Abstract
Executive functions (EFs) are cognitive processes that support flexible goal pursuit. Healthy development of EFs during childhood is critical for later life outcomes including health, wealth and educational attainment. As such it is crucial to understand how EFs can be supported and protected against insult. Here we examine whether there are sensitive periods in the development of EFs, by drawing on deprivation and enrichment studies in humans. While there is suggestive evidence that pre-6 months of age constitutes a sensitive period for EF development, given the higher-order nature of EF, we argue for the possibility of multiple sensitive periods of constituent processes. We identify relevant future questions and outline a research agenda to systematically test for sensitive period in EF development.
Current Opinion in Behavioral Sciences 2020, 36:98–105
This review comes from a themed issue on Sensitive and critical periods
Edited by Catherine A Hartley and Willem E Frankenhuis
For a complete overview see the Issue and the Editorial
Available online 14th September 2020
https://doi.org/10.1016/j.cobeha.2020.08.001
2352-1546/© 2020 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Executive functions
Executive functions (EFs) refer to a category of cognition, which includes working memory, cognitive flexibility, inhibitory control and sustained attention. EFs serve our ability to respond flexibly and adaptively to changes in the environment in the pursuit of long-term goals [1]. EFs undergo critical quantitative and qualitative changes during childhood and are underpinned by neural circuitry that has a protracted developmental time course [2]. Crucially, individual differences in EFs during childhood are a hugely important predictor for later life outcomes such as subjective and physical well-being [3]. Given the importance of EFs for later life outcomes there have been many attempts to train this by means of tailored interventions [4]. One important question in relation to EF development is whether there exist sensitive periods (SPs). Here we discuss the current evidence of core EFs as well as other regulatory processes such as emotion regulation.
Sensitive periods and executive functions
SPs are a point in development during which there is heightened neural sensitivity to specific environmental stimuli, with exposure necessary for typical developmental processes to occur. This ontogenetic process is driven by what is known as ‘experience-expectancy’. After a SP has closed, the ability to learn new skills in that particular domain is constrained by the experience that took place during the SP. In this way, SPs are distinct from ‘critical periods’, as once a critical period has closed further changes are not possible. In contrast to general developmental plasticity or learning processes that continue across life, SPs are associated with a number of specific characteristics [5] (Table 1). SPs are classically associated with primary sensory development, such as the visual [6] and auditory [7] systems, although higher-order functions including language [8] and affective development [9], are also thought to be characterised by SP’s.
Table 1.
Characteristics of sensitive periods and general learning processes
| Sensitive periods | Non-sensitive period learning |
|---|---|
| Experience-expectant | Experience-dependent |
| Temporal window: Maturationally timed onset and closure | Continues across lifespan |
| Ontogenetic constraints | No ontogenetic constraints |
| Formation of a developing system | Reorganisation |
| Parvalbumin cell maturation, GABA, synaptic pruning and remodelling, myelination and perineuronal nets | Synaptogenesis, synaptic strength modulation and pruning |
Why would there be sensitive periods for EFs?
It is not immediately apparent why there would be SPs for EFs. When considering whether SPs exist for EFs, it is useful to remind ourselves of the evolutionary role of SPs, which is to enable a developing organism to become specialised to their particular environment [10]. This is apparent for capacities classically associated with SPs. In the case of language, children are considered genetically predisposed to develop language, but postnatal environmental exposure during a SP is necessary to tune the developing system to the particular language surrounding it. If SPs do exist for EFs, precisely what role would this play? In contrast to domains typically associated with SPs (i.e. vision, language, attachment learning), EFs have a protracted developmental trajectory which continues into adulthood, as does the prefrontal cortex, on which EFs depend (Finn, Sheridan, Hudson Kam, Hinshaw, & D’Esposito, 2010; Peverill, McLaughlin, Finn, & Sheridan, 2016), suggesting an extended period of plasticity. Additionally, the precise type of environmental input required (i.e. social, cognitive, sensory) is not as clearly defined for EFs.
Distinct sensitive periods for distinct components of executive function development
Research into SPs in other higher-order domains, such as emotion processing and language [8,11], report that it is not the case that single SPs exist for these domains. Rather, they rely on numerous other abilities, which may have their own individual SPs, or no SP at all (similar to how there is no sensitive period for vocabulary in language development). We propose a similar approach for investigating SPs for EFs.
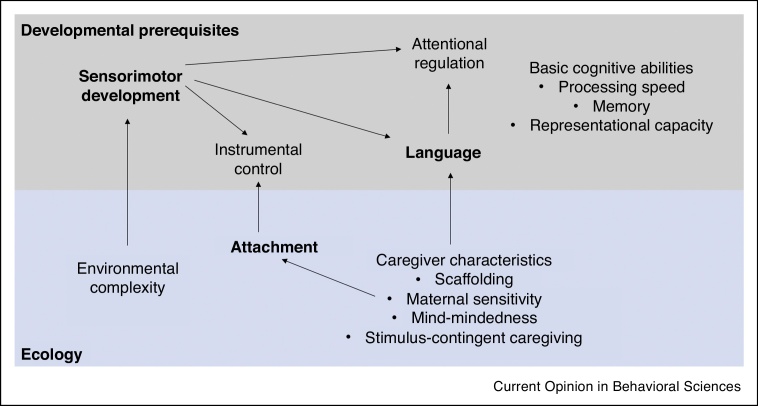
To support an organism in acting to achieve a goal, EFs depend on integration of multiple streams of sensory information. Thus, the development of EFs depends on the earlier development of lower-order systems that supply this information [12], such as the visual and auditory senses. Additionally, EFs are structured hierarchically, with a shared component (which may reflect the ability to bias processing in line with goals) [13], and distinct components that reflect dissociable sub-functions [13,14]. Such organisation is also reflected at the neural level [15]. The ‘shared’ and ‘distinct’ components of EF functions may be associated with separable SPs. Finally, EF development is associated with numerous interacting factors, some of which are internal to the developing individual, and others that exist in the surrounding environment (Figure 1).
Figure 1.
Early executive function development is dependent on multiple interactions of individual-level factors (developmental prerequisites) and environmental factors (ecology). Highlighted in bold are processes that have been associated with sensitive periods, however others may also have their own, distinct sensitive periods (or no sensitive period at all).
Specifically, sensorimotor development may be directly linked to the complexity of the surrounding environment, in terms of presence and variation of visual stimuli (availability of books, toys and other stimuli) and exposure to linguistic complexity [12]. Development of sensorimotor systems in the first few months of life must occur to enable an infant to receive accurate visual input - only after visual input is sufficiently developed, is the infant able to start regulating their attention to this input ([16,12]), which is considered to be a developmental prerequisite of later EF skills [17, 18, 19]. Infant attention regulation is influenced by caregiver interaction, in particular caregiver scaffolding [20], whereby a caregiver directs the infant’s attention to particular items in the environment [21], an interaction that may be mediated by a child’s gaze following [22] and language comprehension abilities [23]. In turn, the parent-infant dynamic is likely influenced by the strength of caregiver attachment, attentiveness of the parent, and reliability in their responding. The development of attachment is dependent on sensitive and stimulus-contingent caregiving [24]. Such caregiving leads to an infant’s increased perception of instrumental control over their environment, which is strongly related to individual differences in EFs [25,26], and may also depend on motor system development [27]. Developmental changes in other cognitive abilities, such as processing speed [19], memory and representational understanding [28], likely also contribute to the above interactions. In this way, a child’s EF development is dependent on numerous interacting factors within their ecological environment. There is evidence that elements of sensorimotor system development [6,7], language [8] and attachment [29] are associated with SPs, however it may also be the case that other individual components of EF development may be associated with their own distinct sensitive period.
As noted above and elsewhere [13], EFs are a collective term for different cognitive functions. It is known that these are subserved by partly overlapping, but also different brain regions [2,30] and that they have subtly distinct developmental trajectories [2]. As such it is reasonable to assume that EFs are potentially underpinned by partially distinct SPs. While to date, there is no evidence in support of this, we also believe this to be unlikely. Cognitive functions (including EFs) become more differentiated with age [31,32] and particularly during early childhood, when SPs are likely to be operational for EFs, these are highly interrelated. This would imply that the same SPs are likely to underpin all EFs even though these ultimately mature into separable functions.
Brain mechanisms
Studying SPs of EFs mandates a close examination of underlying brain circuitry. Thanks to animal models, the mechanisms underlying the operation, opening and closure of SPs are well understood. In comparison, there is a dearth of studies that have shed light on this in humans. Given the crucial role of prefrontal cortical brain regions in EF development it is highly probable that SPs would be subserved by specific developmental mechanisms in subregions of lateral and medial prefrontal cortex and their interaction with subcortical regions. Rather than speculating further on these here, we wish to advance an empirical framework within which these could be studied in humans. Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the central nervous system. The onset of SPs is determined by maturation of particular GABA circuits, namely parvalbumin (PV) cells, which serve as a crucial switch in plasticity [8]. Whereas measuring or even manipulating cell activity in vivo in humans is either challenging or ethically not viable, other proxies might lend themselves to measure such GABAergic activity. GABAergic activity can be measured using both positron emission tomography, and more important for developmental studies using magnetic resonance spectroscopy [33,34]. Further, the maturation of PV inhibitory neurons generates gamma oscillations associated with critical period plasticity [35], and which could be measured using EEG or MEG. Taking an approach informed by animal studies and triangulating across multiple methodologies, albeit proxies of SPs, in longitudinal study designs will be able to advance the study of how and when SPs operate in EF development.
Paradigms to investigate sensitive periods
SPs are typically studied using deprivation and enrichment paradigms or a combination of the two. Most of our mechanistic understanding of SPs has been gained through systematic and controlled manipulation of environmental input in animal models, yet mostly on sensory and attachment systems. In humans, this type of experimental manipulation is not possible, for clear ethical reasons. Instead, we can study the effects of deprivation occurring naturally in contexts such as institutionalisation or adoption, and cognitive training studies as an analogue of enrichment paradigms. To gain an understanding of whether SPs exist for EF development, it is important to consider the effects of dose, timing and duration. These terms reflect the intensity or amount of an experience (dose), at what developmental time-point it occurs (timing) and how long this lasts (duration). To demonstrate that a sensitive period exists, it must be the case that an experience has a particularly large effect at a certain point in development (timing), rather than being accounted for by how much (dose) or how long the experience lasted (duration). It must be noted, that evidence from deprivation studies often are not able to disentangle these effects and therefore do not meet sufficient criteria for determining whether SPs exist (see Woodard 2020, this issue, for discussion). We begin by discussing evidence from deprivation studies.
Cognitive deprivation
Recent studies have shown that cognitive deprivation during development is specifically associated with EF deficits, and that this is distinct to other types of child adversity such as maltreatment [36••,37]. Cognitive deprivation, in comparison to other types of adversity, is characterised by reduced levels of cognitive stimulation. This may be experienced in contexts of poverty, neglect or institutionalisation, all of which are associated with altered EFs, including working memory, cognitive control and shifting [36••,38, 39, 40]. One of the ways that studying these experiences can improve our understanding of SPs is by informing whether it is possible for any early-emerging EF deficits to be remediated by a change in the environment. Studies that look at the long-term effects of a change in environment after institutionalisation (due to adoption or foster home placement) provide a good example of this.
Remediation after early deprivation has been demonstrated across many domains including language, stress reactivity, internalising symptoms, attachment, and brain measures such as white matter connectivity [41, 42, 43, 44, 45]. The evidence for remediation of EF is mixed [38,40,46,47]. One study in particular has been instrumental to this question – the Bucharest Early Intervention Project (BEIP). The BEIP is the only randomised control trial (RCT) of foster care placement. It consists of an original cohort of 136 children, who early in life (at an average age of 22 months), were randomly allocated to either care as usual (in orphanages) or foster care. Another group of children who had never lived in institutions formed a third testing group. This cohort has been studied longitudinally, with testing on various measures at numerous points of development. Due to the RCT design, the BEIP avoids many pitfalls typically associated with institutionalisation studies, such as selection biases associated with adoption and in this way, can more precisely answer questions about the developmental effects of institutionalisation.
Studies from the BEIP cohort report limited benefits of placement into foster care on EFs, measured later in childhood. At age 12, both groups of children who were once institutionalised (i.e. both the ‘care as usual’ and the foster care groups) showed lower performance across a range of cognitive functions including visual-spatial recognition memory, spatial working memory, attention set shifting and rule learning [38], inefficient orienting of attention [40], and error monitoring [47], compared with never-institutionalised children. A recent longitudinal study examining the developmental trajectories in EFs in this cohort from ages 8, 12 and 16, reported that there were persistent deficits across measures of attention, short-term visual memory and spatial working memory at all ages [48••]. The only measures in which there was an advantage of foster care compared with continued institutionalisation were visual-spatial memory and learning. In the context of SPs, this suggests that deprivation experienced very early in life leads to a long-lasting impact on EF development, which does not remediate with enhanced environmental exposure from foster care placement.
Many studies also report that early experience of institutionalisation is associated with heightened rates of psychopathology later in life, with attention-deficit hyperactivity disorder (ADHD) symptoms of inattention and over-activity reported particularly frequently [42,45,48••,49,50,51,52••,53]. This may relate to persistent EF impairments, as working memory and inhibitory control performance mediates the association between institutionalisation and ADHD symptoms at age 8 [54].
Two recent studies from a different cohort, the English and Romanian Adoptees (ERA) cohort, report the long-term psychopathology and neuropsychological outcomes of early institutionalisation [52••,55]. Sonuga-Barke et al. [52••] report that children adopted before 6 months were indistinguishable from peers in adulthood on a range of clinical measures, whereas those that spent greater than 6 months in an institution presented with increased rates of ‘quasi’ autism spectrum disorder, disinhibited social engagement and inattention/over-activity in adulthood.
A further study from the same cohort investigating neuropsychological functioning in adulthood did not report the same 6 month ‘step change’ – rather, even institutionalisation experienced at 3 months was associated with neuropsychological impairments at age 25, including in pro-active inhibitory control, which was accounted for by impairments in IQ [55]. The authors highlight that together these findings point towards a complex relationship between neuropsychological functioning and clinical outcomes, since individuals in the group adopted before 6 months may, in adulthood, have presented with lower inhibitory control and IQ [55] but not ADHD symptoms [52••], whereas deprivation for longer than 6 months appears to be a key for developing clinical symptoms.
Nonetheless, both studies suggest that early severe deprivation occurring specifically within the first 6 months of life has long lasting effects for later EFs, which do not remediate. Other studies have also reported a similar cut off, or ‘step-change’ in terms of later development [56]. Children adopted before 6 [49] and 9 months [57] perform better on measures of inhibitory control than children adopted later in childhood. One study also reports a negative association between inhibitory control performance and age of adoption between 12-78 months, highlighting that an older age of adoption is negatively associated with inhibitory control abilities [58].
Although the importance of age of adoption on later adaptive functioning has long been recognised [59], these findings are intriguing given the protracted developmental period of EFs and the prefrontal cortex. Recent research points towards extensive reorganisation occurring within the prefrontal cortex in early infancy [60]. Factors occurring within the first few months of life (Figure 1), such as parent-child interactions [61], including scaffolding [20] and interaction contingency [62], or levels of cognitive stimulation [63], may critically impact early brain development and the development of capacities such as attentional regulation, which form the ‘building blocks’ of EFs, later leading to altered EF development. Without such input in infancy, as may be the case in institutions, EF development may be perturbed. It remains to be determined whether this period in early infancy constitutes an SP for EF development or whether in fact this is a more general effect across a range of functional domains.
Whilst these findings are intriguing in the context of SPs and may suggest that the first 6 months represent a SP for EFs, it is difficult to determine whether the reported effects relate to SPs or general early developmental effects. Institutionalisation studies often use the age of adoption as a measure of duration (see dose, timing, and duration, above) – however, it is also a measure of timing (i.e. the developmental point at which the insult occurred), and dose (i.e. earlier adoption = lower dose, later adoption = higher dose). This demonstrates the difficulty of disentangling these different aspects in deprivation studies [11].
Enrichment
As stated above, there are inherent limits to the empirical possibilities in researching SPs in humans. Whereas deprivation in animal models is experimentally induced and under total experimental control, in humans such studies rely on naturally occurring circumstances that are reported on retrospectively. This has slowed progress in our understanding and made the research on SPs overly reliant on animal models. Enrichment paradigms offer experimental control over environmental input without the ethical constraint, following the logic that enriched experience-expectant input during SPs will lead to improved sensory or cognitive functions [64,65]. For instance, it has been shown that perceptual mechanisms underlying a range of different stimuli (i.e. faces, language sounds, emotion expressions, racial cues) are initially broadly tuned and become more specialised for specific types of discrimination with experience during infancy [66, 67, 68]. Interestingly, Pascalis et al. [69] showed that increased exposure to non-native faces between the ages of 6-9 months leads to the sustained ability to discriminate novel non-native faces otherwise lost after 9 months of age, providing evidence for a SP for the development of face processing during the first year of life [68]. Similar findings have been reported for the discrimination of difficult speech contrasts during infancy [67].
To be able to infer SPs from enrichment studies the same input needs to be provided across different age groups. The study of enrichment of EFs rests well within a large body of intervention studies aiming to improve EFs and associated domains [4]. While few of these have explicitly studied the interaction between age and EF performance following enrichment, a meta-analysis of EF training studies has demonstrated that the younger such trainings took place (i.e. as young as infancy) the larger the gains and the wider the transfer effects [70•]. It is impossible to infer from one meta-analysis alone, whether the age-related decrease of training effects on EFs constitutes evidence for a SP or a decline in cortical plasticity more generally. While a more systematic research programme is required including large cohorts with a large age-range and longitudinal designs with multiple follow-ups, training studies are a useful device to address questions related to SPs [71].
Viewing enrichment as the positive side to deprivation is perhaps not as straightforward as one might assume. Experience-expectant implies that as long as a minimum of the required input is received development will unfold normally. It may be that enrichment during SPs does not lead to any enhancements and as such may be useless to inform on SPs. Enrichment may also endow the developing organism with increased resilience to potential future stressors. Further detailed and methodically rigorous work will be necessary to solidify the value of this approach.
Adolescence as a sensitive period for EFs?
Adolescence is a period of considerable social, psychological and biological change and characterized by massive structural and functional brain reorganisation [72,73] in part driven by hormonal development. It has been argued that it might also constitute a SP for a range of cognitive functions including affect regulation [71]. This is based on cross-species developmental data where reduced fear extinction was observed in adolescence compared to childhood and adulthood in rodents and humans [74], which was paralleled by absent extinction-learning induced synaptic plasticity in the infralimbic cortex in rodents. Interestingly, rodent studies tracking the development of perineuronal nets (PNN), which preferentially surround GABAergic neurons expressing parvalbumin (i.e. the molecular mechanisms of SPs), found an age-related increase in PNN from juvenile to adult rats in the infralimbic cortex [75•]. There is therefore suggestive evidence that adolescence might indeed be a SP for affective regulatory processes.
Conclusion
To conclude, studies suggest that the early post-natal months constitute a SP for EFs. Social interactions during this period may act as a type of ‘experience-expectant’ input, which facilitates and directs an infant’s developing attention systems, and influences an infant’s sense of control over the environment, without which there may be long-lasting alterations to EF development. At the neuronal level this may be underpinned by early changes in the attachment system and/or the developing prefrontal cortex (and its interaction with other brain regions see [12]), which undergoes substantial changes during infancy. Future studies are needed to conclude whether this, indeed, constitutes a SP, or if this is a more general mechanism. Similarly, training EFs appears to be more effective at younger ages, but at present it is not possible to disentangle whether this is because of a SP or may be explained by normative changes in plasticity associated with development. EFs include several inter-related but distinct sub-domains. It may be that there are distinct trajectories for different sub-domains of EFs, which may themselves have distinct SPs. An example of this may be a distinct SP for affect regulation during adolescence. Disentangling these processes will be important for future studies that aim to enhance EFs.
Conflict of Interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This research is funded by an ERC Starting Grant and a Jacobs Foundation Early Career Research Fellowship.
References
- 1.Munakata Y., Snyder H.R., Chatham C.H. Developing Cognitive Control: Three Key Transitions. Curr Direct Psychol Sci. 2012 doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crone E.A., Steinbeis N. Neural Perspectives on Cognitive Control Development during Childhood and Adolescence. Trends Cogn Sci. 2017 doi: 10.1016/j.tics.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Moffitt T.E., Arseneault L., Belsky D., Dickson N., Hancox R.J., Harrington H.L., Houts R. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Nat Acad Scie USA. 2011 doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond A., Ling D.S. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev Cogn Neurosci. 2016 doi: 10.1016/j.dcn.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knudsen E.I. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 6.Lewis T.L., Maurer D. Multiple sensitive periods in human visual development: Evidence from visually deprived children. Dev Psychobiol. 2005 doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A., Dorman M.F., Spahr A.J. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hearing. 2002 doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Werker J.F., Hensch T.K. Critical periods in speech perception: new directions. Ann Rev Psychol. 2015;66(1):173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- 9.Hartley C.A., Lee F.S. Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology. 2015;40(1):50–60. doi: 10.1038/npp.2014.179. Nature Publishing Group. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fawcett T.W., Frankenhuis W.E. Adaptive explanations for sensitive windows in development. Front Zool. 2015 doi: 10.1186/1742-9994-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodard K., Pollak S.D. Is there evidence for sensitive periods in emotional development? Curr Opin Behav Sci. 2020;36:1–6. doi: 10.1016/j.cobeha.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen M.L., Amso D., McLaughlin K.A. The role of the visual association cortex in scaffolding prefrontal cortex development: A novel mechanism linking socioeconomic status and executive function. Dev Cogn Neurosci. 2019;39 doi: 10.1016/j.dcn.2019.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman N.P., Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. Elsevier Ltd. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake A., Friedman N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr Direct Psychol Sci. 2012 doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenna R., Rushe T., Woodcock K.A. Informing the structure of executive function in children: A meta-analysis of functional neuroimaging data. Front Hum Neurosci. 2017 doi: 10.3389/fnhum.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amso D., Scerif G. The attentive brain: Insights from developmental cognitive neuroscience. Nat Rev Neurosci. 2015 doi: 10.1038/nrn4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blankenship T.L., Slough M.A., Calkins S.D., Deater-Deckard K., Kim-Spoon J., Bell M.A. Attention and executive functioning in infancy: Links to childhood executive function and reading achievement. Dev Sci. 2019;22(6):1–9. doi: 10.1111/desc.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuevas K., Bell M.A. Infant attention and early childhood executive function. Child Dev. 2014 doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose S.A., Feldman J.F., Jankowski J.J. Implications of infant cognition for executive functions at age 11. Psychol Sci. 2012;23:1345–1355. doi: 10.1177/0956797612444902. [DOI] [PubMed] [Google Scholar]
- 20.Bernier A., Carlson S.M., Whipple N. In: Bernier Annie, Carlson Stephanie M., Whipple Natasha., editors. Vol. 81. Wiley on behalf of the Society for Research in Chil; 2010. pp. 326–339. (From External Regulation to Self-Regulation : Early Parenting Precursors of Young Children’s Executive Functioning). [DOI] [PubMed] [Google Scholar]
- 21.Bibok M.B., Carpendale J.I.M., Müller U. New Directions for Child and Adolescent Development. 2009. Parental scaffolding and the development of executive function. [DOI] [PubMed] [Google Scholar]
- 22.Gredebäck G., Astor K., Fawcett C. Gaze following is not dependent on ostensive cues: a critical test of natural pedagogy. Child Dev. 2018;89(6):2091–2098. doi: 10.1111/cdev.13026. [DOI] [PubMed] [Google Scholar]
- 23.Hammond S.I., Müller U., Carpendale J.I.M., Bibok M.B., Liebermann-Finestone D.P. The effects of parental scaffolding on preschoolers’ executive function. Dev Psychol. 2012 doi: 10.1037/a0025519. [DOI] [PubMed] [Google Scholar]
- 24.Beijersbergen M.D., Juffer F., Bakermans-Kranenburg M.J., van Ijzendoorn M.H. Remaining or becoming secure: Parental sensitive support predicts attachment continuity from infancy to adolescence in a longitudinal adoption study. Dev Psychol. 2012 doi: 10.1037/a0027442. [DOI] [PubMed] [Google Scholar]
- 25.Henderson R.K., Snyder H.R., Gupta T., Banich M.T. When does stress help or harm?The effects of stress controllability and subjective stress response on Stroop performance. Front Psychol. 2012 doi: 10.3389/fpsyg.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchís-Ollé M., Fuentes S., Úbeda-Contreras J., Lalanza J.F., Ramos-Prats A., Armario A., Nadal R. Controllability affects endocrine response of adolescent male rats to stress as well as impulsivity and behavioral flexibility during adulthood. Scient Reports. 2019 doi: 10.1038/s41598-019-40061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottwald J.M., Achermann S., Marciszko C., Lindskog M., Gredebäck G. An embodied account of early executive-function development: prospective motor control in infancy is related to inhibition and working memory. Psychol Sci. 2016;27(12):1600–1610. doi: 10.1177/0956797616667447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose S.A., Feldman J.F., Jankowski J.J. The structure of infant cognition at 1 year. Intelligence. 2005;33(3):231–250. [Google Scholar]
- 29.Perry R.E., Blair C., Sullivan R.M. Neurobiology of infant attachment: attachment despite adversity and parental programming of emotionality. Curr Opin Psychol. 2017 doi: 10.1016/j.copsyc.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiske A., Holmboe K. Neural substrates of early executive function development. Dev Rev. 2019;52:42–62. doi: 10.1016/j.dr.2019.100866. Elsevier. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson M.H. Interactive Specialization: A domain-general framework for human functional brain development? Dev Cogn Neurosci. 2011;1(1):7–21. doi: 10.1016/j.dcn.2010.07.003. Elsevier Ltd. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelazo P.D., Anderson J.E., Richler J., Wallner-Allen K., Beaumont J.L., Weintraub S. NIH toolbox cognition battery (CB): Measuring executive function and attention. Monogr Soc Res Child Dev. 2013;78(4):16–33. doi: 10.1111/mono.12032. [DOI] [PubMed] [Google Scholar]
- 33.Duncan N.W., Wiebking C., Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-A review of multimodal imaging studies. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Stagg C.J., Bachtiar V., Johansen-Berg H. What are we measuring with GABA Magnetic Resonance Spectroscopy? Commun Integ Biol. 2011;4(5):573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reh R.K., Dias B.G., Nelson C.A., Kaufer D., Werker J.F., Kolb B., Levine J.D. Critical period regulation across multiple timescales. Proc Nat Acad Sci. 2020 doi: 10.1073/pnas.1820836117. 201820836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Lambert H.K., King K.M., Monahan K.C., McLaughlin K.A. Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Dev Psychopathol. 2017;29(3):929–940. doi: 10.1017/S0954579416000584. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study (and also Sheridan 2017, below) points towards the distinct developmental effects of deprivation and maltreatment, with deprivation having a particularly strong effect on EFs.
- 37.Sheridan M.A., Peverill M., Finn A.S., McLaughlin K.A. Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev Psychopathol. 2017;29(5):1777–1794. doi: 10.1017/S0954579417001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bick J., Zeanah C.H., Fox N.A., Nelson C.A. Memory and Executive Functioning in 12-Year-Old Children With a History of Institutional Rearing. Child Dev. 2018;89(2):495–508. doi: 10.1111/cdev.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackman D.A., Gallop R., Evans G.W., Farah M.J. Socioeconomic status and executive function: Developmental trajectories and mediation. Dev Sci. 2015;18(5):686–702. doi: 10.1111/desc.12246. [DOI] [PubMed] [Google Scholar]
- 40.Lamm C., Troller-Renfree S.V., Zeanah C.H., Nelson C.A., Fox N.A. Impact of early institutionalization on attention mechanisms underlying the inhibition of a planned action. Neuropsychologia. 2018;117:339–346. doi: 10.1016/j.neuropsychologia.2018.06.008. Elsevier Ltd. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bick J., Zhu T., Stamoulis C., Fox N.A., Zeanah C., Nelson C.A. Effect of early institutionalization and foster care on long-term white matter development a randomized clinical trial. JAMA Pediatrics. 2015;169(3):211–219. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humphreys K.L., Gleason M.M., Drury S.S., Miron D., Nelson C.A., Fox N.A., Zeanah C.H. Effects of institutional rearing and foster care on psychopathology at age 12 years in Romania: Follow-up of an open, randomised controlled trial. Lancet Psychiatry. 2015;2:625–634. doi: 10.1016/S2215-0366(15)00095-4. Elsevier Ltd. Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyke A.T., Zeanah C.H., Fox N.A., Nelson C.A., Guthrie D. Placement in foster care enhances quality of attachment among young institutionalized children. Child Dev. 2010 doi: 10.1111/j.1467-8624.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Windsor J., Benigno J.P., Wing C.A., Carroll P.J., Koga S.F., Nelson C.A., Fox N.A. Effect of foster care on young children’s language learning. Child Dev. 2011 doi: 10.1111/j.1467-8624.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeanah C.H., Egger H.L., Smyke A.T., Nelson C.A., Fox N.A., Marshall P.J., Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 2009;166(7):777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- 46.Bos K.J., Fox N., Zeanah C.H., Nelson C.A. Effects of early psychosocial deprivation on the development of memory and executive function. Front Behav Neurosci. 2009;3:1–7. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troller-Renfree S., Nelson C.A., Zeanah C.H., Fox N.A. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. J Child Psychol Psychiatry Allied Disciplines. 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Wade M., Fox N.A., Zeanah C.H., Nelson C.A. Long-term effects of institutional rearing, foster care, and brain activity on memory and executive functioning. Proc Nat Acad Sci USA. 2019;116(5):1808–1813. doi: 10.1073/pnas.1809145116. [DOI] [PMC free article] [PubMed] [Google Scholar]; This provides a longitudinal follow up of the Bucharest Early Intervention Project cohort at ages 8, 12 and 16, reporting on developmental trajectories of EFs, suggesting minimal remediation of EFs due to foster care over this period.
- 49.Colvert E., Rutter M., Kreppner J., Beckett C., Castle J., Groothues C., Hawkins A. Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: Findings from the english and Romanian adoptees study. J Abnormal Child Psychol. 2008;36(7):1057–1068. doi: 10.1007/s10802-008-9232-x. [DOI] [PubMed] [Google Scholar]
- 50.Kessler R. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006 doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreppner J.M., O’Connor T.G., Rutter M., Beckett C., Castle J., Croft C., Dunn J. Can inattention/overactivity be an institutional deprivation syndrome? J Abnorm Child Psychol. 2001 doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- 52••.Sonuga-Barke E.J.S., Kennedy M., Kumsta R., Knights N., Golm D., Rutter M., Maughan B. Child-to-adult neurodevelopmental and mental health trajectories after early life deprivation: the young adult follow-up of the longitudinal English and Romanian Adoptees study. Lancet. 2017;389:1539–1548. doi: 10.1016/S0140-6736(17)30045-4. [DOI] [PubMed] [Google Scholar]; Psychopathology outcomes in young adulthood of children who experienced early severe deprivation. The study reports that early severe deprivation was associated with increased rates of psychopathology age 22-25, but only in those adopted after 6 months of age.
- 53.Spencer T.J., Biederman J., Mick E. Attention-deficit/hyperactivity disorder: Diagnosis, lifespan, comorbidities, and neurobiology. J Pediat Psychol. 2007 doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- 54.Tibu F., Sheridan M.A., McLaughlin K.A., Nelson C.A., Fox N.A., Zeanah C.H. Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychol Med. 2016;46(3):529–541. doi: 10.1017/S0033291715002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golm D., Sarkar S., MacKes N.K., Fairchild G., Mehta M.A., Rutter M., Sonuga-Barke E.J. The impact of childhood deprivation on adult neuropsychological functioning is associated with ADHD symptom persistence. Psychol Med. 2020:1–10. doi: 10.1017/S0033291720001294. [DOI] [PubMed] [Google Scholar]
- 56.Kreppner J.M., Rutter M., Beckett C., Castle J., Colvert E., Groothues C., Hawkins A. Normality andimpairment following profound early institutional deprivation: a longitudinal follow-up into early adolescence. Dev Psychol. 2007 doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- 57.Merz E.C., McCall R.B., Wright A.J., Luna B. Inhibitory control and working memory in post-institutionalized children. J Abnorm Child Psychol. 2013;41(6):879–890. doi: 10.1007/s10802-013-9737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollak S.D., Nelson C.A., Schlaak M.F., Roeber B.J., Wewerka S.S., Wiik K.L., Frenn K.A. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Devt. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Julian M.M. Age at adoption from institutional care as a window into the lasting effects of early experiences. Clin Child Family Psychol Rev. 2013;16(2):101–145. doi: 10.1007/s10567-013-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodel A.S. Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Dev Rev. 2018;48:113–144. doi: 10.1016/j.dr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marciszko C., Forssman L., Kenward B., Lindskog M., Fransson M., Gredebäck G. The social foundation of executive function. Dev Science. 2019:1–25. doi: 10.1111/desc.12924. Online. [DOI] [PubMed] [Google Scholar]
- 62.Gergely G., Watson J.S. Early socio-emotional development: contingency perception and the social-biofeedback model. Early Soci Cognit: Underst Others First Months life. 1999:101–136. Retrieved from https://books.google.nl/books?hl=en&lr=&id=inyOAwAAQBAJ&oi=fnd&pg=PA101&dq=related:80HRfgl2E3sJ:scholar.google.com/&ots=Z0Yi3mGfkF&sig=ZjhsDWlStGMJpcTF2ZnCLzyKpoc. [Google Scholar]
- 63.Rosen M.L., Hagen M.P., Lurie L.A., Miles Z.E., Sheridan M.A., Meltzoff A.N., McLaughlin K.A. Cognitive stimulation as a mechanism linking socioeconomic status with executive function: a longitudinal investigation. Child Dev. 2019;00(0):1–18. doi: 10.1111/cdev.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bavelier D., Levi D.M., Li R.W., Dan Y., Hensch T.K. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox S.E., Levitt P., Nelson C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010 doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heron-Delaney M., Anzures G., Herbert J.S., Quinn P.C., Slater A.M., Tanaka J.W., Lee K. Perceptual training prevents the emergence of the other race effect during infancy. PLoS One. 2011 doi: 10.1371/journal.pone.0019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maye J., Weiss D.J., Aslin R.N. Statistical phonetic learning in infants: Facilitation and feature generalization. Dev Sci. 2008 doi: 10.1111/j.1467-7687.2007.00653.x. [DOI] [PubMed] [Google Scholar]
- 68.Pascalis Olivier, De Haan M., Nelson C.A. Is face processing species-specific during the first year of life? Science. 2002 doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- 69.Pascalis O., Scott L.S., Kelly D.J., Shannon R.W., Nicholson E., Coleman M., Nelson C.A. Plasticity of face processing in infancy. Proc Nat Acad Sci USA. 2005 doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Wass S.V., Scerif G., Johnson M.H. Training attentional control and working memory - Is younger, better? Dev Rev. 2012 [Google Scholar]; A meta-analysis of cognitive training studies demonstrating that cognitive training is more effective at younger ages.
- 71.Fuhrmann D., Knoll L.J., Blakemore S.J. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015 doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 72.Mills K.L., Goddings A.L., Herting M.M., Meuwese R., Blakemore S.J., Crone E.A., Dahl R.E. Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uhlhaas P.J., Roux F., Singer W., Haenschel C., Sireteanu R., Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Nat Acad Sci USA. 2009 doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pattwell S.S., Duhoux S., Hartley C.A., Johnson D.C., Jing D., Elliott M.D., Ruberry E.J. Altered fear learning across development in both mouse and human. Proce Nat Acad Sci USA. 2012 doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Baker K.D., Gray A.R., Richardson R. The development of perineuronal nets around parvalbumin GABAergic neurons in the medial prefrontal cortex and basolateral amygdala of rats. Behav Neurosci. 2017;131(4):289–303. doi: 10.1037/bne0000203. [DOI] [PubMed] [Google Scholar]; This study reports an age-related increased in peri-neuronal nets from juvenile to adult rats in limbic cortices, suggesting adolescence may be a sensitive period for affective processes.