Highlights
-
•
Cytokine release syndrome is a concern for novel antibody therapeutics.
-
•
In vitro cytokine release assays are key for hazard identification.
-
•
Positive and negative control antibodies generated for assay qualification.
-
•
Control antibody panel induced cytokine release in a variety of assay platforms.
Keywords: Cytokine release assay, Hazard identification, Cytokine storm, Antibody therapeutics, Reference reagents
Abstract
Immunomodulatory therapeutics such as monoclonal antibodies (mAb) carry an inherent risk of undesired immune reactions. One such risk is cytokine release syndrome (CRS), a rapid systemic inflammatory response characterized by the secretion of pro-inflammatory cytokines from immune cells. It is crucial for patient safety to correctly identify potential risk of CRS prior to first-in-human dose administration. For this purpose, a variety of in vitro cytokine release assays (CRA) are routinely used as part of the preclinical safety assessment of novel therapeutic mAbs. One of the challenges for the development and comparison of CRA performance is the lack of availability of standard positive and negative control mAbs for use in assay qualification. To address this issue, the National Institute for Biological Standards and Control (NIBSC) developed a reference panel of lyophilised mAbs known to induce CRS in the clinic: human anti-CD52, mouse anti-CD3 and human superagonistic (SA) anti-CD28 mAb manufactured according to the respective published sequences of Campath-1H® (alemtuzumab, IgG1) , Orthoclone OKT-3® (muromonab, IgG2a) and TGN1412 (theralizumab, IgG4), as well as three isotype matched negative controls (human IgG1, mouse IgG2a and human IgG4, respectively). The relative capacity of these control mAbs to stimulate the release of IFN-γ, IL-2, TNF-α and IL-6 in vitro was evaluated in eleven laboratories in an international collaborative study mediated through the HESI Immuno-safety Technical Committee Cytokine Release Assay Working Group. Participants tested the NIBSC mAbs in a variety of CRA platforms established at each institution. This paper presents the results from the centralised cytokine quantification on all the plasma/supernatants corresponding to the stimulation of immune cells in the different CRA platforms by a single concentration of each mAb. Each positive control mAb induced significant cytokine release in most of the tested CRA platforms. There was a high inter-laboratory variability in the levels of cytokines produced, but similar patterns of response were observed across laboratories that replicated the cytokine release patterns previously published for the respective clinical therapeutic mAbs. Therefore, the positive and negative mAbs are suitable as a reference panel for the qualification and validation of CRAs, comparison of different CRA platforms (e.g. solid vs aqueous phase), and intra- and inter-laboratory comparison of CRA performance. Thus, the use of this panel of positive and negative control mAbs will increase the confidence in the robustness of a CRA platform to identify a potential CRS risk for novel immunomodulatory therapeutic candidates.
1. Introduction
Cytokine release syndrome (CRS) is a rapid systemic inflammatory response characterized by the release of pro-inflammatory cytokines (e.g. TNFα, IL-6, IFNγ, IL-2) from immune cells [1]. CRS is characterized by a range of clinical effects from fever and fatigue to multi-organ failure. CRS has been observed after the administration of biotherapeutics, including monoclonal antibodies (mAbs) that target receptors expressed on immune cells, T cell-engaging immunotherapies, and chimeric antigen receptor T cell therapies [2]. Cytokine release is an anticipated risk for some immunomodulatory therapeutics for cancer indications, such as T cell-engagers and chimeric antigen receptor T cells, where cytokine release is expected pharmacology; however, unanticipated CRS is a serious concern for novel biotherapeutics. During the first-in-human (FIH) Phase I clinical trial of TGN1412/theralizumab, (humanized anti-human CD28 superagonist (SA), IgG4), a mAb therapeutic with presumptive anti-inflammatory properties, six healthy volunteers experienced life-threatening, unanticipated CRS [3], [4]. The failure of TGN1412 pre-clinical safety testing to predict a risk for CRS demonstrated the need for a new generation of in vitro cytokine release assays (CRA) that would include the use of human immune cells and allow for hazard identification of potential CRS [5]. Since 2006, a variety of CRA platforms with reported predictivity for a TGN1412-type response are now in use with different sensitivities and capabilities [6], [7], [8], [9]. As previously highlighted in a survey conducted by ILSI–Health and Environmental Sciences Institute (HESI) Immuno-safety Technical Committee CRA Working Group [6], these CRA platforms vary with regards to cell composition (e.g. PBMC, whole blood, endothelial cells), cell density, presentation of mAb therapeutic (e.g. dry or wet-coated adhesion to tissue culture plate wells, in solution, captured to beads) and quantification of cytokine release (e.g. ELISA, multiplex array). These CRA platforms were originally designed for mAb therapeutics for which cytokine release is not part of the expected mechanism of action of the molecule rather than immunomodulatory therapeutics for cancer.
Regardless of the CRA platform employed, the goal to correctly identify unanticipated CRS risk prior to FIH dose administration remains paramount. Confirmation of assay performance, including sensitivity and reproducibility, requires the consistent use of positive and negative control mAbs. Ideally the positive controls would include a panel of mAb therapeutics covering different modalities and with established CRS risk in the clinic. In addition to TGN1412, OKT3®/muromonab (murine anti-human CD3 IgG2a) [10], [11] and Campath-1H®/alemtuzumab (humanized anti-CD52 IgG1) [12] are capable of inducing dose-dependent CRS in patients and cytokine release from human immune cells in vitro [13], [14], [15], [16]. Approximately 50% of the patients treated with muromonab to prevent renal allograft rejection experienced severe CRS if not pre-treated with high dose of corticosteroids [10], [11]. Alemtuzumab, used for the treatment of B-cell chronic lymphocytic leukaemia and multiple sclerosis, also induced moderate to severe CRS in a minority of patients [12]. TGN1412, muromonab, and alemtuzumab have been used to qualify a variety of CRA platforms [6], [7]. However, except for research grade muromonab, it can be difficult to use these positive control mAbs consistently because of high cost and/or restricted availability. Without a common set of positive and negative control mAbs for testing in CRA platforms, it has been challenging to compare the relative strengths and weaknesses of the different CRA formats and to establish confidence in the ability of any novel CRA to identify potential CRS risk.
To address this issue, NIBSC has produced a reference panel of lyophilised recombinant positive control mAbs for CRA qualification, including anti-CD52 mAb, anti-CD3 mAb and anti-CD28(SA) mAb which have been manufactured according to the respective published sequences of alemtuzumab [17], muromonab [18] and TGN1412 [19]. In addition, three isotype-matched negative control mAbs have been generated. The relative capacity of these positive control mAbs to stimulate cytokine release was evaluated in eleven laboratories in an international collaborative study mediated through the HESI Immuno-safety Technical Committee Cytokine Release Assay Working Group. The goals of the study included: 1) comparison of the performance of the NIBSC mAbs to the performance of the positive and negative controls used at each institution, and 2) comparison of cytokine release data across all participants to determine the NIBSC mAb inter-laboratory performance. To help achieve these goals, a centralized analysis of supernatant samples from each participant was performed to further compare the results of NIBSC mAb inter-laboratory performance by removing inter-laboratory variation in methods of cytokine detection. All participants in the study received anti-CD52, anti-CD3, and anti-CD28SA mAbs along with their isotype-matched negative control mAbs from NIBSC. Participants tested the NIBSC mAbs in their standard CRA platforms, which included mAb wet- or dry-coated on tissue culture wells (solid phase) or in solution within the assay (aqueous phase) in the presence of human peripheral blood mononuclear cells (PBMC), whole blood (WB), diluted whole blood (dWB), mAb coated on beads and cultured with PBMC, or peripheral blood leukocytes co-cultured with human umbilical vein endothelial cells (PBL/HUVEC). Data from the first goal (comparison of NIBSC mAbs to institution-specific controls) was used for the benefit of each participant and is not reported in this manuscript. Quantification of NIBSC mAb-induced cytokine release was performed using institution-specific protocols, and the data for 4 cytokines (IFNγ, TNFα, IL-2, and IL-6) were reported back to the study director at NIBSC for statistical analyses. In addition, supernatants from the identical samples measured in-house by each participant were frozen and shipped to NIBSC so that centralized cytokine quantification could be performed using a single multiplex array method on all samples from all CRA platforms.
The data demonstrate that the anti-CD52, anti-CD3 and anti-CD28SA positive control mAbs induced cytokine release from human immune cells in a variety of CRA platforms. There was a high inter-laboratory variability in cytokine levels stimulated by the three positive control mAbs but similar response patterns were observed across laboratories. The isotype (negative) control mAbs consistently induced very low or no cytokine release in the tested CRA platforms. Therefore, the NIBSC positive and negative control mAbs are fit for purpose in a variety of CRA platforms and can be used to improve confidence in assay performance and interpretation.
2. Materials and methods
2.1. Materials
Isotype controls (anti-hapten 4-hydroxy-3-nitrophenyl acetyl) with variable regions from the hybridoma B1-8, anti-CD52 and anti-CD3 mAbs with the respective published sequences of alemtuzumab [17], muromonab [18] were manufactured by Absolute Antibody using AbAb’s recombinant platform (Absolute Antibody Ltd, Oxford, UK). Antibodies were expressed in HEK293 cells using Absolute Antibody proprietary transient expression system and were protein A affinity purified (Absolute Antibody Ltd, Oxford, UK). The anti-CD28SA was prepared at NIBSC using heavy and light chain sequences of TGN1412 as previously described [20]. Expression plasmids were produced by inserting light and heavy chain DNA into pEE12.4 GS (gene expression system, Lonza Biologics, Berkshire, UK). The antibody was expressed in Chinese Hamster Ovary cell line CHO-K1SV and purified using Protein A chromatography followed by size exclusion purification (Superdex 200 10/300 GL column, GE Healthcare).
The positive and negative control mAbs were then formulated in phosphate buffered solution (PBS) and freeze-dried at NIBSC in flame-sealed glass ampoules that were back filled with dry nitrogen to increase the stability and preserve the biological activity of the mAbs. This process ensures a low oxygen and moisture content (Table 1) over the lifespan of the products [21]. The residual moisture content and the percentage of head space O2 of the products are below one percent indicating respectively that drying has been adequate and that the materials are protected against oxidative change [22]. At the time of the writing, no loss of potency has been observed during the accelerated temperature degradation study and the yearly loss estimation could not be calculated. Samples in the accelerated thermal degradation studies will continue to be assessed. Endotoxin concentration in the final reconstituted products was analysed by the Limulus amoebocyte lysate (LAL) gel clot method (European Pharmacopoeia Section 2.6.14 Bacterial endotoxins). Endotoxin level in each antibody preparation was less than 0.12 IU/ml, therefore below the recommended limit of 0.25 IU/ml for water for injection (https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-quality-water-pharmaceutical-use_en.pdf).
Table 1.
Characterisation of positive and negative control mAb preparations.
| Product code | Antibody specificity | Quantity per vial (μg) | Mean fill weight (g) | CV fill weight (%) | Mean residual moisture (%) (n) | CV residual moisture % | Mean headspace oxygen % | CV headspace oxygen % | Monomer(%) |
|---|---|---|---|---|---|---|---|---|---|
| 15/144 | Anti-CD28SA | 100 | 1.0062 | 0.132 | 0.5837 | 19.44 | 0.14 | 68.26 | 89 |
| 15/162 | Anti-CD3 | 200 | 1.0080 | 0.188 | 0.428 | 22.69 | 0.73 | 12.99 | 95 |
| 15/178 | Anti-CD52 | 200 | 1.0080 | 0.169 | 0.351 | 16.45 | 0.45 | 31.66 | 90 |
| 15/198 | IgG1K isotype | 200 | 1.0086 | 0.162 | 0.630 | 11.59 | 0.43 | 14.20 | 90 |
| 15/218 | IgG2a isotype | 200 | 1.0084 | 0.167 | 0.457 | 40.99 | 0.51 | 21.35 | 92 |
| 15/232 | IgG4 isotype | 200 | 1.0084 | 0.156 | 0.454 | 15.50 | 0.29 | 54.36 | 88 |
Determination of aggregate in each positive and negative control mAb preparation was performed by HPLC (TSK gel G3000 SWXL column, TOSOH BIOSCIENCE; 0.2 M sodium phosphate/0.1 M sodium sulphate buffer, pH6; 0.5 ml/min flow rate) and percentage of monomer reported in Table 1.
2.2. Participants
Eleven laboratories from 3 different countries listed in Table 2, participated in the collaborative study.
Table 2.
Participants of the collaborative study.
| Participant | Code | Scientist names and lab addresses |
|---|---|---|
| Amgen | A | Madeline Fort; Melissa Martin; Ruchika Jaisinghani Amgen Inc., 1120 Veterans Blvd, South San Francisco CA 94080, USA |
| Pfizer | B | Lynn O’Donnell1; Marie-Clare St. Rose 2(post-doctoral fellowship completed at Pfizer) 1. Drug Safety Research and Development, Pfizer, Inc., Groton, CT 06340, USA 2.Janssen R&D, 1400 McKean Road, Spring House, PA 19477, USA |
| Merck | C | Aarron Willingham; David Sanden; Bhagyashree Bhagwat MRL, Merck & Co., Inc., 213 E Grand Ave, South San Francisco, CA 94080, USA |
| Roche | D | Heather Hinton; Sebastian Krasniqi, Pharmaceutical Sciences, Roche Innovation Center, Basel, Switzerland. |
| Novartis | E | Babette Wolf Novartis Institutes for BioMedical Research, Klybeckstrasse 141, Basel, CH-4002, Switzerland |
| Janssen | F | Daniel Weinstock; Divya MekalaJanssen R&D, 1400 McKean Road, Spring House, PA 19477, USA |
| Bristol-Myers Squibb | G | Joseph Piccotti; Amber Faust; Henry Chan Bristol-Myers Squibb, 10,300 Campus Point Drive, Suite 100, San Diego, CA 92121, USA |
| AstraZeneca | H | Richard Stebbings; Jose Carlos Chavez AstraZeneca, 121 Oyster Point Blvd, South San Francisco, CA 94080, USA |
| GSK | I | Kimberly Nadwodny; Curtis Maier; Erin Robinson GlaxoSmithKline, 1250 South Collegeville Road, Collegeville, PA 19426, USA |
| Genentech | J | Rod Prell; Aaron Fullerton; Michael Sweeney; Karin Staflin; Genentech1 DNA Way, South San Francisco, CA 94080, USA |
| NIBSC | K | Sandrine Vessillier (Biotherapeutics); Luisa Saraiva (Biotherapeutics); Giles Sharp (Biotherapeutics); Eleanor Atkinson (TDI-Biostatistics) National Institute for Biological Standards and Control, Blanche Lane, South Mimms, Potters Bar, Hertfordshire, EN6 3QG, UK |
2.3. Study design
Participants in the study are members of the HESI Immuno-safety Technical Committee Cytokine Release Assay Working Group. As the goal of the study was to analyse how the panel of recombinant positive and negative control antibodies performed in a variety of CRA platforms, participants were not pre-selected based on the CRA platform(s) used in their respective laboratories. Therefore, data was collected using 6 different assay platforms with some CRA platforms used by multiple participants and others by a single participant (Table 3).
Table 3.
CRA performed by the participants.
| Assay type | IncubationTime | Number of participants | Number of donors (all participants) |
|---|---|---|---|
| PBMC-SP | 18–24 h | 4 | 35 |
| 48 h | 3 | 26 | |
| PBMC-AQ | 24 h | 1 | 8 |
| PBL/HUVEC | 24 h | 1 | 8 |
| WB-AQ | 24 h | 6 | 54 |
| 48 h | 1 | 8 | |
| dWB-AQ | 48 h | 1 | 12 |
| dWB-SP* | 48 h | 1 | 15 |
PBMC: Peripheral Blood Mononuclear Cells; PBL: peripheral blood leukocytes; HUVEC: human umbilical vein endothelial cells; WB: Whole Blood; dWB: diluted Whole Blood; SP: solid phase; AQ: Aqueous Phase; dWB-SP* corresponds to the bead coated method.
Participants were sent ampoules of each of the lyophilised recombinant positive and negative control mAbs to be resuspended in 1 ml of water and asked to evaluate performance using their institution-specific cytokine release assays. Details of the different institution-specific CRAs are summarised in Supplementary Table 1.To find an optimal concentration for cytokine release by the positive control mAbs, some of the participants tested a range of concentrations of the positive and negative mAbs (0.04 to 10 μg/mL) in their respective CRA vs their internal controls (data not shown). In general, 0.25–1 ug/well in solid-phase CRA and 5 ug/mL in aqueous phase CRA induced maximal cytokine production with the positive control mAbs, and these were the concentrations that were chosen for the main comparative study (with the exception of a few participants, see Supplementary Table 1). Participants performed an analysis of cytokine production, including IFN-γ, IL-2, TNF-α and IL-6, using their standard methods (Supplementary Table 1). The data from these four cytokines were selected for comparative analysis due to their known damaging effect during CRS [23]. Supernatants/plasma samples were collected from wells and processed for detection of IFN-γ, IL-2, TNF-α and IL-6 production using both internal methods and by a centralized analysis at NIBSC (see below).
The participants performed their assays in absence of mAb (eg media alone) and in presence of their own positive control mAbs and/or mitogen (eg LPS) to confirm donor response and the assay performance (data not shown). Supernatants from these two conditions was not analysed during the centralised analysis at NIBSC using the MSD platform as the focus of the study was on the assessment of performance of the panel of recombinant reference mAbs with the comparison of the response between the positive and negative control mAbs and not on donor to donor variation.
2.4. Methods
Each of the 6 CRA platforms used in this study had an end point of supernatant or plasma collection after an 18–48 h incubation period (Table 3). More information about each assay can be found in Supplementary Table 1.
2.4.1. PBMC SP (Solid phase) cytokine release assay
PBMC were isolated from freshly heparinized blood that was collected from healthy donors for research purposes in accordance with applicable country government regulations and guidelines. PBMC SP CRA was performed by wet coating wells of microtiter plates with 50–100 μl media/solution containing mAbs (1–5 μg/well) overnight at 4 °C [5], [24], [25] or by drying the coated wells overnight in a biological safety cabinet at room temperature [26]. Plates were then washed with PBS to remove unbound mAb. PBMC were adjusted to a concentration of 1 × 106 cells/ml and 100–200 μl of the cell suspension was added to each well. Plates were incubated for 18–24 h (4 participants) or 48 h (3 participants) in a humidified incubator at 37 °C, 5% CO2.
Data was obtained from independent experiments, each comprising PBMC from different donors. See supplementary table for any variation in the method.
2.4.2. PBMC AQ cytokine release assay
PBMC AQ CRA was performed in Tissue Culture treated 96-well plates by seeding PBMC at a concentration of 1 × 106 cells/ml in 200 μl of cell suspension per well. Cells were treated with mAb at a concentration of 5 μg/ml. Plates were incubated for 24 h in a humidified incubator at 37 °C, 5% CO2.
2.4.3. Whole blood-Aqueous phase (WB-AQ) and diluted WB-AQ cytokine release assay
Whole Blood (WB) cytokine release assays were performed by incubating heparinized WB with the mAbs at 5 μg/mL for 24 h (6 participants) – 48 h (1 participant) in a humidified incubator at 37 °C, 5% CO2 [24], [27], [28]. Data was obtained from independent experiments, each comprising WB from different healthy human donors. See Supplementary Table 1 for details and any variation in the method.
In diluted WB (dWB) cytokine releases assay, 10% diluted blood was incubated with the mAbs at 5 μg/mL for 48 h (1 participant). Heparinized WB was collected from 12 donors. Two ml of WB were diluted 1:6.6 in RPMI 1640 Glutamax containing 1% pen/strep. One hundred μl of PBS, LPS or the appropriately diluted mAbs were aliquoted into 96 well plates. Two hundred μl of dWB was added to each well and incubated at 37 °C, 5% CO2 in a humidified incubator for 48 h. Plates were centrifuged at 500 g for 5 min at 4 °C and supernatants collected and stored frozen at −80 °C until cytokine evaluation.
2.4.4. PBL/HUVEC Co-culture assay
Cryopreserved human umbilical vein endothelial cells (HUVEC, Lonza, Walkers, MD) were thawed and cultured in EGM Growth Media at 37 °C and passaged twice prior to use in the assay [29]. At 70% confluency, HUVEC were harvested, counted, and plated into 96-well flat bottom plates at a seeding density of 6000 cells/well and incubated overnight. On day 1 of the assay, WB was collected from six healthy human donors into sodium heparin vacutainers. Blood components were then isolated into (1) platelet poor plasma (PPP) and (2) peripheral blood leukocytes (PBL), which comprises lymphocytes, monocytes, and granulocytes. PBL were then pelleted and re-suspended in 5 ml of autologous PPP to a final concentration of 1x107 cells/ml. The EGM Growth Media was removed from the wells containing HUVEC cells and replaced with PPP from each donor (100 μl/well). Corresponding autologous PBL were then added for each donor (100 μl/well, for 1x106 PBL/well). Finally, the 5x positive and negative control mAbs were added to the appropriate wells on each plate (50 μl/well) for a final volume of 250 ul/well. A set of control wells denoted as “HUVEC only” and “HUVEC + PBL” were set up for each donor. Plates were incubated at 37 °C/5% CO2 for 20–24 h, centrifuged at 350g for 10 min. Culture supernatants (150 ul/well) were removed and dispensed into new V-bottom 96-well plates, centrifuged at 350 g for 10 min, and ~ 80 μl/well of the supernatants were moved to new V-bottom 96-well plates to ensure removal of all cells. The supernatants were then sealed and stored frozen at −80 °C until cytokine evaluation.
2.4.5. Diluted WB-SP (dWB-SP) bead coated assay
Heparinized WB was collected from 15 donors. Protein A coated beads (0.5% w/v 6.0 – 8.0 μm, Spherotech Inc. Lake Forest, IL) were washed and prepared following manufacturer’s directions. Autologous plasma was coated on beads by adding 1 ml of plasma per donor to 2 ml beads for a final concentration of 6x107 beads/ml. mAbs were immobilized on Protein A beads by adding 2.5 ml of mAb at 200 μg/ml to 3.5 ml beads for a final concentration of 6x107 beads/ml for all mAbs except for NIBSC anti-CD28SA. Five ml of anti-CD28SA at 200 μg/ml was added to 1 ml beads for a final concentration of 6x107 beads/ml. Samples were vortexed and incubated at room temperature on a rocker for 2 h. Beads were washed and resuspended in sterile PBS at 1.7x107 beads/ml. Heparinized WB was collected from 5 donors and 2 ml from each donor was diluted 1:6.6 in RPMI 1640 Glutamax containing 1% pen/strep. One hundred μl of PBS, LPS, autologous plasma coated beads or mAb coated Protein A beads were aliquoted into 96 well plates. dWB (200 ul) was added to each well and incubated at 37 °C, 5% CO2 in a humidified incubator for 48 h. Plates were centrifuged at 500 g for 5 min at 4 °C and supernatants collected and stored frozen at −80 °C until cytokine evaluation [30].
2.4.6. Cytokine measurement
After incubation with positive and negative mAbs, cell-conditioned medium or plasma were obtained by centrifuging the plates. Two aliquots were prepared from each sample; one aliquot was allocated for participant-specific cytokine analysis using internal cytokine panels and the other aliquot was frozen at −70 °C until centralized cytokine analysis was performed at NIBSC. For WB in brief, plate was spun at 1800g at room temperature for 5 min, plasma from duplicates pooled and split into two equal aliquots (aliquot I for internal cytokine measurement, aliquot II for centralized measurement at NIBSC).
Internal cytokine analysis using internal cytokine panels was done at the participant’s labs using either Luminex, Aushon [31] or Meso Scale Discovery (MSD) platforms as stated in Supplementary Table 1 following manufacturer’s instructions.
Centralised cytokine analysis was performed at NIBSC using MSD multiplex plates (Meso Scale Discovery, Gaithersburg, Maryland, USA) to determine IFN-γ, IL-2, TNF-α and IL-6 concentrations in culture supernatants or plasma following the manufacturer’s instructions.
2.4.7. Statistical analysis
Statistical analysis was performed using Minitab 18 software (Minitab). Comparison between positive control and their negative isotype control was performed using linear mixed effect model with log10 (pg/ml) transformed data. Mean results were calculated for each response and treatment method. Analysis was performed with time and treatment as fixed effect and participants included as random effect. A mixed effects model was used to determine intra-laboratory and inter-laboratory variance components. These calculations were performed in R using the R package ‘lme4′ (https://cran.r-project.org/web/packages/lme4/citation.html). Individual laboratory means were also calculated and the absolute difference between these and the median across all laboratory means was calculated for each lab within each response and treatment method.
3. Results
3.1. Performance of the reference reagents in the different assays
The eleven participants used their institution-specific CRA platforms (Supplemental Table 1) to assess the ability of the positive control mAbs (anti-CD28SA, anti-CD3 and anti-CD52 antibodies) and the negative control mAbs (human IgG1, mouse IgG2a and human IgG4) to induce cytokine production from human immune cells relative to internal control mAbs. Comparison between the cytokine quantification performed at the participant site and at NIBSC are reported in supplementary data (Supplementary Fig. 1&2). No significant differences in the levels of cytokine quantifications were noted between the MSD analysis at NIBSC and the other platforms used by the individual participant for their on-site analysis with the exception of the Aushon platform which showed significantly lower IFNγ level (p ≤ 0.005) than the MSD platform during WB-AQ analysis.
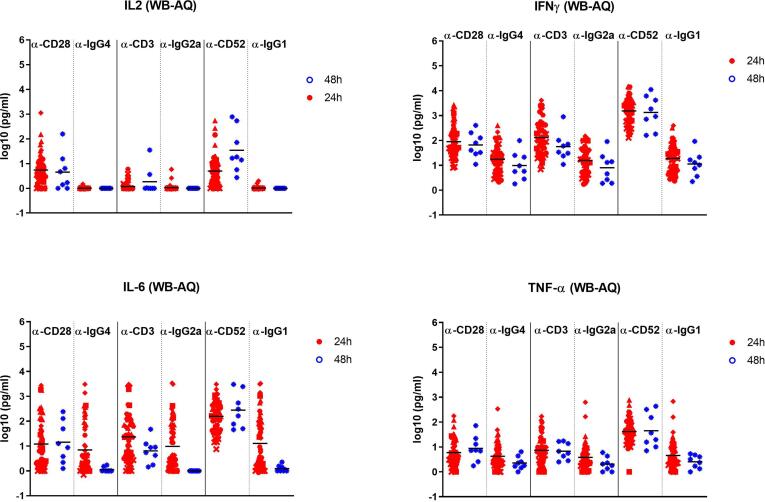
The results from the NIBSC quantification of IFN- γ, IL-2, TNF-α and IL-6 induced by the three positive and negative control mAbs in the eight different CRA formats are reported in Table 4. All positive control mAbs (anti-CD28SA, anti-CD3, and anti-CD52 mAbs) at 0.25–5.0 μg/well in the PBMC-SP and at 5 ug/ml in the WB-AQ CRA platforms induced statistically significant increases in the production of IFN-γ, IL-2, TNF-α, and IL-6 in respect to the isotype control mAbs both at 24 h and 48 h incubation periods (Fig. 1, Fig. 2, and Table 4). The levels of the measured cytokines in response to the three-positive control mAbs were lower in WB-AQ CRA in comparison to PBMC-SP CRA, but still demonstrated significant increases above the isotype control mAbs. (p values are reported in Supplementary Table 2.) Cytokine release in response to the 3 negative control mAbs was generally low in comparison to the positive control mAbs, with notable exceptions for IL-6 in the PBL-HUVEC CRA (Table 4) and IL-6 for some samples in the WB-AQ CRA (Fig. 2).
Table 4.
Comparative analysis of cytokine release with the different CRA methods (n = number of donors). Cytokine release expressed in pg/ml from human cells after 18–48 h incubation with the different antibody controls. Group responses shown are the geometric means and 95% confidence intervals. LOD 1 pg/ml. p values for the comparison of each positive control with its isotype control are reported in supplementary Table 2.
| PBMC-SP 24h |
PBMC-SP 48h |
WB-AQ 24h |
WB-AQ 48h |
dWB-AQ 48h |
dWB-SP 48h |
PBL/HUVEC-AQ 24h |
PBMC-AQ 24h |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 participants (n=35) |
3 participants (n=26) |
6 participants (n=54) |
1 participant (n=8) |
1 participant (n=10) |
1 participant (n=10) |
1 participant (n=8) |
1 participant (n=8) |
||||||||||||||||||
| Treatment | Cytokine | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean | Geometric mean (pg/ml) | Lower 95% CI of geo. mean | Upper 95% CI of geo. mean |
| CD28-SA | IFN-γ | 1521 | 583 | 3965 | 43364 | 19268 | 97594 | 90 | 59 | 136 | 66 | 25 | 175 | 2 | 1 | 2 | 78074 | 65165 | 93540 | 380 | 96 | 1510 | 11 | 5 | 21 |
| IL-2 | 269 | 141 | 512 | 2025 | 805 | 5097 | 5 | 4 | 8 | 5 | 1 | 19 | 1 | 1 | 1 | 159 | 111 | 228 | 20 | 11 | 39 | 9 | 6 | 14 | |
| IL-6 | 125 | 48 | 326 | 263 | 154 | 447 | 12 | 7 | 22 | 14 | 3 | 71 | 1 | 1 | 1 | 351 | 189 | 650 | 2091 | 1162 | 3761 | 6 | 3 | 14 | |
| TNF-α | 346 | 162 | 741 | 5332 | 2608 | 10901 | 6 | 4 | 8 | 9 | 3 | 23 | 1 | 1 | 1 | 2422 | 1606 | 3652 | 28 | 10 | 77 | 6 | 3 | 11 | |
| CD3 | IFN-γ | 33435 | 22590 | 49489 | 71795 | 57259 | 90022 | 130 | 84 | 200 | 56 | 19 | 170 | 161 | 49 | 531 | 3724 | 989 | 14018 | 57 | 19 | 174 | 10807 | 6129 | 19055 |
| IL-2 | 144 | 103 | 201 | 84 | 46 | 152 | 1 | 1 | 1 | 2 | 1 | 5 | 9 | 7 | 11 | 14 | 9 | 21 | 2 | 1 | 3 | 81 | 51 | 128 | |
| IL-6 | 1061 | 673 | 1674 | 520 | 247 | 1094 | 23 | 13 | 42 | 6 | 2 | 16 | 9 | 2 | 37 | 337 | 116 | 981 | 897 | 526 | 1529 | 283 | 160 | 500 | |
| TNF-α | 2870 | 2331 | 3533 | 9326 | 7179 | 12116 | 7 | 5 | 10 | 7 | 4 | 13 | 23 | 9 | 60 | 240 | 131 | 441 | 6 | 2 | 21 | 1371 | 808 | 2327 | |
| CD52 | IFN-γ | 3936 | 2180 | 7105 | 10997 | 4874 | 24812 | 1534 | 1115 | 2112 | 1337 | 360 | 4967 | 15 | 7 | 34 | 783 | 151 | 4078 | 46 | 13 | 157 | 333 | 87 | 1274 |
| IL-2 | 31 | 19 | 50 | 21 | 14 | 30 | 5 | 3 | 7 | 34 | 6 | 190 | 1 | 1 | 1 | 7 | 4 | 11 | 1 | 1 | 2 | 9 | 5 | 17 | |
| IL-6 | 680 | 361 | 1282 | 81 | 48 | 135 | 156 | 107 | 229 | 278 | 70 | 1107 | 2 | 1 | 3 | 566 | 228 | 1403 | 894 | 461 | 1736 | 87 | 27 | 277 | |
| TNF-α | 994 | 717 | 1378 | 2786 | 1508 | 5148 | 42 | 31 | 56 | 45 | 12 | 172 | 3 | 2 | 8 | 141 | 72 | 277 | 5 | 2 | 18 | 163 | 72 | 367 | |
| IgG4 | IFN-γ | 8 | 4 | 16 | 10 | 6 | 18 | 17 | 12 | 25 | 10 | 3 | 29 | 1 | 1 | 2 | 5 | 3 | 9 | 57 | 18 | 180 | 3 | 2 | 8 |
| IL-2 | 1 | 1 | 2 | 6 | 4 | 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 4 | 2 | 1 | 5 | |
| IL-6 | 10 | 6 | 16 | 6 | 4 | 11 | 7 | 4 | 13 | 1 | 1 | 1 | 1 | 1 | 1 | 20 | 7 | 56 | 989 | 480 | 2040 | 2 | 1 | 4 | |
| TNF-α | 30 | 16 | 56 | 16 | 10 | 25 | 4 | 3 | 6 | 2 | 1 | 4 | 1 | 1 | 1 | 11 | 8 | 14 | 6 | 2 | 24 | 2 | 1 | 4 | |
| IgG2a | IFN-γ | 10 | 5 | 19 | 9 | 5 | 13 | 16 | 11 | 22 | 8 | 3 | 25 | 1 | 1 | 1 | 5 | 3 | 9 | 6 | 2 | 17 | 3 | 1 | 8 |
| IL-2 | 1 | 1 | 2 | 5 | 3 | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 5 | 2 | 12 | 2 | 1 | 5 | |
| IL-6 | 14 | 8 | 24 | 7 | 4 | 10 | 10 | 5 | 18 | 1 | 1 | 1 | 1 | 1 | 1 | 26 | 9 | 74 | 9 | 4 | 21 | 2 | 2 | 4 | |
| TNF-α | 45 | 25 | 81 | 26 | 16 | 43 | 4 | 3 | 5 | 2 | 1 | 4 | 1 | 1 | 1 | 9 | 7 | 13 | 38 | 19 | 77 | 2 | 2 | 4 | |
| IgG1 | IFN-γ | 44 | 24 | 79 | 62 | 31 | 126 | 19 | 13 | 26 | 11 | 4 | 29 | 1 | 1 | 2 | 6 | 4 | 10 | 52 | 17 | 163 | 5 | 3 | 10 |
| IL-2 | 2 | 1 | 2 | 6 | 4 | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 3 | 2 | 1 | 4 | 3 | 1 | 6 | |
| IL-6 | 33 | 19 | 58 | 8 | 5 | 13 | 13 | 7 | 25 | 1 | 1 | 2 | 1 | 1 | 1 | 20 | 9 | 48 | 946 | 502 | 1785 | 2 | 1 | 4 | |
| TNF-α | 127 | 74 | 218 | 107 | 50 | 231 | 5 | 3 | 6 | 2 | 1 | 4 | 1 | 1 | 1 | 12 | 9 | 15 | 6 | 2 | 23 | 3 | 2 | 5 | |
Fig. 1.
Analysis of IL-2, IFN γ, TNFα and IL-6 release obtained with PBMC-SP assay. Means of responses of log10 data are reported for each participant (4 participants, 35 donors, time 24 h; 3 participants, 26 donors, time 48 h). All positive controls showed significant higher cytokine levels in comparison to their respective isotype controls in PBMC-SP (p ≤ 0.003).
Fig. 2.
Analysis of IL-2, IFN γ, TNFα and IL-6 release obtained with WB-AQ assay. Means of responses of log10 data are reported for each participant (6 participants, 54 donors, time 24 h; 1 participant, 8 donors, time 48 h). p values for the comparison of each positive control with its isotype control are reported in supplementarytable 2.
The two main CRA methods used by the participants were the PBMC-SP (7 participants) (Fig. 1) and WB-AQ (7 participants) (Fig. 2). Variance analysis was only performed with these two assays as the number of participants for the remaining CRA platforms was too limited. These two CRA were performed with either 24 h (PBMC-SP, 4 participants; WB-AQ, 6 participants) or 48 h (PBMC-SP, 3 participants; WB-AQ, 1 participant) incubation periods. A statistical analysis of the global data (including all participants at a specific time point) showed no significant differences in cytokine production between the two different time points of 24 h and 48 h used by the participants which may be due to the large inter-laboratory variability of each CRA platform (Table 5) and/or the more limited dataset at 48 h.
Table 5.
Variance analysis of PBMC assay, SP presentation, combined 24 & 48 h time points (7 participants, 61 donors) and WB assay at 24 h time point (6 participants, 54 donors). Individual laboratory means of responses of log10 data were calculated and the absolute difference between these and the median across all laboratory means was calculated for each lab within each response and treatment method. A mixed effects model was used to determine intra-laboratory and inter-laboratory variance components. Values from IL6 lab I (outlier’s values) were excluded from the calculation.
| Variance Components |
|||||
|---|---|---|---|---|---|
| PBMC-SP (24 h & 48 h) |
WB-AQ (24 h) |
||||
| Treatment | Cytokine | Intra-Lab (%) | Inter-Lab (%) | Intra-Lab (%) | Inter-Lab (%) |
| CD28-SA | IFN- γ | 29 | 71 | 35 | 65 |
| IL-2 | 12 | 88 | 62 | 38 | |
| IL-6 | 33 | 67 | 38 | 62 | |
| TNF-α | 18 | 82 | 39 | 61 | |
| CD3 | IFN- γ | 38 | 62 | 80 | 20 |
| IL-2 | 22 | 78 | 100 | 0 | |
| IL-6 | 26 | 74 | 48 | 52 | |
| TNF-α | 37 | 63 | 65 | 35 | |
| CD52 | IFN- γ | 63 | 37 | 59 | 41 |
| IL-2 | 40 | 60 | 83 | 17 | |
| IL-6 | 43 | 57 | 45 | 55 | |
| TNF-α | 40 | 60 | 74 | 26 | |
| IgG1 | IFN- γ | 80 | 20 | 75 | 25 |
| IL-2 | 50 | 50 | 86 | 14 | |
| IL-6 | 55 | 45 | 37 | 63 | |
| TNF-α | 40 | 60 | 44 | 56 | |
| IgG2a | IFN- γ | 47 | 53 | 84 | 16 |
| IL-2 | 44 | 56 | 81 | 19 | |
| IL-6 | 72 | 28 | 43 | 57 | |
| TNF-α | 30 | 70 | 57 | 43 | |
| IgG4 | IFN- γ | 50 | 50 | 81 | 19 |
| IL-2 | 44 | 56 | 100 | 0 | |
| IL-6 | 79 | 21 | 35 | 65 | |
| TNF-α | 32 | 68 | 41 | 59 | |
Intra- and inter-laboratory variabilities for cytokine production in response to the three positive and isotype control mAbs in PBMC-SP and WB-AQ are reported in Table 5. For the PBMC-SP platform, inter-laboratory variation in cytokine concentrations measured in response to the tested mAbs was generally higher in comparison to intra-laboratory variation. For example, for the positive control mAbs, the majority of the inter-laboratory variance values were ≥ 50% while the majority of the intra-laboratory variance values were ≤ 50% (Table 5). This could be explained by inter-laboratory differences in protocols for antibody immobilisation in the wells (eg wet vs dry coat) and/or the material and surface coating of different types of plates used (polypropylene vs polystyrene) [26]. The WB-AQ platform showed lower inter-laboratory variance values than the PBMC-SP assay, but generally had higher intra-laboratory variation with majority of variance values ≥ 50%. This may reflect the minimal laboratory manipulation with the WB-AQ CRA but the high variability between individual blood donors. High inter- and intra-variability with the negative control mAbs may reflect the very low cytokine levels in these wells in both assays.
3.2. Ranking of the positive controls
Fold differences in cytokine release between positive control and its isotype were calculated for the different cytokine release assay platforms. Results corresponding to the SP or AQ CRA platforms are reported in Table 6, Table 7, respectively. Each participant CRA was colour graded from green (lower cytokine level) to red (higher cytokine level) to highlight the highest cytokines induced by the three positive controls in the assay. The averages for all similar formats (ALL-SP and WB-AQ) are also reported.
Table 6.
Fold increase in cytokine release between positive controls and their respective isotypes in solid phase assays. Individual laboratory means (log10 data) were calculated for each response and treatment. Fold increase shown were derived from the antilog of the differences in log10 data between positive and isotype control. The column All-SP represents the average fold increase for the 8 PBMC-SP assays. Mean results (log10 data) of all eight assays were calculated for each response and treatment method. Fold increase shown were derived from the antilog of the differences in log10 data between positive and isotype control. Each participant CRA was colour graded from green (lower cytokine level) to red (higher cytokine level) to highlight the highest cytokines induced by the three positive controls in the assay. Results from statistical analyses (p values) are reported in Supplementary Table 2.
 |
Table 7.
Fold increase in cytokine release between positive controls and their respective isotypes in aqueous phase assays. Individual laboratory means (log10 data) were calculated for each response and treatment method. Fold increase shown were derived from the antilog of the differences in log10 data between positive and isotype control. The column All-WB-AQ represents the average fold increase for the 7 WB-AQ assays. Each participant CRA was colour graded from green (lower cytokine level) to red (higher cytokine level) to highlight the highest cytokines induced by the three positive controls in the assay. Results from statistical analyses (p values) are reported in Supplementary Table 2.
 |
Despite differences in fold induction of cytokines between the different SP CRA, a similar profile of cytokine release was obtained for each positive control in all CRA with immobilised mAb presentation (PBMC-SP and diluted WB-SP) (Table 6). Anti-CD28 SA mAb is a strong inducer of IL-2 (35–2796-fold increase with respect to IgG4 isotype control) and IFN- γ (9–23374-fold increase with respect to IgG4 isotype control) when presented in SP CRA at all tested time points. Anti–CD3 mAb is a strong inducer of IFN- γ (617–24493-fold increase with respect to IgG2a isotype control) and TNF-α (7-877-fold increase with respect to IgG2a isotype control) (Table 6), while anti-CD52 mAb is a strong inducer of IFN- γ (25–903903-fold increase with respect to IgG1 isotype control) (Table 6). Diluted WB-SP assay gave a similar profile of cytokine induction as the PBMC-SP in presence of anti-CD28SA with a strong induction of IFN- γ (15180-fold increase with respect to IgG4 isotype control), IL-2 (147-fold increase with respect to IgG4 isotype control) and TNF-α (226-fold increase with respect to IgG4 isotype control). IFN- γ (759-fold increase with respect to IgG2a isotype control) was also strongly induced in the diluted WB-SP in presence of anti-CD3 and anti-CD52. Interestingly, the production of TNF-α in response to either anti-CD28 SA or anti-CD3 mAbs was highest at 48 h in comparison to 24 h in the SP PBMC platform.
The positive control mAbs demonstrated different cytokine release patterns in the various AQ CRA platforms with an overall lower magnitude of cytokine production in comparison to the SP CRA platforms (Table 7). All the WB-AQ assays gave similar profiles of cytokine induction at 24 h or 48 h with the strongest induction of IFN- γ in response to anti-CD52 mAb (28–215-fold increase respect to IgG1 isotype control) (Table 7) as previously described [12]. A different profile of cytokine release was observed with diluted WB-AQ assay, PBL/HUVEC-AQ and PBMC-AQ where the highest cytokine responses were stimulated with anti-CD3 mAb relative to anti-CD28SA and anti-CD52. In the diluted WB assay, anti-CD28SA stimulated no response. In the PBL/HUVEC co-culture assay, the response to anti-CD3 was restricted to IFNγ (10-fold increase respect to IgG2a isotype control) and IL-6 (99-fold increase respect to IgG2a isotype control) and the response to anti-CD28SA was dominated by IL-2 (10-fold induction).
In SP CRA platforms, responses to all three-positive control mAbs were dominated by IFN- γ (120–3858-fold). Anti-CD28SA and anti-CD3 mAbs are respectively the strongest inducers of IL-2 (239-fold) or TNF-α (105-fold) in all the SP CRA. In this CRA platform, the three-positive control mAbs could be ranked as anti-CD28SA > anti-CD3 > anti-CD52 in terms of relative potency to stimulate IL-2 release and ranked as anti-CD3 > anti-CD28SA > anti-CD52 for their relative potency to stimulate TNF-α and IFNγ. The ranking of the different positive controls in AQ CRA varies with the different assays. In WB-AQ assays, responses to anti-CD52 and anti-CD3 mAbs are dominated by IFN- γ.Anti-CD52 mAb is the strongest inducer of IFN- γ (86-fold) and IL-6 (18-fold). Contrary to SP CRA, anti-CD28SA mAb stimulates an equivalent IL-2 and IFNγ responses in WB-AQ CRA. The three-positive control mAbs could be ranked as anti-CD52 > anti-CD3 > anti-CD28SA in the WB-AQ CRA in terms of relative potency to stimulate IFN- γ release. Anti-CD3 induced high levels of IFN- γ in dWB-AQ (130-fold) and in PBMC-AQ (3482-fold) and the three-positive control mAbs could be ranked anti-CD3 > anti-CD52 > anti-CD28SA in terms of relative potency to stimulate IFN- γ release in these two platforms.
4. Discussion
Numerous CRA platforms [6] have been designed for hazard identification of potential CRS; this reflects the optimization of CRA assay design to address the needs of each laboratory, including the mechanism of action of therapeutic candidates and the presence of the target antigen(s) on particular cell types [6], [8], [9]. However, confirmation of the predictive potential of a CRA platform is critical to ensure the reliability of the results. Therefore, the use of appropriate and readily available positive and negative control mAbs in a variety of CRA platforms could enable greater confidence in CRA platform results.
This paper presents the results from the evaluation of a panel of three recombinant mAbs (anti-CD52 mAb, anti-CD3 mAb and anti-CD28SA) and their three respective isotype controls (IgG1, IgG2a and IgG4) in six different CRA platforms. As shown in Table 4, the negative control antibodies mainly induced low levels of cytokines in comparison to the positive control antibodies in the tested CRA platforms. Background levels of cytokines were higher for some cytokines in particular CRA platforms. For example, the upper 95% confidence interval of the geographic mean for IgG1 was > 100 pg/mL for TNFα in the PBMC-SP (24 and 48 hr), IFNγ in the PBMC-SP (48 hr) and PBL/HUVEC, and IL-6 in the PBL/HUVEC. However, as shown in Table 6, Table 7, the fold increase in cytokine release between anti-CD52 mAb and IgG1 was > 10 in 3 of 4 participants for TNFα and 4 of 4 participants for IFNγ in the PBMC-SP (24 and 48 hr). Only in the PBL/HUVEC assay was there no detectable average fold increase in any of the tested cytokines in response to anti-CD52 mAb vs IgG1 control. For some participants, low cytokine release was observed to both the positive and negative control antibodies. For example, Participant I had IL-6 and TNFα levels in a PBMC-SP assay which were higher in response IgG4 than to anti-CD28-SA (Table 6). It is important to note that the values presented in Table 4, Table 6, Table 7 are aggregate data for multiple donors for each participant. Therefore, it is possible that there were detectable fold-differences for individual donors between the positive and negative control antibodies or that relatively high cytokine release in responses to a negative control mAb was skewed by one or more donors for some participants. Each positive control mAb, tested at an optimal concentration for each CRA platform (Supplementary Table 1), induced cytokine release in all CRA tested. Higher cytokine levels were detected when the positive controls mAbs were presented in solid phase than in aqueous phase. Each positive control mAb induced a similar profile of cytokines in all the solid phase CRA tested (PBMC-SP and diluted WB-SP). In the immobilised condition, the three-positive control mAbs could be ranked as anti-CD28SA > anti-CD3 > anti-CD52 in terms of relative potency to stimulate IL-2 release. Anti-CD28 SA mAb in an aqueous phase also induced the most IL-2 production in the PBL/HUVEC co-culture CRA platform in comparison to anti-CD3 and anti-CD52 mAbs. In WB-AQ, the three positive controls could be ranked as anti-CD52 > anti-CD3 > anti-CD28SA in terms of relative potency to stimulate IFN- γ release. with anti-CD52 being the strongest inducer of IFN- γ. as previously described [12], [32], [33]. In this study, the cytokine concentrations detected correspond to the single 5 μg/ml or 0.25–1 μg/well concentration of the positive control mAbs analysed at NIBSC and thus the rankings could possibly shift at lower or higher concentration of mAbs. It is important to note that the goal of this manuscript was to assess the performance of a newly developed panel of control recombinant antibodies for cytokine release assays and not the evaluation of the sensitivity and frequency of responses in the 6 CRA platforms used in this study. In a view of providing the reader with some information on the level of cytokine release expected from the panel of control antibodies in a specific type of assay, all statistical analyses were performed with the donor as a random factor. This approach is statistically less powerful than a paired analysis and some potentially significant differences between positive and negative controls may have been lost. Each cytokine release assay has its own sensitivity for a specific type of antibody, and it is important to know the strength and weakness of an assay. The use of different types of assays is also crucial to cover the mechanism of action of a specific antibody for hazard identification.
These results confirmed the expected behaviours and utility of the three-positive control mAbs in the different CRA platforms. Each CRA platform varies with respect to the cellular components, explaining their different sensitivity for a specific antibody. For mAb therapeutics that target T cells and/or where target clustering is a potentially relevant mechanism of action, PBMC may provide a more sensitive response than WB [8]. WB contains 40–75% of neutrophils and a high concentration of red blood cells (RBC), cells that are depleted during the preparation of the PBMC. It has been previously demonstrated that the presence of glycophorin A on red blood cells can interact with IL-2 and inhibit IL-2-dependent T cell proliferation [34], explaining the poor sensitivity of undiluted WB assay for OKT3 or TGN1412-associated cytokine release [24]. Dilution of WB yielding to the reduction of RBC can restore the sensitivity to anti-CD28SA as shown in diluted WB-SP. It has been previously shown that the response to TGN1412 was dependent of FcγR cross-linking, which can be artificially simulated by SP presentation [5], [25] or be induced by cell contact-dependent priming during high density pre-culture of PBMC in the presence of CD32-expressing cells [35], [36]. The lack of cell contact priming and limited FcγR expressing cells in PBMC-AQ or dWB-AQ CRA may in part explain the poor sensitivity of these two assays for detection of anti-CD28SA mediated cytokine release. T cell responses can also be inhibited by the presence of neutrophils [37] explaining the better sensitivity of dWB-AQ and PBMC-AQ for anti-CD3 in comparison with a rich neutrophil preparation like in WB-AQ or PBL/HUVEC . However, FcγR + neutrophils are important for anti-CD52-mediated cytokine release response and WB-AQ proved to be a much more sensitive method for the detection of neutrophil-mediated cytokine release than diluted WB-AQ [24], [32], [33]. In addition to the influence of the various cell types present, CRA performance with an exposure to a test antibody may be dependent on the maturation state of the responding cell. For example, high IL-2 induction by a CD28 SA mAb may reflect preference for stimulation of central memory T cells [14].
The three positive controls chosen and evaluated in this study have different mechanisms of action and therefore induced cytokine release from different type of immune cells. The performance of the positive control mAbs in the various CRA formats support the hypothesis that no single CRA platform is optimal for every mAb with CRS potential. Within the limitations of the analyses performed, the three positive controls induced cytokine levels above those with the negative control mAbs in the tested CRA platforms, with the exception of anti-CD52 in the PBL/HUVEC platform. However, the relative ability of each positive control mAb to induce either IL-2, TNF-α, IL-6, or IFN-γ varied with each assay platform. Therefore, careful understanding of the mechanism of action of a test antibody is critical for appropriate choice of a CRA platform to identify potential CRA risk. Furthermore, these results emphasize the value of the use of multiple positive and negative control mAbs for the appropriate qualification of a CRA. The development of other controls with a different mechanism of action maybe required in the future to capture for example cytokine release from innate cells.
5. Conclusion
The results of this study confirm that anti-CD3, anti-CD52, and anti-CD28SA mAbs produced by NIBSC induced the release of the pro-inflammatory cytokines IFN-γ, TNFα, IL-2 and IL-6 in a variety of CRA platforms, replicating previously published data generated by the corresponding clinical therapeutic mAbs (ie muromonab, alemtuzumab, and theralizumab) in such assays. Therefore, this panel of antibodies and the isotype control mAbs are suitable for use as positive and negative control antibodies for the qualification and validation of CRAs, comparison of different CRAs (eg solid vs aqueous phase), and intra- and inter-laboratory comparison of CRA performance. Thus, the use of this panel of positive and negative control mAbs will provide information on the reproducibility, robustness and potential limitations of a CRA platform.
CRediT authorship contribution statement
Sandrine Vessillier: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Madeline Fort: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Lynn O’Donnell: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Heather Hinton: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Kimberly Nadwodny: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Joseph Piccotti: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Peter Rigsby: Data curation, Formal analysis, Software, Writing - review & editing. Karin Staflin: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Richard Stebbings: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Divya Mekala: Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing - original draft, Writing - review & editing. Aarron Willingham: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Babette Wolf: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. : .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank Chris Ball for the preparation of the anti-CD28SA, Heather Chambers for technical assistance with the HPLC characterisation, Janet Sutherland for the endotoxin quantification, the Standard processing Division for the preparation of the freeze-dried materials and dispatching the reference reagents. The authors gratefully acknowledge the input from the government, academic, and industry scientists of the HESI ITC Technical Committee for their contributions to this work. We would like to thank Stan Parish for organising the different teleconferences to discuss this standardisation project. This work was funded, in part, by the UK Department of Health’s Policy Research Programme, Grant Number 044/0069. This paper is based on independent research commissioned and funded by the NIHR Policy Research Programme (NIBSC Regulatory Science Research Unit). The views expressed in the publication are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health, ‘arms’ length bodies or other government departments.”
Informed Consent
The human biological samples were sourced ethically, and their research use was in accord with the terms of the informed consents under an IRB/EC approved protocol within each individual laboratory’s organization.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cytox.2020.100042.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Comparison between participant’s analysis (in house) and the centralised analysis performed at NIBSC with MSD platform (PBMC or SP assays). Participant code and analysis methodology are indicated in brackets.
Supplementary figure 2.
Comparison between participant’s analysis (in house) and the centralised analysis performed at NIBSC with Mesoscale platform (WB or AQ assays) (WB assays). Participant code and analysis methodology are indicated in brackets
References
- 1.Bugelski P.J., Achuthanandam R., Capocasale R.J., Treacy G., Bouman-Thio E. Monoclonal antibody-induced cytokine-release syndrome. Expert Rev. Clin. Immunol. 2009;5(5):499–521. doi: 10.1586/eci.09.31. [DOI] [PubMed] [Google Scholar]
- 2.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. ImmunoTherapy Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suntharalingam G., Perry M.R., Ward S., Brett S.J., Castello-Cortes A., Brunner M.D., Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355(10):1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 4.Attarwala H. TGN1412: From Discovery to Disaster. J Young Pharm. 2010;2(3):332–336. doi: 10.4103/0975-1483.66810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.R. Stebbings, L. Findlay, C. Edwards, D. Eastwood, C. Bird, D. North, Y. Mistry, P. Dilger, E. Liefooghe, I. Cludts, B. Fox, G. Tarrant, J. Robinson, T. Meager, C. Dolman, S.J. Thorpe, A. Bristow, M. Wadhwa, R. Thorpe, S. Poole, “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics, Journal of immunology (Baltimore, Md. : 1950) 179(5) (2007) 3325-31. https://doi.org/10.4049/jimmunol.179.5.3325. [DOI] [PubMed]
- 6.Finco D., Grimaldi C., Fort M., Walker M., Kiessling A., Wolf B., Salcedo T., Faggioni R., Schneider A., Ibraghimov A., Scesney S., Serna D., Prell R., Stebbings R., Narayanan P.K. Cytokine release assays: Current practices and future directions. Cytokine. 2014;66(2):143–155. doi: 10.1016/j.cyto.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Grimaldi C., Finco D., Fort M.M., Gliddon D., Harper K., Helms W.S., Mitchell J.A., O'Lone R., Parish S.T., Piche M.S., Reed D.M., Reichmann G., Ryan P.C., Stebbings R., Walker M. Cytokine release: A workshop proceedings on the state-of-the-science, current challenges and future directions. Cytokine. 2016;85:101–108. doi: 10.1016/j.cyto.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Brennan F.R., Kiessling A. In vitro assays supporting the safety assessment of immunomodulatory monoclonal antibodies. Toxicol. In Vitro. 2017;45(Pt 3):296–308. doi: 10.1016/j.tiv.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher E.A.K., Eltahir M., Lindqvist F., Rieth J., Tornqvist G., Leja-Jarblad J., Mangsbo S.M. Extracorporeal human whole blood in motion, as a tool to predict first-infusion reactions and mechanism-of-action of immunotherapeutics. Int. Immunopharmacol. 2018;54:1–11. doi: 10.1016/j.intimp.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Chatenoud L., Ferran C., Legendre C., Thouard I., Merite S., Reuter A., Gevaert Y., Kreis H., Franchimont P., Bach J.F. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids, Transplantation. 1990;49(4):697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Suthanthiran M., Fotino M., Riggio R.R., Cheigh J.S., Stenzel K.H. OKT3-associated adverse reactions: mechanistic basis and therapeutic options. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1989;14(5 Suppl 2):39–44. [PubMed] [Google Scholar]
- 12.Wing M.G., Moreau T., Greenwood J., Smith R.M., Hale G., Isaacs J., Waldmann H., Lachmann P.J., Compston A. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J. Clin. Investig. 1996;98(12):2819–2826. doi: 10.1172/JCI119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung C.H. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist. 2008;13(6):725–732. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood D., Findlay L., Poole S., Bird C., Wadhwa M., Moore M., Burns C., Thorpe R., Stebbings R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br. J. Pharmacol. 2010;161(3):512–526. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gribble E.J., Sivakumar P.V., Ponce R.A., Hughes S.D. Toxicity as a result of immunostimulation by biologics. Expert Opin. Drug Metab. Toxicol. 2007;3(2):209–234. doi: 10.1517/17425255.3.2.209. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein G., Norman D.J., Henell K.R., Smith I.L. Pharmacokinetic study of orthoclone OKT3 serum levels during treatment of acute renal allograft rejection. Transplantation. 1988;46(4):587–589. doi: 10.1097/00007890-198810000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 18.Kung P., Goldstein G., Reinherz E.L., Schlossman S.F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science (New York, N.Y.) 1979;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- 19.Luhder F., Huang Y., Dennehy K.M., Guntermann C., Muller I., Winkler E., Kerkau T., Ikemizu S., Davis S.J., Hanke T., Hunig T. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J. Experim. Med. 2003;197(8):955–966. doi: 10.1084/jem.20021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.C. Ball, B. Fox, S. Hufton, G. Sharp, S. Poole, R. Stebbings, D. Eastwood, L. Findlay, P.W. Parren, R. Thorpe, A. Bristow, S.J. Thorpe, Antibody C region influences TGN1412-like functional activity in vitro, Journal of immunology (Baltimore, Md. : 1950) 189(12) (2012) 5831-40. https://doi.org/10.4049/jimmunol.1201795. [DOI] [PubMed]
- 21.Matejtschuk P., Rafiq S., Johnes S., Gaines Das R. A comparison of vials with ampoules for the storage of biological reference materials. Biolog. J. Int. Assoc. Biolog. Standardization. 2005;33(2):63–70. doi: 10.1016/j.biologicals.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.WHO, Recommendations for the preparation, characterization and establishment of international and other biological reference standards (revised 2004), WHO Technical Report Series (932) (2006) 73-131.
- 23.J. Vidal, T. Kawabata, R. Thorpe, B. silva lima, K. Cederbrant, S. Poole, J. Müller-Berghaus, M. Pallardy, J. Laan, In vitro cytokine release assays for predicting cytokine release syndrome: The current state-of-the-science. Report of a European Medicines Agency Workshop, Cytokine 51 (2010) 213-5. https://doi.org/10.1016/j.cyto.2010.04.008. [DOI] [PubMed]
- 24.Vessillier S., Eastwood D., Fox B., Sathish J., Sethu S., Dougall T., Thorpe S.J., Thorpe R., Stebbings R. Cytokine release assays for the prediction of therapeutic mAb safety in first-in man trials–Whole blood cytokine release assays are poorly predictive for TGN1412 cytokine storm. J. Immunolog. Meth. 2015;424:43–52. doi: 10.1016/j.jim.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eastwood D., Bird C., Dilger P., Hockley J., Findlay L., Poole S., Thorpe S.J., Wadhwa M., Thorpe R., Stebbings R. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. British J. Clin. Pharmacol. 2013;76(2):299–315. doi: 10.1111/bcp.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Findlay L., Eastwood D., Stebbings R., Sharp G., Mistry Y., Ball C., Hood J., Thorpe R., Poole S. Improved in vitro methods to predict the in vivo toxicity in man of therapeutic monoclonal antibodies including TGN1412. J. Immunolog. Meth. 2010;352(1):1–12. doi: 10.1016/j.jim.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Wolf B., Morgan H., Krieg J., Gani Z., Milicov A., Warncke M., Brennan F., Jones S., Sims J., Kiessling A. A whole blood in vitro cytokine release assay with aqueous monoclonal antibody presentation for the prediction of therapeutic protein induced cytokine release syndrome in humans. Cytokine. 2012;60(3):828–837. doi: 10.1016/j.cyto.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Bailey L., Moreno L., Manigold T., Krasniqi S., Kropshofer H., Hinton H., Singer T., Suter L., Hansel T.T., Mitchell J.A. A simple whole blood bioassay detects cytokine responses to anti-CD28SA and anti-CD52 antibodies. J. Pharmacolog. Toxicolog. Meth. 2013;68(2):231–239. doi: 10.1016/j.vascn.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Dhir V., Fort M., Mahmood A., Higbee R., Warren W., Narayanan P., Wittman V. A predictive biomimetic model of cytokine release induced by TGN1412 and other therapeutic monoclonal antibodies. J. Immunotoxicol. 2012;9(1):34–42. doi: 10.3109/1547691X.2011.613419. [DOI] [PubMed] [Google Scholar]
- 30.Walker M.R., Makropoulos D.A., Achuthanandam R., Van Arsdell S., Bugelski P.J. Development of a human whole blood assay for prediction of cytokine release similar to anti-CD28 superagonists using multiplex cytokine and hierarchical cluster analysis. Int. Immunopharmacol. 2011;11(11):1697–1705. doi: 10.1016/j.intimp.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.M.D. Moody, S.W. Van Arsdell, K.P. Murphy, S.F. Orencole, C. Burns, Array-based ELISAs for high-throughput analysis of human cytokines, Biotechniques 31(1) (2001) 186-90, 192-4. https://doi.org/10.2144/01311dd03. [DOI] [PubMed]
- 32.Alakhras N.S., Qiu J., Rocha G.V., Witcher D.R., Koester A., You J., Schaer D.A., Holmgaard R.B., Driscoll K., Willy J.A., Malherbe L.P. FcgammaRIIIa-dependent IFN-gamma release in whole blood assay is predictive of therapeutic IgG1 antibodies safety. MAbs. 2018;10(6):913–921. doi: 10.1080/19420862.2018.1474996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussain K., Hargreaves C.E., Rowley T.F., Sopp J.M., Latham K.V., Bhatta P., Sherington J., Cutler R.M., Humphreys D.P., Glennie M.J., Strefford J.C., Cragg M.S. Impact of Human FcgammaR Gene Polymorphisms on IgG-Triggered Cytokine Release: Critical Importance of Cell Assay Format. Front. Immunol. 2019;10:390. doi: 10.3389/fimmu.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu J.W., Sharom F.J. Glycophorin A interacts with interleukin-2 and inhibits interleukin-2-dependent T-lymphocyte proliferation. Cell. Immunol. 1992;145(2):223–239. doi: 10.1016/0008-8749(92)90327-l. [DOI] [PubMed] [Google Scholar]
- 35.P. Bartholomaeus, L.Y. Semmler, T. Bukur, V. Boisguerin, P.S. Romer, P. Tabares, S. Chuvpilo, D.Y. Tyrsin, A. Matskevich, H. Hengel, J. Castle, T. Hunig, U. Kalinke, Cell contact-dependent priming and Fc interaction with CD32+ immune cells contribute to the TGN1412-triggered cytokine response, Journal of immunology (Baltimore, Md. : 1950) 192(5) (2014) 2091-8. https://doi.org/10.4049/jimmunol.1302461. [DOI] [PubMed]
- 36.Romer P.S., Berr S., Avota E., Na S.Y., Battaglia M., ten Berge I., Einsele H., Hunig T. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood. 2011;118(26):6772–6782. doi: 10.1182/blood-2010-12-319780. [DOI] [PubMed] [Google Scholar]
- 37.McKenna K.C., Beatty K.M., Vicetti Miguel R., Bilonick R.A. Delayed processing of blood increases the frequency of activated CD11b+ CD15+ granulocytes which inhibit T cell function. J. Immunol. Methods. 2009;341(1–2):68–75. doi: 10.1016/j.jim.2008.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.