes stratified by maternal polycystic ovary syndrome (PCOS) diagnosis.
| Outcome | Non-PCOS |
PCOS |
p-value | OR | 95% CI |
|---|---|---|---|---|---|
| N = 35,340 | N = 3626 | ||||
| Cesarean delivery | 9537 (27.4) | 1373 (39.2) | <0.001 | 1.54 | 1.43–1.66 |
| Preterm birtha | 2686 (7.6) | 559 (15.5) | <0.001 | 1.74 | 1.53–1.98 |
| Apgar score at 5 min < 7 | 648 (1.8) | 151 (4.2) | <0.001 | 1.46 | 1.10–1.93 |
| BWT < 2500 gb | 2206 (6.2) | 414 (11.4) | <0.001 | 0.86 | 0.70–1.07 |
| SGA | 2468 (7.2) | 306 (8.7) | 0.001 | 0.98 | 0.84–1.14 |
| BWT > 4000 g | 4473 (13.0) | 477 (13.6) | 0.287 | 1.07 | 0.95–1.20 |
| SCN admissionb | 2783 (7.9) | 502 (14.1) | <0.001 | 1.21 | 1.05–1.40 |
| Stillbirth | 216 (0.6) | 64 (1.8) | <0.001 | 1.23 | 0.80–1.89 |
| Perinatal mortality | 260 (0.7) | 84 (2.3) | <0.001 | 1.49 | 1.02–2.18 |
| ICD diagnoses originating in perinatal period | N = 34,756 | N = 3552 | p-value | OR | 95% CI |
| Any conditions | 5371 (15.5) | 912 (25.7) | <0.001 | 1.39 | 1.25–1.53 |
| Short GA and/or low BWTc | 1974 (5.7) | 417 (11.7) | <0.001 | 1.03 | 0.83–1.27 |
| Infections in perinatal period | 1087 (3.1) | 214 (6.0) | <0.001 | 1.36 | 1.14–1.63 |
| Respiratory distress | 1511 (4.3) | 337 (9.5) | <0.001 | 1.35 | 1.14–1.59 |
| Other pulmonary problems | 997 (2.9) | 234 (6.6) | <0.001 | 1.34 | 1.12–1.61 |
| Feeding problems | 1261 (3.6) | 256 (7.2) | <0.001 | 1.28 | 1.09–1.52 |
| Transitory carbohydrate metabolism disorders | 651 (1.9) | 144 (4.1) | <0.001 | 1.24 | 0.99–1.56 |
| Hemorrhagic, hematological disorders | 2073 (6.0) | 382 (10.8) | <0.001 | 1.26 | 1.08–1.46 |
| Cardiac conditions | 243 (0.7) | 45 (1.3) | <0.001 | 0.77 | 0.52–1.13 |
Source: Reproduced with permission from The American College of Obstetricians and Gynecologists from Doherty et al.6 Published by Wolters Kluwer Health, Inc.
OR: odds ratio; CI: confidence interval; BWT: birth weight; SGA: small-for-gestational age; GA: gestational age; SCN: special care nursery.
Data shown as n (%) and using the adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) summarizing the effects of maternal PCOS on neonatal outcomes while controlling for other risk factors. p-values shown were generated using univariate chi-square test for the univariate comparisons between the groups. All statistically significant effects of PCOS in the adjusted comparisons are shown in boldface.
Recording of a PCOS diagnosis was identified among all women who were hospitalized with a PCOS diagnosis (International Classification of Diseases, 10th Revision (ICD-10): E28.2 or International Classification of Diseases, 9th Revision (ICD-9): 256.4) using the Hospital Morbidity System hospitalization records between 1980 and 2011. Study participants with a recorded PCOS diagnosis were defined as women who had reached 15 years of age in 1980, or later, and who were hospitalized with PCOS as one of the diagnoses noted on their admission between January 1997 and April 2011.
All outcomes adjusted for IVF, Ethnicity (Caucasian, Indigenous, Asian and Other), multiple pregnancy, maternal age (<20, 20–29, 30–39, 40+), parity (0, 1–4, 5+), time epoch (years <1990, 1990–1999, 2000–2011), smoking during pregnancy (yes, no or unreported), maternal hypertension, pre-existing and gestational diabetes and preeclampsia.
Adjustments for additional risk factors—cesarean delivery: maternal mental health disorders, antepartum hemorrhage; preterm birth: maternal mental health disorders, antepartum hemorrhage; Apgar score at 5 min < 7: maternal mental health disorders, gestational age at delivery, fetus small-for-gestational age, fetus large-for-gestational age; BWT < 2500 g and SGA and BWT > 4000 g: antepartum hemorrhage, gestational age at delivery; SCN admission: maternal mental health disorders, antepartum hemorrhage, gestational age at delivery; stillbirth and perinatal mortality: gestational age at delivery.
On 34,650 and 3488 pregnancies non-PCOS and PCOS neonates, respectively, when gestational age at delivery was recorded.
Excluding stillbirths.
High birth weight (ICD diagnoses P08) recorded in 101 (0.3%) and 29 (0.8%) offspring of women with and without PCOS diagnosis, respectively.
Preconception counseling and psychological support
As PCOS commonly affects women of reproductive age, appropriate preconception counseling and advice regarding the impact of lifestyle, obesity and age on fertility should be offered.7 Emphasis should be focused on the impact that weight has on the clinical presentation and outcomes for women affected by the metabolic, psychological and reproductive repercussions of PCOS. Women with PCOS have a lower uptake of contraception, which is thought to be due to perceived lower pregnancy rates.19 Women with PCOS should be counseled regarding the appropriate use of contraception to avoid unplanned pregnancy as the pregnancy rates for women with PCOS are similar to those women without PCOS.19 For those women with PCOS, counseling should be provided that conception may take longer to achieve, hence delaying childbearing may have an accumulative negative impact on conception when age and BMI are factored in Joham et al.19
Interventions to reduce the impact of PCOS on pregnancy outcomes
Given the significant increase in GDM and hypertensive disorders in pregnancy, performing a first trimester oral glucose tolerance test (OGTT) and commencing low-dose aspirin may mitigate the potential for poor placentation often seen in women with PCOS.16 Smoking cessation should be routinely advised and high-dose folate may be of benefit due to the increased risk of congenital anomalies seen in women with PCOS, particularly those that are obese (Table 2).
Table 2.
Developmental anomalies in offspring stratified by the maternal polycystic ovary syndrome (PCOS) diagnosis.
| Anomalies | All offspring |
p-value | Offspring age ⩾6 years |
||
|---|---|---|---|---|---|
| Non-PCOS |
PCOS |
Non-PCOS |
PCOS |
||
| N = 35,340 | N = 3626 | N = 22,094 | N = 2058 | ||
| Any anomalies | 1775 (4.884) | 227 (6.260) | 0.001 | 1234 (5.585) | 160 (7.775) |
| Major anomalies | 1458 (4.012) | 181 (4.992) | 0.013 | 1003 (4.540) | 129 (6.268) |
| Cardiovascular | 371 (1.021) | 56 (1.544) | 0.006 | 242 (1.110) | 43 (2.089) |
| Urogenital | 510 (1.403) | 72 (1.986) | 0.010 | 373 (1.688) | 54 (2.623) |
| Other | 246 (0.677) | 36 (0.993) | 0.045 | 182 (0.824) | 24 (1.166) |
| Non-cardio and urogenital | 1076 (2.961) | 124 (3.420) | 0.206 | 752 (3.404) | 80 (3.887) |
| Musculo-skeletal | 462 (1.271) | 47 (1.296) | 0.992 | 318 (1.439) | 29 (1.409) |
| Gastrointestinal | 218 (0.600) | 28 (0.772) | 0.270 | 143 (0.647) | 18 (0.875) |
| Integument | 133 (0.366) | 12 (0.331) | 0.775 | 118 (0.543) | 8 (0.389) |
| Nervous system | 100 (0.275) | 8 (0.221) | 0.497 | 80 (0.362) | 6 (0.292) |
| Ear, face and neck | 119 (0.327) | 16 (0.441) | 0.298 | 91 (0.412) | 10 (0.486) |
| Eye | 36 (0.099) | 3 (0.083) | 1.000 | 30 (0.136) | 3 (0.146) |
| Respiratory | 26 (0.072) | 4 (0.110) | 0.357 | 15 (0.068) | 4 (0.194) |
| Chromosome defects | 51 (0.140) | 6 (0.165) | 0.651 | 37 (0.167) | 5 (0.242) |
Source: Reproduced with permission from The American College of Obstetricians and Gynecologists from Doherty et al.6 Published by Wolters Kluwer Health, Inc.
All anomalies for offspring are shown and subset of anomalies among offspring who reached the limit of ascertainment (6 years of age and over or death), with data shown as n (%) and percentage accurate to three decimals for greater accuracy when indicating rates per 1000 births. p-values shown were generated using univariate chi-square test for the univariate comparisons between the groups.
Lifestyle factors such as diet and exercise should be addressed to optimize BMI, both for a healthier pregnancy and to reduce exacerbation of PCOS. For those of normal BMI, regular self-monitoring and early action on small increments of weight gain as well as prevention of further weight gain appears to be a feasible approach and more successful than weight loss in women with established obesity.7 Weight reduction of 5%–10% is recommended and has shown to improve ovulation and subsequent conception and reduces metabolic complications.7 The behavioral lifestyle change that has demonstrated efficacy is the use of face-to-face-tailored dietary advice, an energy reduced diet and 150 min of exercise per week. Exercising five times per week for 30 min at a time, three of which are recommended to be aerobic in nature, has been shown to improve clinical outcomes including insulin resistance even when weight is not lost.15 When executed together, exercise and dietary intervention have been shown to improve clinical outcomes compared to diet alone.7
For women with a BMI over 30, who are anovulatory, bariatric surgery can assist with weight loss and improve PCOS markers; however, babies subsequently conceived are at risk of growth restriction, and as such are best managed in a multidisciplinary care setting.15 Weight stabilization should be achieved for 6–12 months prior to attempting conception.38
Depression and anxiety are more common in women with PCOS and is often more severe.14,39,40 The prevalence of depression in women with PCOS varies between 28% and 64%41 and anxiety rates vary from 34% to 57%.42 Psychological disturbances are seen in women regardless of the severity or type of PCOS phenotype exhibited43 and negatively affects quality of life.43,44 This demonstrates that in addition to hirsutism, acne, menstrual irregularities, infertility and obesity women with PCOS have higher rates of anxiety, depression, social phobia, eating disorders and suicide attempts.45 Psychological intervention is an important part of the holistic care approach to women with PCOS and can assist with adapting to stressors encountered at diagnosis, fertility treatment, pregnancy and beyond. Reduction in stress can also minimize its negative impact on the hypothalamic–pituitary–ovarian axis.
Perinatal and childhood outcomes
The perinatal outcomes of infants born to women with PCOS is significantly worse than those born to women without PCOS, and this can be further exacerbated by maternal diabetes, obesity, IVF and medication use.7 The increase in poor perinatal outcomes can be accounted for by earlier gestational age at birth as well as PTD; however, the pathophysiology of these outcomes potentially originates much earlier on. It is possible that poor oocyte, embryo quality and the in utero environment can account for some of the adverse neonatal outcomes found.7 Fetal macrosomia can result due to GDM and the resultant altered glucose metabolism and disturbance in uterine blood flow.14 Babies that are small-for-gestational age (SGA) and of low birth weight are also seen and are due to placental dysfunction, twin birth and an earlier gestational age at delivery.6,25 Evidence suggests that low Apgar scores at 5 min are more common in babies born to women with PCOS.6
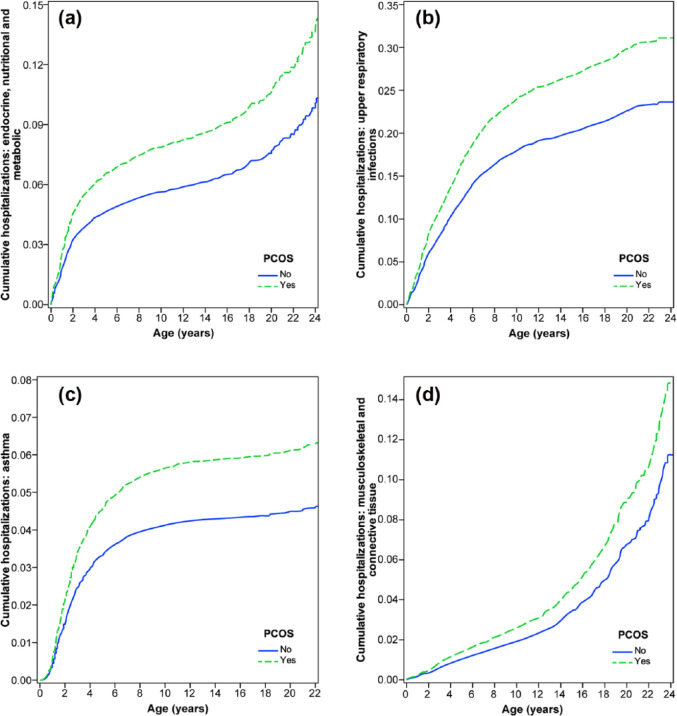
Increased congenital anomalies are seen in infants born to women with PCOS and may include cardiac, neural tube, urogenital and gastrointestinal anomalies such as omphalocele (Table 3).6,14 Perinatal morbidity is also increased, as is postnatal intensive care admission, and the need for pediatric support.24,25 The cause of this is likely to relate to the increased use of infertility treatment, multiple gestation and iatrogenic PTD due to GDM or hypertensive disorders of pregnancy.7 Several studies have demonstrated an increase in PTD before 37 weeks of gestation. In one study, 17% of babies were born prior to 37 weeks of gestation in comparison to a rate of 8% in women without PCOS.15 Within this same population babies, large-for-gestational age (LGA) (reported as >90% for growth) were more commonly found in the PCOS group at 16%, compared to the prevalence within the control population of 6%.15 This finding was consistent even if GDM was absent and was independent of maternal BMI. It is likely that the increase in LGA babies is due to underlying metabolic and endocrine influences.16 After the neonatal period, children born to mothers with PCOS have an increased rate of hospitalizations in childhood and adolescence and had a greater prevalence of metabolic disorder, diseases of the nervous system and asthma (Figure 2).6
Table 3.
Developmental anomalies in offspring stratified by the maternal polycystic ovary syndrome (PCOS) diagnosis.
| Congenital anomalies | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Any anomalies | 1.30 | 1.12–1.51 | 1.20 | 1.03–1.40 |
| Major anomalies | 1.22 | 1.03–1.44 | 1.14 | 0.96–1.35 |
| Cardiovascular | 1.56 | 1.16–2.10 | 1.37 | 1.01–1.87 |
| Urogenital | 1.39 | 1.06–1.82 | 1.36 | 1.03–1.81 |
| Other | 1.43 | 0.98–2.08 | 1.30 | 0.88–1.93 |
| Non-cardio and urogenital | 1.13 | 0.93–1.37 | 1.05 | 0.86–1.28 |
Source: Reproduced with permission from The American College of Obstetricians and Gynecologists from Doherty et al.6 Published by Wolters Kluwer Health, Inc.
OR: odds ratio; CI: confidence interval.
The effects of PCOS on the risk of developmental anomalies summarized with crude and adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) obtained in the logistic regression analyses are shown. All statistically significant effects of PCOS in the adjusted comparisons are shown in boldface.
All outcomes adjusted for IVF, multiple pregnancy, maternal age (<20, 20–29, 30–39, 40+), smoking during pregnancy (yes, no or unreported), gestational diabetes, male gender, current age of offspring in years (<1, 1–2, 3–6, 6+).
Figure 2.
Hospitalizations by age stratified by maternal polycystic ovary syndrome (PCOS) diagnosis: (a) metabolic, nutritional and endocrine disorders; (b) acute and upper respiratory tract infections; (c) asthma; and (d) musculoskeletal and connective tissue. In all analyses, the estimated cumulative admission estimates were adjusted for IVF, Ethnicity (Caucasian, Indigenous, Asian and Other), multiple pregnancy, maternal age (<20, 20–29, 30–39, 40+ years), parity (0, 1–4, 5+), time epoch of birth (years <1990, 1990–1999, 2000–2011), smoking during pregnancy (yes, no or unreported), maternal hypertension, asthma and cardiovascular conditions; pre-existing and gestational diabetes, preeclampsia, male gender, gestational age at delivery (<32, 33–34, 35–36, 37–40, 41+ years), being born small-for-gestational age, major congenital anomalies and neonatal admission to special care nursery after birth.
Source: Reproduced with permission from The American College of Obstetricians and Gynecologists from Doherty et al.6
Education should be given to women with PCOS in regard to lifestyle and dietary measures to ensure that their offspring’s heath and BMI is optimized given the risk of disease in later life.
Future research
Due to the risks that PCOS imposes on women in pregnancy and to their offspring, future research should focus on evidence-based interventions to reduce the pre-pregnancy-related morbidity associated with PCOS, which may lead to improvements in subsequent pregnancy-related outcomes, and techniques to minimize weight gain in pregnancy.
Conclusion
The extent of adverse outcomes for women with PCOS and their children is increased across the board; however, the rates appear to differ according to the features of PCOS present. Adequate support should be offered to institute lifelong lifestyle modifications aiming for a target of a healthy weight, diet and exercise level that can be maintained into later life and potentially considering medical and surgical intervention for weight management and glucose regulation if these other measures fail. Women should be informed of the increased risks of pregnancy complications and the potential for adverse outcomes for their offspring.
Acknowledgments
To the best of our knowledge, the materials included in this chapter do not violate copyright laws. All original sources have been appropriately acknowledged and/or referenced. Where relevant, appropriate permissions have been obtained from the original copyright holders(s).
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.J.H. is the Medical Director of Fertility Specialists of Western Australia and a shareholder in Western IVF; he has received educational sponsorship from MSD, Merck-Serono and Ferring Pharmaceuticals. R.M. has no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R.J.H. has received support from a National Health and Medical Research Council Centre of Research Excellence award for research in PCOS (grant number: 1078444).
References
- 1. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016; 2: 16057. [DOI] [PubMed] [Google Scholar]
- 2. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1): 41–47. [DOI] [PubMed] [Google Scholar]
- 3. Goodman NF, Cobin RH, Futterweit W, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract 2015; 21(12): 1415–1426. [DOI] [PubMed] [Google Scholar]
- 4. Homburg R. Polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2008; 22(2): 261–274. [DOI] [PubMed] [Google Scholar]
- 5. Nelson VL, Qin KN, Rosenfield RL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2001; 86(12): 5925–5933. [DOI] [PubMed] [Google Scholar]
- 6. Doherty DA, Newnham JP, Bower C, et al. Implications of polycystic ovary syndrome for pregnancy and for the health of offspring. Obstet Gynecol 2015; 125(6): 1397–1406. [DOI] [PubMed] [Google Scholar]
- 7. Joham AE, Palomba S, Hart R. Polycystic ovary syndrome, obesity, and pregnancy. Semin Reprod Med 2016. 34(2): 93–101. [DOI] [PubMed] [Google Scholar]
- 8. Lucky AW, Rosenfield RL, McGuire J, et al. Adrenal androgen hyperresponsiveness to adrenocorticotropin in women with acne and/or hirsutism: adrenal enzyme defects and exaggerated adrenarche. J Clin Endocrinol Metab 1986; 62(5): 840–848. [DOI] [PubMed] [Google Scholar]
- 9. van Hooff MH, Voorhorst FJ, Kaptein MB, et al. Predictive value of menstrual cycle pattern, body mass index, hormone levels and polycystic ovaries at age 15 years for oligo-amenorrhoea at age 18 years. Hum Reprod 2004; 19(2): 383–392. [DOI] [PubMed] [Google Scholar]
- 10. Ibanez L, Ferrer A, Ong K, et al. Insulin sensitization early after menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr 2004; 144(1): 23–29. [DOI] [PubMed] [Google Scholar]
- 11. Ibanez L, Valls C, Potau N, et al. Polycystic ovary syndrome after precocious pubarche: ontogeny of the low-birthweight effect. Clin Endocrinol 2001; 55(5): 667–672. [DOI] [PubMed] [Google Scholar]
- 12. Lazar L, Kauli R, Bruchis C, et al. Early polycystic ovary-like syndrome in girls with central precocious puberty and exaggerated adrenal response. Eur J Endocrinol 1995; 133(4): 403–406. [DOI] [PubMed] [Google Scholar]
- 13. Hart R, Norman R. Polycystic ovarian syndrome–prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol 2006; 20(5): 751–778. [DOI] [PubMed] [Google Scholar]
- 14. Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab 2015; 100(3): 911–919. [DOI] [PubMed] [Google Scholar]
- 15. Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 2016; 22(6): 687–708. [DOI] [PubMed] [Google Scholar]
- 16. Sterling L, Liu J, Okun N, et al. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril 2016; 105(3): 791–797. e2. [DOI] [PubMed] [Google Scholar]
- 17. Zeng XL, Zhang YF, Tian Q, et al. Effects of metformin on pregnancy outcomes in women with polycystic ovary syndrome: a meta-analysis. Medicine 2016; 95(36): e4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joham AE, Teede HJ, Ranasinha S, et al. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community-based cohort study. J Womens Health 2015; 24(4): 299–307. [DOI] [PubMed] [Google Scholar]
- 19. Joham AE, Boyle JA, Ranasinha S, et al. Contraception use and pregnancy outcomes in women with polycystic ovary syndrome: data from the Australian longitudinal study on women’s health. Hum Reprod 2014; 29(4): 802–808. [DOI] [PubMed] [Google Scholar]
- 20. ESHRE Capri Workshop Group. Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update 2012; 18(5): 586–599. [DOI] [PubMed] [Google Scholar]
- 21. Shmorgun D, Claman P, Gysler M, et al. The diagnosis and management of ovarian hyperstimulation syndrome. Int J Gynecol Obstet 2012; 116(3): 268–273. [DOI] [PubMed] [Google Scholar]
- 22. Kollmann M, Klaritsch P, Martins WP, et al. Maternal and neonatal outcomes in pregnant women with PCOS: comparison of different diagnostic definitions. Hum Reprod 2015; 30(10): 2396–2403. [DOI] [PubMed] [Google Scholar]
- 23. Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol 2011; 204(6): 558.e1–558.e6. [DOI] [PubMed] [Google Scholar]
- 24. Boomsma CM, Eijkemans MJ, Hughes EG, et al. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006; 12(6): 673–683. [DOI] [PubMed] [Google Scholar]
- 25. Qin JZ, Pang LH, Li MJ, et al. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2013; 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawada M, Masuyama H, Hayata K, et al. Pregnancy complications and glucose intolerance in women with polycystic ovary syndrome. Endocr J 2015; 62(11): 1017–1023. [DOI] [PubMed] [Google Scholar]
- 27. Roos N, Kieler H, Sahlin L, et al. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 2011; 343: d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palomba S, Falbo A, Russo T, et al. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril 2010; 94(5): 1805–1811. [DOI] [PubMed] [Google Scholar]
- 29. Palomba S, Russo T, Falbo A, et al. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab 2012; 97(7): 2441–2449. [DOI] [PubMed] [Google Scholar]
- 30. Palomba S, Russo T, Falbo A, et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod 2013; 28(10): 2838–2847. [DOI] [PubMed] [Google Scholar]
- 31. Palomba S, Falbo A, Chiossi G, et al. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod Biomed Online 2014; 29(3): 370–381. [DOI] [PubMed] [Google Scholar]
- 32. Palomba S, Santagni S, Falbo A, et al. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health 2015; 7: 745–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palomba S, Falbo A, Russo T, et al. Uterine blood flow in pregnant patients with polycystic ovary syndrome: relationships with clinical outcomes. BJOG 2010; 117(6): 711–721. [DOI] [PubMed] [Google Scholar]
- 34. Maliqueo M, Sundstrom Poromaa I, Vanky E, et al. Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod 2015; 30(3): 692–700. [DOI] [PubMed] [Google Scholar]
- 35. Kaptein A, Paillard V, Saunders M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J Biol Chem 1996; 271(11): 5961–5964. [DOI] [PubMed] [Google Scholar]
- 36. Jones HN, Jansson T, Powell TL. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am J Physiol Cell Physiol 2009; 297(5): C1228–C1235. [DOI] [PubMed] [Google Scholar]
- 37. Wax JR. Risks and management of obesity in pregnancy: current controversies. Curr Opin Obstet Gynecol 2009; 21(2): 117–123. [DOI] [PubMed] [Google Scholar]
- 38. Legro RS, Dodson WC, Gnatuk CL, et al. Effects of gastric bypass surgery on female reproductive function. J Clin Endocrinol Metab 2012; 97(12): 4540–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dokras A, Clifton S, Futterweit W, et al. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol 2011; 117(1): 145–152. [DOI] [PubMed] [Google Scholar]
- 40. Veltman-Verhulst SM, Boivin J, Eijkemans MJ, et al. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update 2012; 18(6): 638–651. [DOI] [PubMed] [Google Scholar]
- 41. Deeks AA, Gibson-Helm ME, Teede HJ. Anxiety and depression in polycystic ovary syndrome: a comprehensive investigation. Fertility Steril 2010; 93(7): 2421–2423. [DOI] [PubMed] [Google Scholar]
- 42. Benson S, Arck PC, Tan S, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology 2009; 34(5): 727–735. [DOI] [PubMed] [Google Scholar]
- 43. Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol 1992; 36(1): 105–111. [DOI] [PubMed] [Google Scholar]
- 44. Carmina E. PCOS: metabolic impact and long-term management. Minerva Ginecol 2012; 64(6): 501–505. [PubMed] [Google Scholar]
- 45. Zhang YY, Hou LQ, Zhao TY. Effects of acarbose on polycystic ovary syndrome: a meta-analysis. Exp Clin Endocrinol Diab 2014; 122(6): 373–378. [DOI] [PubMed] [Google Scholar]